Abstract
Background
This systematic review focuses on antiretroviral therapy (ART) for treating human immunodeficiency virus (HIV) infection in ART‐eligible pregnant women. Mother‐to‐child transmission (MTCT) is the primary means by which children worldwide acquire HIV infection. MTCT occurs during three major timepoints during pregnancy and the postpartum period: in utero, intrapartum, and during breastfeeding. Strategies to reduce MTCT focus on these periods of exposure and include maternal and infant use of ART, caesarean section before onset of labour or rupture of membranes, and complete avoidance of breastfeeding. Where these combined interventions are available, the risk of MTCT is as low as 1‐2%. Thus, ART used among mothers who require treatment of HIV for their own health also plays a significant role in decreasing MTCT.
This review is one in a series of systematic reviews performed in preparation for the revision of the 2006 World Health Organization (WHO) Guidelines regarding "Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants" and "Antiretroviral therapy (ART) for HIV Infections in Adults and Adolescents." The findings from these reviews were discussed with experts, key stakeholders, and country representatives at the 2009 WHO guideline review meeting. The resulting WHO 2009 "rapid advice" preliminary guidance on adult and adolescent ART now recommends lifelong treatment for all adults with HIV infection and CD4 counts <350 cells/mm3. These recommendations also apply to pregnant women who are HIV‐infected and they place a high value on early ART to benefit the mother's own health (WHO 2009). The "rapid advice" preliminary guidance also aims to minimize side effects for mothers and their infants (WHO 2009).
Objectives
Our objective was to assess the current literature regarding the treatment of HIV infection in pregnant women who are clinically or immunologically eligible for ART. This review includes an evaluation of the optimal time to start therapy in relation to the woman’s laboratory parameters and/or gestational age. It also includes an analysis of which specific antiretroviral medications to start in women who are not yet on ART and which agents to continue in women who are already on ART.
Search methods
In June 2009, electronic searches were undertaken in these databases: Cochrane's "CENTRAL," EMBASE, PubMed, LILACS, and Web of Science/Web of Social Science. Hand searches were performed of the reference lists of all pertinent reviews and studies identified. Abstracts from relevant conferences were searched. Experts in the field were contacted to locate additional studies. The search strategy was iterative.
Selection criteria
We selected randomized controlled trials and observational studies that evaluated pregnant women with HIV infection who were eligible for ART according to criteria defined by the WHO guideline review committee. Studies were included in the systematic review when a comparison group was clearly defined and where the intervention comprised triple ART. For a study to be considered, each medication in the ART regimen needed to be clearly described.
Data collection and analysis
Two authors independently assessed the selected studies for relevance and inclusion. Relevant data was then extracted from included studies, and the risk of bias assessed. In each included study, the relative risk (RR) for the intervention versus the comparison group was calculated for each outcome, as appropriate, with 95% confidence intervals (CIs).
Main results
To our knowledge, there are no randomized controlled trials or observational studies that address the optimal time to start antiretroviral drugs in ART‐eligible pregnant women in relation to the woman's laboratory parameters and/or gestational age. The medications to continue in ART‐eligible pregnant women who are already receiving ART also have not been evaluated systematically in the current literature. The long‐term mortality of HIV‐positive pregnant women on ART for their own health, and the long‐term virologic or clinical efficacy of ART in treating them, has not been evaluated in randomized clinical trials. In this review, surrogate outcomes for long‐term mortality and virologic and clinical efficacy (e.g. MTCT and infant HIV transmission or death) were evaluated to determine the efficacy of specific antiretroviral regimens to start in women who are not yet on ART.
Three randomized controlled trials and six observational studies were selected. No studies addressed comparative maternal mortality, which regimens to continue in women already on ART, or the laboratory parameters and gestational age at which to start therapy. The use of zidovudine (AZT), lamivudine (3TC) and lopinavir/ritonavir (LPV‐r) starting at 28‐36 weeks gestation in a breastfeeding population reduced infant HIV‐transmission or death at 12 months compared to a short‐course regimen (RR 0.64, 95% CI: 0.44‐0.92) (deVincenzi, 2009). Starting AZT, 3TC, and nevirapine (NVP) at 34 weeks in a mixed‐feeding population reduced infant HIV‐transmission or death at 7 months compared to a short‐course regimen (RR 0.39, 95% CI: 0.12‐0.85) (Bae, 2008).
In the Mma Bana study (a randomized controlled trial in a breastfeeding population) there was no difference in MTCT at six months between the AZT/3TC/LPV‐r and AZT, 3TC, and abacavir (ABC) arms (RR 0.17, 95% CI: 0.02‐1.44) (Shapiro, 2009). Both regimens also showed 92‐95% efficacy in virologic suppression at delivery and during the breastfeeding period. In the Kesho Bora study there was a significant difference in MTCT at 12 months between breastfeeding women who initiated AZT/3TC/LPV‐r starting between 28 and 36 weeks and those receiving a short course regimen (RR 0.58, 95% CI: 0.34‐0.97) (deVincenzi, 2009). MTCT also decreased significantly when AZT/3TC/NVP was compared with a short‐course regimen at seven months in a feeding intervention study (RR 0.15, 95% CI: 0.04‐0.62) (Bae, 2008) and 12 months in a population where either exclusive breastfeeding or replacement feeding was encouraged (RR 0.14, CI: 0.04‐0.47) (Ekouevi, 2008).
In the Mma Bana study, there was increased risk of prematurity among infants born to women receiving AZT/3TC/LPV‐r (RR 1.52, CI: 1.07‐ 2.17) compared with AZT/3TC/ABC (Shapiro, 2009). Ekouevi 2008 showed higher rates of infant low birth weight on AZT/3TC/NVP started at 24 weeks compared to a short course regimen started between 32 and 36 weeks (RR 1.81, 95% CI: 1.09‐ 3.0). Tonwe‐Gold 2007 showed an increase in maternal severe adverse events among the women receiving AZT/3TC/NVP compared with a short‐course regimen (RR 25.33, CI 1.49‐ 340.51).
Authors' conclusions
In ART‐eligible pregnant women with HIV infection, ART is a safe and effective means of providing maternal virologic suppression, decreasing infant mortality, and reducing MTCT. Specifically, AZT/3TC/NVP, AZT/3TC/LPV‐r, and AZT/3TC/ABC have been shown to decrease MTCT. More research is needed regarding the use of specific regimens and their maternal and infant side‐effect profiles.
Keywords: Adolescent; Adult; Female; Humans; Infant, Newborn; Pregnancy; Young Adult; HIV‐1; Anti‐HIV Agents; Anti‐HIV Agents/therapeutic use; Cohort Studies; Drug Therapy, Combination; Drug Therapy, Combination/methods; HIV Infections; HIV Infections/drug therapy; HIV Infections/transmission; Infectious Disease Transmission, Vertical; Infectious Disease Transmission, Vertical/prevention & control; Pregnancy Complications, Infectious; Pregnancy Complications, Infectious/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Therapy for treating HIV infection in pregnant women who require treatment for their own health
Pregnant human immunodeficiency virus‐infected (HIV)‐infected women often need treatment with antiretroviral therapy (ART) for their own health. Mother‐to‐child transmission (MTCT) is the most common way that children worldwide become HIV infected. Treatment of HIV‐infected pregnant women with ART decreases the risk of HIV MTCT. It is possible to decrease the risk of MTCT to 1‐2% with the use of antiretroviral medications, caesarean section before labour begins, and avoiding breastfeeding. When women who require HIV treatment for the benefit of their own health become pregnant, we need to know the most effective therapy, the impact of the drug on the MTCT of HIV, and what the potential complications of the therapy might be for both the mother and her unborn child.
Summary of findings
Summary of findings for the main comparison. Kesho Bora: AZT/3TC/LPV‐r compared to Short‐Course AZT (intrapartum AZT/3TC/sd‐NVP) for HIV‐infected Pregnant Women With CD4 Counts 200‐500 Cells/mm3.
| AZT/3TC/LPV‐r compared to Short‐Course AZT (intrapartum AZT/3TC/sd‐NVP) for HIV‐infected Pregnant Women With CD4 Counts 200‐500 Cells/mm3 | ||||||
| Patient or population: HIV‐infected Pregnant Women With CD4 Counts 200‐500 Cells/mm3 Settings: Resource Limited Intervention: AZT/3TC/LPV‐r 1 Comparison: Short‐Course AZT (intrapartum AZT/3TC/sd‐NVP) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐Course AZT (intrapartum AZT/3TC/sd‐NVP) | AZT/3TC/LPV‐r | |||||
| Infant HIV Transmission or Death at 6 Months | 126 per 1000 | 83 per 1000 (54 to 126) | RR 0.66 (0.43 to 1) | 795 (1 study) | ⊕⊕⊝⊝ low2,3,4,5 | |
| Infant HIV Transmission or Death at 12 months | 163 per 1000 | 104 per 1000 (72 to 150) | RR 0.64 (0.44 to 0.92) | 765 (1 study) | ⊕⊝⊝⊝ very low2,3,4,5,6 | |
| Infant Mortality at 6 Months | 59 per 1000 | 46 per 1000 (25 to 84) | RR 0.78 (0.43 to 1.42) | 781 (1 study) | ⊕⊕⊕⊝ moderate2,3,5,7 | |
| Infant Mortality at 12 months | 100 per 1000 | 63 per 1000 (38 to 103) | RR 0.63 (0.38 to 1.03) | 751 (1 study) | ⊕⊕⊝⊝ low2,3,5,6 | |
| Maternal Grade 3/4 Severe Adverse Events | 131 per 1000 | 143 per 1000 (101 to 200) | RR 1.09 (0.77 to 1.53) | 824 (1 study9) | ⊕⊝⊝⊝ very low3,4,5,7,8 | |
| Infant Grade 3/4 Severe Adverse Events | 325 per 1000 | 309 per 1000 (250 to 377) | RR 0.95 (0.77 to 1.16) | 805 (1 study9) | ⊕⊕⊝⊝ low3,4,7,8 | |
| Prematurity | 109 per 1000 | 134 per 1000 (93 to 195) | RR 1.23 (0.85 to 1.79) | 805 (1 study) | ⊕⊝⊝⊝ very low3,4,5,7,8 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 413 women were randomised to the AZT/3TC/LPV‐r arm, and 411 women were randomised to the short course arm as published in the IAS 2009 abstract. There were 402 live‐born infants in the AZT/3TC/LPV‐r arm and 403 live‐born infants in the short course arm as published in the IAS 2009 abstract. All other denominators were derived from information published in the authors' IAS 2009 abstract. 2 This outcome not deemed subject to risk of bias associated with lack of blinding. 3 As a single study, no comparison study is available to evaluate inconsistency. 4 Kesho Bora's intervention arms evaluated women with CD4 200‐500 cells/mm3. This group contains a subset of women who, using current guidelines, would be eligible for anti‐retroviral therapy for their own health. 5 Small numbers of events. 6 The last baby in the study was enrolled was born November 2008. At the time of the authors' IAS presentation only 28% of the participants had not yet completed 12‐month follow‐up. 7 Confidence interval includes the null. 8 This outcome deemed subject to risk of bias associated with lack of blinding. 9 Per ACTG 1992 Protocol Management Handbook, events of grade 3 or higher classify as "severe".
Summary of findings 2. Kesho Bora continued: AZT/3TC/LPV‐r compared to Short‐Course AZT (intrapartum AZT/3TC/sd‐NVP) for HIV‐infected Pregnant Women With CD4 Counts 200‐500 Cells/mm3 (second part of the preceding Summary of Findings table, showing additional outcomes).
| AZT/3TC/LPV‐r compared to Short‐Course AZT (intrapartum AZT/3TC/sd‐NVP) for HIV‐infected Pregnant Women With CD4 Counts 200‐500 Cells/mm3 | ||||||
| Patient or population: HIV‐infected Pregnant Women With CD4 Counts 200‐500 Cells/mm3 Settings: Resource Limited Intervention: AZT/3TC/LPV‐r 1 Comparison: Short‐Course AZT (intrapartum AZT/3TC/sd‐NVP) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐Course AZT (intrapartum AZT/3TC/sd‐NVP) | AZT/3TC/LPV‐r | |||||
| Low Birth Weight | 77 per 1000 | 112 per 1000 (72 to 173) | RR 1.46 (0.94 to 2.25) | 805 (1 study) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| Stillbirths | 10 per 1000 | 10 per 1000 (2 to 40) | RR 1 (0.25 to 3.95) | 824 (1 study) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| Mother to Child Transmission at Birth | 22 per 1000 | 17 per 1000 (6 to 46) | RR 0.78 (0.29 to 2.07) | 805 (1 study7) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| Mother to Child Transmission at 6 Weeks | 48 per 1000 | 33 per 1000 (16 to 66) | RR 0.69 (0.34 to 1.37) | 789 (1 study) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| Mother to Child Transmission at 6 Months | 85 per 1000 | 48 per 1000 (28 to 84) | RR 0.56 (0.33 to 0.99) | 776 (1 study) | ⊕⊕⊝⊝ low2,3,4,5,8 | |
| Mother to Child Transmission at 12 Months | 95 per 1000 | 55 per 1000 (32 to 92) | RR 0.58 (0.34 to 0.97) | 761 (1 study) | ⊕⊝⊝⊝ very low2,3,4,5,9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 413 women were randomised to the AZT/3TC/LPV‐r arm, and 411 women were randomised to the short course arm as published in the IAS 2009 abstract. There were 402 live‐born infants in the AZT/3TC/LPV‐r arm and 403 live‐born infants in the short course arm as published in the IAS 2009 abstract. All other denominators were derived from information published in the authors' IAS 2009 abstract. 2 This outcome not deemed subject to risk of bias associated with lack of blinding. 3 As a single study, no comparison study is available to evaluate inconsistency. 4 Kesho Bora's intervention arms evaluated women with CD4 200‐500 cells/mm3. This group contains a subset of women who, using current guidelines, would be eligible for anti‐retroviral therapy for their own health. 5 Small numbers of events. 6 Confidence interval includes the null. 7 Preferably, HIV RNA PCR was performed within 72 hours, but was allowed up to 1 week after birth. 8 The overall HIV transmission rate in in the HAART arm of Mma Bana was 1% at 6 months compared with Kesho Bora where transmission was 4.9% in the HAART arm at 6 months. 9 The last baby in the study was enrolled was born November 2008. At the time of the authors' IAS presentation only 28% of the participants had not yet completed 12‐month follow‐up.
Summary of findings 3. Lehman: AZT/3TC/NVP compared to Short Course AZT/sdNVP for HIV‐Infected Pregnant Women With CD4 Counts 200‐500 cells/mm3.
| AZT/3TC/NVP compared to Short Course AZT/sdNVP for HIV‐Infected Pregnant Women With CD4 Counts 200‐500 cells/mm3 | ||||||
| Patient or population: HIV‐Infected Pregnant Women With CD4 Counts 200‐500 cells/mm3 Settings: Resource Limited Intervention: AZT/3TC/NVP Comparison: Short Course AZT/sdNVP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short Course AZT/sdNVP | AZT/3TC/NVP | |||||
| Maternal Resistance | 750 per 1000 | 180 per 1000 (53 to 660)1 | RR 0.24 (0.07 to 0.88) | 27 (1 study6) | ⊕⊝⊝⊝ very low2,3,4,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomization allocated 28 women to the short course arm and 30 women to the HAART arm. Due to loss to follow up and specimen limitations (only samples where 50 cumulative viral copies were tested in 2‐4 independent RT‐PCRs were included), only 16 women in the short course arm and 11 in the HAART arm were included in the author's analysis. 2 Randomization allocated 28 women to the short course arm and 30 women to the HAART arm. Of these participants, 4/28 (14%) were lost to follow up in the short course arm and 6/30 (20%) were lost to follow‐up in the HAART arm. 3 This outcome not subject to risk of bias associated with lack of blinding. 4 This study includes a cohort of women who did not require anti‐retroviral therapy for their own health (CD4 range 200‐ 500). Mean CD4 count in the short course group at 32 weeks gestation was 354 in the short course arm, and 304 in the AZT/3TC/NVP arm. 5 Small numbers of events. 6 Women with K103N or Y181C at 3 months after treatment cessation.
Summary of findings 4. Mma Bana: AZT/3TC/LPV‐r compared to AZT/3TC/ABC for HIV‐Infected Pregnant Women With CD4 Counts >200 cells/mm3.
| AZT/3TC/LPV‐r compared to AZT/3TC/ABC for HIV‐Infected Pregnant Women With CD4 Counts >200 cells/mm3 | ||||||
| Patient or population: HIV‐Infected Pregnant Women With CD4 Counts >200 cells/mm3 Settings: Resource Limited Intervention: AZT/3TC/LPV‐r1 Comparison: AZT/3TC/ABC | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| AZT/3TC/ABC | AZT/3TC/LPV‐r | |||||
| Infant Mortality at 6 Months | 26 per 1000 | 25 per 1000 (9 to 70) | RR 0.95 (0.34 to 2.68) | 553 (1 study) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| Women With Any Grade 3/4 Severe Adverse Event (Laboratory Only) | 147 per 1000 | 116 per 1000 (75 to 178) | RR 0.79 (0.51 to 1.21) | 560 (1 study8,9) | ⊕⊝⊝⊝ very low3,4,5,6,7 | |
| Women With Any Severe Adverse Events Requiring Treatment Modification | 25 per 1000 | 22 per 1000 (8 to 65) | RR 0.89 (0.3 to 2.61) | 560 (1 study) | ⊕⊝⊝⊝ very low3,4,5,6,7 | |
| Infants With Any Grade 3/4 Severe Adverse Events | 410 per 1000 | 463 per 1000 (381 to 562) | RR 1.13 (0.93 to 1.37) | 553 (1 study8,9) | ⊕⊕⊝⊝ low3,4,6,7 | |
| Prematurity | 148 per 1000 | 225 per 1000 (158 to 321) | RR 1.52 (1.07 to 2.17) | 553 (1 study) | ⊕⊕⊝⊝ low2,3,4,5 | |
| Infant Low Birth Weight | 131 per 1000 | 166 per 1000 (111 to 250) | RR 1.27 (0.85 to 1.91) | 553 (1 study) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 275 women were randomised to the AZT/3TC/LPV‐r arm and 285 to the AZT/3TC/NVP arm. There were 270 live‐born infants in the AZT/3TC/LPV‐r arm and 283 live‐born infants in the AZT/3TC/NVP arm. We were unable to account for censoring. 2 This outcome not deemed subject to bias associated with lack of blinding. 3 As a single study, no comparison study is available to evaluate inconsistency. 4 Mma Bana's intervention arms evaluated women with CD4>200. We acknowledge that at the time the study was designed, the randomized intervention arms were designed as prophylaxis of MTCT in women who did not need anti‐retroviral therapy (ART) for their own health. As the WHO treatment guidelines have changes, these arms now include women (CD4 200‐350) who need ART for their own health. Median CD4 in AZT/3TC/LPV‐r arm was 398. Median CD4 in AZT/3TC/ABC arm was 403. 5 Small numbers of events 6 Confidence interval includes the null. 7 This outcome deemed subject to bias associated with lack of blinding. 8 Severe adverse events include any general body, respiratory, gastrointestinal, reproductive, skin, or laboratory (metabolic and hematologic) abnormality. 9 Per ACTG 1992 Protocol Management Handbook, events of grade 3 or higher classify as "severe".
Summary of findings 5. Mma Bana continued: AZT/3TC/LPV‐r compared to AZT/3TC/ABC for HIV‐Infected Pregnant Women With CD4 Counts >200 cells/mm3 (second part of the preceding Summary of Findings table, showing additional outcomes).
| AZT/3TC/LPV‐r compared to AZT/3TC/ABC for HIV‐Infected Pregnant Women With CD4 Counts >200 cells/mm3 | ||||||
| Patient or population: HIV‐Infected Pregnant Women With CD4 Counts >200 cells/mm3 Settings: Resource Limited Intervention: AZT/3TC/LPV‐r1 Comparison: AZT/3TC/ABC | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| AZT/3TC/ABC | AZT/3TC/LPV‐r | |||||
| Stillbirths | 28 per 1000 | 18 per 1000 (6 to 55) | RR 0.65 (0.21 to 1.96) | 560 (1 study) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| Maternal Mortality at Six Months | 4 per 1000 | 1 per 1000 (0 to 34) | RR 0.35 (0.01 to 8.44) | 560 (1 study) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| Mother to Child Transmission In Utero Positive HIV DNA PCR Within 96 Hours | 11 per 1000 | 4 per 1000 (0 to 37) | RR 0.35 (0.04 to 3.34) | 553 (1 study7) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| Mother to Child Transmission Late Postpartum Negative HIV DNA PCR at Birth and One Month, But Positive HIV DNA PCR at Six Months | 7 per 1000 | 1 per 1000 (0 to 30) | RR 0.21 (0.01 to 4.35) | 553 (1 study7) | ⊕⊕⊝⊝ low2,3,4,5,6 | |
| Mother to Child Transmission at Six Months Post‐Partum All Positive HIV DNA PCRs at Six Months | 21 per 10008 | 4 per 1000 (0 to 30) | RR 0.17 (0.02 to 1.44) | 553 (1 study7) | ⊕⊕⊝⊝ low2,3,4,5,6,9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 275 women were randomised to the AZT/3TC/LPV‐r arm and 285 to the AZT/3TC/NVP arm. There were 270 live‐born infants in the AZT/3TC/LPV‐r arm and 283 live‐born infants in the AZT/3TC/NVP arm. We were unable to account for censoring. 2 This outcome not deemed subject to bias associated with lack of blinding. 3 As a single study, no comparison study is available to evaluate inconsistency. 4 Mma Bana's intervention arms evaluated women with CD4>200. We acknowledge that at the time the study was designed, the randomized intervention arms were designed as prophylaxis of MTCT in women who did not need anti‐retroviral therapy (ART) for their own health. As the WHO treatment guidelines have changes, these arms now include women (CD4 200‐350) who need ART for their own health. Median CD4 in AZT/3TC/LPV‐r arm was 398. Median CD4 in AZT/3TC/ABC arm was 403. 5 Small numbers of events 6 Confidence interval includes the null. 7 UN Aids Epidemic Update reports MTCT rates in Botswana of 4‐6% in women receiving HAART and 20‐40% in the untreated population 8 One child with HIV positive PCR who died before 6 months is included in this calculation. 9 Overall HIV transmission at 6 months in the HAART arm was 1% in Mma Bana compared with 4.9% at 6 months in Kesho Bora.
Summary of findings 6. Bae: AZT/3TC/NVP vs. AZT (+/‐ single dose NVP) for HIV‐infected pregnant women with CD4 Counts <200 cells/mm3.
| AZT/3TC/NVP vs. AZT (+/‐ single dose NVP) for HIV‐infected pregnant women with CD4 Counts <200 cells/mm3 | ||||||
| Patient or population: HIV‐infected pregnant women with CD4 Counts <200 cells/mm31 Settings: Resource Limited Intervention: AZT/3TC/NVP vs. AZT (+/‐ single dose NVP) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | AZT/3TC/NVP vs. AZT (+/‐ single dose NVP) | |||||
| HIV‐Transmission or Death at 7 Months | 257 per 1000 | 100 per 1000 (46 to 218) | RR 0.39 (0.18 to 0.85) | 178 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Infant Grade 3/4 Severe Adverse Events at Birth | 46 per 1000 | 58 per 1000 (16 to 209) | RR 1.26 (0.35 to 4.54) | 178 (1 study4) | ⊕⊝⊝⊝ very low2,3 | |
| Prematurity | 128 per 1000 | 72 per 1000 (27 to 192) | RR 0.56 (0.21 to 1.5) | 178 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| Mother to Child Transmission at Birth | 83 per 1000 | 15 per 1000 (2 to 113) | RR 0.18 (0.02 to 1.36) | 178 (1 study) | ⊕⊝⊝⊝ very low2,3,5 | |
| Mother to Child Transmission at 1 Month | 128 per 1000 | 29 per 1000 (6 to 123) | RR 0.23 (0.05 to 0.96) | 178 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Mother to Child Transmission Cumulative by 7 Months | 193 per 1000 | 29 per 1000 (8 to 120) | RR 0.15 (0.04 to 0.62) | 178 (1 study) | ⊕⊝⊝⊝ very low2,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All denominators represent an available case analysis. 2 Small numbers of events. 3 Confidence interval includes the null. 4 Hepatic severe adverse events were not provided at this timepoint, the authors provided hematologic severe adverse events only. 5 Although a large effect, there are few events from one small observational study.
Summary of findings 7. Ekouevi: AZT/3TC/NVP compared to Other Short Course PMTCT Regimen for ART‐Eligible HIV‐infected Pregnant Women.
| AZT/3TC/NVP compared to Other Short Course PMTCT Regimen for ART‐Eligible HIV‐infected Pregnant Women | ||||||
| Patient or population: ART‐Eligible HIV‐infected Pregnant Women Settings: Resource Limited Intervention: AZT/3TC/NVP Comparison: Other Short Course PMTCT Regimen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other Short Course PMTCT Regimen | AZT/3TC/NVP | |||||
| Stillbirths | 29 per 1000 | 34 per 1000 (10 to 114) | RR 1.16 (0.34 to 3.93) | 326 (1 study) | ⊕⊝⊝⊝ very low1,2,3,4 | |
| Infant Low Birth Weight | 124 per 1000 | 224 per 1000 (135 to 372)5 | RR 1.81 (1.09 to 3) | 309 (1 study) | ⊕⊝⊝⊝ very low1,2,3,6 | |
| Mother to Child Transmission at 12 Months | 147 per 1000 | 21 per 1000 (6 to 69)5 | RR 0.14 (0.04 to 0.47) | 311 (1 study) | ⊕⊝⊝⊝ very low1,2,3,7 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 This study compares two sequential cohorts (short course group from 2001‐ 2003 and HAART group from 2003‐2007) instead of two contemporaneous groups. 2 Comparability of cohorts is limited, given that one tier differed statistically from another in age, parity, and WHO stage (see p. 1817). 3 Small numbers of events 4 Confidence interval includes the null. 5 These denominators represent an available case analysis. 6 The length of therapy is different in the short course and HAART groups. Women receiving short course anti‐retroviral therapy (ART) got 4.9 weeks of ART, while the HAART group got 11.7 weeks of ART. 7 Evaluation of this outcome must take late‐postnatal HIV transmission through breastfeeding into account.
