Abstract
Metal-chelating noncanonical amino acids (ncAAs) are uniquely functional building blocks for proteins, peptide catalysts, and small molecule sensors. However, catalytic asymmetric approaches to synthesizing these molecules are hindered by their functional group variability and intrinsic propensity to ligate metals. In particular, bipyridyl-L-alanine (BpyAla) is a highly sought ncAA, but its complex, inefficient syntheses have limited utility. Here, we develop a chemoenzymatic approach to efficiently construct BpyAla. Three enzymes that can be produced in high titer together react to convert Gly and an aldehyde into the corresponding β-hydroxy ncAA, which is subsequently deoxygenated. We explore approaches to synthesizing biaryl aldehydes and show how the three-enzymatic cascade can access a range of α-amino acids with bulky side chains, including a variety of metal-chelating amino acids. We show that newly accessible BpyAla analogues are compatible with existing amber suppression technology, which will enable future merging of traditional synthetic and biosynthetic approaches to tuning metal reactivity.
Keywords: Biocatalysis, Genetic code expansion, Noncanonical amino acid, Pyridoxal phosphate
1. Introduction
Metal-chelating amino acids have a wide variety of applications, including protein assembly, metal sensors, and catalysis.[1–6] Of these metal-chelating noncanonical amino acids (ncAAs), the 2,2′-bipyridyl alanine (BpyAla) is preeminent. This side chain can bind a variety of metals, and the twisted bite angle of the two pyridine rings promotes a variety of highly sought catalytic transformations.[7] Synthetic biology efforts with BpyAla have been facilitated by the high-quality aminoacyl-tRNA synthetase/tRNA pairs, which allow for incorporation through amber stop codon suppression (Figure 1).[8–9] Artificial metalloenzymes featuring BpyAla have existed for over a decade, and yet only a handful of transition metal reactions have been translated to the enzyme active site.[10–14] The limited implementation of BpyAla sharply contrasts 2,2′ bipyridine’s vast utility in organic synthesis. One recurring obstacle to the application of BpyAla is that previous syntheses are multi-step, low-yielding, and sufficiently tedious so as to limit implementation. Were a more streamlined methodology available to access BpyAla and related metal-chelating amino acids, research groups could more easily translate the immense reactivity space afforded by transition metals into protein active sites.
