Abstract
Malaria remains a pressing global health challenge, with rising drug resistance threatening current treatment strategies. Partial resistance to dihydroartemisinin-piperaquine (DHA-PPQ) has emerged in Southeast Asia, particularly in Plasmodium falciparum strains from Cambodia. While artemisinin partial resistance is associated with mutations in kelch13, reduced PPQ sensitivity has been linked to increased copy numbers of the aspartic protease genes plasmepsin II and III and mutations in the chloroquine resistance transporter. In this study, we demonstrate the effective use of CRISPR-Cas9 technology to generate single knockouts (KO) of plasmepsin II and plasmepsin III, as well as a double KO of both genes, in two isogenic Cambodian parasites with varying numbers of plasmepsin gene copies. The deletion of plasmepsin II and/or III increased parasite sensitivity to PPQ. We explored several hypotheses to understand how an increased plasmepsin gene copy number might influence parasite survival under high PPQ pressure. Our findings indicate that protease inhibitors have a minimal impact on parasite susceptibility to PPQ. Additionally, parasites with higher plasmepsin gene copy numbers did not exhibit significantly increased hemoglobin digestion, differences in peptide composition, nor did they produce different amounts of free heme following PPQ treatment compared to wildtype (single copy) parasites. Interestingly, hemoglobin digestion was slowed in parasites with plasmepsin II deletions. We also found that culturing parasites with different plasmepsin II and III copies in amino acid-limited media had little impact on parasite sensitivity to high-dose PPQ. By treating parasites with modulators of digestive vacuole (DV) homeostasis, we found that changes in DV pH potentially affect their response to PPQ. Our research highlights the crucial role of increased plasmepsin II and III gene copy numbers in modulating response to PPQ and begins to uncover the molecular and physiological mechanisms underlying the contribution of plasmepsin II and III amplification to PPQ resistance in Cambodian parasites.
Author summary
Global malaria control has plateaued, with drug-resistant Plasmodium falciparum posing a significant challenge. Artemisinin-based combination therapies (ACTs) are becoming less effective, especially in Southeast Asia, where resistance to dihydroartemisinin-piperaquine (DHA-PPQ) resulted in high rates of treatment failures, notably in Cambodia. Genome-wide association studies link artemisinin partial resistance to kelch13 mutations, while decreased PPQ sensitivity is associated with higher plasmepsin II and III gene copies and mutations in the chloroquine resistance transporter PfCRT.
We previously showed a connection between increased plasmepsin II and III gene copy number and reduced PPQ sensitivity. In this study, we try to understand the biological role of the Plasmepsins in PPQ sensitivity. Therefore, we knocked out plasmepsin II and/or III genes in Cambodian strains using CRISPR-Cas9, and found increased PPQ sensitivity, confirming these genes’ roles in resistance. Plasmepsins are proteases that participate in the hemoglobin degradation cascade in the digestive vacuole of the parasites. However, we detected no evidence that hemoglobin digestion was significantly altered in parasites with increased plasmepsin II and III copy numbers. Altogether, this work elaborates on our current understanding of how plasmepsin II and III amplifications contribute to PPQ resistance and highlights the need for further research into PPQ resistance mechanisms.
Introduction
Despite remarkable strides toward global malaria control since the early 2000s, progress in reducing the total number of malaria cases has plateaued [1]. Drug resistance in Plasmodium falciparum parasites, particularly to first- and second-line artemisinin (ART)-based combination therapies (ACTs), remains a major challenge to malaria elimination. In regions of Southeast Asia, resistance to both potent but short-lived ART-derived compounds and long-lasting partner drugs is emerging and rapidly spreading [2]. Widespread parasite resistance to dihydroartemisinin-piperaquine (DHA-PPQ) has resulted in clinical treatment failures throughout western Cambodia, where DHA-PPQ was adopted in 2008 [3–7]. PPQ resistance is marked by parasite recrudescence 42 days post-treatment [3]. The spread of PPQ-resistant parasites in Southeast Asia has significantly shortened the usable lifespan of DHA-PPQ, resulting in Cambodia’s 2016 reversal to artesunate-mefloquine at a first-line antimalarial [8].
There is a well-established association between mutations in the propeller region of kelch13 and ART treatment failure in P. falciparum parasites, including those collected from patient samples in Southeast Asia by the Tracking Resistance to Artemisinin Collaboration (TRAC) used in this study [9–14]. GWAS of Cambodian P. falciparum isolates have revealed that decreased PPQ sensitivity is associated with increased copy numbers of the genes coding for the aspartic proteases Plasmepsin II and III in combination with a single copy of the multidrug resistance-1 (pfmdr1) gene [15,16]. In vitro studies in the laboratory strain 3D7 identified a slight sensitization to PPQ when plasmepsin II and III were deleted [17], but no susceptibility change was observed when plasmepsin II and III were overexpressed [18]. Furthermore, SNPs in the chloroquine resistance transporter (pfcrt) gene have also been associated with PPQ resistance [5,15,16,19]. Mutations in pfcrt have been shown to confer PPQ resistance when introduced into the Dd2 laboratory strain [20–23]. In vitro PPQ resistance has also been observed in South America, where field isolates from Guiana and Suriname carried a PfCRT C350R variant in combination with plasmepsin II and III amplifications [24]. To date, parasites with mutations in kelch13, increased plasmepsin copy numbers, and pfcrt mutations are now almost fixed in Southeast Asia [25–28], the treatment failure rate increased up to 70% in Western Cambodia [2], and surveillance of these markers is now underway in Africa and South America.
Despite the identification of promising molecular markers, the characterization of PPQ resistance in parasite field isolates has proved challenging. When subjected to a standard drug susceptibility assay, PPQ-resistant parasites yield bimodal dose-response curves, with increased parasite survival at high PPQ concentrations. These bimodal curves cannot be described using traditional non-linear regression analysis and yield non-interpretable EC50 values when a curve fit is forced [4,7]. As a result, many practitioners rely on estimates of the EC90 instead. A PPQ survival assay (PSA) was developed, wherein parasite survival after 48 h of a single PPQ treatment (200 nM) is compared to that of parasites cultured in vehicle control [5]. However, PSA analysis is labor and time intensive. To better describe the bimodal response, we increased the concentration range of PPQ and utilized the area under the curve (AUC) of the high-dose peak to quantify the PPQ response [29]. Culture-adapted TRAC isolates showed a correlation between increased plasmepsin II and plasmepsin III copy numbers and AUC. A panel of clonal isogenic lines, all with single copies of pfmdr1 and identical pfcrt loci, showed decreased sensitivity to PPQ with increasing copy numbers of plasmepsin II and plasmepsin III, implicating these copy number variations (CNVs) as potential drivers of PPQ resistance [29]. Analysis of plasmepsin II and plasmepsin III KOs in the relevant genetic background of Cambodian isolates is key to further understanding the role of genetic background in modulating Plasmepsin II and III action under PPQ pressure.
Plasmepsin II and III, along with Plasmepsin I and IV, are aspartic proteases that participate in the hemoglobin degradation cascade in the digestive vacuole (DV) [30–34]. RNAseq data suggests that Plasmepsin III is maximally transcribed in late ring stages while Plasmepsin II peaks later during the early trophozoite stage, which might reflect a greater role for Plasmepsin III earlier in the intra-erythrocytic developmental cycle [35]. Targeted genetic disruptions of the plasmepsin genes - either individually or in combination - yield viable parasites lacking dramatic changes in morphology or growth, suggesting functional redundancy between these aspartic proteases and other proteases in the DV [34,36]. The digestion of hemoglobin crucially provides a source of amino acids for parasites; however, the breakdown of hemoglobin releases toxic free heme, which aggregates as inert, crystalline hemozoin within the DV [37–39]. As an aminoquinoline, PPQ is thought to impede the degradation of hemoglobin within the parasite DV, leading to a build-up of toxic free heme and parasite death [21,39]. However, the biological mode of PPQ action is not well understood.
Here, we show successful CRISPR-Cas9-mediated plasmepsin II single KO, plasmepsin III single KO, and plasmepsin II and plasmepsin III double KO in two isogenic lines of Cambodian parasites with variable plasmepsin copy numbers. Disruption of plasmepsin II and/or plasmepsin III resulted in increased parasite sensitivity to PPQ, as measured by the AUC. We tested several hypotheses as to how increased plasmepsin copy number could influence survival under high PPQ pressure. We show that protease inhibitors have a minimal effect on parasite susceptibility to PPQ. In addition, hemoglobin digestion was not significantly increased in parasites with higher plasmepsin copy numbers nor did these parasites produce different amounts of free heme upon PPQ treatment. However, hemoglobin digestion was slowed in parasites with plasmepsin II deletions. To explore how the physiological conditions of the DV contribute to PPQ susceptibility, we treated parasites with modulators of DV function, with results suggesting that fluctuations in DV pH affect parasite response to PPQ. Thus, in Cambodian parasites, we describe the critical role of plasmepsin II and plasmepsin III CNVs in modulating parasite response to PPQ and begin to probe the molecular and physiological underpinnings of PPQ resistance.
Results
Generation of plasmepsin KOs in TRAC isolates
We have previously shown that clones from the TRAC isolate KH001_053 contain variations in plasmepsin II and III copy numbers that correlate with their PPQ resistance phenotype, as measured by the AUC in PPQ growth assays. These clones are genetically identical, including the pfcrt (Dd2 like) and the pfmdr1 loci (single copy); the only difference is the plasmepsin II/III CNV [29]. We confirmed the tandem arrangement of the duplicated plasmepsin II and III locus [16,40], where the break points of the duplication are in the 3’ regions of plasmepsin I and plasmepsin III, resulting in a chimeric plasmepsin III/I between two plasmepsin II copies, followed by an intact plasmepsin III copy (Fig 1A).
Fig 1. Correct disruption of the duplicated plasmepsin locus by KO constructs.
(A) Schema of the single and duplicated plasmepsin loci predicted by Amato et al. [16]. (B) Schema of duplicated locus, edited loci and homology plasmids used for integration into the locus. Restriction enzyme sites and expected band sizes for Southern blots are indicated in the schema. gDNA was digested [AflII (A) and NciI (N)], run on a gel, transferred to a membrane, and hybridized with three different probes indicated by colored bars (orange: plasmepsin II, blue: plasmepsin III, and purple: hdhfr cassette). Arrows indicate expected band sizes. Southern blots for additional clones and the single locus parasites (KH001_053G10) are shown in S1–S3 Figs.
To determine if either plasmepsin II or plasmepsin III is responsible for the increased AUC in PPQ growth assays, we generated plasmepsin II and plasmepsin III single KOs and a plasmepsin II/III double KO in the relevant genetic background of a TRAC isolate. A PPQ-susceptible clone of KH001_053 with a single copy of each plasmepsin II and III (KH001_053G10) and a PPQ-resistant clone with two copies of both plasmepsin II and plasmepsin III (KH001_053G8) served as parental lines [29]. We used CRISPR-Cas9 technology to introduce double-strand breaks in the plasmepsin genes and provided the parasites with a template to disrupt either plasmepsin II (G10PMII_KO and G8PMII_KO) or plasmepsin III (G10PMIII_KO and G8PMIII_KO) alone or both genes simultaneously (G10PMII/III_KO and G8PMII/III_KO) with an hdhfr selectable marker as described previously [34]. Gene editing in the duplicated KH001_053G8 locus resulted in the same outcome as the KH001_053G10 single locus due to Cas9 cutting both copies of the duplicated plasmepsin genes and fusion of the two remaining pieces with the hdhfr marker (Figs 1B and S1–S3).