Summary of findings 8. Jamisse (What to Start): AZT/3TC/NVP compared to d4T/3TC/NVP for HIV‐Infected Pregnant Women With CD4<350.
| AZT/3TC/NVP compared to d4T/3TC/NVP for HIV‐Infected Pregnant Women With CD4<350 | ||||||
| Patient or population: HIV‐Infected Pregnant Women With CD4<350 Settings: Resource Limited Intervention: AZT/3TC/NVP 1 Comparison: d4T/3TC/NVP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| d4T/3TC/NVP | AZT/3TC/NVP | |||||
| Maternal Grade 3/4 Severe Adverse Events | 85 per 1000 | 134 per 1000 (51 to 350) | RR 1.58 (0.6 to 4.12) | 146 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All denominators represent an available case analysis. 2 Rates of grade 3/4 hepatotoxicity in Phanuphak study were 1/102 (1%) in the <200 arm and 4/142 (2.8%) in the >200 arm. In the Jamisse study, rates of grade 3/4 hepatotoxicity were 0/79 (0%) in the <250 arm and 4/67 (6%) in the >250 arm. 3 Small numbers of events and confidence interval includes the null.
Summary of findings 9. Jamisse (When to Start): AZT/3TC/NVP for CD4 250‐350 compared to AZT/3TC/NVP for CD4 <250 for HIV‐Infected Pregnant Women.
| AZT/3TC/NVP for CD4 250‐350 compared to AZT/3TC/NVP for CD4 <250 for HIV‐Infected Pregnant Women | ||||||
| Patient or population: HIV‐Infected Pregnant Women1 Settings: Resource Limited Intervention: AZT/3TC/NVP for CD4 250‐350 Comparison: AZT/3TC/NVP for CD4 <250 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| AZT/3TC/NVP for CD4 <250 | AZT/3TC/NVP for CD4 250‐350 | |||||
| Maternal Grade 3/4 Severe Adverse Events | 101 per 1000 | 119 per 1000 (47 to 300) | RR 1.18 (0.47 to 2.97) | 146 (1 study5) | ⊕⊝⊝⊝ very low2,3,4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 All denominators represent an available case analysis. 2 Rates of grade 3/4 hepatotoxicity in Phanuphak study were 1/102 (1%) in the <200 arm and 4/142 (2.8%) in the >200 arm. In the Jamisse study, rates of grade 3/4 hepatotoxicity were 0/79 (0%) in the <250 arm and 4/67 (6%) in the >250 arm. 3 For the CD4<250 arm, 52% of the participants got d4T/3TC/NVP, while 55% of the 250‐350 got AZT/3TC/NVP. 4 Small numbers of events and confidence interval includes the null. 5 In the CD4 250‐350 exposure group, median gestational age at HAART onset was 26 weeks. In the CD$ <250 group, median gestational age at HAART onset was 27 weeks.
Summary of findings 10. Marazzi: AZT/3TC/NVP for HIV Infection in Pregnant Women With CD4 <250 vs. >250 cells/mm3.
| AZT/3TC/NVP for HIV Infection in Pregnant Women With CD4 <250 vs. >250 cells/mm3 | ||||||
| Patient or population: HIV‐infected Pregnant Women Settings: Resource‐Limited Intervention: AZT/3TC/NVP in CD4>250 Comparison: AZT/3TC/NVP in CD4<250 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| AZT/3TC/NVP in CD4<250 | AZT/3TC/NVP in CD4>250 | |||||
| Maternal Grade 3/4 Hepatotoxicity | 93 per 1000 | 60 per 1000 (32 to 112) | RR 0.64 (0.34 to 1.2) | 703 (1 study) | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 11. Phanuphak: AZT/3TC/NVP (PMTCT) compared to AZT/3TC/NVP (ART) for HIV‐1 Infected Pregnant Women Who Have CD4>200 Compared with Those Who Have CD4<200 Cells/mm3.
| AZT/3TC/NVP (PMTCT) compared to AZT/3TC/NVP (ART) for HIV‐1 Infected Pregnant Women Who Have CD4>200 Compared with Those Who Have CD4<200 Cells/mm3 | ||||||
| Patient or population: HIV‐1 Infected Pregnant Women Who Have CD4>200 Compared with Those Who Have CD4<200 Cells/mm3 Settings: Resource Limited Intervention: AZT/3TC/NVP (PMTCT)1 Comparison: AZT/3TC/NVP (ART) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| AZT/3TC/NVP (ART) | AZT/3TC/NVP (PMTCT) | |||||
| Maternal Grade 3/4 Severe Adverse Events | 39 per 1000 | 77 per 1000 (25 to 235) | RR 1.98 (0.65 to 6.03)2 | 244 (1 study) | ⊕⊝⊝⊝ very low3,4,5,6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The denominators represent an available case analysis. 2 This outcome reflects pooled results of grade 3/4 skin rash and hepatotoxicity. We are awaiting information from the authors to elucidate if events were mutually exclusive. 3 This study showed a statistically significant relationship between NVP use and grade 3/4 hepatotoxicity among hiv‐positive pregnant women with CD4>200 vs. CD4<200 (p 0.023 per 100 person years). This is comparable to other studies, although more fatal events were noted in PACTG 1022, where the NVP arm mean CD4 count was 359. 4 Rates of grade 3/4 hepatotoxicity in Phanuphak study were 1/102 (1%) in the <200 arm and 4/142 (2.8%) in the >200 arm. In the Jamisse study, rates of grade 3/4 hepatotoxicity were 0/79 (0%) in the <250 arm and 4/67 (6%) in the >250 arm. 5 Per the methods, "pregnant hiv‐infected women with CD4 counts >200 were prescribed NVP‐containing HAART for PMTCT until delivery... pregnant hiv‐infected women with CD4 cell counts <200 or <350 with WHO stage >2 were provided with NVP‐containing regimens for therapy". The CD4 cell count range in the short course arm was 211‐1169, and 2‐252 in the HAART arm. 6 Small numbers of events, and confidence interval includes the null.
Summary of findings 12. Tonwe‐Gold: AZT/3TC/NVP compared to Other Short Course Regimen for HIV‐Infected Pregnant Women.
| AZT/3TC/NVP compared to Other Short Course Regimen for HIV‐Infected Pregnant Women | ||||||
| Patient or population: HIV‐Infected Pregnant Women Settings: Resource Limited Intervention: AZT/3TC/NVP 1 Comparison: Other Short Course Regimen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other Short Course Regimen | AZT/3TC/NVP | |||||
| HIV Transmission or Death at 1 Month | 49 per 1000 | 42 per 1000 (12 to 146)2 | RR 0.86 (0.25 to 2.97)3 | 218 (1 study) | ⊕⊝⊝⊝ very low4,5,6,7,8 | |
| HIV Transmission or Death at 12 Months | 147 per 1000 | 126 per 1000 (62 to 260)2 | RR 0.86 (0.42 to 1.77)9 | 189 (1 study) | ⊕⊝⊝⊝ very low4,5,7,8 | |
| Infant Mortality at 1 Month | 23 per 1000 | 31 per 1000 (6 to 149) | RR 1.33 (0.27 to 6.47) | 231 (1 study) | ⊕⊝⊝⊝ very low4,5,7,8 | |
| Maternal Grade 3/4 Severe Adverse Events Requiring Treatment Modification | 0 per 1000 | 0 per 1000 (0 to 0) | RR 25.33 (1.49 to 430.51) | 250 (1 study) | ⊕⊝⊝⊝ very low4,5,7 | |
| Infant Low Birth Weight | 121 per 1000 | 263 per 1000 (149 to 461) | RR 2.17 (1.23 to 3.81) | 231 (1 study) | ⊕⊝⊝⊝ very low4,5,7 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In the HAART arm, 5/107 women received d4T/3TC/NVP. In the short course (sc) arm, 14/143 women received sdNVP, 26/143 women received scAZT/sdNVP, and 103/143 women received sc (AZT+3TC)/sdNVP. 2 These denominators represent an available‐case analysis. 3 To account for censoring, log‐rank analysis was performed. The resulting hazard ratio for HIV Transmission or Death at 1 Month is HR 0.86 (0.24‐ 3.08). 4 There were statistically significant differences between the participants' ages, gestational ages at enrollment, WHO clinical staging, CD4 counts at enrollment, and eligibility for anti‐retroviral therapy (ART), making comparability of the cohorts limited. 5 Comparisons between the short course ART and HAART cohorts are indirect based on differences in CD4 counts and WHO clinical staging. 6 This outcome also reflects breastfeeding transmission of HIV. 7 Small numbers of events. 8 Confidence interval includes the null. 9 To account for censoring, log‐rank analysis was performed. The resulting hazard ratio for HIV Transmission or Death at 12 Months is HR 0.84 (0.37‐ 1.93).
Summary of findings 13. Tonwe‐Gold continued: AZT/3TC/NVP compared to Other Short Course Regimen for HIV‐Infected Pregnant Women (second part of the preceding Summary of Findings table, showing additional outcomes).
| AZT/3TC/NVP compared to Other Short Course Regimen for HIV‐Infected Pregnant Women | ||||||
| Patient or population: HIV‐Infected Pregnant Women Settings: Resource Limited Intervention: AZT/3TC/NVP 1 Comparison: Other Short Course Regimen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other Short Course Regimen | AZT/3TC/NVP | |||||
| Stillbirths | 43 per 1000 | 38 per 1000 (11 to 132)2 | RR 0.89 (0.26 to 3.08) | 241 (1 study) | ⊕⊝⊝⊝ very low3,4,5,6 | |
| Maternal Mortality at Delivery | See comment | See comment | Not estimable | 241 (1 study) | ⊕⊝⊝⊝ very low3,4,5 | |
| Mother to Child Transmission of HIV at 1 Month | 33 per 1000 | 11 per 1000 (1 to 93)2 | RR 0.32 (0.04 to 2.83)7 | 217 (1 study) | ⊕⊝⊝⊝ very low3,4,5,6 | |
| Mother to Child Transmission of HIV at 12 Months | 88 per 1000 | 35 per 1000 (10 to 124)2 | RR 0.4 (0.11 to 1.41)8 | 188 (1 study) | ⊕⊝⊝⊝ very low3,4,5,6,9 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In the HAART arm, 5/107 women received d4T/3TC/NVP. In the short course (sc) arm, 14/143 women received sdNVP, 26/143 women received scAZT/sdNVP, and 103/143 women received sc (AZT+3TC)/sdNVP. 2 These denominators represent an available‐case analysis. 3 There were statistically significant differences between the participants' ages, gestational ages at enrollment, WHO clinical staging, CD4 counts at enrollment, and eligibility for anti‐retroviral therapy (ART), making comparability of the cohorts limited. 4 Comparisons between the short course ART and HAART cohorts are indirect based on differences in CD4 counts and WHO clinical staging. 5 Small numbers of events. 6 Confidence interval includes the null. 7 To account for censoring, log‐rank analysis was performed. The resulting hazard ratio for Mother to Child Transmission at 1 Month is HR 0.37 (0.06‐ 2.22). 8 To account for censoring, log‐rank analysis was performed. The resulting hazard ratio for Mother to Child Transmission at 12 Months is HR 0.41 (0.13‐ 1.33). 9 This outcome also reflects breastfeeding transmission of HIV.
Background
In 2008 there were 430,000 new infections with the human immunodeficiency virus (HIV) in children younger than 15 years (UNAIDS 2009). This raised the total number of infected children in 2008 to an estimated 2.1 million worldwide. Although the HIV infection rates in children are decreasing worldwide, the global disease burden is still extremely large, with 33.4 million people worldwide living with HIV at the end of 2008. According to the UNAIDS Epidemic Update, most of the HIV infections in children under the age of 15 were acquired via preventable means (UNAIDS 2009).
This review was one of a series of reviews prepared at the request of the World Health Organization (WHO) to inform the development of the 2010 guidelines on preventing MTCT. WHO's 'rapid advice' preliminary guidance is as follows for the use of antiretroviral therapy (ART) for pregnant women with confirmed HIV serostatus: 1) initiation of ART is recommended at CD4 counts <350 cells/mm3, irrespective of WHO clinical staging or at WHO clinical stage 3 or 4 irrespective of CD4 cell count, 2) ART should be initiated irrespective of gestational age in ART eligible women, 3) first line ART for ART‐eligible women should include a backbone of zidovudine (AZT) and lamivudine (3TC) with the use of either nevirapine (NVP) or efavirenz.
In this review, we focus on ART for treating HIV infection in ART‐eligible pregnant women. ART is defined as the treatment of HIV infection with a combination of three or more antiretroviral drugs. Antiretroviral prophylaxis refers to the use of antiretroviral medications for the prevention of mother‐to‐child transmission (MTCT).
Description of the condition
Mother‐to‐Child Transmission of HIV
In the absence of preventive interventions, published MTCT rates for non‐breastfeeding populations in industrialized countries range between 14‐23%, while the rates in breastfeeding populations in resource‐limited settings range between 25‐48% (De Cock 2000). The risk of MTCT of HIV is multi‐factorial, and maternal plasma HIV RNA levels, mode of delivery and gestational age are independently associated with HIV transmission (European Collaborative Study [ECS], 1997). Evidence from microbiology, pathology, and clinical experience suggests that MTCT occurs during three major timepoints during pregnancy and the postpartum period: in utero, intrapartum, and through breastfeeding. Therefore, interventions to prevent MTCT need to target these specific timepoints.
In UteroTransmission
The proposed empiric definition for in utero HIV transmission in a non‐breastfeeding population requires detection of the HIV genome by either polymerase chain reaction (PCR) or other isolation method on a sample obtained from an infant within 48 hours after delivery (Bryson 1992). Studies have shown that MTCT can occur early in pregnancy, with HIV infection identified in fetuses as early as eight weeks of gestation (Langston 1995). HIV can infect the placenta directly and has been detected in fetal autopsy tissue during all three trimesters (Langston 1995). The virus is thought to spread cell‐to‐cell through the placenta, while another possible mechanism involves the passage of maternal mononuclear cells into the fetal circulation (Langston 1995).
Factors that decrease the placenta’s integrity have also been implicated in utero transmission. This mechanism of action may explain the association between the use of heroin and cocaine and MTCT in the second and third trimester (Bulterys 1997).
Intrapartum Transmission
Up to 80% of MTCT is thought to occur during the intrapartum period, defined as the time period during labour and delivery. However, exact relative proportions of intrauterine and intrapartum transmission have not been defined (Mofenson 1997). Presumptive intrapartum transmission is defined by infant blood samples that are negative for HIV by culture, serum p24 antigen assay, or RNA PCR when obtained during the first week of life. These samples become positive by the same methods between days 7 to 90 after delivery (Bryson 1992) in the absence of breastfeeding.
In infected women, HIV is detected in cervicovaginal secretions (Rasheed 1996). Thus, infant skin and mucosal contact with cervicovaginal secretions or blood in the maternal genital tract, and duration of contact with these secretions are modifiable risk factors. Longer duration of rupture of maternal membranes, and thus fetal contact with cervicovaginal secretions, has been associated with increased MTCT, with an odds ratio of 2% increased transmission risk for every additional hour of ruptured membranes (International Perinatal HIV Group (IPHG) 2001). The timing of membrane rupture is important to consider if the mode of delivery is being considered as a means to avoid MTCT. Two individual patient data meta‐analyses showed that mode of delivery is not associated with a decreased risk of MTCT once membrane rupture occurs (International Perinatal HIV Group (IPHG) 1999 and International Perinatal HIV Group (IPHG) 2001).
Although there is a large drive to decrease maternal serum viral load with antiretroviral drugs as a means of decreasing MTCT, cervicovaginal HIV viral load and the response to antiretroviral drugs do not always correlate with maternal serum viral load (Rasheed 1996). Another current hypothesis about the mechanism of intrapartum MTCT involves a breakdown in the maternal‐fetal barrier followed by maternal to fetal micro‐transfusions through the placenta. These are thought to occur mostly during the first stage of labour (Kwiek 2006).
Late Postnatal/Breastfeeding Transmission
Late postnatal breastfeeding transmission is defined as HIV infection in breastfeeding infants who seroconvert after 4 weeks of age with proof of negative HIV testing prior to 4 weeks of age. The risk of HIV transmission through breast milk is of particular importance as most of the world’s HIV‐infected women live in areas where formula feeding is not affordable, feasible, available, sustainable, or safe. Breastfeeding HIV transmission is responsible for a large burden of MTCT. This is illustrated by a randomized controlled trial in a resource‐limited, breastfeeding population which showed that among all infants exposed to breast milk, breastfeeding transmission accounted for approximately 44% of infant HIV infections (Nduati 2000). In this same study, the rate of HIV transmission rates among breastfeeding infants was significantly higher than those in the non‐breastfeeding arm at the six‐week timepoint, with a substantial number of infections occurring early in breastfeeding (Nduati 2000).
HIV has been detected in both cell‐free and cellular fractions of human breast milk and colostrum (Nicoll 1995). With constant infant exposure to HIV through breastfeeding, the proportion of HIV‐infected infants through MTCT correlates with the duration of breastfeeding (Taha 2007). MTCT is enhanced in women with higher maternal breast milk viral loads and lower maternal CD4 counts (Richardson 2003). Maternal breast abnormalities such as cracked nipples or breast abscesses (Nicoll 1995) also contribute to MTCT, as well as younger maternal age and higher parity (Horvath 2009).
Description of the intervention
As our understanding evolves of how to prevent MTCT optimally, interventions previously thought to be the standard of care may no longer be recommended. This section describes historical interventions that have sequentially lead up to the use of ART, the current standard of care, to prevent MTCT.
Caesarean Section
Because exposure of fetal skin and mucous membranes to cervicovaginal secretions and maternal blood in the genital tract is implicated in intrapartum MTCT, delivery via caesarean section would be expected to decrease MTCT through avoiding these exposures. An individual patient data meta‐analysis evaluated a population of pregnant women that primarily did not receive antiretroviral drugs during pregnancy (only 30% of women received antiretroviral prophylaxis). This study showed that compared to no intervention, elective caesarean section (ECS)‐ defined as caesarean section before labor and before ruptured membranes ‐ was associated with more than a 50% lower rate of MTCT (adjusted OR [aOR] 0.43, 95% confidence interval [CI]: 0.33‐0.56) compared to other modes of delivery. However, in the study subset that received antiretroviral drugs (likely AZT prophylaxis alone), the association between ECS and MTCT of HIV persisted even after adjusting for receipt of antiretroviral drugs, maternal HIV disease stage, and infant birth weight (International Perinatal HIV Group (IPHG) 1999). Women in this study who received antiretroviral drugs and who underwent ECS had an 87% lower rate of MTCT (aOR 0.13, 95% CI: 0.09‐0.19).
When the question of ECS was further examined in a randomized controlled trial of a population that primarily received antiretroviral prophylaxis during pregnancy, similar findings were reported. The women in the study population were relatively immunocompetent (over 90% of the women in both arms had a CD4 count of >200 cells/mm3), and primarily received AZT prophylaxis (only 36% did not receive antiretroviral drugs during pregnancy). The participants were then randomized to caesarian section before labor and before rupture of membranes versus vaginal delivery. In an intention‐to‐treat analysis, ECS decreased the odds of MTCT by 80%, (OR 0.2, 95% CI: 0.1‐0.6) (European Mode of Delivery Collaboration‐ EMDC 1999). Both the meta‐analysis on individual patient data and the clinical trial were conducted before plasma HIV‐1 RNA concentrations (viral load) were routinely available.
In considering ECS as a means of preventing MTCT, one must remember the risk of morbidity to mother and infant. In the EMDC study above, there were no significant differences in postpartum complications between arms (European Mode of Delivery Collaboration‐ EMDC 1999). ECS is also not widely available in resource‐limited settings. Generally, however, where caesarean section is available and safe, ECS for reducing MTCT outweighs the risks of complications in women whose viral loads are poorly controlled or who have not taken antiretrovirals (Read 2005). In fact, the United States Public Health Task Force recommends consideration of a scheduled caesarean section near the time of delivery for HIV‐1 infected pregnant women who have a plasma viral load greater than 1000 copies/mL (Perinatal HIV Guidelines Working Group, 2009).
It is important to note that the studies presented above suggesting a reduction in MTCT with ECS utilized data obtained before data on maternal viral load were routinely collected. They also were not done in the era of combination antiretroviral regimens for prophylaxis or treatment. Some analyses suggest a continued benefit of ECS among women with low viral loads or women receiving combination antiretroviral regimens. For example, in a retrospective cohort study from the United Kingdom, when modeling adjusted for viral load compared planned and unplanned vaginal deliveries against ECS (the reference group, aOR=1), there was an increase in odds of MTCT in those with any vaginal delivery (aOR 2.4, 95% CI: 1.08‐5.35, P=0.033) (Townsend 2008). The utility of ECS in women with ART and/or undetectable viral loads was also addressed in a prospective cohort study from 29 centers in Europe. A subgroup analysis of the mother‐infant pairs showed that among 560 women with undetectable HIV RNA levels, ECS (when compared with vaginal or emergency caesarean delivery) resulted in a 93% reduction in MTCT (OR 0.07; 95% CI: 0.02‐0.31, P=0.0004) (European Collaborative Study (ECS) 2005). While an impressive effect size, due to small numbers of infected children, the values were not adjusted for maternal antiretroviral use. When this factor was considered (none vs. any), there was a 48% reduction in MTCT when ECS was compared with vaginal delivery or emergency caesarian section, although the difference between groups was no longer statistically significant (aOR 0.52; 95% CI: 0.14‐2.03, P= 0.358). However, other studies from North America have not shown statistically significant differences in transmission according to mode of delivery when controlling for antiretroviral use and/or maternal viral load. Thus, the benefit of ECS among women receiving combination antiretroviral regimens or among women with low viral loads remains unclear (Read 2005).
Single Antiretroviral Drug Regimens for Prophylaxis of MTCT
The landmark PACTG 076 study showed that maternal antepartum and intrapartum antiretroviral prophylaxis, as well as postnatal antiretroviral prophylaxis to the infant could reduce MTCT (Connor 1994). Published in 1994, this study showed that use of AZT prophylaxis during the antepartum, intrapartum, and postnatal periods decreased MTCT by 67.5% (95% CI: 40.7% ‐82.1%) (Connor 1994). Specifically, AZT prophylaxis was initiated in HIV‐infected pregnant women from 14 to 34 weeks gestation, during labour and delivery, and then was given post‐partum to their infants until six weeks of age (Connor 1994). This study confirmed the theory that maternal antiretroviral prophylaxis to HIV‐infected women during pregnancy might provide a form of pre‐ and post‐exposure prophylaxis to the infant by reducing maternal viral load and/or by decreasing fetal exposure to HIV in utero and at delivery (Connor 1994). However, the PACTG 076 regimen was felt to be cumbersome and too costly for developing‐world settings and other more feasible strategies for preventing MTCT were investigated. A randomized controlled trial of short course AZT in a non‐breastfeeding population in Thailand showed that starting AZT at 36 weeks decreased MTCT by 50% (from 18.9% to 9.4%) compared with placebo (P=0.006) (Shaffer 1999). This study suggested that practical, effective, shorter, and more affordable regimens also offered some degree of protection against MTCT.
The question of the ideal time to start AZT prophylaxis remained, however, as well as whether antepartum antiretroviral prophylaxis to the mother was more important than postnatal antiretroviral prophylaxis to the infant. A randomized controlled trial in a non‐breastfeeding population in Thailand evaluated the equivalence of four regimens of AZT prophylaxis starting at 28 weeks of gestation, and six weeks postnatal infant therapy (the long‐long regimen) versus AZT starting at 35 weeks gestation, with three days of postnatal infant therapy (the short‐short regimen) (Lallemant 2000). A long‐short and a short‐long regimen also were evaluated. The short‐short regimen was stopped early when, at interim analysis, it showed statistically more transmissions than the long‐long arm (10.5% vs. 4.1%, respectively) (P=0.004). The efficacy of long‐long, long‐short, and short‐long were all equivalent in preventing MTCT, but a subanalysis indicated that there were significantly more in utero MTCT events in the short‐long regimen (5.1%) compared with the longer maternal prophylaxis (1.6%). This finding suggested that a longer infant regimen could not be a substitute for a longer maternal prophylaxis (Lallemant 2000).
In an attempt to expand the antiretroviral prophylaxis options for MTCT, other drug regimens were evaluated. The HIVNET 012 randomized controlled trial (Guay 1999) was performed in a breastfeeding population of HIV‐1 infected pregnant women in Uganda with a median CD4 count of 426 cells/mm3 (AZT arm) and 461 cells/mm3(NVP arm). This study compared the efficacy of single‐dose nevirapine (sdNVP) to AZT at the onset of labour in preventing MTCT. There was no difference in transmission in the two arms at birth, but there were significantly fewer infected infants at age six to eight weeks in the NVP versus AZT arms (21.3% vs. 11.9% respectively, P=0.0027). This regimen was found to be useful for preventing MTCT in women who were diagnosed with HIV during labour and delivery (Guay 1999).
Dual Antiretroviral Drug Regimens for Prophylaxis of MTCT
The search for practical and effective antiretroviral regimens to prevent MTCT for the developing world expanded to include dual drug regimens for prophylaxis of MTCT. The PETRA study, a randomized controlled trial performed in three breastfeeding populations in Africa, showed that a combination of 3TC and AZT was effective at six weeks in decreasing MTCT to 5.7%. However, this transmission benefit disappeared by 18 months and was thought to be due to late‐postnatal MTCT via breastfeeding (PETRA 2002). Because a prolonged transmission benefit up to 12 months was seen in the HIVNET 012 NVP regimen, the Petra AZT/3TC intra/post partum regimen was compared with HIVNET 012 NVP regimen in the SAINT trial (Moodley 2003) to identify if NVP offered protection against early breastfeeding transmission. There was no difference in MTCT between these single and dual prophylaxis regimens (Moodley 2003).
Triple Antiretroviral Drug Regimens for Prophylaxis of MTCT
In the search for effective means of lowering maternal viral load during pregnancy, triple antiretroviral prophylaxis became the standard of care to prevent MTCT in HIV‐infected pregnant women in resource‐rich settings. Cohort studies evaluated combinations of interventions to reduce MTCT and confirmed that any antiretroviral use in pregnancy provided a gradient effect: three drug combination antiretroviral prophylaxis regimens were the most effective in preventing MTCT (transmission 1.2%, 95% CI: 0‐2.5%), then dual prophylaxis (3.8%, 95% CI: 1.1‐6.5%), followed by AZT prophylaxis alone (10.4%, 95% CI: 8.2‐12.6%), and finally no antiretroviral drugs (20.0%, 95% CI: 16.1‐ 23.9%) (Cooper 2002).