Figure 1.
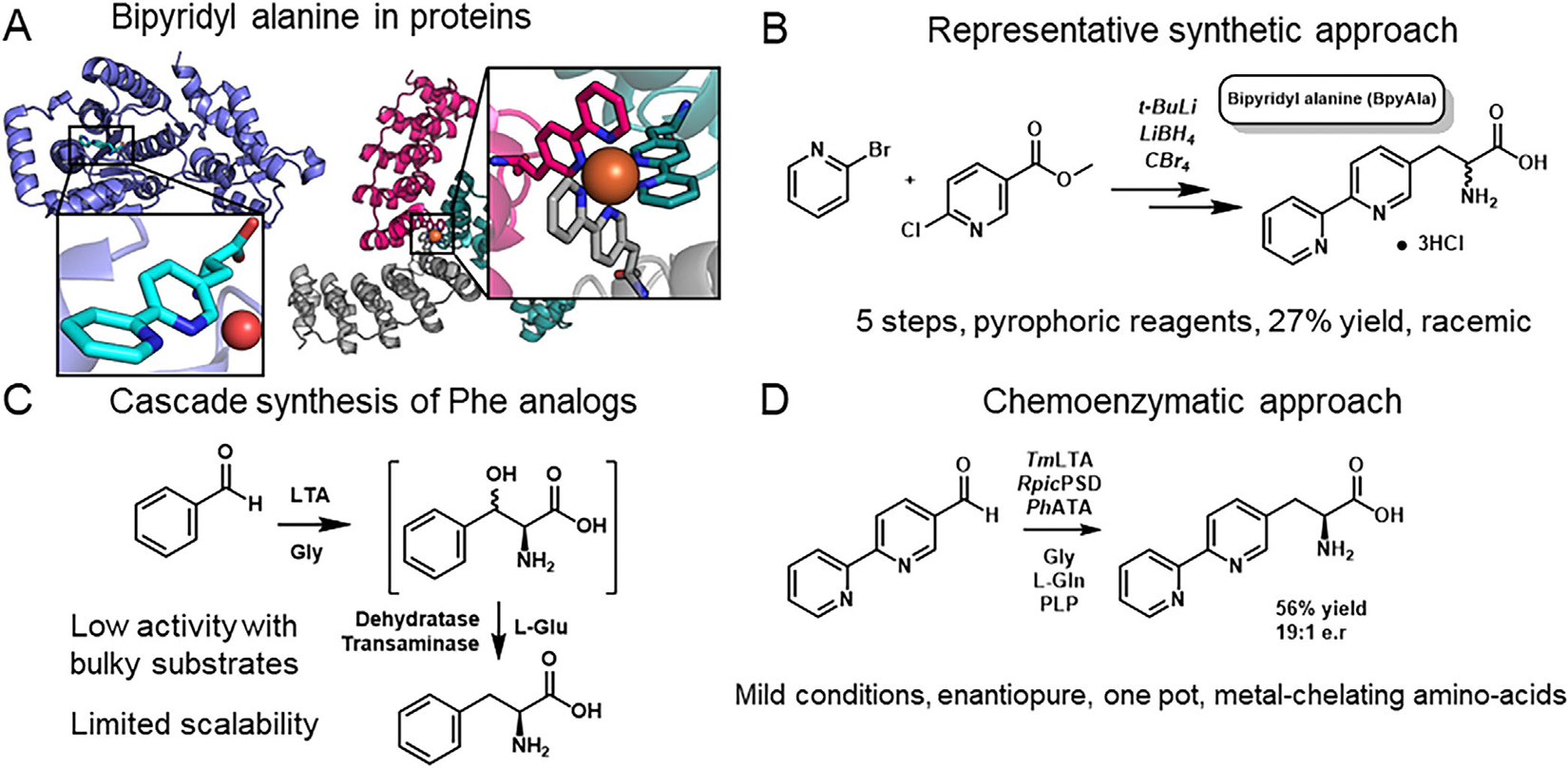
Overview of bipyridyl alanine and previous synthetic approaches. (A) Structure of l-BpyAla and proteins containing this unique metal-chelating moiety. PDB ID right: 2PXH and left: 5EIL. (B) Previous synthetic route deployed by Xie et al.[9] (C) Synthetic biology route to in vivo production of amino acids by Song et al.[24] (D) Strategy proposed here.
The current approach to noncanonical amino acid incorporation involves supplementing a modest concentration (typically 1 mM) of the desired ncAA to media upon induction of the corresponding noncanonical tRNA synthetase.[8,9] For small-scale protein expressions <100 mL, traditional synthetic approaches, such as the Gabriel synthesis or Negishi coupling, are sufficient.[9,15–17] These syntheses require 4–5 steps, rely upon pyrophoric reagents, and tend to yield modest quantities of racemic amino acid.[9,16,18] The intense material demands of larger (>100 mL) protein expression cultures, which are essential for preparative-scale biocatalysis, make previous approaches to ncAA synthesis tedious, at best, and untenable at worst. We posit that these material limitations to the production of BpyAla underlie the current underrepresentation of novel genetically encoded metal-coordinating protein scaffolds.
An alternative approach to amino acid synthesis is to use enzymes. A variety of single-enzyme ncAA synthases have been developed in the past decade and have delivered a variety of ncAAs with good scale, yield, and selectivity.[19–23]
Crucially, when these enzymes afford access to aromatic amino acids, they are typically limited in size to substituted L-tryptophans and smaller aromatic rings. We recently reported two PLP-dependent aldolases that react with the bulky biphenylaldehyde to access β- and γ -hydroxy ncAAs.[25,26] The extension of reactivity to access undecorated BpyAla using a single enzyme is an attractive target but would require the development of new mechanisms. An alternative approach is to combine multiple enzymes together into a single cascade to access metal-chelating ncAAs.