Transgenic parasites were cloned, and subsequently, the integration status was verified by PCR, quantitative PCR (qPCR) (S1 Table), and Southern blots for at least two clones per transfection (Figs 1 and S1–S3).
Loss of plasmepsin duplication in field isolates abolishes PPQ bimodal response
We tested the KH001_053G10 and KH001_053G8 parental lines and the engineered plasmepsin KO parasites in PPQ growth assays, compared the initial Hill slope, and measured the AUC (Fig 2A–E and S2 Table). It should be noted that the KO parasites generated from KH001_053G8 reduced the plasmepsin copy numbers from two copies, and the KH001_053G10 KO parasites from one copy, resulting in genetically identical KOs. Consequently, the engineered parasites from KH001_053G10 and KH001_053G8 were also indistinguishable in their phenotypic response (Fig 2E, one-way ANOVA with Tukey post-test p < 0.05). We therefore used the KH001_053G8 KO lines for further phenotypic analysis. G8PMII_KO, G8PMIII_KO, and G8PMII/III_KO had indistinguishable initial Hill slopes but had a strong significant reduction of the AUC when compared to the KH001_053G8 parent with two copies of both plasmepsin II and III (Fig 2A–D, one-way ANOVA with Tukey post-test p < 0.0001). The same was true for G10PMII_KO, G10PMIII_KO, and G10PMII/III_KO compared to KH001_053G8. However, only G8PMIII_KO, G8PMII/III_KO, G10PMIII_KO, and G10PMII/III_KO showed significantly reduced AUC compared to the KH001_053G10 single locus (Fig 2E and S2 Table, one-way ANOVA with Tukey post-test p < 0.05). Taken together, these data confirm the role of duplicated plasmepsin genes in survival of parasites at high concentrations of PPQ and that expression of plasmepsin III appears to be the dominant driver of this phenotype.
Fig 2. Disruption of plasmepsin loci leads to loss of AUC.
Parasites were exposed to increasing levels of PPQ for 84 h and survival was measured by increased DNA content. Shown is an example of three biologically independent experiments run in triplicate for the KH001_053G8 parental locus (A) and parasites with disruption of either plasmepsin II (G8PM II_KO, B), plasmepsin III (G8PM III_KO, C), or a double KO of plasmepsin II/III (G8PM II/III_KO, D). The areas under the curve (AUC) between the local minima were calculated and the average and SD are shown in for KH00_053G10 and KH001_053G8 parents as well as KOs (E). Statistics show one-way ANOVA with Tukey post-test: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. Standard EC50 values for the PPQ partner drug DHA (F) and three PPQ analogs (mefloquine (G), chloroquine (H), and amodiaquine (I)) are shown as the average and SD for at least three biological replicates run in triplicate. The two parental lines and their relative plasmepsin KO lines are shown. No statistically significant differences were detected between the lines.
Loss of plasmepsins does not affect susceptibility to other antimalarials
To understand if plasmepsin CNVs modulate susceptibility to other antimalarials, we also subjected the engineered plasmepsin KO lines to a panel of related quinolines and DHA (i.e., the partner drug of PPQ in ACTs) in standard in vitro drug susceptibility assays. There were no significant differences in EC50 between the parental lines and the clonal plasmepsin KO lines for chloroquine (CQ), mefloquine (MEF), amodiaquine (AQ) or DHA (Fig 2F–I and S2 Table; one-way ANOVA with Tukey post-test, p > 0.05). We used EC50 assays rather than ring-stage survival assays (RSA) to test DHA susceptibility as we did not see a correlation between plasmepsin II and III copy numbers and RSA phenotype in parasites with the same genetic background as the parental lines we used in previous studies [29]. As expected for parasites with a Dd2-like pfcrt, the parental lines and plasmepsin KO lines were all resistant to CQ compared to the CQ-sensitive laboratory strain 3D7 (EC50: 12 nM, S2 Table). These data suggest that the role of plasmepsin copy numbers in increased survival is specific to PPQ and does not extend to other closely related quinolines.
Aspartic and cysteine protease inhibitors do not impact the PPQ AUC
Given that deletion of plasmepsin II and plasmepsin III abolished the PPQ-resistance phenotype, we wondered if direct inhibition of the protease function of Plasmepsin II and III might reduce the AUC. We demonstrated that the protease inhibitors E64 (a broad-band cysteine protease inhibitor shown to inhibit Falcipain-2 in the DV [41] and block parasite egress from the host red blood cell [42]) and pepstatin A (pepA) (an aspartic protease inhibitor shown to inhibit Plasmepsin function in cell lysates and to bind to Plasmepsin II [43]) had no differential activity in parasites regardless of plasmepsin copy number (S2 Table).
We next investigated if there was an additive effect of the protease inhibitors in combination with different PPQ concentrations in parasites with increased plasmepsin copy numbers. We identified two different drug concentrations where parasite growth was affected but not severely inhibited for each protease inhibitor (69–89% growth, S4 Fig). We then exposed parasites to a constant concentration of each protease inhibitor (7.5 μM and 5 μM for pepA; 2.6 μM and 3.9 μM for E64) in the presence of increasing concentrations of PPQ. Measurements of parasite growth in these assays were normalized to parasite growth with only the protease inhibitor present. We tested the parental lines with the single and the duplicated locus as well as an additional TRAC isolate with an even higher AUC (KH004_057, AUC = 88 ± 21 [29]). The tested protease inhibitor concentrations had no significant effect on the AUC (p > 0.05, one-way ANOVA followed by Tukey post-test), suggesting that disruption of hemoglobin catabolism by protease inhibitors does not modulate PPQ susceptibility under the conditions tested here (S5 Fig, Table A in S3 Table).
PPQ-induced heme accumulation is not impacted by plasmepsin II or III copy numbers
To further explore the possible mechanism of PPQ resistance, we tested the effect of PPQ on hemozoin biocrystallization using a pyridine-labeled heme fractionation assay [44,45]. This assay uses a series of cellular fractionation steps to extract the different heme species in the parasites (i.e., hemoglobin, free heme, and hemozoin) and subsequently measures the Fe3+-heme-pyridine absorbance. It has been demonstrated previously that parasites exposed to increasing CQ or PPQ concentrations show increased levels of free heme (and to a lesser degree hemoglobin) and reduced levels of hemozoin [21].
To understand if the additional copies of plasmepsin II and III influence the generation of free heme or the degradation of hemoglobin under PPQ pressure, we exposed highly synchronized ring-stage parasites (0–6 h post-invasion) from the KH001_053G8 and KH001_053G10 parental lines to a range of PPQ concentrations for 32 h and fractionated the different heme species. We then determined the percentage of each heme species present in the total amount of iron extracted. On average, both parental lines showed similar percentages of all heme species in the absence of drug (average of 13% free heme, 4% hemoglobin, and 83% hemozoin, Fig 3A–C and S4 Table). As shown previously [21], parasites exposed to 200 nM or 2 μM PPQ showed a significant increase in free heme (and to a lesser extent hemoglobin) compared to the untreated control, as well as a reduction in hemozoin formation (unpaired Student’s t-test p < 0.05). These findings are consistent with an inhibition of heme detoxification and hemozoin formation by PPQ treatment. There was no significant difference in the proportion of any heme species between the parental lines containing one (KH001_053G10) or two (KH001_053G10) copies of plasmepsin II and III. This suggests that while both parasite lines experience similar levels of toxic free heme, parasites with increased plasmepsin copy numbers seem to be better adapted to survive these extremely high concentrations of PPQ or free heme.
Fig 3. Heme fractionation of PPQ-treated and untreated parasites with variable plasmepsin copy numbers.
Different heme species were extracted from parasites by subsequent cellular fractionation steps. (A-C): Tightly synchronized KH001_053G10 (grey) and KH001_053G8 (black) ring stage parasites were exposed to various PPQ concentrations for 32 h. Average and SD of percentage of hemozoin Fe (A), hemoglobin (B), and free heme Fe (C) are shown for three independent experiments run in quadruplicate. Statistical comparisons of the drug-treated lines to their untreated controls were performed using two-tailed unpaired Student’s t-tests *p < 0.05; **p < 0.01. (D-F): KH001_053G10 and KH001_053G8 parental lines as well as G8PMII_KO, G8PMIII_KO, and G8PMII/III_KO were incubated with 2 μM PPQ from 24 to 36 h post-synchronization and harvested at 36 h. Statistical comparisons of treated to untreated parasites at the 36 h timepoint from three independent biological replicates were performed using two-tailed unpaired Student’s t-tests *p < 0.05; **p < 0.01; ***p < 0.001.
We next exposed parental and KH001_053G8 KO parasites (G8PMII_KO, G8PMIII_KO, and G8PMII/III_KO) to a single dose of 2 μM PPQ for 12 h between 24 and 36 h post-invasion when hemoglobin digestion is highest. As seen before, even a 12 h exposure of parasites to 2 μM PPQ led to a significant decrease in hemozoin, and an increase in free heme and hemoglobin compared to the 36–42 h untreated control in all lines tested (Fig 3D–F and S5 Table, unpaired Student’s t-test, p < 0.05). However, free heme accumulation was higher in the parental lines (>60%) than in the KO lines (<50%), suggesting that less Plasmepsin II and III activity leads to less free heme. This demonstrates that PPQ is inhibiting hemozoin formation regardless of Plasmepsin II and III abundance or presence.
Hemoglobin-derived peptides are not altered in parasites with increased genomic copies of plasmepsin II and III
While the heme fractionation assay can determine the relative abundance of different heme-iron species, it is not quantitative. We examined whether the abundance of hemoglobin-derived peptides differed between the lines, as previously shown for parasites carrying PfCRT mutations that confer PPQ resistance [22]. The P. falciparum RF7 clinical isolate has three copies of each plasmepsin II and III and a pfcrt M343L mutation; this mutant residue is located on the cytosolic side of PfCRT and is associated with decreased PPQ sensitivity and altered peptide abundance as compared to Dd2-like PfCRT [22]. To explore whether plasmepsin CNV could influence peptide composition, we subcloned RF7, generating two clonal lines, one with a single copy of plasmepsin II and III (B9) and a second clonal line with three copies of plasmepsin II and III (D4). The PfCRT M343L mutation is present in each clone. We performed peptidomic analysis on these clones and ran the analysis in both positive and negative mode. We detected a total of 35 putative endogenous hemoglobin-derived peptides (dipeptides to 13-mers) that could be mapped to either the α or β chains of hemoglobin (S6 Table). We did not observe any significant difference in hemoglobin-derived peptide composition or abundance when comparing parasite clones with single (B9) or triple (D4) plasmepsin II and III copy numbers (S6A and S6B Fig). These data further suggest that hemoglobin digestion is not significantly altered in the presence of increased plasmepsin copy numbers.