Different antiretroviral regimens have been evaluated for efficacy, and cohort studies have not shown any difference in MTCT rates between non‐nucleoside reverse transcriptase inhibitor‐based and protease inhibitor (PI)‐based regimens where antiretroviral drugs are used for prophylaxis of MTCT (60% of the women in Townsend 2008 had CD4 counts >350 cells/mm3). Townsend 2008 also showed that even after adjusting for viral load and mode of delivery, antiretroviral drugs initiated at conception or earlier in delivery were associated with reduced MTCT, with an adjusted OR of 0.90 for each week of antiretrovirals received (Townsend 2008).
The Evolution of Antiretroviral Therapy for a Pregnant Woman's Own Health
As discussed above, the landmark Pediatric Aids Clinical Trial Group (PACTG) Protocol 076 study published in 1994 showed that use of AZT prophylaxis during the antepartum (starting at 14 weeks), intrapartum, and postnatal periods (AZT was given to infants until six weeks of age) decreased MTCT by 67.5% (95% CI: 40.7%‐82.1%) (Connor 1994). This study confirmed an important theory that maternal antiretroviral prophylaxis during pregnancy might provide a form of pre‐ and post‐exposure prophylaxis to the infant by reducing maternal viral load and/or by decreasing fetal exposure to HIV in utero and at delivery (Connor 1994).
A subsequent analysis of data from a randomized controlled trial suggested that maternal plasma viral load was the best predictor of MTCT, and antiretroviral prophylaxis that decreased maternal viral load to less than 500 copies/mL minimized the risk of vertical HIV transmission while improving the mother’s own health (Mofenson 1999). Further studies evaluated MTCT at low viral loads (<1000 copies/mL) and found that with relative viral suppression, MTCT occurred in only 1% of women who received antiretroviral prophylaxis. These findings argued for the use of triple antiretroviral prophylaxis in pregnancy to reduce MTCT. This finding was especially important as antiretroviral prophylaxis in pregnant women was found to offer additional clinical protection against MTCT beyond the reduction in viral load (Ioannidis 2001). However, it is important to note that despite the protective effect of antiretroviral drugs, MTCT still can occur at any viral load, making no degree of viral suppression a reliable predictor of protection from MTCT (European Collaborative Study (ECS) 1999).
The optimal timing for initiation of therapy in adults has been controversial. Recent results from the CIPRA‐HT001 randomized controlled trial in Haiti suggest there are both mortality and morbidity benefits for initiating ART in adults before CD4 cell counts fall below 200 cells/mm3 (Fitzgerald 2009). CIPRA‐HT001 compared death and tuberculosis rates in patients with CD4 counts between 200‐ 350 cells/mm3 with either immediate or. delayed (CD4 <200 cells/mm3) ART. There was a mortality benefit of immediate therapy, with a hazard ratio [HR] of 4 for death (P=0.0011) and a HR of 2 for the development of tuberculosis (delayed vs. early therapy, P=0.0125) (Fitzgerald 2009). Although this was not a pregnant population, the results are still widely applicable and influenced the revision of the recent WHO guidelines to recommend ART for the pregnant woman's own health at any gestational age with a maternal CD4 count of less than 350 cells/mm3 (WHO 2009).
Infant‐Only Prophylaxis
Infant‐only prophylaxis has been evaluated in settings where antepartum HIV counseling and testing are not available and where timing does not allow NVP to be given more than two hours before delivery. In the NVAZ randomized, open‐label study, infants whose mothers had not received antiretrovirals were randomized to sdNVP at birth versus sdNVP + AZT for 7 days. In the infants who were HIV‐negative at birth, transmission rates were lower in the combination arm versus NVP (15.3% vs. 20.9%, P=0.03) at birth and at 6‐8 weeks (7.7% vs. 12.1%, P=0.03) (Taha (NVAZ) 2003). However, the benefit in decreasing transmission with dual infant prophylaxis after delivery is not present if the mother received intrapartum sdNVP prophylaxis (Taha 2004).
Maternal Side Effects from Antiretrovirals
Although grouped by class, each individual antiretroviral medication has an independent side‐effect profile, and some maternal side‐effects are specific to the regimen received. A full discussion of antiretroviral‐related side‐effects is beyond the scope of this review, but salient adverse effects are reviewed briefly.
Overall, maternal antiretrovirals during pregnancy are well‐tolerated. A large U.S. clinical trial found that moderate symptoms or laboratory abnormalities occurred in only 5% of HIV‐infected pregnant women who receive ART (Watts 2004). Major concerns with the use of antiretrovirals in pregnancy are anemia, hepatotoxicity, hyperglycemia/gestational diabetes, and resistance when single agents are used. AZT is often associated with treatment‐limiting anemia; however, a large cohort study also showed in a multivariate analysis that any antiretroviral use in pregnancy (both early and late) was associated with anemia (OR 1.35, 95% CI: 1.06‐2.42) (Tuomala 2005). In a toxicity analysis from an international randomized controlled trial, 4.6% of patients receiving long‐term protease inhibitor (PI)‐based therapy developed gestational diabetes, which was significantly higher than in the late monotherapy reference group (1.4%, P=0.038) (Watts 2004). Also, maternal use of single antiretroviral agents was associated with a greater risk of maternal resistance than were combination regimens (McIntyre 2009).
Use of NVP‐based regimens is cautioned in women with a CD4 count greater than 250 cells/mm3 because of the risk of hepatoxicity (Hitti 2004). In a clinical trial of NVP with AZT and 3TC, all NVP‐related treatment limiting toxicities (5/17, 29% of NVP recipients) occurred in women with CD4 counts greater than 250 cells/mm3, with one case of fatal fulminant hepatic failure and death (Hitti 2004). However, a retrospective Italian analysis of pregnant HIV‐infected women did not confirm the relation between high CD4 counts and increased rates of hepatotoxicity. This study showed that rates of grade 3‐4 hepatic toxicity actually occurred in fewer women with CD4 counts greater than 250 cells/mm3 (5.9% [34 of 573] patients) than in women with CD4 counts of less than 250 cells/mm2 (9.4% [12 of 128] patients) (Marazzi 2006). It is important to note that like the Hitti study, the only NVP‐related fatality in the Marazzi study occured in a women whose CD4 count was greater than 250 cells/mm3. It is currently not known whether pregnancy increases the risk of NVP‐related hepatotoxicity.
Infant Side Effects from Maternal Antiretrovirals
There is no evidence to suggest that exposure to ART in utero causes congenital abnormalities, but cohort studies suggest that infant exposure to maternal antiretrovirals can be associated with preterm birth (European Collaborative Study (ECS) 2000) and anemia (European Collaborative Study (ECS) 2003). The relation between antiretrovirals during pregnancy and infant low birth weight is disputed. The majority of cohort studies suggest that antiretrovirals are not associated with infant low birth weight (Szyld 2006 and Schulte 2007); however, some studies have shown that PI‐based antiretroviral regimens are associated with very low birth weight (Tuomala 2002). Infant resistance to antiretrovirals is another side effect that can occur when single‐drug regimens are given to the infant to prevent intrapartum and post‐natal transmission (McIntyre 2009). Because many of these studies cannot identify specific regimens or eliminate confounding, it is important to gain further information from randomized controlled trials.
Other Interventions
Previous Cochrane reviews have critically evaluated other interventions to decrease MTCT. There was no evidence of an effect of vaginal disinfection on the risk of MTCT (Wiysonge 2005). Observational data have suggested an association between vitamin A deficiency and MTCT; however, a Cochrane review of this subject did not show that antenatal vitamin A supplementation had an effect on MTCT. Given the wide confidence intervals of the review's pooled effect estimate, neither a beneficial nor a harmful effect could be excluded (Wiysonge 2005a). Hyperimmune HIV immunoglobulin, when used with AZT, did not have any additional effect on decreasing MTCT above AZT alone (Bond 2007). Complete avoidance of breastfeeding has also been advocated as a means of preventing MTCT; however, the risk of MTCT needs to be balanced with the infant’s risk of malnutrition and non‐HIV‐related morbidity (WHO (Infant Feeding) 2009).
How the intervention might work
Evidence from microbiology, pathology, and clinical experience suggests that MTCT occurs during three major timepoints during pregnancy and the postpartum period: in utero, intrapartum, and during breastfeeding. Strategies to reduce MTCT focus on these timepoints and include maternal and infant use of antiretrovirals, caesarean section before onset of labour or rupture of membranes, and complete avoidance of breastfeeding.
Why it is important to do this review
Now that ART is the standard of care for preventing MTCT in women who need therapy for their own health (WHO 2009) it is important to investigate individual regimens, their efficacy in the pregnant HIV‐infected population, and their specific side‐effect profiles in both the mother and the infant. The cohort studies discussed above, although valuable for their insights on the global efficacy of combination therapy in preventing MTCT, were not able to distinguish between the efficacies of specific regimens and their side effects. It is also important to evaluate what is known from the current literature about what ART regimen to start and when to start ART in eligible HIV‐infected pregnant women in terms of specific laboratory parameters and gestational age.
Objectives
Our objective was to assess the current literature regarding the treatment of HIV infection in clinically or immunologically eligible pregnant women with ART, the current standard of care. This assessment includes an evaluation of the optimal time to start therapy in relation to the woman’s laboratory parameters and gestational age. It also includes an analysis of which specific antiretroviral medications to start in women who are not yet on therapy, and which agents to continue in women who are already on therapy.
Methods
Criteria for considering studies for this review
Types of studies
1) Randomized controlled trials
2) Observational studies (including case series) when randomized controlled trial data were limited, incomplete, or unavailable
Types of participants
HIV‐infected pregnant women who require ART for their own health * according to guidelines current at the time the search strategy was designed (WHO 2006):
1) CD4 testing available:
‐start ART when CD4 count <200 cells/mm3 and WHO Clinical Stages 1 or 2
‐start ART when CD4 count <350 cells/mm3 and WHO Clinical Stage 3
2) CD4 testing not available
‐start ART at WHO Clinical Stages 3 or 4
* given the paucity of randomized controlled clinical trial data for this population, randomized controlled trials were accepted that included women with CD4 counts ranging from 200‐500 cells/mm3 since this population contained the pre‐specified population of interest
Types of interventions
1) Use of a clearly defined ART regimen
2) Use of a comparison group
Types of outcome measures
Outcomes of interest
Maternal outcomes:
1. Maternal mortality at 1 and 2 years, and later time points when available
2. Severe adverse events (excluding death) including: hepatotoxicity in women given NVP (CD4 250‐350 cells/mm3 and >350 cells/mm3), renal toxicity with tenofovir, all other grade 3 or 4 severe adverse events
3. Response to antiretrovirals: clinical, immunological, virological
4. Development of resistance resulting in antiretroviral discontinuation or virological failure, as defined by the study authors
5. Adherence and tolerability to treatment, and retention in care
Infant outcomes:
1. All grade 3 or 4 severe adverse events as well as prematurity, stillbirth, low birth weight, and teratogenicity
2. Infant HIV‐free survival at 6 weeks and 18 months
3. MTCT of HIV at 6 weeks and 18 months, noting other time points as defined by study authors
4. Resistance to subsequent antiretrovirals
Search methods for identification of studies
See Cochrane HIV/AIDS Group search strategy.
Electronic searches
See Table 14 for our strategies in searching Cochrane CENTRAL, EMBASE and PubMed, LILACS, and Web of Science/Web of Social Science.
1. Search Strategies for Full‐Text Articles.
| PMTCT searches |
| Publication Date from 1994/01/01 to 2009/06/17 |
|
PubMed: HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) AND randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR observational [tw] OR cohort studies [mh] OR case‐control studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR controll* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) AND (MOTHER‐TO‐CHILD TRANSMISSION) OR (MOTHER TO CHILD TRANSMISSION) OR (ADULT TO CHILD TRANSMISSION) OR (ADULT‐TO‐CHILD TRANSMISSION) OR (MATERNAL TO CHILD TRANSMISSION) OR (MATERNAL‐TO‐CHILD TRANSMISSION) OR (VERTICAL TRANSMISSION) OR (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR PMTCT OR (Infectious Disease Transmission, Vertical/prevention and control[Mesh] AND HIV Infections/prevention and control[Mesh]) OR (mother[tw] AND HIV Infections/prevention and control[Mesh]) OR ((infant[tw] OR baby[tw]) AND HIV Infections/prevention and control[Mesh]) 8081 |
|
EMBASE: (((('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp)) OR (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp))) OR ((('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp)) OR (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/exp)))) OR ((((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp) OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp)) OR (('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp) OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp))) OR ((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp) OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp)) OR (('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp) OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/exp))))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) AND 'mother‐to‐child transmission' OR 'mother to child transmission' OR 'adult‐to‐child transmission' OR 'adult to child transmission' OR 'maternal‐to‐child transmission' OR 'maternal to child transmission' OR ('vertical transmission'/exp OR 'vertical transmission'/exp) OR ('vertical disease transmission' OR mtct OR pmtct OR 'perinatal transmission') AND [embase]/lim AND [1994‐2009]/py 5815 |
|
WEB OF SCIENCE, WEB OF SOCIAL SCIENCE: (TS=HIV OR TS=HIV/AIDS OR TS=AIDS) AND (TS=Mother‐to‐child transmission OR TS=vertical transmission OR TS=mother OR TS=infant OR TS=baby OR TS=perinatal OR TS=postnatal OR TS=prenatal OR TS=antenatal) AND Document Type=(Article OR Meeting Abstract OR Meeting Summary OR Meeting‐Abstract OR Proceedings Paper) Databases=SCI‐EXPANDED, SSCI Timespan=1994‐2009 4697 |
|
LILACS: (HIV OR VIH OR AIDS OR SIDA OR HIV/AIDS) AND (mother‐to‐child OR PMTCT OR MTCT OR mother OR baby OR infant OR vertical) 942 |
|
COCHRANE “CENTRAL”: (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (VIRAL SEXUALLY TRANSMITTED DISEASES) AND (MOTHER‐TO‐CHILD TRANSMISSION) OR (MOTHER TO CHILD TRANSMISSION) OR (ADULT‐TO‐CHILD TRANSMISSION) OR (ADULT TO CHILD TRANSMISSION) OR (MATERNAL‐TO‐CHILD TRANSMISSION) OR (MATERNAL TO CHILD TRANSMISSION) OR (MTCT OR PMTCT) OR (PERINATAL TRANSMISSION) OR (VERTICAL TRANSMISSION) OR (VERTICAL DISEASE TRANSMISSION) 225 |
|
Gross: 13,945 Net, after duplicates removal and first broad cut: 3,335 |
Electronic searches were undertaken using the following databases: CENTRAL, EMBASE and PubMed, LILACS, and Web of Science/Web of Social Science. Hand searches of the reference lists of all pertinent reviews and studies found also were undertaken. Abstracts from numerous relevant conferences, including the International AIDS Conferences and the annual Conferences on Retroviruses and Opportunistic Infections, were searched. Experts in the field of HIV prevention were contacted to locate any further studies or relevant conference proceedings not included in the databases to ensure that unpublished studies were included. The search strategy was iterative. There were no restrictions on language.
With regard to the electronic literature search, the optimal sensitive search strategy developed by The Cochrane Collaboration and detailed in the Cochrane Reviewers’ Handbook (Higgins 2008) was used, in conjunction with search terms identified in Table 14, to identify relevant studies from January 1, 1994 to June 17, 2009.
Searching other resources
A comprehensive electronic search was undertaken to find relevant conference abstracts. Using a comprehensive database of HIV/AIDS conference abstracts, we searched 1st‐4th IAS Pathogenesis (2001‐2009); 10th‐17th IAC (1994‐2008); 1st‐16th CROI (1994‐2009); US National HIV Prevention Conference ('99, '03, '05); 7th‐14th BHIVA (2001‐2008); and 8th‐9th European AIDS Society Conference (2001, 2003), using these terms:“3TC, ABC, AZT, ZDV, d4T, TDF, FTC, NRTI, NNRTI, nucleoside, nucleotide, protease, DLV, EFV, ETR, NVP, APV, ATV, DRV, IDV, LPV, RTV, NFV, TPV, T‐20, MVC, Atripla, lamivudine, abacavir, zidovudine, stavudine, zalcitabine, didanosine, emtricitabine, epzicom, kivexa, Trizivir, Combivir, Truvada, delavirdine, efavirenz, nevirapine, amprenavir, fosamprenavir, atazanavir darunavir, indinavir, lopinavir, ritonavir, saquinavir, tipranavir, enfurvitide, maraviroc, raltegravir, tenofovir, breast, mother, infant, baby, pregnant, pregnancy, perinatal, postnatal, feeding, breastfeeding, vertical, mtct, pmtct, "when to start" OR timing OR ("early" AND "initia*")”
Data collection and analysis
The search for studies was performed with the assistance of the Cochrane HIV/AIDS Group. No tables of comparisons describing qualitative or quantitative methods used to combine data were necessary as the individual studies were too diverse to pool and were each analyzed independently.
Selection of studies
The authors performed the selection of potentially relevant studies. The titles, abstracts and descriptor terms of all downloaded material from the electronic searches were read and irrelevant reports discarded to create a pool of potentially eligible studies. All identified citations then were evaluated independently to establish relevance of the article and whether or not the full article should be acquired. If there was uncertainty, the full article was obtained.
Data extraction and management
Using a pre‐designed data abstraction form, the authors extracted relevant data from each of the included studies.
Assessment of risk of bias in included studies
The quality of randomized controlled trials was assessed independently by the authors [A.S.S., E.K.D.] using guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) and is displayed in Figure 1 and Figure 2. The quality of observational studies was assessed independently by the authors [A.S.S., E.K.D.] using the Newcastle‐Ottawa Quality Assessment Scale (Newcastle‐Ottawa). Where data was incomplete, A.S.S. and E.K.D. attempted to contact the study’s primary authors.
1.
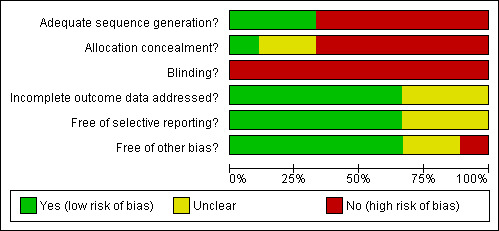
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
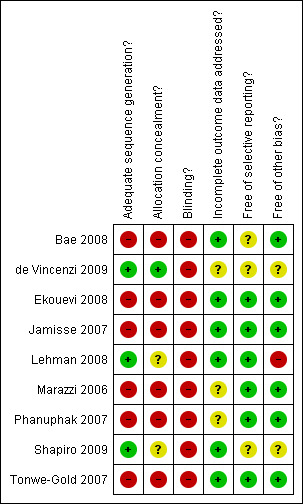
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Data synthesis
Data synthesis was performed independently by the authors [A.S.S., E.K.D.] and discussed with a statistician. Unpublished data for the Mma Bana and Kesho Bora trials were provided to the authors of this review to facilitate presentations to the WHO guideline review committee. However, only data presented in authors' published abstracts at the 2009 International AIDS Society meeting were used in this publication. In the case of the Kesho Bora abstract where only numerators and proportions were available, denominators were estimated statistically with the assistance of a statistician (Higgins 2008).
Results
Description of studies
Randomized Controlled Trials
The Kesho Bora study conducted by deVincenzi and colleagues included a randomized controlled trial (RCT). The total enrolment was 1140 HIV‐infected pregnant women in five sites in Burkina Faso, Kenya and South Africa, of whom 824 were enrolled in the clinical trial (de Vincenzi 2009). Women with CD4 counts between 200 and 500 cells/mm3 were randomized to triple antiretroviral prophylaxis during pregnancy and breastfeeding (AZT/3TC/LPV‐r from 28‐36 weeks of pregnancy until six months post partum) compared to short‐course prophylaxis (AZT from 28‐36 weeks until labour, AZT/3TC and sdNVP at onset of labour, with AZT/3TC one week after delivery) to prevent MTCT of HIV‐1. The mean CD4 count in both arms was 335 cells/mm3. Cohort groups were comprised of 119 women with CD4 <200 cells/mm3 or HIV Stage 4 who received AZT/3TC/NVP from 16 weeks + infant sdNVP, and 129 women with CD4 >500 cells/mm3 and HIV Stage <4 who received AZT from 28‐36 weeks until labour with maternal/infant sdNVP at onset of labour. The remaining 31 women were allocated to short‐course prophylaxis. The authors’ primary endpoints were infant HIV‐free survival at 6 weeks and 12 months (Table 1 and Table 2).
Lehman and colleagues conducted a RCT of HIV‐1‐infected pregnant women who presented to an antenatal clinic in Nairobi, Kenya with a primary outcome of comparing the effect of two antiretroviral regimens on breast milk HIV‐1 viral load (Lehman 2008). The reported study is a secondary analysis of resistance in the initial RCT. Pre‐specified inclusion criteria were: intent to breastfeed, hemoglobin greater than 8g/dL, no previous exposure to antiretrovirals, and age greater than 18 years. Exclusion criteria included having a CD4 count of <200 or >500 cells/mm3. At 34 weeks gestation, women were randomized to one of two arms. In the triple antiretroviral arm, AZT/3TC/NVP were given twice daily from 34 weeks until 6 months after delivery. In the AZT/sdNVP arm, AZT was given twice daily from 34 weeks gestation and intrapartum, while NVP was given as a single oral dose at the onset of labour with 2mg/kg to the infant after delivery in a single dose within 72 hours of delivery. Fifty‐eight women were randomized, and mean CD4 counts for each arm were not provided. During follow‐up, maternal blood was collected at 35 and 37 weeks gestation, delivery, 1, 3, 6, 9, and 12 months post‐partum. Women were included in the sub‐study if they had a plasma sample available from three months after treatment cessation. Plasma samples were frozen and shipped to Seattle, WA where allele‐specific PCR was performed to detect K103N and Y181C mutations (Table 3).
The Mma Bana study is a RCT comparing two different triple antiretroviral regimens in HIV‐infected pregnant women who intended to breastfeed their infants (Shapiro 2009). This study was performed at four sites in Botswana. Enrolled were 730 women, with 560 in the randomized arms and 170 in the observational arm. HIV‐infected pregnant women with CD4 counts greater than or equal to 200 cells/mm3 were randomized to AZT, 3TC, and abacavir (ABC) vs. AZT/3TC/LPV‐r from 26‐34 weeks gestation. An observational group was composed of women with CD4 counts of <200 cells/mm3, who received AZT/3TC/NVP from 18‐34 weeks gestation. All regimens were continued through delivery with plans for weaning by six months post‐partum. All infants received sdNVP and one month of AZT. Inclusion criteria for randomization included: a CD4 count of 200‐500 cells/mm3 and absence of an AIDS‐defining illness. The mean CD4 count in the AZT/3TC/ABC arm was 398 cells/mm3 and the mean CD4 count in the AZT/3TC/LPV‐r arm was 403 cells/mm3. The authors' primary endpoints were maternal HIV‐1 RNA suppression to <400 copies/mL among randomized arms at delivery and through breastfeeding, and MTCT rates in the overall study population (Table 4 and Table 5).
Cohort Studies
In Botswana (Bae 2008), researchers conducted a nested cohort study within a RCT in which HIV‐1 infected pregnant women were referred for enrollment in the Mashi study (Thior 2006). Eligibility criteria included gestational age of 33‐35 weeks, WHO clinical stage 2 or 3 and CD4 T cell count of <350 cells/mm3, or WHO clinical stage 4 or CD4 cell count <200 cells/mm3. Once ART became available in October 2002, ART‐eligible women were assigned to the AZT/3TC/NVP arm. The 69 women in the AZT/3TC/NVP arm had a median CD4 count at enrollment of 132 cells/mm3, and the 109 women in the ART‐unexposed arm had a median CD4 count of 146 cells/mm3. Of the ART‐eligible women, 69 received AZT/3TC/NVP twice daily at 34 weeks with supplemental AZT during delivery and 109 received short‐course regimens (AZT twice daily starting at 34 weeks, with either sdNVP or placebo in labour) while ART availability was delayed. All live‐born infants of these women were selected for the analysis. Both ART exposed and unexposed (the short‐course arm) infants were divided into subgroups based on feeding strategies. Infants received either one month of AZT prophylaxis with formula feeding or AZT for the duration of breastfeeding. A complete blood count was obtained on all infants at birth and at one, four, and seven months of age. The authors' primary outcome was to assess hematologic and hepatic toxicities associated with in utero and breastfeeding exposure to maternal triple ART (Table 6).
Ekouevi 2008 is an observational study in a breastfeeding population in the Ivory Coast. This study enrolled HIV‐1 infected pregnant women who were referred to the ANRS Ditrame‐Plus or MTCT‐Plus projects. Eligibility criteria included WHO clinical stages 2 or 3 and CD4 T cell count of <350 cells/mm3, or WHO clinical stage 4 or CD4 cell count of <200 cells/mm3. Of 326 HIV‐infected pregnant women, 175 received a short‐course regimen and 151 received ART. There were 168 women who received ART, with a mean CD4 count of 182 cells/mm3. There were 190 women who received a short course regimen, with a mean CD4 count of 177 cells/mm3. In the Ditrame‐Plus Study, ART was not yet available for pregnant women, thus eligible mothers received either intrapartum sdNVP after short course AZT initiated at 36 weeks gestation, or short course AZT/3TC initiated at 32 weeks gestation until 3 days postpartum (short course group). In the MTCT‐Plus project, eligible women received AZT/3TC/NVP (or stavidine [d4T], 3TC, and NVP) antepartum, during labour and after delivery (ART group). In both groups, infants received AZT syrup for 7 days + sdNVP syrup on day 2 or 3. Women were counseled to either replacement feed or to practice exclusive breastfeeding for 4‐6 months (Table 7).
The Jamisse 2007 observational study was conducted in Maputo, Mozambique. All pregnant women were offered HIV‐1 testing and HIV‐1 seropositive women were referred to the study hospital for CD4 cell count testing. Women who met eligibility criteria were offered enrollment in an observational study. Eligibility criteria included: greater than ≥16 weeks gestational age, >18 years of age, ART naive, and CD4 cell count < 350 cells/mm3. From August 2004 through June 2005, 163 HIV‐1 positive pregnant women were enrolled. Study participants returned 2 weeks after enrollment to begin antiretrovirals. 146 women received AZT/3TC/NVP as a first‐line regimen. For women with hemoglobin <8.5g/mL, d4T was substituted for AZT. This substitution was also made if AZT was not available. Efavirenz replaced NVP in the case of NVP toxicity. Participants were seen monthly until 34 weeks of gestation, after which follow‐up occurred every 2 weeks until delivery. Post‐partum visits occurred 1 week after delivery and then monthly until 6 months from the time of the initiation of antiretrovirals. Blood samples were taken at 1‐2 weeks and 1, 2, and 6 months after ART initiation. Outcomes in women with CD4<250 cells/mm3 were compared with those with CD4> 250 cells/mm3. The authors’ primary endpoint was to evaluate toxicity associated with NVP‐based regimens. The data from this study were used to evaluate both when to start antiretrovirals and what regimen to use (Table 8 and Table 9).