Biocatalytic cascades leverage a series of coupled enzymatic reactions, which enables a one-pot reaction scheme.[27] Products of one reaction serve as substrates to the next, thus minimizing losses associated with workup and isolation. Further, a key advantage of biocatalytic cascades is the coupling of low yielding reactions to thermodynamically favorable reactions to drive yields through Le-Chatelier’s principle.[28] While none of the previous cascades have been demonstrated to access amino acids with the large steric profile of the BpyAla sidechain,[27] Song et. al made a major advance in the synthesis of unbranched ncAAs.[24] They identified aldehydes as an ideal staring material, as they are cheap, stable, and diverse analogues are available.[27] Aldol addition using a pyridoxal phosphate (PLP)-dependent l-threonine aldolase readily affords a β-hydroxy amino acid.[29] The β-hydroxy amino acid is then dehydrated with a PLP-dependent threonine dehydratase,[30] which catalyzes elimination of the hydroxy group to generate a dehydroamino acid that subsequently tautomerizes and hydrolyzes to yield an α-keto acid.[31] Last, the α-amine is re-introduced either by enzymatic reductive amination or transamination. The PLP-dependent transaminase relies on a variety of sacrificial amine donors to directly yield the desired α-amino acid product. This retrosynthetic strategy represents a significant conceptual advance in amino acid synthesis. However, the cascade was demonstrated with a modest set of aldehydes and none that had large bicyclic or biaryl side chains associated with privileged metal chelating motifs. Additionally, enzymes were deployed in a whole-cell context that required dosing substrate over 40 h.[27] Identification of enzymes that can operate with bulky, metal-chelating motifs on the scales required for practical use in heterologous expression remains an outstanding challenge.
Herein, we identify a set of complementary PLP-dependent enzymes that synthesize a variety of metal-chelating ncAAs in a one-pot tandem cascade reaction. Each enzyme can operate under synthetically demanding conditions and tolerate high concentrations of aldehyde (50 mM) as the side chain donor. As this synthesis proceeds without protecting groups, the crude cascade mixture could be used as a source of bipyridyl alanine without further purification for use in ribosomal translation using genetic code expansion technology. Additionally, some of the novel ncAAs showed promiscuity with the Bpy synthetase-tRNA system in vivo expanding the toolkit of metal ligands in proteins.
2. Results and Discussion
2.1. Synthesis of Bipyridylaldehyde
We began our chemoenzymatic pursuit of BpyAla with consideration of the corresponding [2,2′-bipyridine]-5-carbaldehyde (Bpy aldehyde). While this molecule is commercially available, the cost is relatively high, and use on the scale would benefit from ready access. Traditional approaches for synthesis of biaryl compounds, such as Suzuki–Miyora coupling, rely on aryl boronic acids.[32] However, 2-pyridyl boronic acids undergo rapid proto-deborylation, preventing this route.[33] We instead relied on a simple, albeit slow, one-step Stille coupling yielding the corresponding aldehyde in 60% isolated yield from readily available starting materials (see Supporting Information).[34] While this route is serviceable, the use of a neurotoxic organotin reagent leaves much to be desired. An alternative, less toxic route was explored leveraging cross-electrophile coupling. An acetal-protected 6-bromonicotinaldehyde and 2-pyridyl trifluoromethanesulfonate were coupled using Ni–Pd chemistry, affording 1 in 28% yield, providing a greener alternative synthetic route. With the aldehyde in hand, we investigated the ability of enzymes to engage this bulky aldehyde.