To better understand what effects PPQ treatment has on parasite metabolism, we used targeted metabolite analysis of 3D7 trophozoites treated for 2.5 h with 140 nM PPQ or 10 nM ATQ (as a control, S7 Table and S6C and S6D Fig). Parasites treated with ATQ, a known inhibitor of the electron transport chain, showed a strong increase in N-carbamoyl-L-aspartate and dihydroorotate as expected (S6D Fig) [46]. In contrast, there were no significant changes in our targeted list of metabolites in parasites treated with PPQ compared to untreated parasites (S6C Fig). Similarly, untargeted analysis of all putative hemoglobin-derived peptides (≤13 amino acids in length) clearly shows that there is little difference in the putative peptide signals between PPQ-treated and mock-treated parasites (S6E and S6F Fig and S8 Table).
Hemoglobin digestion is slowed in plasmepsin KO parasites
To further explore the role of Plasmepsins in hemoglobin degeneration, we investigated whether parasites with increased copy numbers or deletions of plasmepsin II and III differ in their composition or accumulation of heme species during their life cycle. We tightly synchronized the two parental lines KH001_053G10 and KH001_053G8 as well the KO clones G8PMII_KO, G8PMIII_KO, and G8PMII/III_KO and performed heme fractionation assays at timepoints 24, 36, and 42 h post-synchronization (Fig 4). As expected, the hemozoin percentage increased over time for all lines, while hemoglobin and free heme concentrations decreased (Fig 4A–C and S5 Table). Throughout the lifecycle, there was a significantly lower proportion of hemozoin in the G8PMII_KO and G8PMII/III_KO lines compared to the KH001_053G10 single copy line (Fig 4A, unpaired Student’s t-test p < 0.05). Concordantly, hemoglobin and free heme levels were significantly higher in the G8PMII_KO and G8PMII/III_KO lines compared to KH001_053G10, suggesting that hemoglobin digestion is slowed in the absence of plasmepsin II. G8PMIII_KO parasites showed an intermediate phenotype between the single copy parent KH001_053G10 line and the G8PMII_KO and G8PMII/III_KO lines (Fig 4A-C).
Fig 4. Plasmepsin KO parasites have slowed hemoglobin metabolism.
Tightly synchronized parasites were harvested at different time-points throughout the life cycle. The average and SD of percentage of hemozoin Fe (A), hemoglobin (B), and free heme Fe (C) are shown for three independent experiments for parasites with a single plasmepsin locus (KH001_053G10, grey), duplicated plasmepsin locus (KH001_053G8, black), G8PMII_KO (orange), G8PMIII_KO (blue), or G8PMII/III_KO (red). Statistical comparisons at each time point were performed between the single copy KH001_053G10 line and all other lines using two-tailed unpaired Student’s t-tests *p < 0.05; **p < 0.01. (D) Cell cycle progression was measured by the number of nuclei present per cell using flow cytometry and SYBRGreen staining of DNA. Parasites were defined as trophozoites when they had at least three nuclei and had started DNA replication. Shown is the percentage of trophozoites at each time point (24 h, 36 h and 42 h post-invasion). Statistical comparisons were performed between the single copy KH001_053G10 line and all other lines at the same time point using two-tailed unpaired Student’s t-tests *p < 0.05; ***p < 0.001; ****p < 0.0001.
A similar increase in undigested hemoglobin has been observed in PPQ-resistant parasite lines carrying the PfCRT G353V or F145I variants [22] as well as in plasmepsin II KOs examined by Western blot [36]. There was no difference between the two parental lines, suggesting that hemoglobin digestion efficiency is not increased in parasites with additional plasmepsin copy numbers. There was no statistically significant difference in individual heme species of a parasite line between the time point at 36 and 42 h (by unpaired Student’s t-test), indicating that most of the hemoglobin digestion was completed by 36–42 h post-invasion. The reduced hemoglobin digestion efficiency in KO parasites could explain the observation that less free heme is released under PPQ treatment (Fig 3F).
We next asked whether the delay in hemozoin formation seen in G8PMII_KO and G8PMII/III_KO was correlated to a delay in overall life cycle progression. To measure cell cycle progression, we determined the percentage of trophozoites in each sample by flow cytometry prior to the heme-iron species extraction (Fig 4D). In agreement with the heme-iron species data, trophozoite formation was delayed in the plasmepsin KO parasites compared to both KH001_053G10 and KH001_053G8 parental lines, with the G8PMII_KO and G8PMII/III_KO lines being more delayed than the G8PMIII_KO line. It remains unclear whether the delay in hemoglobin digestion is the result or the cause of slowed progression through the life cycle.
Growth in amino acid-limited media does not change PPQ sensitivity
We compared PPQ sensitivity between parasites cultured in regular media and those cultured in amino acid-limited media. We used a mixture of 25% complete RPMI and 75% RPMI free of all amino acids except isoleucine, methionine, and glutamine as amino acid-limited media (S7 Fig). We then performed PPQ drug inhibition assays with the KH001_053G10, KH001_053G8, G8PMII/III_KO and KH004_057 lines in regular and amino acid-limited media. All four lines showed similar PPQ phenotypes in amino acid-limited media compared to regular media. The KH001_053G8 and KH004_057 parasite lines with increased plasmepsin II and III copies had significantly higher AUC than the plasmepsin double KO parasite G8PMII/III_KO in both media conditions (Table B in S3 Table and Fig 5, two-way ANOVA with Šidák correction). The size of the AUC therefore still correlates with plasmepsin II and III copy number under amino acid-limited conditions.
Fig 5. Low amino acid conditions do not markedly impact the PPQ resistance phenotype.
Parasites were exposed to increasing levels of PPQ in regular (dots and solid lines) or amino acid-limited media (circles and dotted lines) for 84 h and survival was measured by increased DNA content. Shown is an example of at least four biologically independent experiments run in triplicate for the single copy plasmepsin clone KH001_053G10 (A), the duplicated plasmepsin locus clone KH001_053G8 (B), the double KO of plasmepsin II/III (G8PMII/III_KO, C), and the multicopy plasmepsin locus clone KH004_057 (D). The area under the curve (AUC) between the local minima was calculated and the average and SD is shown in (E). Statistics show two-way ANOVA with Šidák correction: *p < 0.05; **p < 0.01; ***p < 0.001; ns: not significant.
We also tested replication levels of KH001_053G10, KH001_053G8, G8PMII/III_KO, and KH004_057 in regular media and amino acid-limited media, and all four lines showed similar replication patterns to each other (S8 Fig). In regular media, parasitemia increased 6-to-7-fold with no statistical difference between the lines (one-way ANOVA with Tukey post-test). In contrast, all parasite lines grew significantly less in amino acid-limited media indicated by an increase in parasitemia of 2-fold only (S8 Fig). These replication rates suggest that all lines still rely similarly on amino acid uptake from the media regardless of plasmepsin copy number.
PPQ activity is not dependent on external pH
Drug uptake and availability to the parasite can be affected by the drug’s protonation status and its ability to cross membranes. Changes in the pH of the extracellular environment have been shown to affect CQ potency [47,48]. CQ is membrane permeable at neutral pH, but loses its permeability once protonated in the low pH of the DV [49] and concentrates within the DV via ‘weak-base trapping’ and subsequent binding to ferriprotoporphyrin IX [50]. Decreasing the pH of the extracellular environment reduces the overall pH gradient between the DV and extracellular environment and leads to reduced CQ accumulation in the DV and increased survival of the parasites.
Indeed, in media with a pH adjusted to 6.74, EC50 values for CQ are dramatically increased when compared to the EC50 values at a neutral pH of 7.5 (S9A–F Fig and S9 Table). Moreover, Dd2, KH001_53G8, and KH001_53G10 were not completely killed at the highest CQ concentration tested (2 μM). Increasing the pH of the media to 8.24 had the opposite effect, significantly reducing the EC50 of CQ when compared to media at a pH of 7.5 (p > 0.01, paired Student’s t-test, S9 Fig and S9 Table). This is consistent with a larger pH gradient between the extracellular medium (at pH 8.24) and the DV leading to increased CQ accumulation within the DV.
PPQ, like CQ, is a weak base, and we hypothesized that its protonation could be affected by the pH of its surrounding medium. In in vitro PPQ susceptibility assays, parasites showed only marginal higher sensitivity to high PPQ concentrations at increased extracellular pH, and the differences in AUC between the different pH conditions were not statistically significant (Fig 6A–D and S9 Table). This suggests that the activity of PPQ is less affected by external pH than the CQ activity.
Fig 6. Effect of pH on PPQ AUC.
A-D: Parasites were exposed to increasing levels of PPQ for 84 h in acidic (pH = 6.74), normal (pH = 7.5) or basic (pH = 8.24) media. Shown is an example of three biologically independent experiments run in triplicate for KH001_053G10 (A), KH001_053G8 (B) or KH004_057 (C), as well as the average and SD of the AUC for all three biological replicates (D). There were no statistically significant differences detected. (E-L): Parasites were exposed to increasing levels of PPQ in the presence of DMSO or E-H) CCCP at either 5 μM or 15 μM concentrations or I-L) concanamycin A (ConA) at either 0.1 nM or 0.2 nM. Shown is one example of three biologically independent experiments run in triplicate for KH001_53G10 (E, I), KH001_53G8 (F, J), and KH004_057 (G, K). The area under the curve (AUC) between the local minima was calculated and the average and SD are shown for CCCP (H) and ConA (L). Statistical comparisons of the drug-treated lines and DMSO-treated controls were performed using one-way ANOVA with Dunnett’s post-test: *p < 0.05 and **p < 0.01.
Alkalization of the DV affects PPQ AUC
The hemoglobin digestion activity of DV lysates [32,51], as well as recombinant Plasmepsins [30,31], is maximal at low pH, consistent with conditions within the acidic DV (DV pH of about 4.5–5.5 [52–54]). We therefore reasoned that perturbations to the DV pH might also impact Plasmepsin activity and thereby alter the PPQ resistance phenotype of parasites with increased plasmepsin II and III copy numbers. We used concanamycin A (ConA, an inhibitor that blocks the V-type ATPase from pumping H+ ions into the DV lumen, thereby leading to an alkalization of the DV [55]) and carbonyl cyanide m-chlorophenyl hydrazone (CCCP, a proton ionophore dissipating H+ gradients across membranes in general) to interrogate the role of the DV pH in PPQ resistance.
We first determined the EC50 for ConA and CCCP. While we did not detect any differences in susceptibility to ConA, G8PMII_KO and G8PMII/III_KO parasites were less susceptible to CCCP than KH001_053G10 (p < 0.05, one-way ANOVA followed by Dunnett’s post-test, Table C in S3 Table). Previous work demonstrated that vacuolar pH impacted CQ efficacy [48,56], and we similarly found a significant increase in susceptibility to CQ when we tested CQ in the presence of CCCP at a concentration of either 5 μM or 15 μM against parasites lines with various plasmepsin copy numbers (p < 0.01, one-way ANOVA followed by Tukey post-test, S9G-L Fig and Table D in S3 Table). Interestingly, the decreased survival was specific for the CQ-resistant PfCRT isoform and had non-significant effect on the CQ-sensitive 3D7 parasite line.