In Marazzi's study, all women enrolled in the Drug Resources Enhancement against AIDS and Malnutrition cohort from 5/1/2002 through 7/30/2004 were evaluated retrospectively (Marazzi 2006). All pregnant, HIV‐infected women were eligible, but charts were only reviewed if the women kept follow‐up appointments through delivery, agreed to take antiretrovirals, and had been on antiretrovirals for more than 14 days at the time of study closure. 703 women were included. All pregnant HIV‐infected women were prescribed AZT/3TC/NVP from (on average) 27 weeks gestation until delivery. Women with CD4 cell counts <200 cells/mm3, a viral load of <55,000 copies/mL, or WHO stage 3 or 4 were given AZT/3TC/NVP starting from 15 weeks of gestation and indefinitely following delivery. Women with a viral load of <55,000 copies/mL and CD4 counts >200 cells/mm3 continued AZT/3TC/NVP for up to 6 months post‐partum. Pregnant HIV‐infected women with a hemoglobin of <8g/100mL were given d4T/3TC/NVP. Laboratory monitoring was obtained at baseline, at weeks 2 and 4 during the first month and every 4 weeks thereafter until delivery. The authors' primary outcome was to assess the incidence and consequences of adverse reactions when HIV‐infected pregnant women in Africa are given NVP‐based ART (Table 10).
In an observational study conducted in Thailand (Phanuphak 2007), patients (78 men, 244 pregnant women, and 87 non‐pregnant women) with HIV infection were prescribed NVP‐based regimens. Participants were deemed to be ART eligible if they had CD4 cell counts <200 cells/mm3 or <350 cells/mm3 at WHO stage >2. Pregnant HIV‐infected women with CD4>200 were prescribed NVP‐containing ART from >28 weeks until delivery (PMTCT group) with AZT/3TC continued 1 week post‐partum. Pregnant HIV‐infected women with CD4 cell counts <200 cells/mm3 or <350 cells/mm3 at WHO stage >2 were given NVP‐based ART starting from 14 weeks of gestation and indefinitely following delivery (the ART group). ART comprised either AZT/3TC/NVP or d4T/3TC/NVP. For pregnant women, laboratory monitoring was obtained at baseline, at weeks 2, 4, 6, and 8, and then every 4 weeks until delivery with clinical assessments at regular intervals (Table 11).
Tonwe‐Gold's study identified pregnant women at two community based antenatal clinics in two low‐income urban districts of the Ivory Coast were referred for enrolment in the MTCT‐Plus initiative observational study (Tonwe‐Gold 2007). Eligibility for ART included: WHO clinical stage 4 irrespective of CD4 T cell count; WHO stage 2 or 3 and CD4 T cell count <350 cells/mm3; or CD4 cell count <200 cells/mm3. 107 women began ART with the plan to continue medications post‐partum (median gestational age 30 weeks), with 102 women on AZT/3TC/NVP; 143 women received short course regimens to prevent mother to child transmission of HIV, 103 of them with short course AZT/3TC and sdNVP during labour. Pregnant women meeting eligibility criteria initiated treatment (as early as 24 weeks of gestation) with AZT/3TC/NVP (the ART group). Treatment continued during labour and postnatally. Pregnant women who were not eligible for ART received short course regimens (usually AZT/3TC) with sdNVP in labour from 32 weeks of gestation until 3 days postpartum, or short course AZT from 28 weeks, or sdNVP alone, or both short course AZT and sdNVP (short course group). Irrespective of maternal regimen, all infants received AZT syrup for 7 days and sdNVP syrup on day 3. The authors’ primary endpoint was MTCT at 4 weeks and 12 months (Table 12 and Table 13).
Results of the search
Full‐Text Article Search
After screening 13,945 citations, we removed 6,592 irrelevant citations (animal, behavioral, in vitro studies, etc.). We also removed 4,018 duplicate references. The remaining 3,335 references were hand‐screened by 2 authors. We identified 59 potentially relevant full‐text articles for closer review (Figure 3).
3.
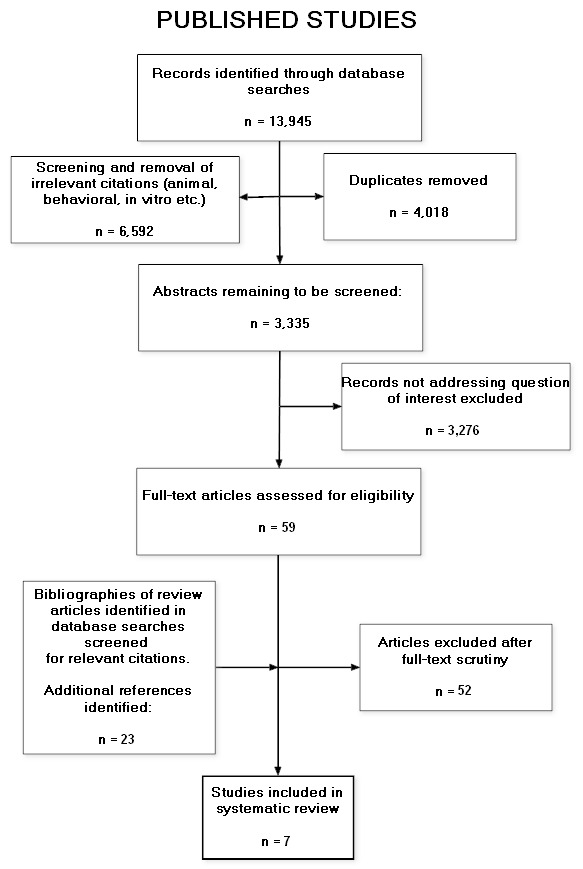
Flowsheet‐ Full‐text Articles
Conference Abstract Search
The search of conference abstracts yielded 6099 results (Figure 4).
4.
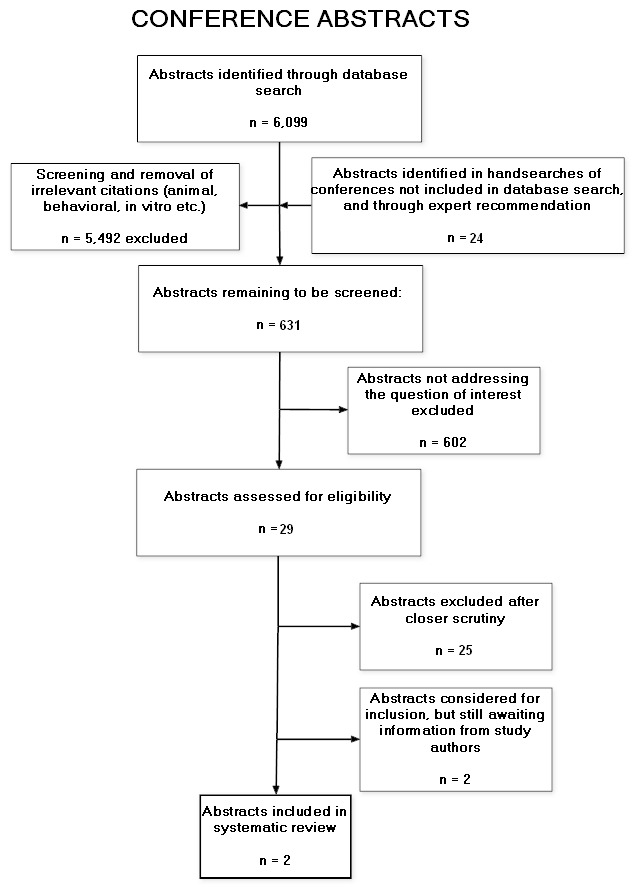
Flowsheet‐ Abstracts
Included studies
Full‐Text Inclusion
After reviewing the 59 complete references for the studies we determined that 7 studies met our inclusion criteria (Figure 3).
Conference Abstract Inclusion
We searched the 6099 conference abstract results for terms relevant to animal research, behavioural research, etc. (e.g. simian, feline, monkey, baboon, chimpanzee, rhesus, macaque, MSM, condom, behavio/ural, couples, husband, wife, boyfriend, girlfriend, violent, violence, cocaine, heroin, methamphetamine, in vitro, murine, mouse). We scanned the titles of and then excluded the resulting 5492 citations. There were then 607 net conference abstract results remaining to be screened (Figure 4). Other pertinent conference websites were hand‐searched for relevant abstracts. From this search, 24 abstracts were identified, and 4 were eligible for inclusion in the systematic review. However, 2 authors did not respond to requests for information. As such, only 2 abstracts were ultimately included in the systematic review.
Excluded studies
Full‐Text Article Exclusion
We excluded 52 full‐text articles from the systematic review, for reasons such as the following: there was no comparison group, the antiretroviral regimens were not clearly defined, some members of the study group were not pregnant (Figure 3).
Conference Abstract Exclusion
Of the 607 abstracts screened, 0 were included in the systematic review (Figure 4). From the 24 abstracts identified in the hand‐search of pertinent conference websites, 20 abstracts were excluded. Two authors did not respond to requests for information and thus their two abstracts were also excluded.
Risk of bias in included studies
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13
Infant HIV Transmission or Death
In the Kesho Bora study, there was a significant risk reduction in infant HIV transmission or death at 12 months (relative risk [RR] 0.58, CI 0.34‐0.97) in the triple antiretroviral arm (AZT/3TC/LPV‐r at 28‐36 weeks of pregnancy until 6 months post partum) compared to short course (AZT from 28‐36 weeks until labour, AZT/3TC/sd‐NVP at onset of labour, AZT/3TC one week after delivery) (de Vincenzi 2009). However, there was no significant difference in infant HIV transmission or death between the two groups at 6 months.
In Tonwe‐Gold 2007 women in the ART group received AZT/3TC/NVP post‐partum and all infants received AZT syrup for 7 days. Infant HIV transmission or death at 12 months was 12.6% (11/87) in the AZT/3TC/NVP group and 14.7% (15/102) in the short course group (either AZT, sdNVP, AZT+sdNVP, or AZT/3TC+sdNVP), (RR 0.86, CI 0.42‐ 1.77).
In Bae 2008, women in the ART group received AZT/3TC/NVP post‐partum and infants received AZT for one month vs. the duration of breastfeeding depending on randomization. This study showed a 61% reduction (RR 0.39, CI 0.18‐ 0.85) in HIV transmission or death at 7 months in the ART arm (AZT/3TC/NVP twice daily at 34 weeks with supplemental AZT during delivery) compared to a short course regimen (AZT twice daily starting at 34 weeks, with either sdNVP or placebo in labour).
Mother‐to‐Child Transmission
In Kesho Bora, there was no difference in mother‐to‐child transmission at birth (RR 0.78, CI 0.3‐ 2.1) or at 6 weeks after delivery (RR 0.69, CI 0.34‐ 1.37) between women on AZT/3TC/LPV‐r started between 28‐ 36 weeks and those on a short course regimen (de Vincenzi 2009). However, there was a significant difference in mother‐to‐child transmission at 6 (RR 0.56, CI 0.33‐ 0.99) and 12 months (RR 0.58, CI 0.34‐ 0.97) between women on AZT/3TC/LPV‐r started between 28‐ 36 weeks and those on a short course regimen.
In Shapiro 2009, there was no difference in mother‐to‐child transmission in utero (RR 0.35, CI 0.04‐ 3.34), late postpartum (RR 0.21, CI 0.01‐ 4.35), and at 6 months (RR 0.17, CI 0.02‐ 1.44) between the AZT/3TC/LPV‐r and AZT/3TC/ABC arms.
In Ekouevi 2008, mother‐to‐child transmission of HIV at 12 months occurred in 2.1% (3/141) of infants in the ART group, and 14.7% (25/170) in the short course group who were no longer receiving MTCT prophylaxis, RR 0.14 (0.04‐0.47). In Tonwe‐Gold 2007, however, mother‐to‐child transmission of HIV at 12 months occurred in 3.5% (3/86) of infants in the ART group, and 8.8% (9/102) of infants in the short course group (either AZT/3TC with sdNVP in labour from 32 weeks of gestation, AZT from 28 weeks, sdNVP alone, or AZT from 28 weeks with sdNVP) who were no longer receiving MTCT prophylaxis, RR 0.4 (0.11‐1.41). In Bae 2008, mother‐to‐child transmission also decreased significantly at 7 months when AZT/3TC/NVP was compared with a short course regimen (AZT twice daily starting at 34 weeks, with either sdNVP or placebo in labour) where women were no longer receiving antiretrovirals post‐partum (RR 0.15, 0.04‐ 0.62).
Maternal Severe Adverse Events
(Maternal severe adverse events included any general body, respiratory, gastrointestinal, reproductive, skin, or laboratory abnormality)
In the Kesho Bora study, there was no significant difference in maternal severe adverse events (RR 1.09, CI 0.77‐ 1.53) between the triple antiretroviral arm (AZT/3TC/LPV‐r at 28‐36 weeks of pregnancy until 6 months post partum) compared to short course (AZT from 28‐36 weeks until labour, AZT/3TC/sd‐NVP at onset of labour, AZT/3TC one week after delivery) (de Vincenzi 2009).
In Shapiro 2009, 14.7% (42/285) of women in the AZT/3TC/ABC arm and 11.6% (32/275) of women in the AZT/3TC/LPV‐r arm experienced a grade 3 or 4 severe adverse laboratory event, RR 0.79, (0.51‐1.21). Severe adverse events requiring treatment modification occurred in 2.5% (7/285) of women in the AZT/3TC/ABC arm and 2.2% (6/275) of women in the AZT/3TC/LPV‐r arm (RR 0.89, CI 0.3‐ 2.61).
In Jamisse 2007, all four women with severe hepatotoxicity had CD4 counts >250 cells/mm3 (p=0.02). Rates of peripheral neuropathy, or skin and hematologic toxicity did not differ by CD4 cell count group. When women on AZT/3TC/NVP were compared with d4T/3TC/NVP there was no difference in adverse events (RR 1.58, 0.6‐ 4.12). When all grade 3 or 4 severe adverse events were pooled (peripheral neuropathy, hepatotoxicity or skin and hematologic toxicity), there was no significant difference between CD4 strata (RR 1.18, CI 0.47‐ 2.97).
In Phanuphak 2007, pregnant women who received antiretrovirals for prophylaxis of MTCT (CD4 cells >250 cells/mm3) had a significantly higher rate of symptomatic hepatotoxicity (p=0.0003) than pregnant women who received ART for their own health (CD4<250 cells/mm3). When rash and hepatic grade 3 or 4 severe adverse events were combined, there was no significant difference in events between groups, RR 1.98 (0.65‐ 6.03).
In Marazzi 2006, when women on AZT/3TC/NVP who had CD4 counts of <250 cells/mm3 were compared with women with CD4 counts of >250 cells/ mm3 there was no significant difference in hepatotoxicity between the groups (RR 0.64, CI 0.34‐ 1.2).
When comparing maternal ART to short course regimens, Tonwe‐Gold 2007 showed an increase in maternal severe adverse events in the AZT/3TC/NVP arm (RR 25.33, CI 1.49‐ 430.51).
Maternal Mortality and Resistance
In Shapiro 2009, maternal mortality at six months after delivery was 0% (0/275) among women in the AZT/3TC/LPV‐r arm and 0.4% (1/285) among women in the AZT/3TC/ABC arm (RR 0.35, CI 0.01‐ 8.44).
In Lehman 2008 there was a significantly different decrease in maternal resistance with AZT/3TC/NVP administration compared to a short‐course regimen (RR 0.24, CI 0.07‐ 0.88).
Infant Severe Adverse Events
(Infant severe adverse events included any general body, respiratory, gastrointestinal, reproductive, skin, laboratory or developmental abnormality, including prematurity, stillbirth, or teratogenicity)
In the Kesho Bora study, prematurity occurred in 13.4% (54/402) of infants in the triple antiretroviral arm (AZT/3TC/LPV‐r at 28‐36 weeks of pregnancy until 6 months post partum) and 10.9% (44/403) of infants in the short course prophylaxis arm (AZT from 28‐36 weeks until labour, AZT/3TC/sd‐NVP at onset of labour, AZT/3TC one week after delivery) (RR 1.23, CI 0.85‐ 1.79) (de Vincenzi 2009). There were 30.8% (124/402) of infants in the triple antiretroviral arm and 32.5% (131/403) of infants in the short course arm with a grade 3 or 4 severe adverse event (RR 0.95, CI 0.77‐ 1.16). There was no difference in low birth weight (RR 1.46, CI 0.94‐ 2.25) or stillbirths (RR 1, CI 0.25‐ 3.95) between the arms. There was no difference in the rate of infant mortality between either arm at 6 months (RR 0.78, CI 0.43‐ 1.42) and 12 months with AZT/3TC/LPV‐r compared to short course (RR 0.63, CI 0.38‐ 1.03).
In Shapiro 2009, there was a statistically significant difference in prematurity between the arms. There were significantly more infants born prematurely in the AZT/3TC/LPV‐r arm (61/270) when compared with the AZT/3TC/ABC (42/283) arm (RR 1.52, CI 1.07‐ 2.17). Stillbirths accounted for 2.8% (8/285) of the deliveries in the AZT/3TC/ABC arm and 1.8% (5/275) of the deliveries in the AZT/3TC/LPV‐r arm, RR 0.65, CI 0.21‐ 1.96). There was no difference in low birth weight (RR 1.27, CI 0.85‐ 1.91) between the arms. Grade 3 or 4 severe adverse events occurred in 41% (116/283) of infants in the AZT/3TC/ABC arm and 46.3% (125/270) of infants in the AZT/3TC/LPV‐r arm (RR 1.13, CI 0.93‐ 1.37).
In Bae 2008, in utero exposure to maternal ART in the presence of infant AZT prophylaxis was associated with increased risk of neutropenia in infants between birth and 1 month of age (21.7% who received AZT/3TC/NVP arm vs. 5.5% who received a short course regimen, p<0.01), although there was no difference in infant severe adverse events between the two groups at birth (RR 1.26, 0.35‐ 4.54).
In Ekouevi 2008, stillbirths occurred in 3.3% (5/151) of the deliveries in the ART arm and 2.9% (5/175) of the deliveries in the short course arm, RR 1.16, (0.34‐ 3.93). Low birth weight was significantly higher in the ART arm with 22.3% (31/139), vs. 12.4% (21/170) in the short course arm, RR 1.81, (1.09‐3.0).
In Tonwe‐Gold 2007, low birth weight occurred significantly more often in the ART arm (26.3%) than in the short course group (8.8%), RR 2.17, (1.23‐ 3.81). However, there was no difference between the arms in the number of stillbirths (RR 0.89, CI 0.26‐3.08)
Discussion
Summary of main results
Three randomized clinical trials and six intervention cohort studies were included in this review of ART for treating HIV infection in ART‐eligible pregnant women, evaluating the evidence for when to initiate ART and what regimen(s) to use.
When considering the RCTs included in this review, it is important to note that when both the Mma Bana and the Kesho Bora studies were designed, the triple antiretroviral arms were designed as prophylaxis interventions. With changes in the MTCT guidelines, the evaluated groups (Kesho Bora: women with CD4 200‐500 cells/mm3, Mma Bana: women with CD4 >200 cells/mm3) now also contain a subset women who require ART for their own health (WHO 2009). As such, these studies were evaluated in our review. Four additional studies compared maternal antiretroviral regimens to short‐course regimens (de Vincenzi 2009; Bae 2008; Ekouevi 2008; Tonwe‐Gold 2007) . When reviewing the results of these studies that compare triple antiretroviral regimens with short‐course regimens, it is important to note that the short‐course regimens provided postnatal therapy to the mothers for no more than seven days. Thus, in these studies, a comparison in the late postnatal period between triple antiretroviral regimens and short‐course regimens represents a comparison of antiretroviral drugs and no drug.
From our review, there is evidence to support the use of ART to prevent MTCT of HIV in women who require ART for their own health. One of the RCTs compared two triple antiretroviral regimens (Shapiro 2009). The following regimens, when compared to another triple antiretroviral regimen (Shapiro 2009) or a short‐course regimen (de Vincenzi 2009; Bae 2008; Ekouevi 2008; Tonwe‐Gold 2007) decreased rates of MTCT to 5% or less (time frame at which outcome was measured is noted with reference): AZT/3TC/LPV‐r (deVincenzi, 2009 [6 months], Shapiro 2009 [6 months]), AZT/3TC/ABC (Shapiro 2009 [6 months]) and AZT/3TC/NVP (Ekouevi 2008 [12 months]; Bae 2008 [7 months]; Tonwe‐Gold 2007 [6 months]). In situations where maternal anemia is problematic, our review suggests efficacy when d4T is substituted for AZT (Ekouevi, 2007). It is important to note that in studies that evaluated MTCT at birth (consistent with in utero transmission), rates of in utero transmissions are not statistically different between triple antiretroviral regimens and short‐course regimens (de Vincenzi 2009 and Bae 2008). However, it is important for breastfeeding populations to consider the superiority of triple antiretroviral regimens over short‐course regimens for preventing late‐postnatal MTCT (de Vincenzi 2009; Tonwe‐Gold 2007 ; Bae 2008).
The Kesho Bora RCT showed that in utero transmission rates were similar between a triple antiretroviral regimen (started in the second trimester of pregnancy between 26‐ 28 weeks and continued through 6 months of breastfeeding) and a short‐course regimen (started at 28‐36 weeks and continued one week postpartum). However, by decreasing late postnatal transmission of HIV, the triple antiretroviral regimen significantly lowered HIV‐transmission or death at 12 months when compared with short‐course regimens (see discussion in previous paragraph regarding the comparison in the late postnatal period between triple antiretroviral regimens and short‐course regimens representing a comparison of triple antiretroviral therapy vs. no drug) (de Vincenzi 2009). The Mma Bana study likewise concluded that maternal antiretrovirals were safe and effective in preventing MTCT while providing infants the benefit of breastfeeding (Shapiro 2009). Both studies suggest that AZT/3TC/LPV‐r is an effective regimen for use in the HIV‐infected pregnant population.
The Mma Bana study also suggests that AZT/3TC/LPV‐r and AZT/3TC/ABC are effective regimens in providing maternal virologic suppression at delivery, and throughout breastfeeding, important outcomes for women requiring ART for their own health (Shapiro 2009). There was no difference in infant HIV‐free survival at six months between the study arms (AZT/3TC/LPV‐r and AZT/3TC/ABC) (Shapiro 2009) suggesting many types of ART may be equivalent in preventing MTCT.
Neither the Kesho Bora study nor the Mma Bana study showed a difference between arms in rates of in utero MTCT. The difference in overall MTCT rates between the two studies (1%, 95% CI: 0.5‐2.0% in Mma Bana and 4.6%, 95% CI: 2.9‐7.2% in Kesho Bora) may be related to the lower CD4 counts at enrolment in Kesho Bora participants (398‐403 cells/mm3 Mma Bana, 335 cells/mm3 Kesho Bora), shorter duration of antepartum antiretroviral regimens in Kesho Bora (median 11 weeks Mma Bana vs. 40% <6 weeks in Kesho Bora), and lower rates of adherence in Kesho Bora (94% Mma Bana, 89% Kesho Bora).
When given to women requiring ART for their own health, ART is effective in providing virologic suppression with a low risk of side effects. As previously mentioned, the Mma Bana study showed no significant difference between the two triple antiretroviral regimens in providing maternal virologic suppression (<400 copies/mL) at delivery and throughout breastfeeding (Shapiro 2009). There were also no significant differences in maternal grade 3 or 4 severe adverse events between the AZT/3TC/ABC and AZT/3TC/LPV‐r arms (Shapiro 2009). The Tonwe‐Gold study showed an increase in maternal severe adverse events in the AZT/3TC/NVP arm (8.4%, 9/107) compared with the short‐course arm (0%, 0/143). However, the median gestational age at onset of therapy was 27 weeks in the ART arm and 32 weeks in the short‐course arm (Tonwe‐Gold 2007) and is likely related to exposure to increased lengths of therapy in the AZT/3TC/NVP arm. The study authors note that adverse events resolved rapidly with treatment change. This further suggests that ART is safe during pregnancy in a population of women with advanced disease who require therapy for their own health (median CD4 count 189 cells/mm3).
NVP‐related hepatotoxicity was evaluated in our review in the Jamisse, Marazzi, and Phanuphak publications. The rates of grade 3 or 4 hepatotoxicity in pregnant women with CD4 counts <250 cells/mm3 were lower than those women with CD4 counts >250 cells/mm3 in both the Jamisse (0% in women <250 cells/mm3 and 5.9% in women >250 cells/mm3) and Phanuphak (0.98% in women <250 cells/mm3 and 3.5% in women >250 cells/mm3) studies. This finding is consistent with other published studies (Hitti 2004). However, there were higher rates of grade 3 or 4 hepatotoxicity in the Marazzi study in women with lower CD4 counts (9.4% in women <250 cells/mm3 and 5.9% in women >250 cells/mm3). It is important to note that the women with CD4 cell counts of <250 cells/ mm3 started therapy at 15 weeks of gestation, compared with 25 weeks in the group with CD4 cell counts >250. Thus, the length of therapy may account for the difference in events between groups. In this same study, 36/46 of the grade 3 or 4 events were laboratory abnormalities that resolved spontaneously without medication adjustments. In this cohort, they also observed a sooner onset of hepatic toxicity in women with CD4 counts >250 cells/ mm3 (Marazzi 2006).
There were no grade 3‐4 hepatotoxic events in women with CD4 <250 cells/mm3 in the Jamisse publication and 1/102 (0.98%) grade 3‐4 hepatotoxic events in this population the Phanuphak publication. These rates are similar to those published in PACTG 1022, where there were no grade 3‐4 hepatotoxic events in women with CD4 <250 cells/mm3 (Hitti 2004). Grade 3‐4 hepatotoxicity rates in women with CD4 >250 cells/mm3 were 5.9% (34/574) in the Marazzi study. Similarly, hepatotoxicity rates in women with CD4 >250 cells/mm3 were 5.9% (4/67) in the Jamisse study, and 3.5% (5/192) in the Phanuphak study. Although these rates are low, caution should still be exercised when NVP is prescribed to pregnant women with CD4 counts greater than 250 cells/ mm3 because there have been cases of fatal hepatotoxicity with NVP use in this population (Marazzi 2006 and Hitti 2004).