2.2. Identification of a β-Hydroxy BpyAla Synthase
Previous in vivo cascades utilized L-threonine aldolase (LTA) from Pseudomonas aeruginosa.[27] While this enzyme is highly stereoselective,[35] it has only modest thermal stability and expression in E. coli. To address these limitations, we considered two alternative enzymes. A homologous LTA from Thermatoga maritima (TmLTA) was selected for its exceptional thermal stability and high expression in E. coli. This enzyme was shown to have modest activity with nicotinaldehyde, but activity with large biaryl aldehydes was unknown.[36] We also considered an L-threonine transaldolase, ObiH, originally found in the obafluorin biosynthetic pathway in Pseudomonas fluorescens.[37,38] We and others have shown that ObiH has high expression titers in E. coli, albeit with comparatively moderate thermostability.[25,39–42] Crucially, ObiH was previously reported to react with a bulky biphenyl 4-carboxaldehyde, which has a similar steric profile to Bpy aldehyde.[43]
TmLTA and ObiH were heterologously expressed and purified to assess activity with the Bpy aldehyde. Analytical scale reactions were evaluated by LC-MS analysis, which indicated ObiH reacted to form β-hydroxy-BpyAla (Figure S1). We tested the impact of an acetaldehyde reduction system (NAD+, ammonium formate, alcohol dehydrogenase, and formate dehydrogenase) and assessed the impact on product formation via Marfey’s analysis.[42] In line with previous work,[44] we have observed a dramatic increase in product formation upon the addition of a two-enzyme system to reduce the acetaldehyde byproduct of the ObiH reaction. This reducing system (RS) provides a strong thermodynamic driving force and provides a kinetic benefit by preventing inhibition by acetaldehyde. When TmLTA was assayed under analogous conditions, it produced the corresponding β-hydroxy-ncAA, albeit in lower yield. Nevertheless, consideration of the next steps in the target cascade and prior literature on these families of enzymes strongly recommended the use of TmLTA for subsequent development.
The target cascade relies on a PLP-dependent dehydratase that could act on either Thr or β-hydroxy-BpyAla. Activity on Thr, however, would deplete starting material and generate α-keto butyrate, which would subsequently compete in the final transamination step (Figure 2). In this context, TmLTA has a unique advantage because it uses Gly as the donor amino acid, which avoids potential downstream incompatibilities (Figure 2). Dehydratases are also syn-stereoselective,[30] and each aldolase produces both isomers.[25,36] While the slow epimerization of ObiH is advantageous for stereoselective β-hydroxy-ncAA synthesis, it may interfere with the envisioned cascade. Based on these considerations, TmLTA was chosen for subsequent cascade development, and we next searched for β-hydroxy amino acid dehydratases that could operate under compatible reaction conditions.
Figure 2.

Selection of C─C bond-forming pyridoxyal-dependent aldolase. Both TmLTA and ObiH catalyzed the formation of β-hydroxy BpyAla with an appropriate amino acid donor. Addition of a reducing system (RS) to remove acetaldehyde from the ObiH reaction improved yields. The potential for formation of diverse shunt products from ObiH catalysis (purple) directed us to use TmLTA for subsequent work.
2.3. Cascade Synthesis of α-Keto Acids
Three dehydratases were evaluated for cascade reactivity with TmLTA. Two threonine deaminases, from Thermotoga maritima (TmTDA) and Thermus thermophilus (TtTDA), as well as a phenylserine dehydratase from Ralstonia pickettii (RpicPSD), were expressed in BL21 (DE3). No TmTDA was isolated; however, RpicPSD and TtTDA were expressed in high titer, >100 mg protein/L culture. These soluble dehydratases were subsequently screened in cascade with TmLTA using three equivalents of glycine. TtTDA was attractive because, as a thermostable enzyme, it has high activity on Thr at elevated temperatures (Figures 3 and S2).
Figure 3.

Dehydratase screening for formation of Bpy α-keto acid. Two dehydratases were tested in a cascade with TmLTA. TtPSD was screened at 65 °C, where threonine dehydration was maximized, while RpicPSD was screened at 37 °C. TtPSD was found to stall at Bpy β-hydroxy amino acid, while RpicPSD carried the reaction to near completion and high yield, 85%.
However, TtTDA had only trace activity on the bulky β-hydroxy BpyAla (Figure 3).[45] In contrast, cascade reactions of RpicPSD at 37 °C had nearly complete conversion of aldehyde, and BpyAla was formed with an analytical yield of 85% (Figure 3). RpicPSD was therefore selected for a brief reaction yield optimization. We found that product formation was relatively insensitive to catalyst loadings >1 mol % and that the equivalents of Gly could be reduced to two (Figure S3).