Similarly, when treated with 15 μM CCCP and normalized to growth in 15 μM CCCP without PPQ, parasites tested in an PPQ susceptibility assay exhibited reduced AUC values compared to parasites not treated with CCCP. This finding indicates that intracellular pH also affects the PPQ resistance phenotype (p < 0.05 one-way ANOVA followed by Dunnett’s post-test, Fig 6E–H and Table A in S3 Table). When we tested ConA in combination with PPQ, we also found a significant reduction in the AUC for KH001_053G10 and KH001_053G8 but not for KH004_057 (Fig 6I–L and Table A in S3 Table). We next wanted to test whether PPQ itself could affect the alkalization of the DV.
PPQ does not influence DV pH
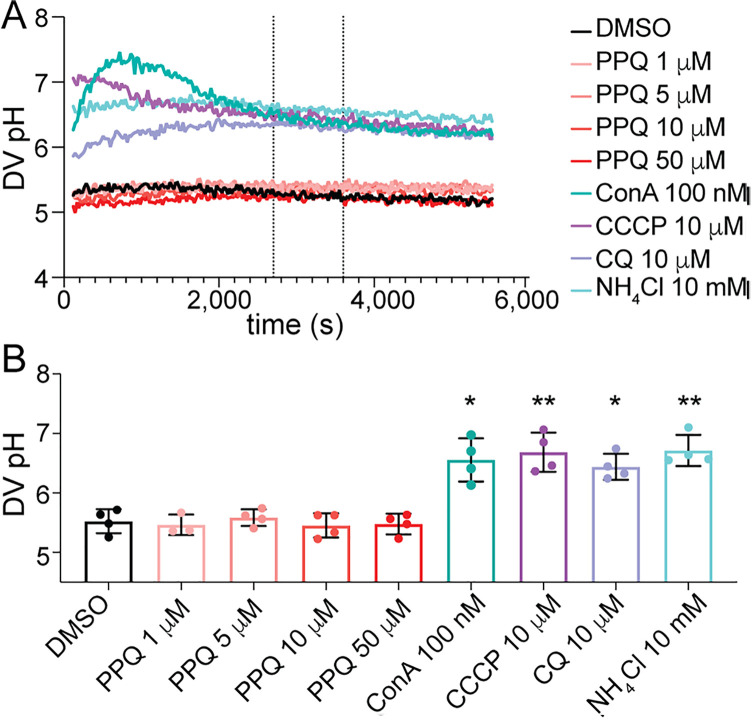
The weak-base trapping effect of CQ can cause alkalinization of acidic compartments [57]. The activities of Plasmepsin enzymes are pH-sensitive and are most active at low pH. Hence, we hypothesized that if PPQ also acts as a weak base accumulator, sufficiently high concentrations of PPQ in the DV might disrupt the DV pH, thereby reducing Plasmepsin activity, and the production of free heme that leads to parasite death. Amplification of plasmepsin II and III could therefore rescue this reduced activity due to increased protein expression, leading to the survival of PPQ-resistant parasites under high PPQ concentrations. To test this hypothesis, we used ratiometric fluorescence-based measurements of saponin-isolated Dd2 trophozoite parasites with fluorescein-dextran loaded DVs as a way to monitor DV pH in the presence or absence of PPQ. Fluorescence traces for pH calibration buffers indicated resting DV pH values of 5.5 ± 0.2 (mean ± SD, n = 4; Fig 7), consistent with previous studies [53,58]. The V-type ATPase inhibitor ConA (100 nM), proton ionophore CCCP (10 µM), and weak-base NH4Cl (10 mM) all caused rapid alkalinization of parasite DVs, resulting in significantly elevated DV pH compared to the vehicle control following 45 min of exposure (6.5–6.7; p < 0.05, one-way ANOVA with Dunnett’s post-test, Fig 7 and S10 Table). CQ at 10 µM also caused significant alkalinization, resulting in an average DV pH of 6.4 ± 0.2 (p < 0.05, one-way ANOVA, Dunnett’s post-test). By contrast, concentrations of up to 50 µM of PPQ had no impact on DV pH (Fig 7), suggesting that PPQ does not exert an effect via pH modulation over this time-course. This provides further evidence that PPQ and CQ have distinct effects on DV physiology.
Fig 7. Digestive vacuole pH remains unchanged with exposure to various concentrations of PPQ.
(A) DV pH traces of Dd2 parasites exposed to PPQ and control treatments of concanamycin A (ConA, 100 nM), CCCP (10 µM), CQ (10 µM) and NH4Cl (10 mM). Shown are the averaged results of internal technical duplicates from a representative experiment. DV pH was quantified for each treatment as an average of the measurements taken between 45 and 60 mins of compound exposure (dashed vertical lines), (B) average DV pH measurements following 45 to 60 mins of exposure to PPQ at 1, 5, 10 or 50 µM and to known DV pH modulators ConA, CQ, CCCP and NH4Cl. The data are the average and SD of three to four independent experiments (performed with blood from different donors). The asterisks denote a significant difference from the DMSO solvent control: *p < 0.05; **p < 0.01, (one-way ANOVA). Raw data are provided in S10 Table.
Discussion
We explored the contribution of plasmepsin CNVs to the bimodal resistance phenotype to PPQ as measured by the AUC in P. falciparum parasites, independent of the known contributions of pfcrt mutations. We generated plasmepsin II, III, and double KO parasites in the clinically relevant genetic background of Cambodian isolates. Consistent with previous findings in different genetic backgrounds, Plasmepsins are not essential for parasite survival under in vitro conditions [34,37,59,60]. In PPQ dose-response experiments however, the resulting plasmepsin KO parasite lines had lost their secondary survival peak, clearly demonstrating that Plasmepsins are a main driver of the AUC phenotype.
KO lines demonstrated a slower rate of hemoglobin degradation and delayed cell cycle progression compared to wildtype parasites but were similarly susceptible to the protease inhibitors E64 and pepA, consistent with prior studies [30–34,36]. While other Plasmodium species have only one aspartic protease in the DV, P. falciparum has expanded the repertoire from the homolog plasmepsin IV to three additional paralogs including plasmepsin II and III [61]. Earlier studies showed that there is no significant change in expression of the other Plasmepsins or falcipains in individual plasmepsin KO lines [34]. It is likely that the paralogous Plasmepsins can perform similar or overlapping roles; as such, we expect that Plasmepsin I and/or IV can partially compensate for Plasmepsin II and/or III loss.
We investigated several potential biochemical and metabolic mechanisms underlying the AUC phenotype. However, we were unable to define a specific mechanism explaining the genetic results. Multiple copies of plasmepsin II and III did not increase hemozoin formation and parasites with plasmepsin CNVs showed no difference in levels and composition of hemoglobin-derived peptides. Furthermore, the addition of PPQ showed similarly high levels of free heme and reduced hemozoin formation in single and multicopy plasmepsin parasites. This lack of impact of plasmepsin II and III copy numbers on hemoglobin digestion was also evident when parasites were grown under amino acid-constrained conditions, which places greater reliance on hemoglobin degradation for amino acid metabolism. There was little difference in growth between parasite lines grown in amino acid-limited media, and susceptibility to PPQ was not impacted by amino acid-restriction in parasites with increased copy numbers or plasmepsin KO. Together, these observations suggest that hemoglobin degradation is tightly regulated by the parasite, and simple upregulation of this pathway by plasmepsin II and III amplification cannot explain the PPQ resistance mechanism.
We also hypothesized that PPQ accumulation might impact DV pH and thereby modulate the activity of the aspartic proteases in the DV. In accordance with this hypothesis, survival under PPQ pressure was slightly reduced when the DV pH was disturbed by proton ionophores or V-type ATPase inhibitors. However, in contrast to the pH buffering effect observed by us and others for CQ [57], PPQ had no effect on DV pH at a range of physiological concentrations. These data suggest that PPQ does not exert a pH buffering effect on the DV. In contrast, we observed that CQ sensitivity is highly responsive to perturbations of the parasite proton gradient by altered extracellular pH or the addition of proton ionophores or V-type ATPase inhibitors, consistent with previous studies [48,50,62]. CQ and PPQ are weakly basic and are expected to accumulate to high concentrations in the DV, however, PPQ is substantially more lipophilic than CQ (logP of 6.1 for PPQ and 4.7 for CQ), and has a lower mean pKa value than CQ (6.1 vs 9.3) [63,64]. Together, this leads to an 8 orders-of-magnitude greater predicted lipid accumulation ratio for PPQ (~973,000) compared to CQ (8.25) under the acidic conditions of the DV [63,64]. Thus, it is possible that high concentrations of PPQ are sequestered within membranes and lipids of the DV, perhaps including the lipid droplets within which hemozoin formation is thought to occur [65,66]. This would be consistent with the lack of a direct effect of PPQ on DV pH, in contrast to CQ.
The unusual biphasic curve exemplified by PPQ-resistant parasites suggests that multiple competing processes may be underlying this complex phenotype. Indeed, plasmepsin copy number amplification is linked to two other determinants of drug resistance, mutations in pfcrtand kelch13. The Cambodian isolates studied here possess the Dd2 isoform of PfCRT (which provides CQ resistance but not PPQ resistance) and carry the Kelch13 C580Y allele that confers delayed clearance by DHA. Both genes are implicated in DV-related processes. Kelch13 has been localized to the cytostome where it plays a role in hemoglobin endocytosis and trafficking to the DV, and the C580Y mutation present in the Cambodian isolates studied here has been shown to reduce hemoglobin uptake in early-stage parasites [67,68]. It is possible that the increase in plasmepsin copy numbers may have arisen in this background due to DHA-PPQ treatment pressure in Southeast Asia and could play a compensatory role by increasing hemoglobin degradation in early rings. Indeed, the inheritance of Kelch13 C580Y has been linked with increased plasmepsin copy numbers in a genetic cross between a wildtype Malawian parasite and a Cambodian line [69]. Furthermore, increased plasmepsin copy numbers have been observed in field isolates in Southeast Asia in a large diversity of genetic backgrounds, independent of DHA-PPQ selection pressure [27], as early as 2007 [25,70]. Unfortunately, we were unable to reliably measure hemozoin formation in early ring stages and therefore could not directly test whether plasmepsin amplification compensates for delayed hemoglobin degradation in kelch13 mutants.
PfCRT is a DV-resident transporter required for the transport of hemoglobin-derived peptides out of the DV [22,71–73]. Mutations in PfCRT can mediate drug efflux from the DV [22,74–77]. The CQ-resistant, PPQ-sensitive Dd2 haplotype of pfcrt is present in all parasites in this study and carries 8 amino acid differences from the canonical 3D7 wildtype allele (i.e., M74I, N75E, K76T, A220S, Q271E, N326S, I356T, and R371I). No additional pfcrt mutations exist in these parasites, so differences in PPQ resistance between plasmepsin KO, plasmepsin single-copy, and plasmepsin multicopy parasites are attributed to plasmepsin CNVs and not to pfcrt mutations. We hypothesize that plasmepsin amplifications need a mutated Dd2-like pfcrt background to confer PPQ resistance, as plasmepsin amplification in wildtype pfcrt parasites does not lead to PPQ resistance [78]. Novel mutations added to the Dd2-isoform of PfCRT (e.g., C101F, T93S, H97Y, F145I, I218F, M343L, and G353V) can also mediate PPQ resistance in the absence of plasmepsin copy number changes in natural isolates [2,5,19,25] and engineered parasites [20,21,28,79], and can be additive or synergistic with plasmepsin CNVs. Two genetic crosses with a sensitive parasite and a PPQ-resistant parasite harboring increased plasmepsin copy number and a novel pfcrt mutation have been generated and analyzed for their PPQ phenotypes [69,80]. While the major contributor to high PSA survival was mapped to pfcrt, higher copy numbers of plasmepsin II and III increased the resistance even further. When AUC was used as a phenotype for QTL mapping, the main peak was pfcrt but a second strong peak was found around plasmepsins, suggesting an epistatic link between PfCRT and Plasmepsins in conferring PPQ resistance [80].