Our review also shows that when ART is taken for a woman’s own health, there is a risk of side effects for the unborn child. Two cohort studies suggested a correlation of low birth weight (LBW) with antiretroviral use. However, it is reassuring that this relation was not observed in the RCTs evaluated. The Ekouevi observational study showed that infants born to women in the AZT/3TC/NVP arm had higher rates of LBW (22.3%, 31/139) than infants born to women in the short course arm (12.4%, 21/170) (RR 1.81, CI 1.09‐ 3.0). The Tonwe‐Gold cohort also showed evidence of increased LBW in the AZT/3TC/NVP arm (26.3%, 26/99) compared to the short course arm (12.1%, 16/132) (RR 2.17, 1.23‐ 3.81). Because both of these cohort studies were performed from data collected in the MTCT‐Plus initiative, one must consider that the presence of similar results in both studies simply represents the use of the same patients, rather than a reproducible event. One must also take the duration of medication exposure between groups into consideration. The women in the Ekouevi ART arm were exposed to antiretrovirals for an average of 11.7 weeks, as compared to women in the short‐course arm who were exposed to therapy for 4.9 weeks of pregnancy. In the Tonwe‐Gold study, the length of study medications was significantly different with the AZT/3TC/NVP arm initiating therapy at 30 weeks and the short‐course arm initiating therapy at 34 weeks (P<0.001). It is well known that substance use and no previous exposure to ART contribute to infant LBW (Floridia 2008). Since none of these variables were directly assessed in the Ekouevi or Tonwe‐Gold studies, it is not possible to rule out that the relation found between ART and LBW is related to confounding.
When discussing the safety of ART on the unborn child, it is important to acknowledge that in the Kesho Bora and Mma Bana trials many of the infants experienced severe adverse events. In the Mma Bana trial 41% of infants in the AZT/3TC/ABC arm experienced less than one grade 3 or 4 laboratory event, compared with 46% in the AZT/3TC/LPV‐r arm (RR 1.13, CI 0.93‐ 1.37). Primarily, the laboratory events included anemia, neutropenia, and hyperbilirubinemia (Shapiro 2009). However, it is interesting to note that in the RCTs this phenomenon was not specific to triple antiretroviral regimens. In Kesho Bora, the rates of infants who had less than one severe adverse event was 30.8% in the triple prophylaxis arm and 32.5% in the short course arm (de Vincenzi 2009).
The Mma Bana study also raises a long‐standing concern about whether in utero exposure to PI‐based ART leads to preterm birth. Cohort studies showing both positive and negative associations of in utero exposure to PIs have been previously reported in the literature (European Collaborative Study (ECS) 2000, Tuomala 2002). However, the detection of an association in a RCT deserves further focus and investigation. Certainly the risk of disease progression in the mother where other therapies are not available, as well as MTCT, should be weighed against the risks of preterm birth.
Overall completeness and applicability of evidence
With regard to our original objectives, there was no evaluable evidence from the systematic review to recommend the optimal time to start ART therapy in relationship to the woman’s laboratory parameters and gestational age as well as which ART regimens to continue in women who are already on ART. There was also no evaluable evidence about the long‐term mortality benefits of ART for HIV‐infected pregnant women who initiate ART for their own health. Similarly, long‐term ART‐related virologic, immunologic, and clinical efficacy data were also lacking in this population.
This review is limited by the available literature on ART for treating HIV infection in ART‐eligible pregnant women. The RCTs meeting criteria for inclusion in this review evaluate cohorts of women who required ART for their own health, in combination with women requiring antiretrovirals for prophylaxis of MTCT (de Vincenzi 2009, Lehman 2008, Shapiro 2009).
Authors' conclusions
Implications for practice.
The recent WHO update on the use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants recommends that all pregnant women with HIV infection should be started on life‐long ART for their own health when their CD4 count is <350 cells/mm3, regardless of clinical symptoms. In addition, it is now recommended that HIV‐infected pregnant women who need ART for their own health should be started on treatment with ART regardless of gestational age. The aim of these recommendations is to improve the health of HIV‐infected pregnant women and the HIV‐free survival of their infants (WHO 2009).
This recommendation highlights the importance of appropriate health care for pregnant women. An established system of HIV testing, treatment and monitoring needs to be incorporated into routine prenatal care. With such, when pregnant women are found to have HIV infection they can receive appropriate ART for their own health and prevent the transmission of HIV infection to their child. In addition, healthcare providers need to be knowledgeable about when to initiate ART and what regimens to use for maximum efficacy while preserving future treatment options. They also need to be aware of potential side effects and toxicities of ART in pregnant women and their infants.
Implications for research.
The expanded eligibility criteria for HIV‐infected pregnant women who should receive ART will undoubtedly lead to an increase in the population of pregnant women who are eligible to receive treatment for their own health. There is a need for implementation research to determine the best approaches to expand access to HIV‐infected pregnant women as well as cost‐effective strategies to provide quality HIV care and ART for eligible pregnant women, especially in resource‐limited settings.
What's new
| Date | Event | Description |
|---|---|---|
| 5 May 2010 | Amended | Minor correction to the citation of one of the references (Bond 2007) |
Notes
To see the GRADE Evidence Profiles for the comparisons analyzed in this review's Summary of Findings tables, please refer to this page on the web site of the Cochrane Review Group on HIV/AIDS: http://www.igh.org/Cochrane/GRADE
Acknowledgements
For Financial Support: The World Health Organization and the Centers for Disease Control and Prevention (see "External sources of support").
Jaco Homsy, MD, MPH; Eliza Humphreys, MD, MPH; Tara Horvath, MA; Gail Kennedy, MPH; Rachel King, MPH; Joy Mirjahangir, MPH; George Rutherford, MD; Karen Schlein, MPH, MBA; Rose Whitmore, BA; Gavrilah Wells, BA; Institute for Global Health, Global Health Sciences, University of California, San Francisco.
Andy T. Anglemyer, MPH, University of California, Berkeley.
Jennifer S. Read, MD, MS, MPH, DTM&H, National Institute of Child Health and Human Development, National Institutes of Health.
The authors wish to acknowledge the time and GRADE expertise contributed by:
Holger Schünemann, MD, PhD, McMaster University, Ontario, Canada.
Nancy Santesso, RD, MLIS, McMaster University, Ontario, Canada.
Data and analyses
Comparison 1. Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infant Mortality at 6 Months | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.34, 2.68] |
| 2 Grade 3/4 Maternal Severe Adverse Events (Laboratory) | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.21] |
| 3 Maternal Severe Adverse Events Requiring Treatment Modification | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.30, 2.61] |
| 4 Maternal Mortality at Six Months | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.44] |
| 5 Grade 3/4 Infant Severe Adverse Events | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.93, 1.37] |
| 6 Prematurity | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.07, 2.17] |
| 7 Infant Low Birth Weight | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.85, 1.91] |
| 8 Mother to Child Transmission In Utero | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.34] |
| 9 Mother to Child Transmission Late Post‐partum | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.35] |
| 10 Mother to Child Transmission at Six Months Post‐Partum | 1 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.44] |
| 11 Stillbirths | 1 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.21, 1.96] |
1.1. Analysis.
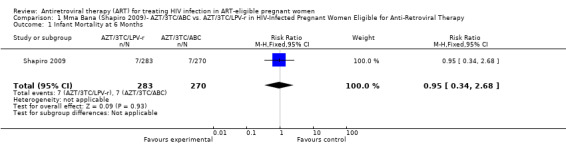
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 1 Infant Mortality at 6 Months.
1.2. Analysis.
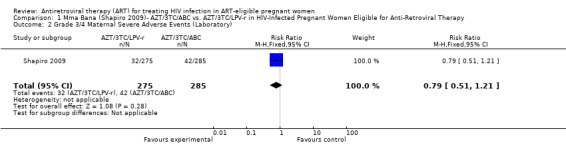
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 2 Grade 3/4 Maternal Severe Adverse Events (Laboratory).
1.3. Analysis.
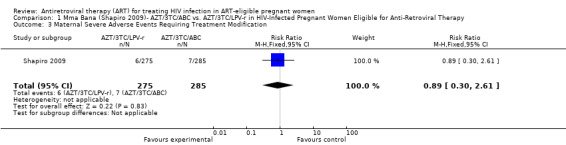
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 3 Maternal Severe Adverse Events Requiring Treatment Modification.
1.4. Analysis.
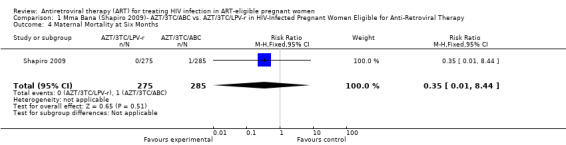
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 4 Maternal Mortality at Six Months.
1.5. Analysis.
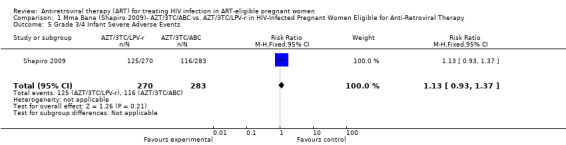
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 5 Grade 3/4 Infant Severe Adverse Events.
1.6. Analysis.
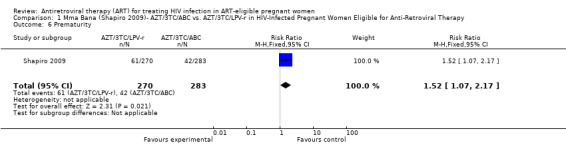
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 6 Prematurity.
1.7. Analysis.
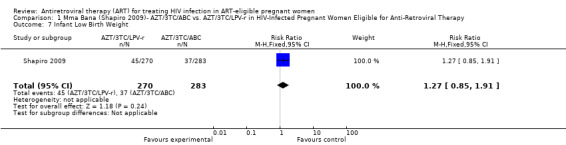
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 7 Infant Low Birth Weight.
1.8. Analysis.
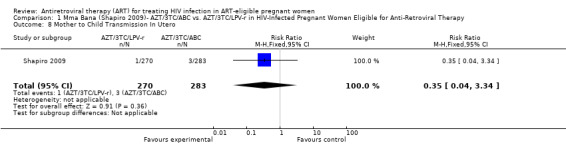
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 8 Mother to Child Transmission In Utero.
1.9. Analysis.
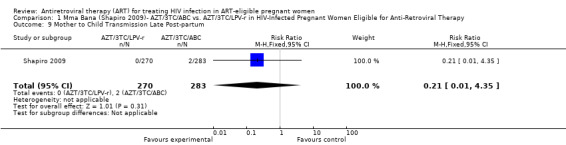
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 9 Mother to Child Transmission Late Post‐partum.
1.10. Analysis.
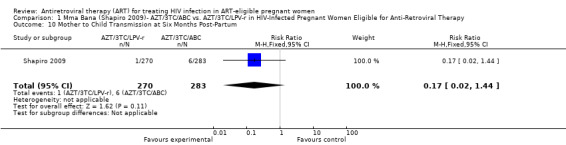
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 10 Mother to Child Transmission at Six Months Post‐Partum.
1.11. Analysis.
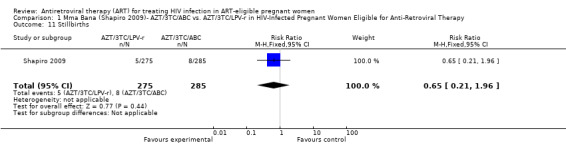
Comparison 1 Mma Bana (Shapiro 2009)‐ AZT/3TC/ABC vs. AZT/3TC/LPV‐r in HIV‐Infected Pregnant Women Eligible for Anti‐Retroviral Therapy, Outcome 11 Stillbirths.
Comparison 2. Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infant HIV Transmission or Death at 6 Months | 1 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.43, 1.00] |
| 2 Infant HIV Transmission or Death at 12 Months | 1 | 765 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.92] |
| 3 Infant Mortality at 6 Months | 1 | 781 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.42] |
| 4 Infant Mortality at 12 months | 1 | 751 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.38, 1.03] |
| 5 Maternal Grade 3/4 Severe Adverse Events Not Requiring Treatment Modification | 1 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.77, 1.53] |
| 6 Infant Grade 3/4 Severe Adverse Events | 1 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.16] |
| 7 Prematurity | 1 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.85, 1.79] |
| 8 Low Birth Weight | 1 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.94, 2.25] |
| 9 Stillbirths | 1 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.25, 3.95] |
| 10 Mother to Child Transmission at Delivery | 1 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.29, 2.07] |
| 11 Mother to Child Transmission at 6 Weeks | 1 | 789 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.34, 1.37] |
| 12 Mother to Child Transmission at 6 Months | 1 | 776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.33, 0.99] |
| 13 Mother to Child Transmission at 12 Months | 1 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.34, 0.97] |
2.1. Analysis.
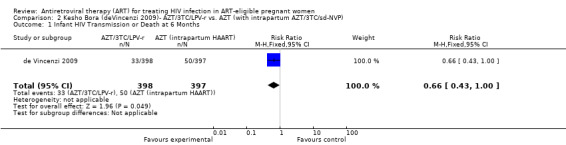
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 1 Infant HIV Transmission or Death at 6 Months.
2.2. Analysis.
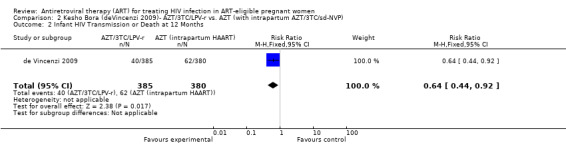
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 2 Infant HIV Transmission or Death at 12 Months.
2.3. Analysis.
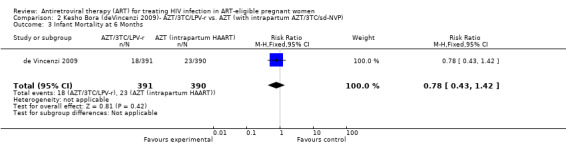
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 3 Infant Mortality at 6 Months.
2.4. Analysis.
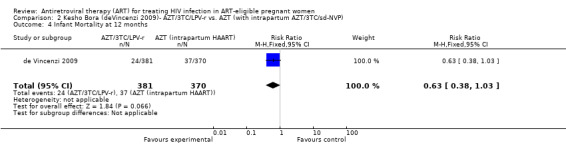
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 4 Infant Mortality at 12 months.
2.5. Analysis.
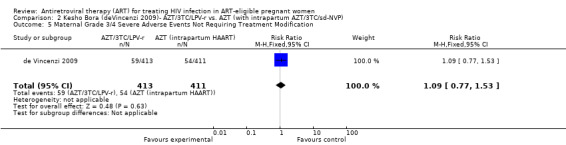
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 5 Maternal Grade 3/4 Severe Adverse Events Not Requiring Treatment Modification.
2.6. Analysis.
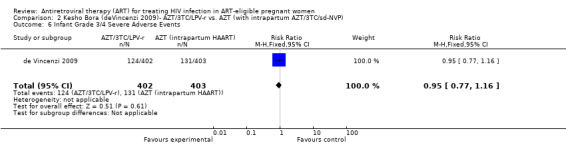
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 6 Infant Grade 3/4 Severe Adverse Events.
2.7. Analysis.
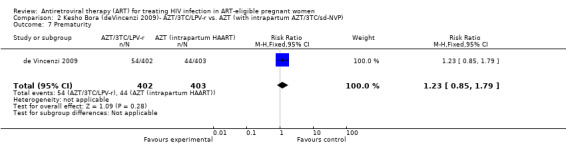
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 7 Prematurity.
2.8. Analysis.
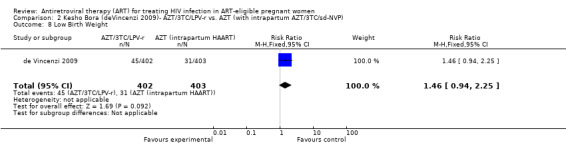
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 8 Low Birth Weight.
2.9. Analysis.
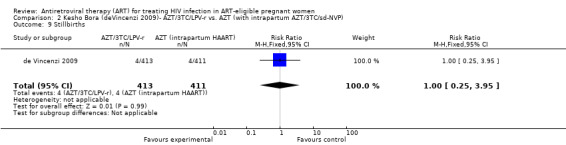
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 9 Stillbirths.
2.10. Analysis.
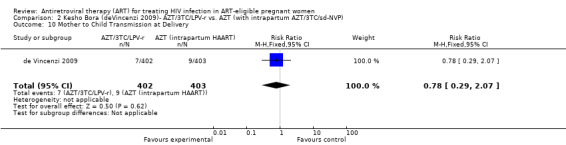
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 10 Mother to Child Transmission at Delivery.
2.11. Analysis.
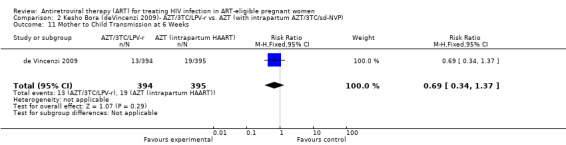
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 11 Mother to Child Transmission at 6 Weeks.
2.12. Analysis.
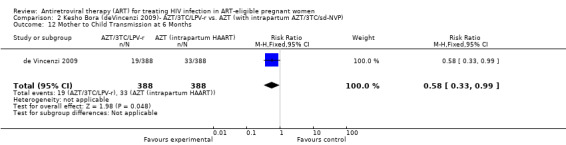
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 12 Mother to Child Transmission at 6 Months.
2.13. Analysis.
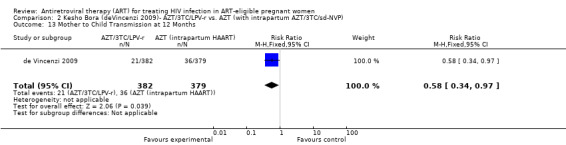
Comparison 2 Kesho Bora (deVincenzi 2009)‐ AZT/3TC/LPV‐r vs. AZT (with intrapartum AZT/3TC/sd‐NVP), Outcome 13 Mother to Child Transmission at 12 Months.
Comparison 3. Lehman 2008‐ Resistance After AZT/3TC/NVP vs. Short Course AZT + sdNVP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal Resistance | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.07, 0.88] |
3.1. Analysis.
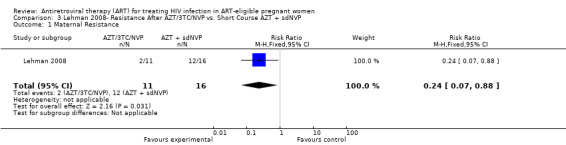
Comparison 3 Lehman 2008‐ Resistance After AZT/3TC/NVP vs. Short Course AZT + sdNVP, Outcome 1 Maternal Resistance.
Comparison 4. Ekouevi 2008‐ All Eligible: AZT/3TC/NVP vs. Other Short Course PMTCT Regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mother to Child Transmission at 12 Months | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.47] |
| 2 Infant Low Birth Weight | 1 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.09, 3.00] |
| 3 Stillbirths | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.34, 3.93] |
4.1. Analysis.
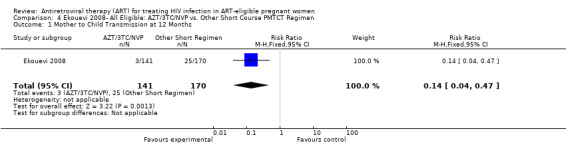
Comparison 4 Ekouevi 2008‐ All Eligible: AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 1 Mother to Child Transmission at 12 Months.
4.2. Analysis.
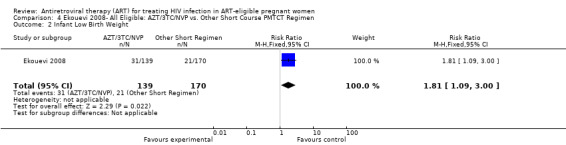
Comparison 4 Ekouevi 2008‐ All Eligible: AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 2 Infant Low Birth Weight.
4.3. Analysis.
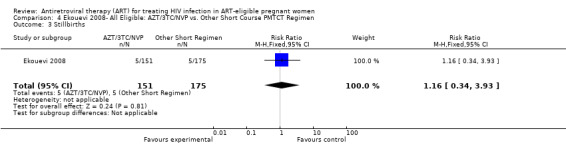
Comparison 4 Ekouevi 2008‐ All Eligible: AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 3 Stillbirths.
Comparison 5. Tonwe‐Gold 2007: Eligible vs. Ineligible˜ AZT/3TC/NVP vs. Other Short Course PMTCT Regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV Transmission or Death at 1 Month | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.25, 2.97] |
| 2 HIV Transmission or Death at 1 Year | 1 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.42, 1.77] |
| 3 Infant Mortality at 1 Month | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.27, 6.47] |
| 4 Maternal Grade 3/4 Severe Adverse Events Requiring Treatment Modification | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 25.33 [1.49, 430.51] |
| 5 Infant Low Birth Weight | 1 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.23, 3.81] |
| 6 Stillbirths | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.26, 3.08] |
| 7 Mother to Child Tranmsission of HIV at 1 Month | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.04, 2.83] |
| 8 Mother to Child Transmission of HIV at 1 Year | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.11, 1.41] |
5.1. Analysis.
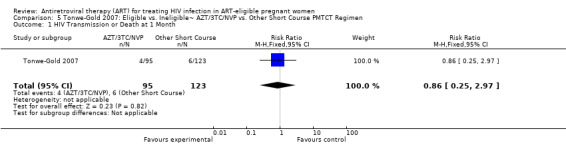
Comparison 5 Tonwe‐Gold 2007: Eligible vs. Ineligible˜ AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 1 HIV Transmission or Death at 1 Month.
5.2. Analysis.
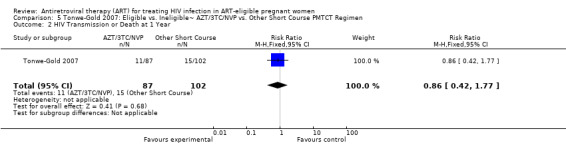
Comparison 5 Tonwe‐Gold 2007: Eligible vs. Ineligible˜ AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 2 HIV Transmission or Death at 1 Year.
5.3. Analysis.
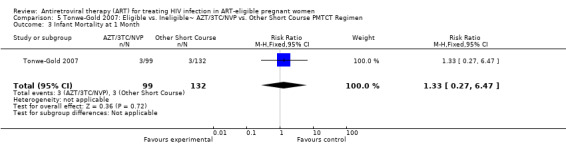
Comparison 5 Tonwe‐Gold 2007: Eligible vs. Ineligible˜ AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 3 Infant Mortality at 1 Month.
5.4. Analysis.
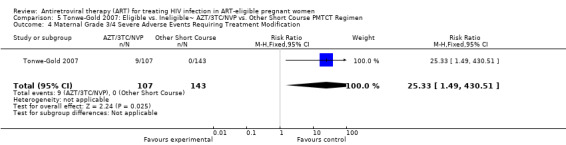
Comparison 5 Tonwe‐Gold 2007: Eligible vs. Ineligible˜ AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 4 Maternal Grade 3/4 Severe Adverse Events Requiring Treatment Modification.
5.5. Analysis.
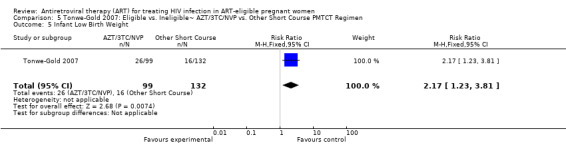
Comparison 5 Tonwe‐Gold 2007: Eligible vs. Ineligible˜ AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 5 Infant Low Birth Weight.
5.6. Analysis.
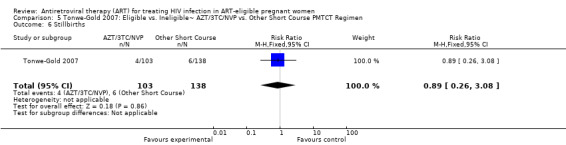
Comparison 5 Tonwe‐Gold 2007: Eligible vs. Ineligible˜ AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 6 Stillbirths.
5.7. Analysis.
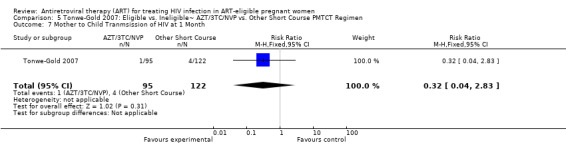
Comparison 5 Tonwe‐Gold 2007: Eligible vs. Ineligible˜ AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 7 Mother to Child Tranmsission of HIV at 1 Month.
5.8. Analysis.
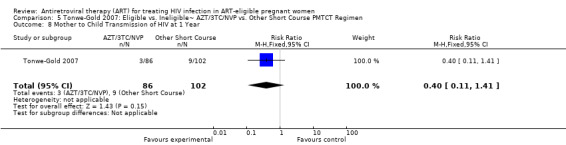
Comparison 5 Tonwe‐Gold 2007: Eligible vs. Ineligible˜ AZT/3TC/NVP vs. Other Short Course PMTCT Regimen, Outcome 8 Mother to Child Transmission of HIV at 1 Year.
Comparison 6. Jamisse 2007: AZT/3TC/NVP in CD4<250 vs. CD4>250.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal Grade 3/4 Severe Adverse Events | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.47, 2.97] |
6.1. Analysis.
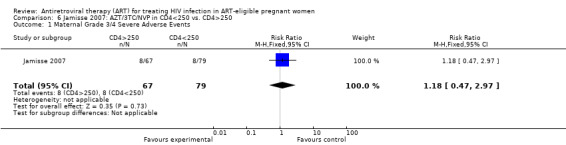
Comparison 6 Jamisse 2007: AZT/3TC/NVP in CD4<250 vs. CD4>250, Outcome 1 Maternal Grade 3/4 Severe Adverse Events.
Comparison 7. Jamisse 2007: AZT/3TC/NVP vs. d4T/3TC/NVP in Women With CD4<350.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal Grade 3/4 Severe Adverse Events | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.60, 4.12] |
7.1. Analysis.
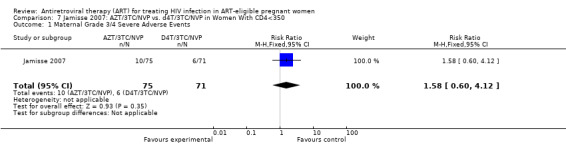
Comparison 7 Jamisse 2007: AZT/3TC/NVP vs. d4T/3TC/NVP in Women With CD4<350, Outcome 1 Maternal Grade 3/4 Severe Adverse Events.