2.4. Identification and Optimization of a Cascade-Compatible Transaminase for BpyAla Synthesis
We pursued a transaminase approach to convert Bpy α-keto acid into the target BpyAla and searched recent literature on preparative scale biocatalysis with aromatic amino acid transaminases. Two exemplary candidates were identified, Pyrococcus horikoshii aromatic amino-acid transaminase (PhATA) and Thermus thermophilus aromatic amino-acid transaminase (TtATA). Each transaminase was tested in a cascade with TmLTA and RpicPSD on Bpy aldehyde. Transamination is often close to thermoneutral, relying on high concentrations of a sacrificial amino donor. We screened glutamine as a “smart” amine donor, whose corresponding α-keto acids undergoe subsequent cyclization that serves as a thermodynamic driving force for the reaction.[46]. Using Gln, TtATA was found to have high activity at low (0.025%) catalyst loading. However, PhATA was found to express at a 4-fold higher titer and was able to achieve comparable yields at 0.05% catalyst loading (Figure S4) and was therefore selected as the final enzyme in the cascade (Figure 4).
Figure 4.

Time course analysis of complete three-enzyme cascade. Interconversion of intermediates was observed for the full cascade across 24 h. Bpy aldehyde (1) was observed to rapidly deplete with no significant buildup of β-hydroxy amino acid. α-ketoacid (3) conversion to BpyAla (4) is the rate-limiting step of the cascade.
Optimization of catalyst loading and glutamine equivalents, along with a pH and temperature screen (Figure S5), resulted in a cascade that delivered the desired BpyAla in 66% assay yield. To better understand the limitations of this cascade, we conducted a time course over 24 h. At 6 h, all aldehydes had been consumed with only the corresponding Bpy α-keto acid and BpyAla mass peaks appearing. The reaction reaches equilibrium within 12 h with only α-keto acid remaining as an intermediate (Figure 4). These data, therefore, reveal that transaminase equilibrium as the bottleneck to be addressed in future studies. For the present work, the 56% yield using only three high-expressing PLP-dependent enzymes represents a substantial advance over previous methods, and we continued to preparative scale demonstrations of reactivity with Bpy aldehyde and other, related metal-chelating motifs.
2.5. Preparative Scale Synthesis of BpyAla
With a functional cascade in hand, we scaled up a set of representative ncAAs. Our first target, BpyAla, was produced on multimmole scale in moderate yield, 508 mg in 56% yield, with great enantioselectivity. Electronic and steric variations in the metal-chelating moiety of the amino-acid are of special interest, as tuning these factors leads to enhanced activity in a variety of synthetically useful transformations. A pyrazine analogue 2 of BpyAla was selected due to an additional nitrogen that, were it incorporated into a protein, could serve as a potential H-bond acceptor to tune electronic effects of the ligand. The pyrazole analogue 3 has a smaller terminal ring system that offers a wider bite angle for a potential metal. Deviating from a biraryl steric profile, we also considered an 8-hydroxy-quinoline motif, which was also readily accepted into the cascade. A phenanthroline and terpyridine side chain were explored; however, both motifs were poorly tolerated and primarily remained as the corresponding aldehydes with trace product formation using terpyridine (Figure S6). Several haloaryls were also generated as a potential common synthon for generating a large diversity of metal-chelating biaryl amino acids. All products were made with a >19:1 enantiomeric ratio (e.r.) ranging in low to good yields, 20%–70% (Figure 5). We additionally synthesize two nonmetal chelating ncAAs, for which tRNA-synthetase pairs were previously developed.[9,47] Biphenylalanine was produced in good yield, 73%, despite the low aqueous solubility of the starting aldehyde (<1 mg/mL). Reaction with m-nitro benzaldehyde yielded photoactive Phe analogue, albeit in low yield.
Figure 5.

Preparative-scale cascade synthesis of noncanonical amino acids.