Heterologous expression studies show that Dd2 PfCRT is capable of transporting PPQ, albeit poorly, and PPQ resistance-conferring mutations in PfCRT increase this transport activity [77,81]. Furthermore, PPQ-resistant pfcrt mutations confer substantial fitness costs that are associated with accumulation of hemoglobin-derived peptides [22,23,82]. It remains possible that plasmepsin amplification could modulate interactions between Dd2 isoforms of PfCRT and hemoglobin-derived peptide substrates or between PfCRT and PPQ (e.g., a competitive substrate of PfCRT) [77], leading to increased fitness and/or PPQ resistance. Regardless of the underlying mechanism, there is strong genetic evidence for an epistatic interaction between novel pfcrt mutations and plasmepsin CNVs in PPQ-resistant parasites.
In Southeast Asia – where increased copy numbers of plasmepsins have been described as early as 2007, alongside kelch13 mutations – mutations in pfcrt appear to have emerged after 2010 [83] and to have risen toward fixation by 2016 in an increased plasmepsin copy number background [20]. However, the opposite observation was made in South America, where the PfCRTC350R variant has been detected since 2002, while plasmepsin amplifications were only detected after 2007 [24]. A follow-up study found no significant association between the PfCRTC350R variant and plasmepsin copy numbers and observed a decrease in the prevalence of both between 2016–2021 [84]. In Africa, where the proportion of pfcrt CQ resistant haplotypes is not fixed, increased plasmepsin II copy numbers have been detected in parasites collected between 2014–2015, notably in Burkina Faso and Uganda (>30%) [85]. It remains to be seen whether plasmepsin CNV confers some degree of resistance to PPQ in these African backgrounds, in the absence of pfcrt or kelch13 mutations.
The precise mechanism for plasmepsin amplification-mediated PPQ resistance remains unclear. One possibility is that high levels of unbound heme or heme-drug conjugates induced by high PPQ concentrations might cause oxidative stress and impair hemoglobin degradation, such that plasmepsin II and III amplification in PPQ-resistant parasites could restore sufficient hemoglobin degrading activity for continued parasite growth in the presence of PPQ. Indeed, it has been suggested that Plasmepsin II and III exist in a hemozoin-detoxifying complex that is inhibited by CQ at both the hemoglobin degradation and hemozoin formation steps [86]. Plasmepsin II and III amplification could therefore influence the rate-limiting steps of this process, leading to the complex concentration-response curve observed for PPQ-resistant parasites. Furthermore, high concentrations of PPQ could potentially interfere with the transport of hemoglobin-derived peptides via PfCRT. Thus, in addition to ensuring continued hemoglobin degradation under high free heme, plasmepsin II and III amplification might also ensure sufficient flux of hemoglobin-derived peptides to outcompete PPQ for PfCRT-mediated transport, further ensuring continued supply of amino acids to the parasite cytosol under high PPQ exposure.
Overall, in the natural setting, plasmepsin CNV has a role in conferring PPQ resistance. Here, we demonstrate that the AUC phenotype (i.e., increased parasite survival under high PPQ pressure) is mediated by increased plasmepsin copy number in TRAC isolates. While we document the importance of plasmepsins in mediating increased AUC in PPQ-resistant parasites, further studies are required to arrive at a complete mechanistic understanding of the biological role of plasmepsins in PPQ resistance.
Materials and methods
Ethics statement
The Plasmodium falciparum isolates used in this study were derived from samples collected as part of the Tracking Resistance to Artemisinin Collaboration (TRAC), which was approved by local Institutional Review Boards (IRBs) and the Oxford Tropical Research Ethics Committee (OxTREC). Written informed consent was obtained from all participants or their guardians as part of the original TRAC studies.
All P. falciparum cultures were maintained in human erythrocytes and serum obtained from healthy anonymous donors, purchased through Interstate Blood Bank (Memphis, TN, USA), a commercial vendor licensed to provide de-identified human biological materials for research use. No individuals were recruited or sampled as part of this study.
All laboratory procedures involving genetically modified P. falciparum parasites were conducted under approved Biosafety Level 2 (BSL-2) conditions in accordance with the guidelines of the Institutional Biosafety Committee (IBC) at the Harvard T.H. Chan School of Public Health.
Parasite culture
The parental parasite lines (KH001_053 and KH004_057) were collected from Pursat and Pailin, Cambodia in 2011 through the Tracking Resistance to Artemisinin Collaboration (TRAC), and culture adapted and subcloned as previously described resulting in KH001_053G10 and KH001_053G8 [29]. All parasites were grown in fresh human erythrocytes (O+) at 4–5% hematocrit in Roswell Park Memorial Institute (RPMI) 1640 media supplemented with 26.6 mM NaHCO3, 27.7 mM HEPES, 0.41 mM hypoxanthine, 10% O+ human serum, and 25 μg/mL gentamicin. Human serum (heat inactivated and pooled) and human erythrocytes were supplied by Interstate Blood Bank, Inc., Memphis, TN. For in vitro drug susceptibility assays, parasites were cultured in media containing 0.5% Albumax (Invitrogen, Carlsbad, CA), in place of human serum. Cultures were incubated at 37°C with rotation (55 RPM) under 1% O2/5% CO2/balance N2 gas. For amino acid-limited conditions, Albumax media was generated as described with the exception that amino acid-free RPMI (United States Biological, Life Sciences, Salem, MA) supplemented with glutamine, isoleucine and methionine (300 mg/L, 50 mg/L, and 15 mg/L, respectively, Sigma-Aldrich, St Louis, MO) was mixed with complete RPMI in a 3:1 ratio to make amino acid-limited media.
Design and construction of CRISPR-Cas9 plasmid vectors for transfection
Transfections were performed using a three-plasmid strategy. The first plasmid contained homology regions (HRs) to the gene of interest (GOI) flanking a hdhfr positive selectable marker, while the other plasmids contained the coding sequence for Cas9 and a single guide RNA (gRNA) specific to each GOI, targeting two sites in the GOI in total.
To generate the basic template vector pGEMhdhfr + , the hdhfr positive selectable marker cassette was PCR amplified from the vector pL-6_eGFP [87] with primers hdhfr 5’ F and hdhfr 3’ R and cloned into pGEM-3Z (Promega, Madison, WI) digested with HincII. Two flanking regions corresponding to the GOI were then cloned into this basic vector to generate a template for homologous recombination and disruption of the GOI. The HRs were amplified from 3D7 gDNA with the following primer sets from Liu et al., [34] - plasmepsin II 5’ HR: PMII 5’ HR Fwd and PMII 5’ HR Rev; plasmepsin II 3’ HR: PMII 3’ HR Fwd and PMII 3’ HR Rev; plasmepsin III 5’ HR: PMIII 5’ HR Fwd and PMIII 5’ HR Rev; and plasmepsin III 3’ HR: PMIII 3’ HR Fwd and PMIII 3’ HR Rev. The 3’ HRs were digested with PstI and SphI and ligated into the appropriately digested pGEMhdfr+ basic template vector. The coordinating 5’ HRs were digested with AflII and XmaI and ligated into the pGEMhdfr+ vector already containing the 3’ HR. The plasmepsin II/III double KO construct contains a plasmepsin II 5’ HR and a plasmepsin III 3’ HR flanking the hdhfr cassette. The HR plasmids were transfected into parasites in either their circular or BglI-linearized form. GOI-specific gRNA sequences were generated using Benchling (https://benchling.com) and ligated into the BbsI digested pDC2-Cas9-U6-hDHFR vector [88]. All primers used are described in S11 Table.
Parasite transfection and selection
Parental KH001_053G8 and KH001_053G10 parasites were synchronized using 5% D-sorbitol (Sigma-Aldrich, St. Louis, MO) and early ring-stage parasites were mixed with 50 μg of each transfection plasmid in cytomix (120 mM KCl, 0.15 mM CaCl2, 2 mM EGTA, 5 mM MgCl2, 10 mM K2HPO4/KH2PO4 (pH 7.6), 25 mM HEPES (pH 7.6) and electroporated in 2 mm cuvettes at 0.31 kV and 950 μF [89]. Electroporated cells were then placed in O+ media with fresh erythrocytes at 5% hematocrit. After 24–48 h of drug-free recovery, cultures were continuously treated with 5 nM WR99210, which positively selects for parasites containing hdhfr. Recovered parasites were genotyped as bulk cultures and subcloned by limiting dilution. Subsequently, clonal lines were genotyped by PCR, qPCR, and Southern blotting. All primers used are described in S11 Table.
Polymerase chain reaction (PCR) for genotyping transfectants
Genomic DNA was isolated from bulk transfectant cultures and from the resulting clonal lines using the QIAamp Blood Mini Kit (Qiagen, Hilden, Germany). To detect the presence of hdhfr integration at the GOI, PCR was performed upon isolated gDNA using the Phusion High-Fidelity PCR Mastermix (New England BioLabs, Ipswich, MA), according to the manufacturer’s instructions. For screening purposes, PCR reactions were also performed direct-from-culture, using a 1:100 dilution of parasitized packed human erythrocytes in the final PCR reaction.
Parasites that were wildtype at the plasmepsin II locus were detected using primers located upstream and downstream of the gene (PM2 5’ Upstream Fwd and PM2 3’ Downstream Rev), respectively. Integration of the hdhfr cassette at the plasmepsin II locus was detected using a forward primer upstream of the gene and a reverse primer located in the hdhfr cassette (PM2 5’ Upstream Fwd and hdhfr cassette 5’ Rev). To detect parasites that were wildtype at the plasmepsin III locus, a forward primer specific for the sequence between the homology regions and a reverse primer located downstream of the gene (PM3 Btwn HRs Fwd and PM3 3’ Downstream Rev) were used. Integration of the hdhfr cassette at the plasmepsin III locus was detected using a forward primer found in the hdhfr cassette and a reverse primer located downstream of the gene (hdhfr cassette 3’ Fwd and PM3 3’ Downstream Rev). All plasmepsin II/III KO transfectants were probed for integration at both the plasmepsin II and plasmepsin III loci. The presence of plasmid was probed using primers specific to the backbone of the pGEMhdhfr+ template vector (pGEM_hdhfr+ Fwd and pGEM_hdhfr+ Rev) for all transfections. All primer sequences are listed in S11 Table.