Comparison 8. Phanuphak 2007: AZT/3TC/NVP in CD4<200 vs. CD4>200.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal Grade 3/4 Severe Adverse Events | 1 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.65, 6.03] |
8.1. Analysis.
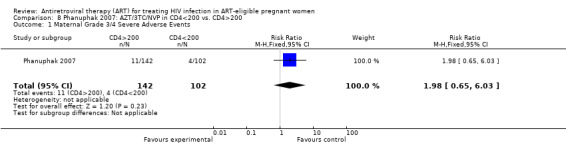
Comparison 8 Phanuphak 2007: AZT/3TC/NVP in CD4<200 vs. CD4>200, Outcome 1 Maternal Grade 3/4 Severe Adverse Events.
Comparison 9. Bae 2008: AZT/3TC/NVP vs. AZT in CD4<200.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV‐Transmission or Death at 7 Months | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.18, 0.85] |
| 2 Infant Grade 3/4 Severe Adverse Events at Birth | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.35, 4.54] |
| 3 Prematurity | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.21, 1.50] |
| 4 Mother to Child Transmission at Birth | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.36] |
| 5 Mother to Child Transmission at 1 Month | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 0.96] |
| 6 Mother to Child Transmission Cumulative by 7 Months | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
9.1. Analysis.
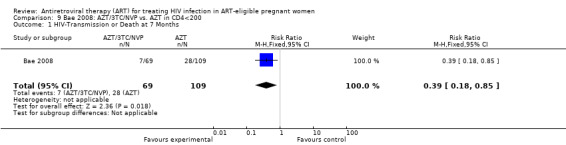
Comparison 9 Bae 2008: AZT/3TC/NVP vs. AZT in CD4<200, Outcome 1 HIV‐Transmission or Death at 7 Months.
9.2. Analysis.
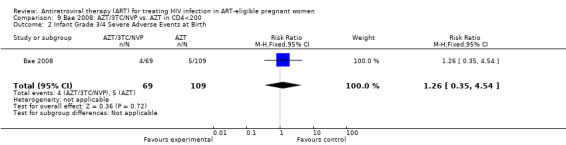
Comparison 9 Bae 2008: AZT/3TC/NVP vs. AZT in CD4<200, Outcome 2 Infant Grade 3/4 Severe Adverse Events at Birth.
9.3. Analysis.
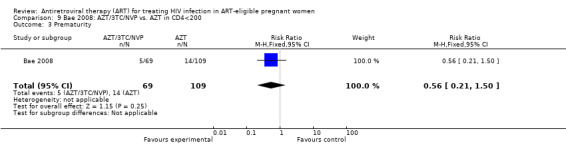
Comparison 9 Bae 2008: AZT/3TC/NVP vs. AZT in CD4<200, Outcome 3 Prematurity.
9.4. Analysis.
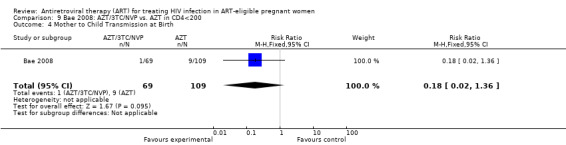
Comparison 9 Bae 2008: AZT/3TC/NVP vs. AZT in CD4<200, Outcome 4 Mother to Child Transmission at Birth.
9.5. Analysis.
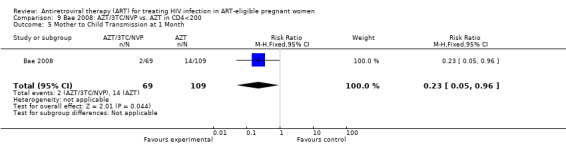
Comparison 9 Bae 2008: AZT/3TC/NVP vs. AZT in CD4<200, Outcome 5 Mother to Child Transmission at 1 Month.
9.6. Analysis.
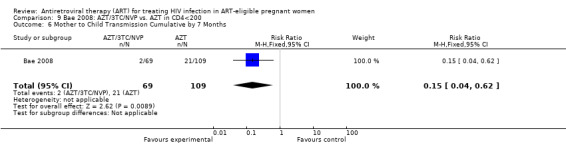
Comparison 9 Bae 2008: AZT/3TC/NVP vs. AZT in CD4<200, Outcome 6 Mother to Child Transmission Cumulative by 7 Months.
Comparison 10. Marazzi 2006: AZT/3TC/NVP in <250 vs. >250.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hepatotoxicity in NVP‐Based Regimens With CD4>250 vs. CD4<250 | 1 | 703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.34, 1.20] |
10.1. Analysis.
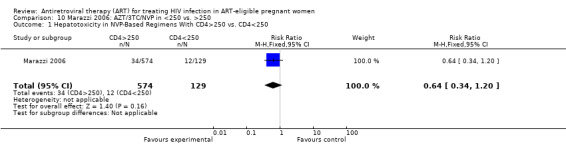
Comparison 10 Marazzi 2006: AZT/3TC/NVP in <250 vs. >250, Outcome 1 Hepatotoxicity in NVP‐Based Regimens With CD4>250 vs. CD4<250.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bae 2008.
| Methods | Observational Study (Nested Cohort Study Within a Randomized Clinical Trial) | |
| Participants | HIV‐1 infected pregnant women in Botswana were referred for enrollment in the randomized controlled Mashi study. Eligibility criteria included: gestational age of 33‐35 weeks, WHO clinical stage 2 or 3 and CD4 T cell count <350 cells/mm3, or WHO clinical stage 4 or CD4 cell count <200 cells/mm3. Once ART became available in October 2002, ART‐eligible women were assigned to the AZT/3TC/NVP arm. The 69 women in the ART arm had a median CD4 count at enrollment of 132 cells/mm3, and the 109 women in the ART‐unexposed arm had a median CD4 count of 146 cells/mm3. | |
| Interventions | Of the ART eligible women, 109 received short course prophylaxis while AZT/3TC/NVP availability was delayed and 69 received AZT/3TC/NVP. All live‐born infants of these women were selected for analysis. Women in the ART group received AZT/3TC/NVP twice daily at 34 weeks with supplemental AZT during delivery. In the short course arm, women were given AZT twice daily starting at 34 weeks, with either sdNVP or placebo in labour. Both ART exposed and unexposed (the short course arm) were divided into subgroups based on feeding strategies. Infants received either 1 month of AZT prophylaxis with formula feeding, or AZT for the duration of breastfeeding. A CBC was obtained on all infants at birth, and 1, 4, and 7 months of age. The author's primary outcome was to assess hematologic and hepatic toxcities associated with in utero and breastfeeding exposure to maternal AZT/3TC/NVP. | |
| Outcomes |
Primary Outcome:In utero exposure to maternal AZT/3TC/NVP was associated with increased risk of neutropenia in infants up to 1 months of age (21.7% AZT/3TC/NVP vs. 5.5% short course regimen, p<0.01). This difference was not present at birth or at 7 months. At 1 month it was seen in breast‐fed, but not formula‐fed infants. There were also no significant differences between groups with respect to anemia or thrombocytopenia. Postnatal ART exposure was not associated with hematologic or hepatic toxicities. Infant HIV‐Transmission or Death: 61% reduction in HIV transmission at 7 months in the AZT/3TC/NVP arm (RR 0.84, CI 0.18‐ 0.85) Infant Severe Adverse Events: No signficant difference between groups in hematological outcomes at birth. |
|
| Notes | Cohort study was nested within MASHI trial (Thior, JAMA 2006; 296: 794‐ 805). By the Newcastle Ottawa Scoring System this study obtained 4 of 4 stars for selection, 1 of 2 stars for comparability, and 2 of 3 stars for outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Observational Study |
| Allocation concealment? | High risk | Observational Study |
| Blinding? All outcomes | High risk | Observational Study |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Unclear risk | The author's primary outcomes are hematologic and hepatic toxicities, but no hepatic toxicities are presented in tabular form, and are addressed in text only (with no birth outcomes given). |
| Free of other bias? | Low risk | By the Newcastle Ottawa Scoring System this study obtained 4 of 4 stars for selection, 1 of 2 stars for comparability, and 1 of 3 stars for outcomes. |
de Vincenzi 2009.
| Methods | Randomized Controlled Trial | |
| Participants | 1140 HIV‐infected pregnant women in five sites in Burkina Faso, Kenya and South Africa were enrolled in the Kesho Bora clinical trial of triple‐antiretroviral regimen during pregnancy and breastfeeding compared to short‐course prophylaxis to prevent mother‐to‐child transmission of HIV‐1. The mean CD4 count in both arms was 335 cells/mm3. Cohort groups were comprised of 119 women with CD4 <200 cells/mm3 or HIV Stage 4, and 129 women with CD4 >500 cells/mm3 and HIV Stage <4. Of the remaining 855 women, 31 were allocated to short course prophylaxis and the remaining 824 were randomized to ART or short course arms. | |
| Interventions |
RANDOMIZATION (CD4 200‐500): Triple ART Arm: AZT/3TC/LPV‐r (28‐36 weeks pregnancy until 6 months post partum) Short Course Arm: AZT (from 28‐36 weeks until labour) + AZT/3TC/sdNVP (at onset of labour) + AZT/3TC (one week after delivery) COHORT (CD4 <200 cells/mm3): AZT/3TC/NVP (from 18‐ 36 wks + infant sdNVP) COHORT (CD4 >500 cells/mm3): AZT (from 34‐36 weeks until labour) + maternal/infant sdNVP (at onset of labour) |
|
| Outcomes |
Primary Outcome: HIV‐free infant survival at 6 weeks and 12 months (calculated in this review as HIV‐transmission or death). Risk reduction 36% at 12 months in the AZT/3TC/LPV‐r arm (RR 0.64, 0.44‐ 0.92). Secondary Outcome: AIDS‐free survival of mothers at 18 months post‐partum (not yet reported). Maternal and infant severe adverse events. Maternal Severe Adverse Events: No significant difference between arms (RR 1.09, CI 0.77‐ 1.53) Infant Severe Adverse Events: Prematurity occurred in 13.4% (54/402) in AZT/3TC/LPV‐r arm and 10.9% (44/403) in the short course arm, (RR 1.23, CI 0.85‐ 1.79). There were 124/402 (30.8%) infants in the AZT/3TC/LPV‐r arm and 131/403 (32.5%) of infants in the short course arm with a grade 3 or 4 severe adverse event, (RR 0.95, CI 0.77‐ 1.16). There were no differences between arms in low birth weight (RR 1.46, CI 0.94‐ 2.25) or stillbirths (RR 1, CI 0.25‐ 3.95). |
|
| Notes | Kesho Bora Study, IAS 2009 abstract LBPEC01 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Sequence were generated by a computer. |
| Allocation concealment? | Low risk | Assignments allocated in sequentially numbered, opaque, sealed envelopes. |
| Blinding? All outcomes | High risk | Participants and providers were not blinded to treatment, but outcomes were assessed in a blinded manner and lab‐based data were assessed by masked investigators. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Unpublished data |
| Free of selective reporting? | Unclear risk | Unpublished data |
| Free of other bias? | Unclear risk | Unpublished data |
Ekouevi 2008.
| Methods | Observational Study | |
| Participants | HIV‐1 infected pregnant women in the Ivory Coast were referred for enrolment in the ANRS Ditrame Plus or MTCT‐Plus projects. Eligibility criteria included WHO clinical stage 2 or 3 and CD4 T cell count <350 cells/mm3, or WHO clinical stage 4 or CD4 cell count <200 cells/mm3. Of 326 HIV‐infected pregnant women, 175 received a short course regimen and 151 received ART. | |
| Interventions | There were 190 women who received a short course regimen, the mean CD4 count in this arm was 177 cells/mm3. There were 168 women who received ART, the mean CD4 count in this arm was 182 cells/mm3. In the Ditrame Plus Study, ART was not yet available for pregnant women, thus eligible mothers received either: intrapartum sdNVP after short course AZT initiated at 36 weeks gestation, or short course AZT/3TC initiated at 32 weeks gestation until 3 days postpartum (short course group). In the MTCT‐Plus project, eligible women received AZT/3TC/NVP (or d4T/3TC/NVP) antepartum, during labour and after delivery (ART group). In both groups, infants received AZT syrup for 7 days + sdNVP syrup on day 2 or 3. Women were counseled to either replacement feed or to practice exclusive breastfeeding for 4‐6 months. The authors' primary outcome was to evaluate pregnancy outcomes in women with advanced HIV disease. | |
| Outcomes |
Primary Outcome: Stillbirths accounted for 3.3% (5/151) of the deliveries in the AZT/3TC/NVP group and 2.9% (5/175) of the deliveries in the short course group, RR 1.16, (0.34‐ 3.93). Low birth weight occured in 22.3% (31/139) in the AZT/3TC/NVP group and 12.4% (21/170) in the short course group, RR 1.81, (1.09‐ 3.0). Mother to child transmission of HIV (MTCT) at 12 months: In a setting with minimal breastfeeding prophylaxis in the short course arm, transmission occurred 3/141 (2.1%) infants in the AZT/3TC/NVP arm, and 25/170 (14.7%) in the short course arm, RR 0.14 (0.04‐ 0.47). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Observational study |
| Allocation concealment? | High risk | Observational study |
| Blinding? All outcomes | High risk | Observational study |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | By the Newcastle Ottawa Scoring System this study obtained 3 of 4 stars for selection, 0 of 2 stars for comparability, and 2 of 3 stars for outcomes. |
Jamisse 2007.
| Methods | Observational Study | |
| Participants | All pregnant women in Maputo, Mozambique were offered HIV‐1 testing. HIV‐1 seropositive women were referred to the study hospital for CD4 cell count testing. Women who met eligibility criteria were offered enrollment in an observational study. Study eligibility included: greater than 18 weeks gestational age, ≥18 years of age, ART naive, and CD4 cell count < 350 cells/mm3. | |
| Interventions | From August 2004 through June 2005, 163 HIV‐1 positive pregnant women were enrolled. Study participants returned 2 weeks after enrollment to begin ART. 146 received antiretroviral therapy, and as a first‐line regimen AZT/3TC/NVP was offered. For women with hemoglobin <8.5g/mL , d4T was substituted. This substitution was also made if AZT was not available. Nelfinavir replaced NVP in the case of toxicity. Participants were seen monthly until 34 weeks of gestation, after which follow‐up occurred every 2 weeks until delivery. Post‐partum visits occurred 1 week after delivery and then monthly until 6 months from the time of AZT/3TC/NVP initiation. Blood samples were taken at 1‐2 weeks and 1, 2, and 6 months after ART initiation. Outcomes in women with CD4<250 cells/mm3 were compared with those with CD4> 250 cells/mm3. The authors' primary endpoint was to evaluate toxicity associated with NVP‐based regimens. | |
| Outcomes | Primary Outcome: All four women with severe hepatotoxicity has CD4 counts >250 (p=0.02). Rates of peripheral neuropathy, or skin and hematologic toxicity did not differ by CD4 cell count group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Observational Study |
| Allocation concealment? | High risk | Observational Study |
| Blinding? All outcomes | High risk | Observational Study |
| Incomplete outcome data addressed? All outcomes | Low risk | Loss to follow‐up adequately specified. All subjects accounted for at delivery, 14 lost to follow up (163 enrolled, 12 never returned to start antiretroviral therapy, 2 lost after starting antiretroviral therapy). |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | By the Newcastle Ottawa Scoring System this study obtained 3 of 4 stars for selection, 1 of 2 stars for comparability, and 2 of 3 stars for outcomes. |
Lehman 2008.
| Methods | Randomized Controlled Trial | |
| Participants | HIV‐1 infected pregnant women who presented to a Nairobi, Kenya clinic. Pre‐specified inclusion criteria were: intent to breastfeed, hemoglobin greater than 8g/dl, no previous exposure to antiretrovirals, and age greater than 18. Exclusion criteria included having a CD4 count of <200 or >500 cells/mm3. | |
| Interventions | At 34 weeks gestation women were randomized to one of two arms. In the ART arm, AZT/3TC/NVP was given twice daily from 34 weeks until 6 months after delivery. In the AZT/sdNVP arm, AZT was given twice daily from 34 weeks gestation and intrapartum, while NVP was given as a single oral dose at the onset of labour with 2mg/kg in a single dose to the infant within 72 h of delivery. 58 women were randomized, mean CD4 counts for each arm were not provided. During follow‐up, maternal blood was collected at 35 and 37 weeks gestation, delivery, 1, 3, 6, 9, and 12 months post‐partum. Women were included in the substudy if they had a plasma sample available from 3 months after treatment cessation. Plasma samples were frozen and shipped to Seattle, WA where allele‐specific PCR was performed to detect K103N and Y181C mutations. | |
| Outcomes |
Primary outcome: Reported in: Chung, MH. Antiviral Therapy 2008; 13: 799‐807 Secondary outcome (Resistance): Low levels of resistant virus (K103N and Y181C) were detected in 75% of women treated with AZT/sdNVP (12/16) and only 18% of women treated with AZT/3TC/NVP (2/11) (p= 0.007). Y181C was more prevalent than K103N at 3 months and showed little evidence of decay by 12 months. |
|
| Notes | Parent study: Chung MH, et al. HAART vs AZT/NVP effects on early breast milk HIV‐1 type‐1 RNA: a phase II randomized controlled trial. Antiviral Therapy 2008; 13: 199‐807. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | " Randomization was performed using computer‐generated block randomization". |
| Allocation concealment? | Unclear risk | Not addressed. |
| Blinding? All outcomes | High risk | "Study investigators and participants were not blinded to the interventions". |
| Incomplete outcome data addressed? All outcomes | Low risk | Plasma was available on 23/28 (82%) women in the short course arm and 17/30 (56%) of the AZT/3TC/NVP arm. Of women with plasma available, in the short course arm 16/23 (70%) of samples were used and 11/17 (65%) were used in the AZT/3TC/NVP arm. Per the authors, "we limited this analysis to the 27 samples (16 and 11 in the AZT/sdNVP and AZT/3TC/NVP arms, respectively) in which at least 50 cumulative viral RNA copies were tested in the 2‐4 independent RT‐PCRs. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | High risk | Plasma was not available on many of the enrolled women, introducing potential ascertainment bias. |
Marazzi 2006.
| Methods | Observational Study | |
| Participants | All women enrolled in the DREAM cohort from 5/1/2002 through 7/30/2004 were evaluated retrospectively. All pregnant, HIV‐infected women were eligible, but charts were only reviewed if the women kept follow‐up appointments through delivery, agreed to take ART, and had been on therapy for more than 14 days at the time of study closure. 703 women were included. | |
| Interventions | All pregnant HIV‐infected women were prescribed AZT/3TC/NVP from ≥25 weeks until delivery. Women with CD4 cell counts <200 cells/mm3, a viral load of <55,000 copies/mL, or WHO stage 3 or 4 were given AZT/3TC/NVP starting from 15 weeks of gestation and indefinitely following delivery. Women with a viral load of <55,000 copies/mL and CD4 counts >200 cells/mm3 continued AZT/3TC/NVP for up to 6 months post‐partum. Pregnant HIV‐infected women with a hemoglobin of <8g/100mL were given d4T/3TC/NVP. Laboratory monitoring was obtained at baseline, at weeks 2 and 4 during the first month and every weeks thereafter until delivery. The author's primary outcome was to assess the incidence and consequences of adverse reactions when HIV‐infected pregnant women in Africa are given NVP‐based ART. | |
| Outcomes | Primary Outcome: The incidence of grade 3‐4 adverse reactions (hepatoxicity, skin rashes, and Stevens‐Johnson syndrome) was 6.5, 2.4 and 1.1% respectively. Five women died during pregnancy, but only 1 death could be associated with ART. Only hepatic grade 3 or 4 severe adverse events were evaluated by CD4 strata. There was no signifcant difference in events between groups, RR 0.64 (0.34‐ 1.20). | |
| Notes | DREAM program= Drug Resources Enhancement against AIDS and Malnutrition. A program designed and run by the Community of Sant'Egidio in Mozambique as well as other sub‐Saharan African countries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Observational Study |
| Allocation concealment? | High risk | Observational Study |
| Blinding? All outcomes | High risk | Observational Study |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Retrospective chart review. Of 999 patients whose charts were examined, 137 patients either refused AZT/3TC/NVP or were lost‐to‐follow up. Reasons for loss to follow‐up were not addressed. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | By the Newcastle Ottawa Scoring System this study obtained 3 of 4 stars for selection, 1 of 2 stars for comparability, and 2 of 3 stars for outcomes. |
Phanuphak 2007.
| Methods | Observational Study | |
| Participants | Patients with HIV infection were prescribed NVP‐containing regimens (78 men, 244 pregnant, and 87 non‐pregnant women). Participants were eligible for ART if they had CD4 cell counts <200 cells/mm3 or <350 cells/mm3 at WHO stage >2. | |
| Interventions | Pregnant HIV‐infected women with CD4>200 were prescribed NVP‐based antiretroviral prophylaxis from ≥28 weeks until delivery (PMTCT group) with AZT/3TC continued 1 week post‐partum. Pregnant HIV‐infected women with CD4 cell counts <200 cells/mm3 or <350 cells/mm3 at WHO stage >2 were givenAZT/3TC/NVP starting from 14 weeks of gestation and indefinitely following delivery (the ART group). ART comprised AZT/3TC/NVP or d4T/3TC/NVP. For pregnant women, laboratory monitoring was obtained at baseline, at weeks 2, 4, 6, and 8, and then every 4 weeks until delivery with clinical assessments regular intervals. Participants were stratified by CD4 cell count at study initiation. The authors' primary outcome was to determine the incidence of, and risk factors for, NVP‐associated hepatotoxicity and rash. | |
| Outcomes | Primary Outcome: Pregnant women who received antiretroviral prophylaxis for PMTCT (CD4 cells >250 cells/mm3) had a significantly higher rate of symptomatic hepatotoxicity (p=0.0003) than pregnant women receiving AZT/3TC/NVP for their own health. When rash and hepatic grade 3 or 4 severe adverse events were combined, there was no significant difference in events between groups, RR 1.98 (0.65‐ 6.03). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Observational study |
| Allocation concealment? | High risk | Observational study |
| Blinding? All outcomes | High risk | Observational study |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No statement about incomplete outcome data. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | By the Newcastle Ottawa Scoring System this study obtained 3 of 4 stars for selection, 1 of 2 stars for comparability, and 1 of 3 stars for outcomes. |
Shapiro 2009.
| Methods | Randomized controlled trial | |
| Participants | HIV‐infected pregnant women who intended to breastfeed their infants. The study was performed at four clinical sites in Botswana. 730 women were enrolled: 560 to the randomized arms, 170 to the observational arm. | |
| Interventions | HIV‐infected pregnant women with CD4 greater than or equal to 200 cells/mm3 were randomized to AZT/3TC/ABC (Arm A) vs. AZT/3TC/LPV‐r (Arm B) from 26‐34 weeks gestation. An observational group was composed of women with CD4 < 200 cells/mm3 who received AZT/3TC/NVP from 18‐34 weeks gestation. All regimens were continued through planned weaning by 6 months post‐partum. All infants received sdNVP and one month of AZT. Inclusion criteria for randomization included: a CD4 count of 200‐500 cells/mm3 and absence of an AIDS‐defining illness. The mean CD4 count in the AZT/3TC/ABC arm was 398 cells/mm3 and the mean CD4 count in the AZT/3TC/LPV‐r arm was 403 cells/mm3. The author's primary endpoints were maternal HIV‐1 RNA suppression to <400 copies/mL among randomized arms at delivery and through breastfeeding, and MTCT rates in the overall study population. | |
| Outcomes |
Primary Outcome: Reported in the author's IAS abstract, but no clear denominators for reliable data‐entry. HIV‐1 RNA suppression (<400 copies/mL) at delivery occurred in 96% of arm A and 93% of Arm B. Throughout breastfeeding there was 92% suppression reported for arm A vs. 93% for arm B. Secondary Outcome: MTCT rates at 6 months were: 2.1% arm A and <1% in arm B, RR 0.17, (0.02‐ 1.44). Maternal Severe Adverse Events:There were 42 of 285 (14.7%) women in arm A and 32/275 (11.6%) in arm B with a grade 3 or 4 severe adverse event, RR 0.79, (0.51‐1.21). Severe adverse events requiring treatment modification occurred in 2.5% (arm A) and 2.2% (arm B), RR 0.89, (0.3‐ 2.61). Infant Severe Adverse Events: Stillbirths accounted for 2.8% (8/285) of the deliveries in arm A and 1.8% (5/275) of the deliveries in arm B, RR 0.65, (0.21‐ 1.96). Prematurity occured in 14.8% (42/283) in arm A and 22.6% (61/270) in arm B, RR 1.52, (1.07‐ 2.17). There were 116/283 (41%) infants in arm A and 125/270 (46.3%) of infants in arm B with a grade 3 or 4 severe adverse event, RR 1.13, (0.93‐ 1.37). |
|
| Notes | Mma Bana Study, IAS 2009 abstract WELBB101. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | The random assignments were computer generated using permuted blocks procedure stratified by site. |
| Allocation concealment? | Unclear risk | Likely, but not specified |
| Blinding? All outcomes | High risk | |
| Incomplete outcome data addressed? All outcomes | Low risk | |
| Free of selective reporting? | Unclear risk | Unpublished data |
| Free of other bias? | Unclear risk | Unpublished data |
Tonwe‐Gold 2007.