2.6. Bpy Synthetase Promiscuity for Metal-Chelating Amino Acids
We closed our exploration of metal-chelating ncAAs by probing the ability of these new BpyAla analogues to interface with existing amber suppression technology.[8,9] As a readout of ncAA incorporation, a point mutant of sfGFP was generated at position D190 with an amber-stop-codon, D190*. Induction of sfGFP(190*) in the absence of ncAA yielded little to no fluorescence due to early termination during translation. In agreement with previous works, the introduction of BpyAla to the medium along with the induction of the corresponding BpyAla tRNA/synthetase leads to the readthrough of full sfGFP and subsequent fluorescence. The fluorescence counts of the sfGFP incorporating BpyAla closely matched the wild-type sfGFP fluorescence, indicating high levels of BpyAla incorporation (Figure 6). We screened the BpyRS/tRNA pair with two BpyAla analogues, 2 and 3, and found that we were able to incorporate them into sfGFP, albeit to a lesser extent (Figure 6). Mass spectrometry analysis of sfGFP(190*) containing 1, 2, and 3 shows peaks corresponding to the expected masses of sfGFP with the initiator methionine removed (Figures S7–S9). By simply adopting previously developed technology, we were able to integrate new metal-chelating ncAAs into ribosomal translation. The ease of our biocatalytic method opens new avenues for varying the electronic and geometric features at the metal sites of artificial metalloproteins.
Figure 6.

Amber suppression with new ncAAs bearing metal-chelating motifs. Fluorescence of superfolder GFP (sfGFP) with the ncAA BpyA incorporated at position 190. In the absence of amino acids, no fluorescence is observed. In the presence of BpyAla (1), fluorescence is restored. Weaker, but observable amber suppression was also observed in the presence of analogues 2 and 3. Incorporation was confirmed through top-down mass spectrometry. See Supporting Information for further details.
3. Conclusion
We report a three-enzyme cascade that converts readily available amino acids and synthetically accessible aromatic biaryl metal-chelating aldehydes to yield novel metal-chelating amino acids. Each enzyme can be accessed in high titer, >100 mg protein L-1 culture, and this chemoenzymatic approach operates with minimal workup. Further, the aromatic amino acids produced are easily isolated through reverse-phase chromatography. This modular chemoenzymatic approach to BpyAla may lower the barriers to future work with this costly amino acid. This work also enables the development of artificial metalloenzymes to tune the electronic and steric environment of the metal-chelating moiety. Promisingly, we found that two such BpyAlal analogues can be incorporated into a model protein using amber stop codon suppression with the system previously developed for BpyAla. This work opens the way for more streamlined production of this privileged ligand and enables new opportunities to develop tunable metal-chelating motifs in protein engineering.
Supplementary Material
Supporting Information
The authors have cited additional references within the Supporting Information.[48–53]
Supporting information for this article is available on the WWW under https://doi.org/10.1002/cctc.202401958
Acknowledgements
We thank Debashrito Deb and Prof. Amy Weeks for their assistance with protein mass spectrometry experiments. We are grateful to Samantha Bruffy, Dr. Meghan Campbell, and the Buller Buddies for many insightful discussions. This work was supported by the Office of the Vice Chancellor for Research and Graduate Education at the University of Wisconsin-Madison with funding from the Wisconsin Alumni Research Foundation. This work was initiated under the support of NIH DP2-GM137417 and completed under R35GM153276 (to Andrew R. Buller). James S. Andon was supported by a Steenbock Predoctoral Graduate Fellowship administered by the University of Wisconsin-Madison, Department of Biochemistry. Seth H. Young was supported by a T32-GM008349 Biotechnology Training Program, and the Bruker AVANCE III-500 NMR spectrometers were supported by the Bender Fund. This material is based upon work supported by the National Science Foundation under Grant No. CHE-2154698 (to Daniel J. Weix). The Bruker AVANCE 400 NMR spectrometer was supported by NSF grant CHE-1048642.