Quantitative PCR
To determine copy numbers of pfmdr1, plasmepsin II, and plasmepsin III, qPCR was performed on genomic DNA (extracted with QIAmp Blood Mini Kit, Qiagen, Hilden, Germany) as previously described [29] with the following modifications: amplification reactions were done in MicroAmp 384-well plates in 10 μl SYBR Green master mix (Applied Biosystems, Foster City, CA), 150 nM of each forward and reverse primer and 0.4 ng template. Forty cycles were performed in the Applied Biosystems ViiATM 7 Real-time PCR system (Life Technologies, Carlsbad, CA). pfmdr1 primers were designed after Price et al.[90] whereas β- tubulin primers for the endogenous control were designed after Ribacke et al.[91]. To test for integration of the hdhfr cassette into the plasmepsin locus, two primer sets were designed located between the HRs for plasmepsin II (RTPCR PMII forw and rev) and III (RTPCR PMIII forw and rev) as well as on the hdhfr gene (hdhfr_RTPCR_F and R). Technical replicates were run in quadruplicate. Copy numbers were considered increased (>1) when the average of three biological replicates was above 1.6. Primers are listed in S11 Table.
Southern blotting
Parasite-infected red blood cells were lysed with 0.15% saponin (Sigma-Aldrich, St. Louis, MO) in PBS and gDNA was isolated from freed parasites via phenol-chloroform extraction. For each clone, 5 ug gDNA was digested with the following restriction enzymes in the KH001_053G10 lines: EcoO1091 and XmnI for the plasmepsin II KO clones; KpnI and NsiI for the plasmepsin III KO clones; EcoO1091, XmnI, KpnI and NsiI for the plasmepsin II/III double KO clones; AflII and Ncil for the plasmepsin II KO and plasmepsin III KO clones in a KH001_053G8 parental background and all plasmepsin II/III KO clones. As controls, gDNA from the parental lines (KH001_053G8 and KH001_053G10) and corresponding plasmid vectors were also digested. Digested gDNA was resolved on a 1% agarose gel in TAE and transferred to Amersham Hybond – N+ nylon transfer membrane (GE Healthcare, Chicago, IL). Probe hybridization and horseradish peroxidase-mediated signal detection were performed using the ECL Direct Nucleic Acid Labeling and Detection System (GE Healthcare, Chicago, IL), following the manufacturer’s instructions. DNA probes were designed to detect hdhfr, plasmepsin II 3’ HR, or plasmepsin III 3’ HR. The hdhfr probe was PCR amplified from plasmid vector, while the plasmepsin II 3’ HR and plasmepsin III 3’ HR probes were PCR amplified from KH001_053G10 gDNA; the corresponding primers are listed in S11 Table.
In vitro drug susceptibility assays by SYBR Green I staining
Drug susceptibility assays were performed using the SYBR Green I method as previously described [92]. In brief, tightly synchronized 0–6 h post-invasion rings (by a Percoll gradient and D-sorbitol synchronization 6 h later, Sigma-Aldrich, St. Louis, MO) at 1% parasitemia and 1% hematocrit in 40 uL of 0.5% Albumax-complemented RPMI media were grown for 84 h in 384-well clear bottom plates, in the presence of different drug concentrations. Drug assays were extended from the standard 72 h drug exposure to 84 h due to the tight synchronization of the parasites. All drug conditions were performed in three technical replicates, with at least three biological replicates. Drugs were dispensed in 24-point dilution series of PPQ (0.07 nM–50 μM) and 12-point dilution series of all other drugs (CQ, E64, MQ, DHA, WR99210, pepstatin A methyl ester (pepA), and AQ; Sigma-Aldrich, St. Louis, MO) into 384-well plates by an HP D300e Digital Dispenser (Hewlett Packard, Palo Alto, CA). Growth at 84 h was quantified by staining parasite DNA with SYBR Green I (Lonza, Visp, Switzerland) for 24 h, and measuring relative fluorescence units at an excitation of 494 nm and an emission of 530 nm using a SpectraMax M5 (Molecular Devices, San Jose, CA). Half-maximal effective concentration (EC50) values, for all drugs except PPQ, were calculated using a nonlinear regression with the log(inhibitor) vs. response-Variable slope curve-fitting algorithm using GraphPad Prism version 8–10 (GraphPad Software, La Jolla, CA). PPQ susceptibility was quantified using area under the curve (AUC) between the two local minima of the high-dose peak, as previously described [29].
PPQ stocks were resuspended in a 0.5% lactic acid/0.1% Triton X-100 aqueous solution, CQ stocks were resuspended in a 0.1% Triton X-100 aqueous solution, and all other drugs were resuspended in dimethyl sulfoxide (DMSO).
For drug susceptibility assays performed in amino acid-limited conditions, amino acid-limited media was generated as described above and parasites were transferred to amino acid-limited media right before the start of the drug assay.
To test the effects of DV stress in modulating PPQ response, parasites were treated with a 24-point dilution series of PPQ in combination with a fixed concentration of the additional inhibitor (either E64: 2.6 μM or 3.9 μM; pepstatin A methyl ester (pepA): 5 μM or 7.5 μM; CCCP: 5 μM or 15 μM; concanamycin A (ConA): 0.1 nM or 0.2 nM; or DMSO control (Sigma-Aldrich, St. Louis, MO). Relative growth was normalized in comparison to parasite growth in the presence of E64, pepA, CCCP, or ConA at the test concentration alone.
To determine the role of pH in drug susceptibility, parasites were exposed to a dilution series of PPQ or CQ in pH-adjusted Albumax-complemented media for 84 h at pH values of ~6.75, ~ 7.5, or ~8.25.
Cellular heme fractionation assay
Baseline levels of different heme species in the parasite lines were determined using pyridine-based detergent-mediated cellular heme fractionation assays described in detail by Combrinck et al. [44,45] For the drug exposure experiments with KH001_053G10 and KH001_053G8, parasites were synchronized to ring stages using two to three cycles of D-sorbitol treatment and early rings were incubated at 37˚C at 5% parasitemia and 2% hematocrit in 24-well plates. After 32 h, late trophozoites were harvested by lysing red blood cells with 0.05% saponin followed by multiple washes with 1 × PBS (pH 7.5). Pellets were then resuspended in 1 × PBS and stored at −80°C before further analysis. An aliquot of the trophozoite suspension was stained by SYBR Green I and quantified via flow cytometry (described in detail below) to determine the total number of trophozoites. For determining the heme composition throughout the life cycle, we increased the starting culture volume to 10 mL of 5% parasitemia at 2.5% hematocrit and further tightened the synchronization to 0–6 h post-erythrocyte-invasion rings by a Percoll gradient and D-sorbitol synchronization 6 h later. Parasites were harvested at 24, 36 and 42 h post D-sorbitol synchronization. One set of parasites was exposed to 2 μM PPQ between 24 and 36 h and harvested at 36 h.
Samples were thawed and DV content released from trophozoites by hypotonic lysis and sonication (53 kHz, 320 W). Parasite fractions corresponding to digested hemoglobin, free heme-Fe, and hemozoin were then carefully recovered through centrifugation and treatment with HEPES buffer (pH 7.4), 4% SDS, 25% pyridine solution, 0.3 M HCl and 0.3 M NaOH (Sigma-Aldrich, St. Louis, MO).
The UV-visible spectrum of each heme fraction was measured as a Fe3+-heme-pyridine complex using a multi-well SpectraMax M5 plate reader (Molecular Devices, San Jose, CA). The total amount of each heme-Fe species in a sample was quantified using a heme standard curve and interpolation. The percentage of each species per sample was compared between the different strains and conditions. Two-tailed unpaired Student’s t-tests were used for comparing PPQ-treated vs untreated lines at the same time point or between KH001_053G10 and the other strains.
Flow cytometry to quantify parasitemia and stage
Parasites were stained in 10X SYBR Green I in 1xPBS for 30 min in the dark at 37°C. The staining solution was removed, and cells were resuspended in five times the volume of the initial volume of PBS. Flow cytometry data acquisition was performed on a MACSQuant VYB (Miltenyi Biotec) with a 488 nm laser and a 525 nm filter and analyzed with FlowJo 2. Red blood cells were gated on the forward light scatter and side scatter and infected red blood cells were detected in channel B1. At least 100,000 events were analyzed per sample. Parasites were considered trophozoites when the DNA content was 3 times higher than the ring-stage signal.
Metabolite extraction for HPLC-MS data collection
Synchronized cultures of trophozoite stage (24–28 h post-invasion) parasites (5–10% parasitemia, 2% hematocrit) were purified from uninfected red blood cells by VarioMacs Magnet using a MACS CS column (Miltenyi Biotec. Charlestown, MA). The purified parasite pellet was resuspended at 5x107 – 1x108 cells/mL in Albumax media, and allowed to recover for 1 h at 37°C. For comparison between R7 clones B9 and D4, parasites were processed directly. For drug exposure experiments, following recovery, purified trophozoites were incubated in 6-well plates with either 10 nM atovaquone (ATQ, positive control), 140 nM PPQ, or no-drug control for 2.5 h at 37°C. Immediately following treatment, parasite pellets were washed with 1 mL 1x ice-cold PBS, before being resuspended in 1 mL prechilled 90:10 methanol-water and placed on ice. Samples were vortexed, resuspended and centrifuged for 10 min at 15,000 rpm and 4°C. Samples were stored at −80°C before being dried down under nitrogen flow for UHPLC-MS analysis. The dried metabolites were resuspended in HPLC-grade water (Chromasolv; Sigma-Aldrich, St. Louis, MO), containing chlorpropamide as an internal control, to a concentration between 1x105 and 1x106 cells/mL, based on hemocytometer counts of purified parasites.
HPLC-MS data collection
All samples were processed in triplicate with method blanks to reduce technical variation and account for background signal. Samples were randomized with pooled quality control samples and blanks run regularly throughout the runs.
For untargeted putative-hemoglobin peptide analysis, 5 μL of each sample was injected for analysis. Metabolites were separated using a reversed phase method on a HPLC Prominence 20 UFLCXR system (Shimadzu, Marlborough, MA) using a Waters BEH C18 column (100 mm x 2.1 mm 1.7 μm particle size) at 55 °C and an aqueous acetonitrile gradient run for 20 min at a flow rate of 250 μL/min. Solvent A was HPLC grade water with 0.1% formic acid, and Solvent B was HPLC grade acetonitrile with 0.1% formic acid. The solvent gradient was at 0.0 min 3% of B, 10.0 min 45% of B, 12.0 min 75% of B, 17.5 min 75% of B, and 18.0–20.0 min: 3% of B. Eluate was delivered into a (QTOF) 5600 TripleTOF using a DuoSprayTM ion source (AB Sciex, Toronto Canada). Capillary voltage was 5.5 kV in positive and 3.8 kV in negative ion mode with declustering potentials of 80V and −80V respectively. The TripleTOF was scanning 50–1000 m/z, and 16 MS/MS product ion scans (100 ms) per duty cycle using collision energy of 50V with a 20V spread.