| Methods | Observational Study | |
| Participants | Pregnant women identified at two community based antenatal clinics in two low‐income urban districts of the Ivory Coast were referred for enrollment in the MTCT‐Plus initiative. Eligibility for ART included: WHO clinical stage 4 irrespective of CD4 T cell count; WHO stage 2 or 3 and CD4 T cell count <350 cells/mm3; or CD4 T cell count <200 cells/mm3. 107 women began triple ART with the plan to continue medications post‐partum (median gestational age 30 weeks), with 102 on AZT/3TC/NVP; 143 women received short course antiretroviral prophylaxis to prevent mother to child transmission of HIV, 103 women with short course AZT/3TC with sdNVP during labour. | |
| Interventions | Pregnant women meeting eligibility criteria initiated triple ART (as early as 24 weeks of gestation) with AZT/3TC/NVP (ART group). Treatment continued during labour and postnatally. Pregnant women who only required prophylaxis for MTCT received short course prophylaxis regimens (usually AZT/3TC) with sdNVP in labour from 32 weeks of gestation until 3d postpartum, or short course AZT from 28 weeks, or sdNVP alone, or both short course AZT and sdNVP (short course group). Irrespective of maternal regimen, all infants received AZT syrup for 7d and sdNVP syrup on d3. | |
| Outcomes |
Primary Outcome: Mother to child transmission at 12 months was 3.5% (3/86) in the ART group and 8.8% (9/102) in the short course group, RR 0.4, (0.11‐ 1.41). Infant HIV‐Transmission and Death: At 12 months the outcome had occured in 12.6% infants (11/87) in the ART group and 14.7% (15/102) in the short course group, (RR 0.86, CI 0.42‐ 1.77). Infant Severe Adverse Events: Low birth weight occured in 26.3% (26/99) in the ART group and 12.1% (16/132) in the short course group, RR 2.17, (1.23‐ 3.81). |
|
| Notes | This study was not designed to compare ART to short course regimens directly. By the Newcastle Ottawa Scoring System this study obtained 3 of 4 stars for selection, 0 of 2 stars for comparability, and 2 of 3 stars for outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Observational Study |
| Allocation concealment? | High risk | Observational Study |
| Blinding? All outcomes | High risk | Observational Study |
| Incomplete outcome data addressed? All outcomes | Low risk | For data up to delivery. |
| Free of selective reporting? | Low risk | |
| Free of other bias? | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alimenti 2003 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Arrive 2007 | Did not evaluate a triple‐therapy regimen. |
| Arrive 2008 | Review article. Pertinent references from the bibliography were individually evaluated. |
| Bardeguez 2008 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Bauer 2006 | There was no comparison group. Also, only one mother got triple therapy, making this like a case report (which were excluded). |
| Bellon 2004 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Black 2008 | There was a priority regimen, but no comparison group. |
| Black 2008a | There was a priority regimen, but no comparison group. |
| Briand 2009 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Bucceri 2002 | Exact regimens specified, but outcomes are not provided by regimen. Mean CD4 418 at study entry, not representing population of interest. |
| Bunders 2005 | Only 82% of women received triple therapy and outcomes are not given by type of therapy. |
| Chi 2007 | Does not evaluate a triple therapy intervention. |
| Ciaranello 2008 | Exact antiretroviral triple therapy regimen not specified. All enrolled women had CD4 counts greater than 200 cells/mm3 and no history of AIDS. |
| Clayden 2009 | Review article. Pertinent references from the bibliography were individually evaluated. |
| Cooper 2002 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Cotter 2006 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Dabis 2005 | Evaluates short‐course antepartum regimens. The triple therapy regimen used intrapartum is for prophylaxis and not for the mother's own health. |
| Deschamps 2009 | There was a defined regimen, but no comparison group. |
| Dinsmoor 2002 | Exact triple therapy antiretroviral regimens administered are not defined. Not all included patients received triple therapy. |
| Dorenbaum 2002 | Exact triple therapy antiretroviral regimens administered are not defined. Half of the participants did not received triple antiretroviral therapy. |
| Duran 2006 | Exact triple therapy antiretroviral regimens administered are not defined. |
| El 2006 | This cohort study enrolled a population (CD4<500 cells/mm3) was not specifically the targeted population of interest. |
| El 2007 | This cohort study enrolled a population (CD4<500 cells/mm3) was not specifically the targeted population of interest. Infant outcomes reported did not meet DAIDS criteria for severe adverse events. |
| European Collaborative Study (ECS) 2000 | Exact triple therapy antiretroviral regimens administered are not defined. |
| European Collaborative Study (ECS) 2003 | Only 18% of women received triple antiretroviral therapy. Exact triple therapy antiretroviral regimens administered are not defined. |
| European Collaborative Study (ECS) 2005 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Fiore 2006 | Exact triple therapy antiretroviral regimens administered are not defined. Outcome evaluated not felt to be a severe adverse event. |
| Garcia‐Tejedor 2009 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Giuliano 2003 | Does not evaluate a triple therapy intervention. |
| Goetghebuer 2009 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Grosch‐Woerner 2008 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Hitti 2004 | There was no restriction to maternal CD4 count at study entry. |
| Hitti 2007 | Exact triple therapy antiretroviral regimens administered are not defined although components are identified. Outcomes are not given by regimen. |
| Hoffman 2009 | Study was considered for inclusion in the systematic review, but authors did not reply to requests for additional information. |
| Jackson 2007 | Does not evaluate a triple therapy intervention. |
| Justman 2003 | Does not evaluate a pregnant population. |
| Kourtis 2007 | Meta‐Analysis that combines triple and mono/dual therapy interventions. Triple therapy intervention references selected and evaluated individually. |
| Kowalska 2003 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Livingston 2007 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Lopez‐Cortes 2007 | There was a regimen defined for each patient, but no comparison group. Also, no entry restriction regarding CD4 cell counts. |
| Lorenzi 1998 | There was a regimen defined for each patient, but no comparison group. |
| Marti 2007 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Martin 2006 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Martin 2007 | Exact triple therapy antiretroviral regimens administered are not defined. |
| McIntyre 2002 | Review article. Pertinent references from the bibliography were individually evaluated. |
| McIntyre 2006 | Review article. Pertinent references from the bibliography were individually evaluated. |
| Mellins 2008 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Mofenson 2002 | Review article. Pertinent references from the bibliography were individually evaluated. |
| Mussi‐Pinhata 2007 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Nurutdinova 2008 | There was a regimen defined for each patient, but no comparison group. |
| Onen 2008 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Ono 2008 | The primary outcome was not felt to be a severe adverse event. |
| Pacheco 2006 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Palacios 2009 | Targeted population of interest was women with CD4 greater than 300 cells/mm3. |
| Peltier 2009 | Compares two different triple therapy regimens, but is primarily an evaluation of breastfeeding with maternal antiretrovirals vs. formula feeding to prevent late‐postnatal MTCT. It does not address when or what to start for triple ART‐eligible pregnant women who require triple ART for their own health. |
| Resino 2001 | This cohort study enrolled a population (vertically infected children over the age of 2) that was not the targeted population of interest |
| Schulte 2007 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Stek 2009 | Review article. Pertinent references from the bibliography were individually evaluated. |
| Szyld 2006 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Taha 2009 | In the PEPI‐Malawi trial, women with unknown HIV status were screened at the time of delivery or late during pregnancy. With some women initiating ART after their delivery, this study population does not uniformly meet inclusion criteria for the systematic review (HIV‐infected pregnant women). |
| Tang 2006 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Tempelman 2004 | The complete triple therapy regimens administered were not always defined (Nelfinavir in 44%, NVP in 23%, AZT/3TC in 85%). |
| Thomas 2008 | Study was considered for inclusion in the systematic review, but authors did not reply to requests for additional information. |
| Thorne 2004 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Timmermans 2005 | Exact NRTI regimens administered with NVP and Nelfinavir are not defined. |
| Townsend 2006 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Townsend 2007 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Townsend 2009 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Tuomala 2002 | Combination therapy not clearly defined as triple therapy, and exact antiretroviral regimens administered are not defined. |
| Tuomala 2005 | Exact triple therapy antiretroviral regimens administered are not defined. |
| van der Merwe 2006 | Exact triple therapy antiretroviral regimens administered are not defined. |
| van Schalkwyk 2008 | Retrospective chart review did not provide CD4 entry criteria. Non‐nevirapine regimen not specified and outcomes not given by specified regimen. |
| Vithayasai 2002 | Not all patients receive triple therapy. No comparison group. |
| Volmink 2007 | Review article. Pertinent references from the bibliography were individually evaluated. |
| Watts 2004 | Exact triple therapy antiretroviral regimens administered are not defined. |
| Watts 2004a | Individual antiretroviral components identified, but exact triple therapy antiretroviral regimens administered are not defined. |
| Weinberg 2009 | No CD4 or immunologic entry criteria to ensure the population assessed fit the population of interest. Exact triple therapy antiretroviral regimens administered are not defined. |
| Zorrilla 2007 | There was a defined regimen, but no comparison group. |
Differences between protocol and review
None.
Contributions of authors
A.S.S. and E.K.D. collaborated on the GRADE evidence profiles and manuscript.
Sources of support
Internal sources
Global Health Sciences, University of California, San Francisco, USA.
External sources
-
World Health Organization #200123310, Switzerland.
Systematic reviews and development of GRADE profiles, based on the new WHO GRC guidelines, for the "WHO Guidelines on Antiretroviral Drugs for Treating Pregnant Women Living with HIV and Preventing HIV Infection in Infants ‐ 2009 revision"
-
Cooperative Agreement #U2GPS001468, "Atlanta HQ UCSF Technical Assistance to Support the President's Emergency Plan for AIDS Relief" from the Centers for Disease Control and Prevention (CDC), with funds from National Center for HIV, Viral Hepatitis, STDs and TB Prevention (NCHSTP)., USA.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC.
Declarations of interest
There are no conflicts of interest to report.
Edited (no change to conclusions)
References
References to studies included in this review
Bae 2008 {published data only}
- Bae WH, Wester C, Smeaton LM, Shapiro RL, Lockman S, Onyait K, et al. Hematologic and hepatic toxicities associated with antenatal and postnatal exposure to maternal highly active antiretroviral therapy among infants. AIDS (London, England) 2008;22(13):1633‐40. [PUBMED: 18670224] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Vincenzi 2009 {published data only (unpublished sought but not used)}
- deVincenzi I, et al. Triple antiretroviral prophylaxis during pregnancy to prevent mother‐to‐child transmission of HIV‐1 (The Kesho Bora Study). International AIDS Society Conference. Cape Town, South Africa;July 2009:Abstract LBPEC01. [Google Scholar]
Ekouevi 2008 {published data only}
- Ekouevi DK, Coffie PA, Becquet R, Tonwe‐Gold B, Horo A, Thiebaut R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d'Ivoire. AIDS (London, England) 2008;22(14):1815‐20. [PUBMED: 18753864] [DOI] [PubMed] [Google Scholar]
Jamisse 2007 {published data only}
- Jamisse L, Balkus J, Hitti J, Gloyd S, Manuel R, Osman N, et al. Antiretroviral‐associated toxicity among HIV‐1‐seropositive pregnant women in Mozambique receiving nevirapine‐based regimens. Journal of acquired immune deficiency syndromes (1999) 2007;44(4):371‐6. [PUBMED: 17259905] [DOI] [PubMed] [Google Scholar]
Lehman 2008 {published data only}
- Lehman DA, Chung MH, John‐Stewart GC, Richardson BA, Kiarie J, Kinuthia J, et al. HIV‐1 persists in breast milk cells despite antiretroviral treatment to prevent mother‐to‐child transmission. AIDS (London, England) 2008;22(12):1475‐85. [PUBMED: 18614871] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marazzi 2006 {published data only}
- Marazzi MC, Germano P, Liotta G, Guidotti G, Loureiro S, Cruz Gomes A, et al. Safety of nevirapine‐containing antiretroviral triple therapy regimens to prevent vertical transmission in an African cohort of HIV‐1‐infected pregnant women. HIV medicine 2006;7(5):338‐44. [PUBMED: 16945080] [DOI] [PubMed] [Google Scholar]
Phanuphak 2007 {published data only}
- Phanuphak N, Apornpong T, Teeratakulpisarn S, Chaithongwongwatthana S, Taweepolcharoen C, Mangclaviraj S, et al. Nevirapine‐associated toxicity in HIV‐infected Thai men and women, including pregnant women. HIV medicine 2007;8(6):357‐66. [PUBMED: 17661843] [DOI] [PubMed] [Google Scholar]
Shapiro 2009 {published data only (unpublished sought but not used)}
- Shapiro R, et al. A randomized trial comparing highly active antiretroviral therapy regimens for virologic efficacy and the prevention of mother‐to‐child transmission among breastfeeding women in Botswana (The Mma Bana Study). International AIDS Society Conference. Cape Town, South Africa;July 2009:Abstract WELBB101. [Google Scholar]
Tonwe‐Gold 2007 {published data only}
- Tonwe‐Gold B, Ekouevi DK, Viho I, Amani‐Bosse C, Toure S, Coffie PA, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two‐tiered approach. PLoS medicine 2007;4(8):e257. [PUBMED: 17713983] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alimenti 2003 {published data only}
- Alimenti A, Burdge DR, Ogilvie GS, Money DM, Forbes JC. Lactic acidemia in human immunodeficiency virus‐uninfected infants exposed to perinatal antiretroviral therapy. The Pediatric infectious disease journal 2003;22(9):782‐9. [PUBMED: 14506368] [DOI] [PubMed] [Google Scholar]
Arrive 2007 {published data only}
- Arrive E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, Masquelier B, et al. Prevalence of resistance to nevirapine in mothers and children after single‐dose exposure to prevent vertical transmission of HIV‐1: a meta‐analysis. International journal of epidemiology 2007;36(5):1009‐21. [PUBMED: 17533166] [DOI] [PubMed] [Google Scholar]
Arrive 2008 {published data only}
- Arrive E, Dabis F. Prophylactic antiretroviral regimens for prevention of mother‐to‐child transmission of HIV in resource‐limited settings. Current opinion in HIV and AIDS 2008;3(2):161‐5. [PUBMED: 19372960] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bardeguez 2008 {published data only}
- Bardeguez AD, Lindsey JC, Shannon M, Tuomala RE, Cohn SE, Smith E, et al. Adherence to antiretrovirals among US women during and after pregnancy. Journal of acquired immune deficiency syndromes (1999) 2008;48(4):408‐17. [PUBMED: 18614923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauer 2006 {published data only}
- Bauer GR, Colgrove RC, Larussa PS, Pitt J, Welles SL. Antiretroviral resistance in viral isolates from HIV‐1‐transmitting mothers and their infants. AIDS (London, England) 2006;20(13):1707‐12. [PUBMED: 16931934] [DOI] [PubMed] [Google Scholar]
Bellon 2004 {published data only}
- Bellon Cano JM, Sanchez‐Ramon S, Ciria L, Leon JA, Gurbindo D, Fortuny C, et al. The effects on infants of potent antiretroviral therapy during pregnancy: a report from Spain. Medical science monitor : international medical journal of experimental and clinical research 2004;10(5):CR179‐84. [PUBMED: 15114266] [PubMed] [Google Scholar]
Black 2008 {published data only}
- Black V, Hoffman RM, Sugar CA, Menon P, Venter F, Currier JS, et al. Safety and efficacy of initiating highly active antiretroviral therapy in an integrated antenatal and HIV clinic in Johannesburg, South Africa. Journal of acquired immune deficiency syndromes (1999) 2008;49(3):276‐81. [PUBMED: 18845949] [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2008a {published data only}
- Black V, Rees H. Incidence of nevirapine‐associated hepatitis in an antenatal clinic. South African medical journal = Suid‐Afrikaanse tydskrif vir geneeskunde 2008;98(2):116‐8. [PUBMED: 18350206] [PubMed] [Google Scholar]
Briand 2009 {published data only}
- Briand N, Mandelbrot L, Chenadec J, Tubiana R, Teglas JP, Faye A, et al. No relation between in‐utero exposure to HAART and intrauterine growth retardation. AIDS (London, England) 2009;23(10):1235‐43. [PUBMED: 19424054] [DOI] [PubMed] [Google Scholar]
Bucceri 2002 {published data only}
- Bucceri AM, Somigliana E, Matrone R, Ferraris G, Rossi G, Grossi E, et al. Combination antiretroviral therapy in 100 HIV‐1‐infected pregnant women. Human reproduction (Oxford, England) 2002;17(2):436‐41. [PUBMED: 11821291] [DOI] [PubMed] [Google Scholar]
Bunders 2005 {published data only}
- Bunders MJ, Bekker V, Scherpbier HJ, Boer K, Godfried M, Kuijpers TW. Haematological parameters of HIV‐1‐uninfected infants born to HIV‐1‐infected mothers. Acta paediatrica (Oslo, Norway : 1992) 2005;94(11):1571‐7. [PUBMED: 16303696] [DOI] [PubMed] [Google Scholar]
Chi 2007 {published data only}
- Chi BH, Chintu N, Lee A, Stringer EM, Sinkala M, Stringer JS. Expanded services for the prevention of mother‐to‐child HIV transmission: field acceptability of a pilot program in Lusaka, Zambia. Journal of acquired immune deficiency syndromes (1999) 2007; Vol. 45, issue 1:125‐7. [PUBMED: 17460478] [DOI] [PubMed]
Ciaranello 2008 {published data only}
- Ciaranello AL, Seage GR 3rd, Freedberg KA, Weinstein MC, Lockman S, Walensky RP. Antiretroviral drugs for preventing mother‐to‐child transmission of HIV in sub‐Saharan Africa: balancing efficacy and infant toxicity. AIDS (London, England) 2008;22(17):2359‐69. [PUBMED: 18981776] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clayden 2009 {published data only}
- Clayden, P. Safety Of Antiretrovirals In Pregnancy. Southern African Journal Of Hiv Medicine 2009;33:15‐18. [Google Scholar]
Cooper 2002 {published data only}
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV‐1‐infected women and prevention of perinatal HIV‐1 transmission. Journal of acquired immune deficiency syndromes (1999) 2002;29(5):484‐94. [PUBMED: 11981365] [DOI] [PubMed] [Google Scholar]
Cotter 2006 {published data only}
- Cotter AM, Garcia AG, Duthely ML, Luke B, O'Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth?. The Journal of infectious diseases 2006;193(9):1195‐201. [PUBMED: 16586354] [DOI] [PubMed] [Google Scholar]
Dabis 2005 {published data only}
- Dabis F, Bequet L, Ekouevi DK, Viho I, Rouet F, Horo A, et al. Field efficacy of zidovudine, lamivudine and single‐dose nevirapine to prevent peripartum HIV transmission. AIDS (London, England) 2005;19(3):309‐18. [PUBMED: 15718842] [PMC free article] [PubMed] [Google Scholar]
Deschamps 2009 {published data only}
- Deschamps MM, Noel F, Bonhomme J, Devieux JG, Saint‐Jean G, Zhu Y, et al. Prevention of mother‐to‐child transmission of HIV in Haiti. Revista panamericana de salud publica = Pan American journal of public health 2009;25(1):24‐30. [PUBMED: 19341520] [DOI] [PubMed] [Google Scholar]
Dinsmoor 2002 {published data only}
- Dinsmoor MJ, Forrest ST. Lack of an effect of protease inhibitor use on glucose tolerance during pregnancy. Infectious diseases in obstetrics and gynecology 2002;10(4):187‐91. [PUBMED: 12648312] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorenbaum 2002 {published data only}
- Dorenbaum A, Cunningham CK, Gelber RD, Culnane M, Mofenson L, Britto P, et al. Two‐dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA : the journal of the American Medical Association 2002;288(2):189‐98. [PUBMED: 12095383] [DOI] [PubMed] [Google Scholar]
Duran 2006 {published data only}
- Duran AS, Ivalo SA, Hakim A, Masciottra FM, Zlatkes R, Adissi L, et al. Prevention of mother to child HIV transmission. Medicina 2006;66(1):24‐30. [PUBMED: 16555724] [PubMed] [Google Scholar]
El 2006 {published data only}
- Beitune P, Duarte G. Antiretroviral agents during pregnancy: consequences on hematologic parameters in HIV‐exposed, uninfected newborn infant. European journal of obstetrics, gynecology, and reproductive biology 2006;128(1‐2):59‐63. [PUBMED: 16876310] [DOI] [PubMed] [Google Scholar]
El 2007 {published data only}
- Beitune P, Duarte G, Campbell O, Quintana SM, Rodrigues LC. Effects of antiretroviral agents during pregnancy on liver enzymes and amylase in HIV‐exposed, uninfected newborn infants. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases 2007;11(3):314‐7. [PUBMED: 17684631] [DOI] [PubMed] [Google Scholar]
European Collaborative Study (ECS) 2000 {published data only}
- European Collaborative Study. Combination antiretroviral therapy and duration of pregnancy. AIDS (London, England) 2000;14(18):2913‐20. [PUBMED: 11398741] [DOI] [PubMed] [Google Scholar]
European Collaborative Study (ECS) 2003 {published data only}
- European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV‐infected women. Journal of acquired immune deficiency syndromes (1999) 2003;32(4):380‐7. [PUBMED: 12640195] [DOI] [PubMed] [Google Scholar]
European Collaborative Study (ECS) 2005 {published data only}
- European Collaborative Study. Mother‐to‐child transmission of HIV infection in the era of highly active antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2005;40(3):458‐65. [PUBMED: 15668871] [DOI] [PubMed] [Google Scholar]
Fiore 2006 {published data only}
- Fiore S, Newell ML, Trabattoni D, Thorne C, Gray L, Savasi V, et al. Antiretroviral therapy‐associated modulation of Th1 and Th2 immune responses in HIV‐infected pregnant women. Journal of reproductive immunology 2006;70(1‐2):143‐50. [PUBMED: 16423410] [DOI] [PubMed] [Google Scholar]
Garcia‐Tejedor 2009 {published data only}
- Garcia‐Tejedor A, Maiques V, Perales A, Lopez‐Aldeguer J. Influence of highly active antiretroviral treatment (HAART) on risk factors for vertical HIV transmission. Acta obstetricia et gynecologica Scandinavica 2009;88(8):882‐7. [PUBMED: 19557554] [DOI] [PubMed] [Google Scholar]
Giuliano 2003 {published data only}
- Giuliano M, Palmisano L, Galluzzo CM, Amici R, Germinario E, Okong P, et al. Selection of resistance mutations in pregnant women receiving zidovudine and lamivudine to prevent HIV perinatal transmission. AIDS (London, England) 2003;17(10):1570‐2. [PUBMED: 12824800] [DOI] [PubMed] [Google Scholar]
Goetghebuer 2009 {published data only}
- Goetghebuer T, Haelterman E, Marvillet I, Barlow P, Hainaut M, Salameh A, et al. Vertical transmission of HIV in Belgium: a 1986‐2002 retrospective analysis. European journal of pediatrics 2009;168(1):79‐85. [PUBMED: 18392638] [DOI] [PubMed] [Google Scholar]
Grosch‐Woerner 2008 {published data only}
- Grosch‐Woerner I, Puch K, Maier RF, Niehues T, Notheis G, Patel D, et al. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV‐1‐infected women. HIV medicine 2008;9(1):6‐13. [PUBMED: 18199167] [DOI] [PubMed] [Google Scholar]
Hitti 2004 {published data only}
- Hitti J, Frenkel LM, Stek AM, Nachman SA, Baker D, Gonzalez‐Garcia A, et al. Maternal toxicity with continuous nevirapine in pregnancy: results from PACTG 1022. Journal of acquired immune deficiency syndromes (1999) 2004;36(3):772‐6. [PUBMED: 15213559] [DOI] [PubMed] [Google Scholar]
Hitti 2007 {published data only}
- Hitti J, Andersen J, McComsey G, Liu T, Melvin A, Smith L, et al. Protease inhibitor‐based antiretroviral therapy and glucose tolerance in pregnancy: AIDS Clinical Trials Group A5084. American journal of obstetrics and gynecology 2007;196(4):331.e1‐7. [PUBMED: 17403409] [DOI] [PubMed] [Google Scholar]
Hoffman 2009 {unpublished data only}
- Hoffman R, Black V, Technau K, Merwe K, Currier J, Chersich M, Coovadia A. Impact of highly active antiretroviral therapy regimen and duration of therapy on risk of mother‐to‐child HIV transmission in Johannesburg, South Africa. International AIDS Society Conference. Capetown, South Africa;July 2009:Abstract WEPEB260. [Google Scholar]
Jackson 2007 {published data only}
- Jackson DJ, Chopra M, Doherty TM, Colvin MS, Levin JB, Willumsen JF, et al. Operational effectiveness and 36 week HIV‐free survival in the South African programme to prevent mother‐to‐child transmission of HIV‐1. AIDS (London, England) 2007;21(4):509‐16. [PUBMED: 17301570] [DOI] [PubMed] [Google Scholar]
Justman 2003 {published data only}
- Justman JE, Benning L, Danoff A, Minkoff H, Levine A, Greenblatt RM, et al. Protease inhibitor use and the incidence of diabetes mellitus in a large cohort of HIV‐infected women. Journal of acquired immune deficiency syndromes (1999) 2003;32(3):298‐302. [PUBMED: 12626890] [DOI] [PubMed] [Google Scholar]
Kourtis 2007 {published data only}
- Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV‐infected women and the risk of premature delivery: a meta‐analysis. AIDS (London, England) 2007;21(5):607‐15. [PUBMED: 17314523] [DOI] [PubMed] [Google Scholar]