Footnotes
Conflict of Interests The authors declare no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- [1].Mills JH, Sheffler W, Ener ME, Almhjell PJ, Oberdorfer G, Pereira JH, Parmeggiani F, Sankaran B, Zwart PH, Baker D, Proc. Natl. Acad. Sci. USA 2016, 113, 15012–15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Drienovská I, Rioz-Martínez A, Draksharapu A, Roelfes G, Chem. Sci. 2015, 6, 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yang M, Song WJ, Nat. Commun. 2019, 10, 5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kise KJ, Bowler BE, Tetrahedron Asymmetry 1998, 9, 3319–3324. [Google Scholar]
- [5].Roelfes G, Acc. Chem. Res. 2019, 52, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zubi YS, Seki K, Li Y, Hunt AC, Liu B, Roux B, Jewett MC, Lewis JC, Nat. Commun. 2022, 13, 1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kaes C, Katz A, Hosseini MW, Chem. Rev. 2000, 100, 3553–3590. [DOI] [PubMed] [Google Scholar]
- [8].Wang L, Brock A, Herberich B, Schultz PG, Science 2001, 292, 498–500. [DOI] [PubMed] [Google Scholar]
- [9].Xie J, Liu W, Schultz PG, Angew. Chem. Int. Ed. 2007, 46, 9239–9242. [DOI] [PubMed] [Google Scholar]
- [10].Lewis JC, ACS Catal. 2013, 3, 2954–2975. [Google Scholar]
- [11].Yang M, Song WJ, Nat. Commun. 2019, 10, 5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Drienovská I, Rioz-Martínez A, Draksharapu A, Roelfes G, Chem. Sci. 2015, 6, 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mills JH, Sheffler W, Ener ME, Almhjell PJ, Oberdorfer G, Pereira JH, Parmeggiani F, Sankaran B, Zwart PH, Baker D, Proc. Natl. Acad. Sci. USA 2016, 113, 15012–15017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Day GJ, Zaytsev AV, Brewster RC, Kozhevnikov VN, Jarvis AG, Angew. Chem. Int. Ed. 2024, 52, e202413073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brittain WDG, Cobb SL, Org. Biomol. Chem. 2018, 16, 10–20. [DOI] [PubMed] [Google Scholar]
- [16].Kise KJ, Bowler BE, Tetrahedron Asymmetry 1998, 9, 3319–3324. [Google Scholar]
- [17].Heinisch T, Pellizzoni M, Dürrenberger M, Tinberg CE, Köhler V, Klehr J, Häussinger D, Baker D, Ward TR, J. Am. Chem. Soc. 2015, 137, 10414–10419. [DOI] [PubMed] [Google Scholar]
- [18].Deck K, Brittain WDG, Org. Biomol. Chem. 2024, In press. [DOI] [PubMed] [Google Scholar]
- [19].Buller AR, Brinkmann-Chen S, Romney DK, Herger M, Murciano-Calles J, Arnold FH, Proc. Natl. Acad. Sci. USA 2015, 112, 14599–14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Murciano-Calles J, Romney DK, Brinkmann-Chen S, Buller AR, Arnold FH, Angew. Chem. Int. Ed. 2016, 55, 11577–11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hai Y, Chen M, Huang A, Tang Y, J. Am. Chem. Soc. 2020, 142, 19668–19677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zmich A, Perkins LJ, Bingman C, Acheson JF, Buller AR, ACS Catal. 2023, 13, 11644–11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Goldberg SL, Goswami A, Guo Z, Chan Y, Lo ET, Lee A, Truc VC, Natalie KJ, Hang C, Rossano LT, Schmidt MA, Org. Process Res. Dev. 2015, 19, 1308–1316. [Google Scholar]
- [24].Song W, Wang JH, Wu J, Liu J, Chen XL, Liu LM, Nat. Commun. 2018, 9, 10.1038/s41467-018-06241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Doyon TJ, Kumar P, Thein S, Kim M, Stitgen A, Grieger AM, Madigan C, Willoughby PH, Buller AR, Chem. Bio. Chem. 2022, 23, 1–10. [Google Scholar]