For targeted metabolomics analysis, 10 µL of each sample was injected for analysis. For each sample, 10 µL were injected through a XSelect HSS T3 2.5 µM C18 column (Waters, Millford, MA #186006151) at 30˚ C and eluted using a 200 µL/min 25 min gradient of 3% aqueous methanol, 15 mM acetic acid (Millipore Sigma, Burlington, MA, #A6283), 10 mM tributylamine (Millipore Sigma, Burlington, MA, #90781), 2.5 µM medronic acid ion pairing agent (Millipore Sigma, Burlington, MA, #M9508) (A) and 100% HPLC-grade methanol (B), with the gradients: 0–5.0 min 100% A, 0% B; 5.0–13.0 min 80% A, 20% B; 13.0–15.0 min 45% A, 55% B; 15.0–17.5 min 35% A, 65% B; 17.5–21.0 min 5% A, 95% B; 21.0–25 min 100% A, 0% B. Negative-ion mode, using a scan range of 85–1,000 m/z and a resolution of 140,000 at m/z 200 was used for ion detection.
HPLC-MS data processing
MSConvert of the ProteoWizard software package was used to convert the.wiff/.wiffscan (untargeted) or.raw (targeted) data files to.mzML formatted files compatible with MS-Dial or El-Maven, which were used to align, group, quantify and initially visualize the untargeted and targeted data, respectively. For putative hemoglobin-derived peptides analysis using untargeted data, peak areas from both positive and negative modes were exported, and the resulting feature quantification matrices were used as input for a custom R script designed to putatively annotate peak groups as human hemoglobin-derived peptides of matching m/z value within 15 ppm, considering all potential human hemoglobin peptides up to 13 amino acids long. The resulting table of annotated peptides was then exported to Microsoft Excel for final processing. For targeted analysis, feature quantification matrices were exported directly from El-Maven to Microsoft Excel for final processing.
For both datasets, within each biological replicate, the chlorpropamide control signal was used as an internal control for instrument variation correction. The blank signals were then subtracted from the chlorpropamide-corrected data, and a detection reproducibility filter was applied to the list of metabolites to exclude those that had a relative standard deviation across the pooled quality control samples of greater than 30. Log2 fold changes were calculated relative to no-drug controls, and the resulting values across biological replicates were used to generate volcano plots using GraphPad Prism 10.
All metabolomics data has been made publicly available through the Metabolomics Workbench (https://www.metabolomicsworkbench.org, NIH grant U2C-DK119886) [93] with the following study numbers: ST003904, ST003902, and ST003906.
Digestive vacuole pH determination
Saponin-isolated trophozoite-stage parasites containing the membrane-impermeant pH-sensitive fluorescent indicator fluorescein-dextran (10,000 MW; Invitrogen, Carlsbad, CA) in their DVs were prepared as outlined previously [55,58]. The isolated parasites were washed and suspended in malaria saline (125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 20 mM glucose, 25 mM HEPES; pH 7.1) at a density of ~2–3 × 107 cells/mL. Isolated parasite suspensions were checked for integrity pre- and post-experiment by light microscopy of Giemsa-stained parasites. Prior studies have demonstrated that parasites isolated in this manner maintain ion gradients and membrane potentials for >3 h under the conditions tested here [94–96]. 100 µL of cell suspension was added to an equal volume of malaria saline containing test compounds at 2x test concentrations in a 96-well clear-bottomed black plate. The pH of the DV was monitored at 37°C over 1.5 h using a Molecular Devices M5i plate reader (excitation 490 and 450 nm, emission 520 nm). Fluorescence ratios (490/450) were calibrated to pH units using calibration buffers at pH 4.5, 5.1, 5.7 and 6.3 (130 mM KCl, 1 mM MgCl2, 20 mM glucose, 25 mM HEPES, 180 nM nigericin, 100 nM ConA) as described previously [55,58].
Supporting information
(A) Schema of original loci, homology plasmids used for integration into the locus and resulting edited loci. Restriction enzyme sites and expected band sizes for Southern blots are indicated in the schema in orange for plasmepsin II and in blue for plasmepsin III KOs. (B) Southern blots with different probes, expected band sizes are indicated by arrows. Clones indicated with a star were used for phenotyping. Plasmids were either transfected in circular (cir) or linearized (lin) form. (C) WR99210 primarily targets the plasmodial dhfr and an increase in EC50 is correlated with the presence of one or several hdhfr cassettes present. Shown is the average EC50 and standard deviations of three biological replicates for each clone (one-way ANOVA with Dunnett’s post-test compared to 1D with a single integration of the hdhfr cassette. ****p < 0.0001).
(TIF)
(A) Schema of original parental loci, homology plasmid used for integration into the loci, and resulting edited locus which is identical for both parents. Restriction enzyme sites and expected band sizes for Southern blots are indicated in the schema in orange for plasmepsin II and in blue for plasmepsin III KOs. (B) Southern blots with plasmepsin III probe, expected band sizes are indicated by arrows. Clones indicated with a star were used for phenotyping. (C) Average EC50 and standard deviations of three biological replicates of WR99210 for each clone except 4E (n = 1). nd: not determined.
(TIF)
(A) Schema of original parental loci, homology plasmid used for integration into the loci and resulting edited locus which is identical for both parents. Restriction enzyme sites outside the locus were selected to confirm complete deletion of the regions between the homology regions and expected band sizes for Southern blots are indicated in the schema. Clones indicated with a star were used for phenotyping. (B) The same Southern blots was hybridized three times with the plasmepsin II, plasmepsin III, or hdhfr probe. Expected band size for each probe is indicated with arrows. The loss of hybridization for the plasmepsin II probe confirms the deletion and fusion of plasmepsin II and plasmepsin III in the KO clones.
(TIF)
Ring-stage parasites were either grown in complete media or in complete media with the addition of CCCP (15 or 5 μM), E64 (3.9 or 2.6 μM), pepA (7.5 or 5 μM), ConA (0.1 or 0.2 nM), 10 μM DHA (dead) or 0.5% DMSO for 72 h. Growth was measured by the incorporation of SYBRGreen into DNA, read by a spectrometer and normalized to parasites cultured in media only.
(TIF)
Parasites were exposed to increasing levels of PPQ in the presence of DMSO or (A) E64 at a concentration of either 2.6 μM or 3.9 μM or (B) pepstatin A (pepA) at a concentration of either 5 μM or 7.5 μM. Shown is one example of three biologically independent experiments run in triplicates. (C) Average and SD of the area under the curve (AUC) between the local minima for three biological replicates. No statistically significant difference was detected between PPQ alone and PPQ in combination with either E64 or pepA by ordinary one-way ANOVA with Tukey post-test.
(TIF)
A and B: Two clones from the Cambodian RF7 parasite line [22] with either one copy (B9) or three copies (D4) of plasmepsin II and III were used for small molecule metabolomic analysis, which includes relative quantitation of short peptides. Metabolomic analysis was run in both positive and negative mode and a total of 35 putative endogenous hemoglobin-derived peptides (i.e., dipeptides to 13-mers) were detected based on their m/z match that could be mapped to either the alpha (A) or beta (B) chains of hemoglobin. Shown are the volcano plots combining statistical significance and fold change observed in metabolites from RF7 clones D4 compared to B9. C to F: Effects of PPQ or ATQ treatment on the parasite’s metabolism. Purified P. falciparum 3D7 trophozoites were treated for 2.5 h with 140 nM PPQ or 10 nM ATQ (as a control) and volcano plots comparing metabolites from untreated vs PPQ-treated parasites for targeted metabolite analysis from PPQ (C) and ATQ (D) treated parasites compared to untreated are shown. Volcano plots comparing metabolites from untargeted analysis of all putative hemoglobin-derived peptides of amino acid length 13 or less are shown in (E) (alpha chain) and (F) (beta chain). The dotted lines depict the significance cutoff of p = 0.01 and a two-fold change in metabolite abundance. Only in N-carbamoyl-L-aspartate and dihydroorotate under ATQ treatment were significantly increased in abundance [46].
(TIF)
We determined the minimal amino acid needs of parasites to allow for enough DNA replication to perform drug susceptibility assays by SYBR Green I staining. KH001_053G10, KH001_053G8, G8PMII/III_KO and KH004_057 were synchronized and set up at 1% parasitemia and 2% hematocrit in regular RPMI media or RPMI media with isoleucine, methionine, and glutamine as the only amino acid sources. The two conditions were then mixed in 10% increments (90% regular media plus 10% amino acid-free (except isoleucine, methionine, and glutamine) media, 80% and 20% etc.) in 96 well plates and incubated at 37°C for 72 h. Growth was analyzed by adding SYBR Green to the plates, measuring the fluorescence, and normalizing the signal to parasites grown in regular media. The final assays conditions for drug susceptibility assays were set at 25% full amino acid RPMI and termed amino acid-limited media.
(TIF)
KH001_053G10, KH001_053G8, G8PMII/III_KO, and KH004_057 parasites were synchronized and set up at 0.5% parasitemia in either regular media or amino acid-limited media. Parasite replication was measured by estimating the parasitemia in the second cycle by flow cytometry of SYBR Green-stained parasite samples and dividing it by the initial parasitemia. Shown are the average replication rates for three biological replicates with SD. There were no statistically significant differences detected between the strain grown in either regular or amino acid-limited media by one-way ANOVA followed by Dunnett’s post-test, ns = no significance. The replication rate for all strains was significantly less in amino acid-limited media compared to regular media by Student’s t-test: *p < 0.05.
(TIF)
A-F) Parasites were exposed to increasing levels of CQ in acidic (pH = 6.74), normal (pH = 7.5) or basic (pH = 8.24) media. Shown is one example for each tested line of three biologically independent experiments run in triplicates. G-L) Parasites were exposed to increasing levels of CQ in the presence of DMSO or CCCP at a concentration of either 5 μM or 15 μM. The EC50 was calculated where possible (an # indicates when parasites were not killed completely at the highest concentration), and the average and SD are shown in (F and L). Statistics show one-way ANOVA with Tukey post-test for each strain tested in the presence of CCCP or two tailed paired Student’s t-test for the external pH changes: *p < 0.05; **p < 0.01; ***p < 0.001.
(TIF)
Plasmids were either transfected in circular form (cir) or were linearized with BglI before transfection (lin). Shown are all the clones analyzed for every transfection that was recovered. Integration was confirmed by PCR of the 5’ and 3’ region and appearance of a band at the right size was considered a positive result (green check mark). The absence of the transfection plasmid was screened for with primers targeting the backbone of the plasmid which is lost after correct integration (red cross). Quantitative PCR was also used to confirm copy numbers of plasmepsin II and III as well as the hdhfr gene inserted into the locus. Primers are listed in S11 Table.
(XLSX)
Parasite lines were exposed to various concentration of drug and the EC50 was calculated for each drug. Each experiment was run in triplicate and at least three biological replicates were performed for each parasite line/clone. Shown are the EC50, SD and sample size for each line.
(XLSX)
(A) Combination assays of parasites in the presence of increasing concentrations of PPQ and a constant concentration of E64, pepA, ConA, or CCCP measured as AUC. (B) Parasites were cultured either in regular or amino acid-limited media and exposed to increasing concentrations of PPQ and the AUC was measured. (C) EC50 for ConA and CCCP. (D) Combination assays in the presence of increasing concentrations of CQ and a constant concentration of CCCP measured as EC50.