Kowalska 2003 {published data only}
- Kowalska A, Niemiec T, Midaoui A, Burkacka E. Effect of antiretroviral therapy on pregnancy outcome in HIV‐1 positive women. Medycyna wieku rozwojowego 2003;7(4 Pt 1):459‐68. [PUBMED: 15010556] [PubMed] [Google Scholar]
Livingston 2007 {published data only}
- Livingston EG, Cohn SE, Yang Y, Watts HD, Bardeguez AD, Jones TB, et al. Lipids and lactate in human immunodeficiency virus‐1 infected pregnancies with or without protease inhibitor‐based therapy. Obstetrics and gynecology 2007;110(2 Pt 1):391‐7. [PUBMED: 17666616] [DOI] [PubMed] [Google Scholar]
Lopez‐Cortes 2007 {published data only}
- Lopez‐Cortes LF, Ruiz‐Valderas R, Rivero A, Camacho A, Marquez‐Solero M, Santos J, et al. Efficacy of low‐dose boosted saquinavir once daily plus nucleoside reverse transcriptase inhibitors in pregnant HIV‐1‐infected women with a therapeutic drug monitoring strategy. Therapeutic drug monitoring 2007;29(2):171‐6. [PUBMED: 17417070] [DOI] [PubMed] [Google Scholar]
Lorenzi 1998 {published data only}
- Lorenzi P, Spicher VM, Laubereau B, Hirschel B, Kind C, Rudin C, et al. Antiretroviral therapies in pregnancy: maternal, fetal and neonatal effects. Swiss HIV Cohort Study, the Swiss Collaborative HIV and Pregnancy Study, and the Swiss Neonatal HIV Study. AIDS (London, England) 1998;12(18):F241‐7. [PUBMED: 9875571] [DOI] [PubMed] [Google Scholar]
Marti 2007 {published data only}
- Marti C, Pena JM, Bates I, Madero R, Jose I, Pallardo LF, et al. Obstetric and perinatal complications in HIV‐infected women. Analysis of a cohort of 167 pregnancies between 1997 and 2003. Acta obstetricia et gynecologica Scandinavica 2007;86(4):409‐15. [PUBMED: 17486461] [DOI] [PubMed] [Google Scholar]
Martin 2006 {published data only}
- Martin F, Navaratne L, Khan W, Sarner L, Mercey D, Anderson J, et al. Pregnant women with HIV infection can expect healthy survival: three‐year follow‐up. Journal of acquired immune deficiency syndromes (1999) 2006;43(2):186‐92. [PUBMED: 16940856] [DOI] [PubMed] [Google Scholar]
Martin 2007 {published data only}
- Martin F, Taylor GP. Increased rates of preterm delivery are associated with the initiation of highly active antiretrovial therapy during pregnancy: a single‐center cohort study. The Journal of infectious diseases 2007;196(4):558‐61. [PUBMED: 17624841] [DOI] [PubMed] [Google Scholar]
McIntyre 2002 {published data only}
- McIntyre J, Gray G. What can we do to reduce mother to child transmission of HIV?. BMJ (Clinical research ed.) 2002;324(7331):218‐21. [PUBMED: 11809646] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntyre 2006 {published data only}
- McIntyre J. Strategies to prevent mother‐to‐child transmission of HIV. Current opinion in infectious diseases 2006;19(1):33‐8. [PUBMED: 16374215] [DOI] [PubMed] [Google Scholar]
Mellins 2008 {published data only}
- Mellins CA, Chu C, Malee K, Allison S, Smith R, Harris L, et al. Adherence to antiretroviral treatment among pregnant and postpartum HIV‐infected women. AIDS care 2008;20(8):958‐68. [PUBMED: 18608073] [DOI] [PubMed] [Google Scholar]
Mofenson 2002 {published data only}
- Mofenson LM, Munderi P. Safety of antiretroviral prophylaxis of perinatal transmission for HIV‐infected pregnant women and their infants. Journal of acquired immune deficiency syndromes (1999) 2002;30(2):200‐15. [PUBMED: 12045684] [DOI] [PubMed] [Google Scholar]
Mussi‐Pinhata 2007 {published data only}
- Mussi‐Pinhata MM, Rego MA, Freimanis L, Kakehasi FM, Machado DM, Cardoso EM, et al. Maternal antiretrovirals and hepatic enzyme, hematologic abnormalities among human immunodeficiency virus type 1‐uninfected infants: the NISDI perinatal study. The Pediatric infectious disease journal 2007;26(11):1032‐7. [PUBMED: 17984811] [DOI] [PubMed] [Google Scholar]
Nurutdinova 2008 {published data only}
- Nurutdinova D, Onen NF, Hayes E, Mondy K, Overton ET. Adverse effects of tenofovir use in HIV‐infected pregnant women and their infants. The Annals of pharmacotherapy 2008;42(11):1581‐5. [PUBMED: 18957630] [DOI] [PubMed] [Google Scholar]
Onen 2008 {published data only}
- Onen NF, Nurutdinova D, Sungkanuparph S, Gase D, Mondy K, Overton ET. Effect of postpartum HIV treatment discontinuation on long‐term maternal outcome. Journal of the International Association of Physicians in AIDS Care (Chicago, Ill. : 2002) 2008;7(5):245‐51. [PUBMED: 18812593] [DOI] [PubMed] [Google Scholar]
Ono 2008 {published data only}
- Ono E, Nunes dos Santos AM, Menezes Succi RC, Machado DM, Angelis DS, Salomao R, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV‐1‐infected mothers on HAART. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica ... [et al.] 2008;41(8):700‐8. [PUBMED: 18797705] [DOI] [PubMed] [Google Scholar]
Pacheco 2006 {published data only}
- Pacheco SE, McIntosh K, Lu M, Mofenson LM, Diaz C, Foca M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV‐uninfected children: An analysis of the women and infants transmission study. The Journal of infectious diseases 2006;194(8):1089‐97. [PUBMED: 16991083] [DOI] [PubMed] [Google Scholar]
Palacios 2009 {published data only}
- Palacios R, Senise J, Vaz M, Diaz R, Castelo A. Short‐term antiretroviral therapy to prevent mother‐to‐child transmission is safe and results in a sustained increase in CD4 T‐cell counts in HIV‐1‐infected mothers. HIV medicine 2009;10(3):157‐62. [PUBMED: 19245537] [DOI] [PubMed] [Google Scholar]
Peltier 2009 {published data only}
- Peltier CA, Ndayisaba GF, Lepage P, Griensven J, Leroy V, Pharm CO, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother‐to‐child transmission in Rwanda. AIDS (London, England) 2009;23(18):2415‐23. [PUBMED: 19730349] [DOI] [PMC free article] [PubMed] [Google Scholar]
Resino 2001 {published data only}
- Resino Garcia S, Bellon Cano JM, Navarro Caspistegui J, Gurbindo Gutierrez D, Leon Leal JA, Munoz‐Fernandez MA. T‐cell subsets variation during clinical and immunological progression in vertically HIV‐infected children [Variacion de las subpoblaciones de celulas T segun la progresion clinica e inmunologica en ninos infectados verticalmente por HIV‐1.]. Medicina 2001;61(5 Pt 1):557‐65. [PUBMED: 11721322] [PubMed] [Google Scholar]
Schulte 2007 {published data only}
- Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG. Declines in low birth weight and preterm birth among infants who were born to HIV‐infected women during an era of increased use of maternal antiretroviral drugs: Pediatric Spectrum of HIV Disease, 1989‐2004. Pediatrics 2007;119(4):e900‐6. [PUBMED: 17353299] [DOI] [PubMed] [Google Scholar]
Stek 2009 {published data only}
- Stek AM. Antiretroviral medications during pregnancy for therapy or prophylaxis. Current HIV/AIDS reports 2009;6(2):68‐76. [PUBMED: 19358777] [DOI] [PubMed] [Google Scholar]
Szyld 2006 {published data only}
- Szyld EG, Warley EM, Freimanis L, Gonin R, Cahn PE, Calvet GA, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS (London, England) 2006;20(18):2345‐53. [PUBMED: 17117021] [DOI] [PubMed] [Google Scholar]
Taha 2009 {published data only}
- Taha TE, Kumwenda J, Cole SR, Hoover DR, Kafulafula G, Fowler MG, et al. Postnatal HIV‐1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. The Journal of infectious diseases 2009;200(10):1490‐7. [PUBMED: 19832114] [DOI] [PubMed] [Google Scholar]
Tang 2006 {published data only}
- Tang JH, Sheffield JS, Grimes J, McElwee B, Roberts SW, Laibl V, et al. Effect of protease inhibitor therapy on glucose intolerance in pregnancy. Obstetrics and gynecology 2006;107(5):1115‐9. [PUBMED: 16648418] [DOI] [PubMed] [Google Scholar]
Tempelman 2004 {published data only}
- Tempelman C, Timmermans S, Godfried MH, Dieleman JP, Boer K, Ende ME. Highly active antiretroviral therapy (HAART) in HIV‐positive pregnant women in the Netherlands, 1997‐2003: safe, effective and with few side effects [Krachtige antiretrovirale therapie (HAART) bij HIV‐positieve zwangeren in Nederland, 1997‐2003: veilig, effectief en met weinig bijwerkingen.]. Nederlands tijdschrift voor geneeskunde 2004;148(41):2021‐5. [PUBMED: 15553999] [PubMed] [Google Scholar]
Thomas 2008 {unpublished data only}
- Thomas T, Masaba R, Ndivo R, Zeh C, Borkowf C, Thigpen M, Cock K, Amornkul P, Greenberg A, Fowler M, and Kisumu Breastfeeding Study Team. Prevention of Mother‐to‐Child Transmission of HIV‐1 among Breastfeeding Mothers Using HAART: The Kisumu Breastfeeding Study, Kisumu, Kenya, 2003‐2007. Conference on Retroviruses and Opportunistic Infections Boston MA;February 2008:Abstract 45aLB. [Google Scholar]
Thorne 2004 {published data only}
- Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV‐infected women treated with highly active antiretroviral therapy in Europe. AIDS (London, England) 2004;18(17):2337‐9. [PUBMED: 15577551] [DOI] [PubMed] [Google Scholar]
Timmermans 2005 {published data only}
- Timmermans S, Tempelman C, Godfried MH, Nellen J, Dieleman J, Sprenger H, et al. Nelfinavir and nevirapine side effects during pregnancy. AIDS (London, England) 2005;19(8):795‐9. [PUBMED: 15867493] [DOI] [PubMed] [Google Scholar]
Townsend 2006 {published data only}
- Townsend CL, Tookey PA, Cortina‐Borja M, Peckham CS. Antiretroviral therapy and congenital abnormalities in infants born to HIV‐1‐infected women in the United Kingdom and Ireland, 1990 to 2003. Journal of acquired immune deficiency syndromes (1999) 2006;42(1):91‐4. [PUBMED: 16763496] [DOI] [PubMed] [Google Scholar]
Townsend 2007 {published data only}
- Townsend CL, Cortina‐Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV‐infected women in the United Kingdom and Ireland. AIDS (London, England) 2007;21(8):1019‐26. [PUBMED: 17457096] [DOI] [PubMed] [Google Scholar]
Townsend 2009 {published data only}
- Townsend CL, Willey BA, Cortina‐Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and congenital abnormalities in infants born to HIV‐infected women in the UK and Ireland, 1990‐2007. AIDS (London, England) 2009;23(4):519‐24. [PUBMED: 19165088] [DOI] [PubMed] [Google Scholar]
Tuomala 2002 {published data only}
- Tuomala RE, Shapiro DE, Mofenson LM, Bryson Y, Culnane M, Hughes MD, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. The New England journal of medicine 2002;346(24):1863‐70. [PUBMED: 12063370] [DOI] [PubMed] [Google Scholar]
Tuomala 2005 {published data only}
- Tuomala RE, Watts DH, Li D, Vajaranant M, Pitt J, Hammill H, et al. Improved obstetric outcomes and few maternal toxicities are associated with antiretroviral therapy, including highly active antiretroviral therapy during pregnancy. Journal of acquired immune deficiency syndromes (1999) 2005;38(4):449‐73. [PUBMED: 15764963] [DOI] [PubMed] [Google Scholar]
van der Merwe 2006 {published data only}
- Merwe K, Chersich MF, Technau K, Umurungi Y, Conradie F, Coovadia A. Integration of antiretroviral treatment within antenatal care in Gauteng Province, South Africa. Journal of acquired immune deficiency syndromes (1999) 2006;43(5):577‐81. [PUBMED: 17031321] [DOI] [PubMed] [Google Scholar]
van Schalkwyk 2008 {published data only}
- Schalkwyk JE, Alimenti A, Khoo D, Maan E, Forbes JC, Burdge DR, et al. Serious toxicity associated with continuous nevirapine‐based HAART in pregnancy. BJOG : an international journal of obstetrics and gynaecology 2008;115(10):1297‐302. [PUBMED: 18715416] [DOI] [PubMed] [Google Scholar]
Vithayasai 2002 {published data only}
- Vithayasai V, Moyle GJ, Supajatura V, Wattanatchariya N, Kanshana S, Sirichthaporn P, et al. Safety and efficacy of saquinavir soft‐gelatin capsules + zidovudine + optional lamivudine in pregnancy and prevention of vertical HIV transmission. Journal of acquired immune deficiency syndromes (1999) 2002;30(4):410‐2. [PUBMED: 12138347] [DOI] [PubMed] [Google Scholar]
Volmink 2007 {published data only}
- Volmink J, Siegfried NL, Merwe L, Brocklehurst P. Antiretrovirals for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane database of systematic reviews (Online) 2007;1:CD003510. [PUBMED: 17253490] [DOI] [PubMed] [Google Scholar]
Watts 2004 {published data only}
- Watts DH, Balasubramanian R, Maupin RT Jr, Delke I, Dorenbaum A, Fiore S, et al. Maternal toxicity and pregnancy complications in human immunodeficiency virus‐infected women receiving antiretroviral therapy: PACTG 316. American journal of obstetrics and gynecology 2004;190(2):506‐16. [PUBMED: 14981398] [DOI] [PubMed] [Google Scholar]
Watts 2004a {published data only}
- Watts DH, Covington DL, Beckerman K, Garcia P, Scheuerle A, Dominguez K, et al. Assessing the risk of birth defects associated with antiretroviral exposure during pregnancy. American journal of obstetrics and gynecology 2004;191(3):985‐92. [PUBMED: 15467577] [DOI] [PubMed] [Google Scholar]
Weinberg 2009 {published data only}
- Weinberg A, Forster‐Harwood J, McFarland EJ, Pappas J, Davies JK, Kinzie K, et al. Resistance to antiretrovirals in HIV‐infected pregnant women. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2009;45(1):39‐42. [PUBMED: 19329355] [DOI] [PubMed] [Google Scholar]
Zorrilla 2007 {published data only}
- Zorrilla CD, Dyke R, Bardeguez A, Acosta EP, Smith B, Hughes MD, et al. Clinical response and tolerability to and safety of saquinavir with low‐dose ritonavir in human immunodeficiency virus type 1‐infected mothers and their infants. Antimicrobial agents and chemotherapy 2007;51(6):2208‐10. [PUBMED: 17420209] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bond 2007
- Bond K, Horváth T, Harvey K, Wiysonge CS, Read JS. The Cochrane Library and mother‐to‐child transmission of HIV: an umbrella review. Evidence‐based Child Health: A Cochrane Review Journal 2007;2:4‐24. [Google Scholar]
Bryson 1992
Bulterys 1997
- Bulterys M, Landesman S, Burns DN, Rubinstein A, Goedert JJ. Sexual behavior and injection drug use during pregnancy and vertical transmission of HIV‐1. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association 1997;15(1):76‐82. [PUBMED: 9215658] [DOI] [PubMed] [Google Scholar]
Connor 1994
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, et al. Reduction of maternal‐infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. The New England journal of medicine 1994;331(18):1173‐80. [PUBMED: 7935654] [DOI] [PubMed] [Google Scholar]
Cooper 2002
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV‐1‐infected women and prevention of perinatal HIV‐1 transmission. Journal of acquired immune deficiency syndromes (1999) 2002;29(5):484‐94. [PUBMED: 11981365] [DOI] [PubMed] [Google Scholar]
De Cock 2000
- Cock KM, Fowler MG, Mercier E, Vincenzi I, Saba J, Hoff E, et al. Prevention of mother‐to‐child HIV transmission in resource‐poor countries: translating research into policy and practice. JAMA : the journal of the American Medical Association 2000;283(9):1175‐82. [PUBMED: 10703780] [DOI] [PubMed] [Google Scholar]
European Collaborative Study (ECS) 1999
- European Collaborative Study. Maternal viral load and vertical transmission of HIV‐1: an important factor but not the only one. The European Collaborative Study. AIDS (London, England) 1999;13(11):1377‐85. [PUBMED: 10449292] [PubMed] [Google Scholar]
European Collaborative Study (ECS) 2000
- European Collaborative Study. Combination antiretroviral therapy and duration of pregnancy. AIDS (London, England) 2000;14(18):2913‐20. [PUBMED: 11398741] [DOI] [PubMed] [Google Scholar]
European Mode of Delivery Collaboration‐ EMDC 1999
- European Mode of Delivery Collaboration. Elective caesarean‐section versus vaginal delivery in prevention of vertical HIV‐1 transmission: a randomised clinical trial. The European Mode of Delivery Collaboration. Lancet 1999;353(9158):1035‐9. [PUBMED: 10199349] [DOI] [PubMed] [Google Scholar]
Fitzgerald 2009
- Fitzgerald D and the GHESKIO Centers, Haiti. A randomized clinical trial of early versus standard antiretoviral therapy for HIV‐infected patients with a CD4 T cell count of 200‐350 cells/mL (CIPRAHT001). International AIDS Society Conference. Capetown, South Africa;July 2009:Abstract WESY201. [Google Scholar]
Floridia 2008
- Floridia M, Ravizza M, Bucceri A, Lazier L, Vigano A, Alberico S, et al. Factors influencing gestational age‐adjusted birthweight in a national series of 600 newborns from mothers with HIV. HIV clinical trials 2008;9(5):287‐97. [PUBMED: 18977717] [DOI] [PubMed] [Google Scholar]
Guay 1999
- Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single‐dose nevirapine compared with zidovudine for prevention of mother‐to‐child transmission of HIV‐1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354(9181):795‐802. [PUBMED: 10485720] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S. Cochrane handbook for systematic reviews. Hoboken, NJ;Wiley‐Blackwell:2008. [Google Scholar]
Hitti 2004
- Hitti J, Frenkel LM, Stek AM, Nachman SA, Baker D, Gonzalez‐Garcia A, et al. Maternal toxicity with continuous nevirapine in pregnancy: results from PACTG 1022. Journal of acquired immune deficiency syndromes (1999) 2004;36(3):772‐6. [PUBMED: 15213559] [DOI] [PubMed] [Google Scholar]
Horvath 2009
- Horvath T, Madi BC, Iuppa IM, Kennedy GE, Rutherford G, Read JS. Interventions for preventing late postnatal mother‐to‐child transmission of HIV. Cochrane database of systematic reviews (Online);2009(1):CD006734. [PUBMED: 19160297] [DOI] [PMC free article] [PubMed] [Google Scholar]
International Perinatal HIV Group (IPHG) 1999
- International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1‐‐a meta‐analysis of 15 prospective cohort studies. The International Perinatal HIV Group. The New England journal of medicine 1999;340(13):977‐87. [PUBMED: 10099139] [DOI] [PubMed] [Google Scholar]
International Perinatal HIV Group (IPHG) 2001
- International Perinatal HIV Group. Duration of ruptured membranes and vertical transmission of HIV‐1: a meta‐analysis from 15 prospective cohort studies. AIDS (London, England) 2001;15(3):357‐68. [PUBMED: 11273216] [DOI] [PubMed] [Google Scholar]
Ioannidis 2001
- Ioannidis JP, Abrams EJ, Ammann A, Bulterys M, Goedert JJ, Gray L, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads <1000 copies/ml. The Journal of infectious diseases 2001;183(4):539‐45. [PUBMED: 11170978] [DOI] [PubMed] [Google Scholar]
Kwiek 2006
- Kwiek JJ, Mwapasa V, Milner DA Jr, Alker AP, Miller WC, Tadesse E, et al. Maternal‐fetal microtransfusions and HIV‐1 mother‐to‐child transmission in Malawi. PLoS medicine 2006;3(1):e10. [PUBMED: 16287342] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lallemant 2000
- Lallemant M, Jourdain G, Coeur S, Kim S, Koetsawang S, Comeau AM, et al. A trial of shortened zidovudine regimens to prevent mother‐to‐child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. The New England journal of medicine 2000;343(14):982‐91. [PUBMED: 11018164] [DOI] [PubMed] [Google Scholar]
Langston 1995
- Langston C, Lewis DE, Hammill HA, Popek EJ, Kozinetz CA, Kline MW, et al. Excess intrauterine fetal demise associated with maternal human immunodeficiency virus infection. The Journal of infectious diseases 1995;172(6):1451‐60. [PUBMED: 7594702] [DOI] [PubMed] [Google Scholar]
McIntyre 2009
- McIntyre JA, Hopley M, Moodley D, Eklund M, Gray GE, Hall DB, et al. Efficacy of short‐course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother‐to‐child HIV transmission: a randomized clinical trial. PLoS medicine 2009;6(10):e1000172. [PUBMED: 19859531] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mofenson 1997
- Mofenson LM. Mother‐child HIV‐1 transmission: Timing and determinants. Obstetrics and gynecology clinics of North America 1997;24(4):759‐84. [PUBMED: 9430166] [DOI] [PubMed] [Google Scholar]
Mofenson 1999
- Mofenson LM, Lambert JS, Stiehm ER, Bethel J, Meyer WA 3rd, Whitehouse J, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. The New England journal of medicine 1999;341(6):385‐93. [PUBMED: 10432323] [DOI] [PubMed] [Google Scholar]
Moodley 2003
- Moodley D, Moodley J, Coovadia H, Gray G, McIntyre J, Hofmyer J, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother‐to‐child transmission of human immunodeficiency virus type 1. The Journal of infectious diseases 2003;187(5):725‐35. [PUBMED: 12599045] [DOI] [PubMed] [Google Scholar]
Nduati 2000
- Nduati R, John G, Mbori‐Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV‐1: a randomized clinical trial. JAMA : the journal of the American Medical Association 2000;283(9):1167‐74. [PUBMED: 10703779] [DOI] [PubMed] [Google Scholar]
Newcastle‐Ottawa
- Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta‐analyses. Ottawa Health Research Institute. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
Nicoll 1995
- Nicoll A, Newell ML, Praag E, Perre P, Peckham C. Infant feeding policy and practice in the presence of HIV‐1 infection. AIDS (London, England) 1995; Vol. 9, issue 2:107‐19. [PUBMED: 7718182] [PubMed]
Perinatal HIV Guidelines Working Group, 2009
- The Perinatal HIV Guidelines Working Group. Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV‐Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. April 29, 2009 pp 1‐90;Available at http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf:Accessed January 13, 2010. [Google Scholar]
PETRA 2002
- Petra Study Group. Efficacy of three short‐course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV‐1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double‐blind, placebo‐controlled trial. Lancet 2002;359(9313):1178‐86. [PUBMED: 11955535] [DOI] [PubMed] [Google Scholar]
Rasheed 1996
- Rasheed S, Li Z, Xu D, Kovacs A. Presence of cell‐free human immunodeficiency virus in cervicovaginal secretions is independent of viral load in the blood of human immunodeficiency virus‐infected women. American journal of obstetrics and gynecology 1996;175(1):122‐9. [PUBMED: 8694037] [DOI] [PubMed] [Google Scholar]
Read 2005
- Read JS, Newell MK. Efficacy and safety of cesarean delivery for prevention of mother‐to‐child transmission of HIV‐1. Cochrane database of systematic reviews (Online) 2005;1:CD005479. [PUBMED: 16235405] [DOI] [PubMed] [Google Scholar]
Richardson 2003
- Richardson BA, John‐Stewart GC, Hughes JP, Nduati R, Mbori‐Ngacha D, Overbaugh J, et al. Breast‐milk infectivity in human immunodeficiency virus type 1‐infected mothers. The Journal of infectious diseases 2003;187(5):736‐40. [PUBMED: 12599046] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulte 2007
- Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG. Declines in low birth weight and preterm birth among infants who were born to HIV‐infected women during an era of increased use of maternal antiretroviral drugs: Pediatric Spectrum of HIV Disease, 1989‐2004. Pediatrics 2007;119(4):e900‐6. [PUBMED: 17353299] [DOI] [PubMed] [Google Scholar]
Shaffer 1999
- Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short‐course zidovudine for perinatal HIV‐1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet 1999;353(9155):773‐80. [PUBMED: 10459957] [DOI] [PubMed] [Google Scholar]
Szyld 2006
- Szyld EG, Warley EM, Freimanis L, Gonin R, Cahn PE, Calvet GA, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS (London, England) 2006;20(18):2345‐53. [PUBMED: 17117021] [DOI] [PubMed] [Google Scholar]
Taha (NVAZ) 2003
- Taha TE, Kumwenda NI, Gibbons A, Broadhead RL, Fiscus S, Lema V, et al. Short postexposure prophylaxis in newborn babies to reduce mother‐to‐child transmission of HIV‐1: NVAZ randomised clinical trial. Lancet 2003;362(9391):1171‐7. [PUBMED: 14568737] [DOI] [PubMed] [Google Scholar]
Taha 2004
- Taha TE, Kumwenda NI, Hoover DR, Fiscus SA, Kafulafula G, Nkhoma C, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA : the journal of the American Medical Association 2004;292(2):202‐9. [PUBMED: 15249569] [DOI] [PubMed] [Google Scholar]
Taha 2007
- Taha TE, Hoover DR, Kumwenda NI, Fiscus SA, Kafulafula G, Nkhoma C, et al. Late postnatal transmission of HIV‐1 and associated factors. The Journal of infectious diseases 2007;196(1):10‐4. [PUBMED: 17538877] [DOI] [PubMed] [Google Scholar]
Thior 2006
- Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother‐to‐child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA : the journal of the American Medical Association 2006;296(7):794‐805. [PUBMED: 16905785] [DOI] [PubMed] [Google Scholar]
Townsend 2008
- Townsend CL, Cortina‐Borja M, Peckham CS, Ruiter A, Lyall H, Tookey PA. Low rates of mother‐to‐child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000‐2006. AIDS (London, England) 2008;22(8):973‐81. [PUBMED: 18453857] [DOI] [PubMed] [Google Scholar]
Tuomala 2002
- Tuomala RE, Shapiro DE, Mofenson LM, Bryson Y, Culnane M, Hughes MD, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. The New England journal of medicine 2002;346(24):1863‐70. [PUBMED: 12063370] [DOI] [PubMed] [Google Scholar]
UNAIDS 2009
- Joint United Nations Programme on HIV/AIDS. 2009 AIDS Epidemic Update. Geneva, Geneva: 2009;1:1‐100. [Google Scholar]
Watts 2004
- Watts DH, Balasubramanian R, Maupin RT Jr, Delke I, Dorenbaum A, Fiore S, et al. Maternal toxicity and pregnancy complications in human immunodeficiency virus‐infected women receiving antiretroviral therapy: PACTG 316. American journal of obstetrics and gynecology 2004;190(2):506‐16. [PUBMED: 14981398] [DOI] [PubMed] [Google Scholar]
WHO (Infant Feeding) 2009
- WHO Infant Feeding Collaborative Study Team. Rapid advice: infant feeding in the context of HIV. Geneva October 22‐23, 2009;1:1‐30. [Google Scholar]
WHO 2006
- WHO MTCT Collaborative Study Team. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. Geneva June 28‐29, 2005;1:1‐87. [Google Scholar]
WHO 2009
- WHO MTCT Collaborative Study Team. Rapid Advice: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva October 19‐21, 2009;1:1‐26. [Google Scholar]
Wiysonge 2005
- Wiysonge CS, Shey MS, Shang JD, Sterne JA, Brocklehurst P. Vaginal disinfection for preventing mother‐to‐child transmission of HIV infection. Cochrane database of systematic reviews (Online) 2005;1(4):CD003651. [PUBMED: 16235334] [DOI] [PubMed] [Google Scholar]
Wiysonge 2005a
- Wiysonge CS, Shey MS, Sterne JA, Brocklehurst P. Vitamin A supplementation for reducing the risk of mother‐to‐child transmission of HIV infection. Cochrane database of systematic reviews (Online) 2005;1(4):CD003648. [PUBMED: 16235332] [DOI] [PubMed] [Google Scholar]