- [26].Ellis JM, Campbell ME, Kumar P, Geunes EP, Bingman CA, Buller AR, Nat. Catal. 2022, 5, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Song W, Wang J-H, Wu J, Liu J, Chen X-L, Liu L-M, Nat. Commun. 2018, 9, 3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Huffman MA, Fryszkowska A, Alvizo O, Borra-Garske M, Campos KR, Canada KA, Devine PN, Duan D, Forstater JH, Grosser ST, Halsey HM, Hughes GJ, Jo J, Joyce LA, Kolev JN, Liang J, Maloney KM, Mann BF, Marshall NM, McLaughlin M, Moore JC, Murphy GS, Nawrat CC, Nazor J, Novick S, Patel NR, Rodriguez-Granillo A, Robaire SA, Sherer EC, Truppo MD, Whittaker AM, Verma D, Xiao L, Xu Y, Yang H, Science 2019, 366, 1255–1259. [DOI] [PubMed] [Google Scholar]
- [29].Recombinant D, Aldolases L, Kimura T, Vassilev VP, Shen G-J, Wong C-H, Scripps T, Torrey N, Road P, Jolla L, J. Am. Chem. Soc. 1997, 7863119, 11734–11742. [Google Scholar]
- [30].Möckel B, Eggeling L, Sahm H, Mol. Microbiol. 1994, 13, 833–842. [DOI] [PubMed] [Google Scholar]
- [31].Lambrecht JA, Schmitz GE, Downs DM, mBio 2013, 4, 10.1128/mbio.00033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Miyaura N, Suzuki A, Chem. Rev. 1995, 95, 2457–2483. [Google Scholar]
- [33].Cook XAF, de Gombert A, McKnight J, Pantaine LRE, Willis MC, Angew. Chem. Int. Ed. 2021, 60, 11068–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Heller M, Schubert US, J. Org. Chem. 2002, 67, 8269–8272. [DOI] [PubMed] [Google Scholar]
- [35].Fesko K, Reisinger C, Steinreiber J, Weber H, Schürmann M, Griengl H, J. Mol. Catal. B. 2008, 52–53, 19–26. [Google Scholar]
- [36].Beaudoin SF, Hanna MP, Ghiviriga I, Stewart JD, Enzyme Microb. Technol. 2018, 119, 1–9. [DOI] [PubMed] [Google Scholar]
- [37].Schaffer JE, Reck MR, Prasad NK, Wencewicz TA, Nat. Chem. Biol. 2017, 13, 737–744. [DOI] [PubMed] [Google Scholar]
- [38].Scott TA, Heine D, Qin Z, Wilkinson B, Nat. Commun. 2017, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kumar P, Meza A, Ellis JM, Carlson GA, Bingman CA, Buller AR, ACS Chem. Biol. 2021, 16, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meza A, Campbell ME, Zmich A, Thein SA, Grieger AM, McGill MJ, Willoughby PH, Buller AR, ACS Catal. 2022, 10700–10710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xu L, Wang LC, Xu XQ, Lin J, Catal. Sci. Technol. 2019, 9, 5943–5952. [Google Scholar]
- [42].Xu L, Wang LC, Su BM, Xu XQ, Lin J, Bioresour. Technol. 2020, 310, 123439. [DOI] [PubMed] [Google Scholar]
- [43].Doyon TJ, Kumar P, Thein S, Kim M, Stitgen A, Grieger AM, Madigan C, Willoughby PH, Buller AR, Chem. Bio. Chem. 2022, 23, e202100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bruffy SK, Meza A, Soler J, Doyon TJ, Young SH, Lim J, Huseth KG, Willoughby PH, Garcia-Borràs M, Buller AR, Nat. Chem. 2024, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Doyon TJ, Kumar P, Thein S, Kim M, Stitgen A, Grieger AM, Madigan C, Willoughby PH, Buller AR, Chem. Bio. Chem. 2022, 23, e202100577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Heuson E, Charmantray F, Petit JL, de Berardinis V, Gefflaut T, Adv. Synth. Catal. 2019, 361, 778–785. [Google Scholar]
- [47].Peters FB, Brock A, Wang J, Schultz PG, Chem. Biol. 2009, 16, 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Otte M, Kuijpers PF, Troeppner O, Ivanović-Burmazović I, Reek JNH, de Bruin B, Chem. Eur. J. 2013, 19, 10170–10178. [DOI] [PubMed] [Google Scholar]
- [49].Ingoglia BT, Wagen CC, Buchwald SL, Tetrahedron 2019, 75, 4199–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Borozdina YB, Mostovich EA, Cong PT, Postulka L, Wolf B, Lang M, Baumgarten M, J. Mater. Chem. C. 2017, 5, 9053–9065. [Google Scholar]
- [51].Seganish WM, DeShong P, J. Org. Chem. 2004, 69, 1137–1143. [DOI] [PubMed] [Google Scholar]
- [52].Umemoto T, Tomizawa G, J. Org. Chem. 1989, 54, 1726–1731. [Google Scholar]
- [53].Pöller S, Schuhmann W, Electrochim. Acta 2014. 140, 101–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