(XLSX)
Different heme species were extracted from parasites by subsequent cellular fractionation steps. Tightly synchronized KH001_053G10 and KH001_053G8 ring-stage parasites were exposed to various PPQ concentrations for 32 h. The amount of iron species (Fe) per sample was estimated based on the standard curve run for each biological replicate and the percentages for each species per sample were calculated. Shown is each value from three biological replicates run in quadruplicate with the average and SD of percentage of hemozoin Fe, hemoglobin, and free heme Fe. Statistical comparisons of the drug-treated lines to their untreated controls were performed using two-tailed unpaired Student’s t-tests *p < 0.05; **p < 0.01.
(XLSX)
Tightly synchronized parasites were harvested at different time-points throughout the life cycle; the average and SD of percentage of hemozoin Fe, hemoglobin, and free heme Fe are shown for three independent experiments for parasites with a single locus (KH001_053G10), duplicated locus (KH001_053G8), G8PMII_KO, G8PMIII_KO, or G8PMII/III_KO. Statistical comparisons at each time point were performed between the single copy KH001_053G10 parasites and all other lines using two-tailed unpaired Student’s t-tests *p < 0.05; **p < 0.01. Additionally, parasites were incubated with 2 μM PPQ from 24 to 36 h post synchronization and harvested at 36 h. Statistical comparisons of PPQ-treated to untreated parasites at the 36 h timepoint from three independent biological replicates were performed using two-tailed unpaired Student’s t-tests *p < 0.05; **p < 0.01; ***p < 0.001.
(XLSX)
Two clones from the Cambodian RF7 parasite line with either one copy (B9) or three copies (D4) of plasmepsin II and III were used for metabolomic analysis, which includes the relative quantitation of short peptides [22]. High performance liquid chromatography-mass spectrometry (HPLC-MS)-based metabolomic analysis was run in both positive and negative mode, and a total of 35 putative endogenous hemoglobin derived peptides (dipeptides to 13-mers) based on m/z match were detected that could be mapped to either the alpha or beta chains of hemoglobin. The log2 fold changes of D4/B9 of all detected hemoglobin peptides are shown for three biological replicates each by positive and negative mode. The combined average log2 of D4/B9 and the -log10(p-value) of all putative peptides were plotted in S6A and S6B Fig.
(XLSX)
3D7 trophozoites were treated for 2.5 h with 140 nM PPQ or 10 nM ATQ and the log2 fold changes for PPQ/no drug and ATQ/no drug are listed for three and six independent biological replicates, respectively. Data for this targeted approach is collected in negative mode and uses a retention-time-validated reference set of 115 targeted metabolites [46]. Only in N-carbamoyl-L-aspartate and dihydroorotate under ATQ treatment were significantly upregulated, as expected from previous studies [46]. The combined average log2 of PPQ/no drug and ATQ/no drug and the -log10(p-value) of all samples were plotted in S6C and S6D Fig.
(XLSX)
3D7 trophozoites were treated for 2.5 h with 140 nM PPQ and the log2 fold changes for PPQ/no drug are listed for three independent biological replicates. High performance liquid chromatography-mass spectrometry (HPLC-MS)-based metabolomic analysis was run in both positive and negative mode and a total of 220 putative endogenous hemoglobin derived peptides (dipeptides to 13-mers) were detected based on m/z match that could be mapped to either the alpha or beta chains of hemoglobin. The combined average log2 of PPQ/no drug and the -log10(p-value) of all samples were plotted in S6E and S6F Fig.
(XLSX)
Parasites were exposed to increasing levels of CQ or DHA for 72 h or PPQ for 84 h in acidic (pH = 6.74), normal (pH = 7.5) or basic (pH = 8.24) media. Included are the EC50 or AUC data for four biologically independent experiments run in triplicates for Dd2, 3D7, KH001_053G10, KH001_053G8, and KH004_057 including the average and SD of the AUC or EC50. Unpaired Student’s t-test between pH at 7.5 and lower or higher pH if more than three values could be determined: **p < 0.01, ***p < 0.001.
(XLSX)
A) DV pH traces of Dd2 parasites exposed to PPQ (50 μM, 10 μM, 5 μM and 1 μM) and control treatments Concanamycin A (ConA, 100 nM), CCCP (10 µM), CQ (10 µM) and NH4Cl (10 mM) over 90 min run in technical duplicates. B) DV pH was quantified for each treatment as an average of the measurements taken between 45 and 60 mins of compound exposure. Shown is the average pH for three to four independent experiments (performed with blood from different donors).
(XLSX)
This table includes all primers used in this study.
(XLSX)
Data Availability
All metabolomics data has been made publicly available through the Metabolomics Workbench (93) (https://www.metabolomicsworkbench.org,) with the following study numbers: ST003904, ST003902, and ST003906. This work is supported by Metabolomics Workbench/National Metabolomics Data Repository (NMDR) (grant# U2C-DK119886), Common Fund Data Ecosystem (CFDE) (grant# 3OT2OD030544) and Metabolomics Consortium Coordinating Center (M3C) (grant# 1U2C-DK119889). All other data produced in this manuscript is provided in the supplementary tables and figures.
Funding Statement
DFW received funding from the ExxonMobil Foundation. https://corporate.exxonmobil.com DAF received partial funding support from the NIH (R01 AI124678, R37 AI050234, and R01 AI147628). SM is grateful for the support by the Human Frontiers Science Program Long-Term Fellowship (LT000976/2016-L). ML and GR were supported by grant R21 AI174085 from the NIH. ML was also supported by the Eberly College of Science and the Huck Institutes of the Life Sciences at The Pennsylvania State University. GR was supported by a Penn State Eberly Research Fellowship. The authors would like to acknowledge the Huck Institutes' Metabolomics Core Facility (RRID:SCR_023864) for maintenance of the Thermo Exactive Plus. Funder did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Organization WH. World malaria report 2023. 2023.
- 2.van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. 2019. Epub 2019 Jul 28. 10.1016/s1473-3099(19)30391-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, et al. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob Agents Chemother. 2013;57(2):818–26. doi: 10.1128/AAC.00686-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leang R, Taylor WRJ, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in Western Cambodia: Dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59(8):4719–26. doi: 10.1128/AAC.00835-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duru V, Khim N, Leang R, Kim S, Domergue A, Kloeung N, et al. Plasmodium falciparum dihydroartemisinin-piperaquine failures in Cambodia are associated with mutant K13 parasites presenting high survival rates in novel piperaquine in vitro assays: retrospective and prospective investigations. BMC Med. 2015;13:305. doi: 10.1186/s12916-015-0539-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spring MD, Lin JT, Manning JE, Vanachayangkul P, Somethy S, Bun R, et al. Dihydroartemisinin-piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis. 2015;15(6):683–91. Epub 2015 April 17. 10.1016/s1473-3099(15)70049-6 [DOI] [PubMed] [Google Scholar]
- 7.Chaorattanakawee S, Lon C, Jongsakul K, Gawee J, Sok S, Sundrakes S, et al. Ex vivo piperaquine resistance developed rapidly in Plasmodium falciparum isolates in northern Cambodia compared to Thailand. Malar J. 2016;15(1):519. doi: 10.1186/s12936-016-1569-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organization WH. World malaria report 2018. 2018.
- 9.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371(5):411–23. doi: 10.1056/NEJMoa1314981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A-C, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505(7481):50–5. doi: 10.1038/nature12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straimer J, Gnädig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP, et al. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015;347(6220):428–31. doi: 10.1126/science.1260867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson TJ, Nair S, McDew-White M, Cheeseman IH, Nkhoma S, Bilgic F, et al. Population parameters underlying an ongoing soft sweep in Southeast Asian malaria parasites. Mol Biol Evol. 2017;34(1):131–44. 10.1093/molbev/msw228 [DOI] [PMC free article] [PubMed]
- 13.Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis. 2015;211(5):670–9. doi: 10.1093/infdis/jiu491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui FA, Boonhok R, Cabrera M, Mbenda HGN, Wang M, Min H, et al. Role of Plasmodium falciparum kelch 13 protein mutations in P. falciparum populations from northeastern Myanmar in mediating artemisinin resistance. mBio. 2020;11(1). Epub 2020 February 25. 10.1128/mBio.01134-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study. Lancet Infect Dis. 2017;17(2):174–83. Epub 2016 November 8. 10.1016/s1473-3099(16)30415-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, et al. Genetic markers associated with dihydroartemisinin-piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis. 2017;17(2):164–73. doi: 10.1016/S1473-3099(16)30409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee A, Gagnon D, Wirth DF, Richard D. Inactivation of Plasmepsins 2 and 3 sensitizes Plasmodium falciparum to the antimalarial drug piperaquine. Antimicrob Agents Chemother. 2018;62(4):e02309-17. doi: 10.1128/AAC.02309-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loesbanluechai D, Kotanan N, de Cozar C, Kochakarn T, Ansbro MR, Chotivanich K, et al. Overexpression of plasmepsin II and plasmepsin III does not directly cause reduction in Plasmodium falciparum sensitivity to artesunate, chloroquine and piperaquine. Int J Parasitol Drugs Drug Resist. 2019;9:16–22. doi: 10.1016/j.ijpddr.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal S, Moser KA, Morton L, Cummings MP, Parihar A, Dwivedi A, et al. Association of a novel mutation in the Plasmodium falciparum chloroquine resistance transporter with decreased piperaquine sensitivity. J Infect Dis. 2017;216(4):468–76. doi: 10.1093/infdis/jix334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross LS, Dhingra SK, Mok S, Yeo T, Wicht KJ, Kumpornsin K, et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat Commun. 2018;9(1):3314. doi: 10.1038/s41467-018-05764-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhingra SK, Redhi D, Combrinck JM, Yeo T, Okombo J, Henrich PP, et al. A variant PfCRT isoform can contribute to Plasmodium falciparum resistance to the first-line partner drug piperaquine. MBio. 2017;8(3). Epub 2017 May 11. 10.1128/mBio.00303-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okombo J, Mok S, Qahash T, Yeo T, Bath J, Orchard LM, et al. Piperaquine-resistant PfCRT mutations differentially impact drug transport, hemoglobin catabolism and parasite physiology in Plasmodium falciparum asexual blood stages. PLoS Pathog. 2022;18(10):e1010926. doi: 10.1371/journal.ppat.1010926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wicht KJ, Small-Saunders JL, Hagenah LM, Mok S, Fidock DA. Mutant PfCRT can mediate piperaquine resistance in African Plasmodium falciparum with reduced fitness and increased susceptibility to other antimalarials. J Infect Dis. 2022;226(11):2021–9. doi: 10.1093/infdis/jiac365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Florimond C, de Laval F, Early AM, Sauthier S, Lazrek Y, Pelleau S, et al. Impact of piperaquine resistance in Plasmodium falciparum on malaria treatment effectiveness in The Guianas: a descriptive epidemiological study. Lancet Infect Dis. 2024;24(2):161–71. doi: 10.1016/S1473-3099(23)00502-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton WL, Amato R, van der Pluijm RW, Jacob CG, Quang HH, Thuy-Nhien NT, et al. Evolution and expansion of multidrug-resistant malaria in southeast Asia: a genomic epidemiology study. Lancet Infect Dis. 2019;19(9):943–51. doi: 10.1016/S1473-3099(19)30392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, et al. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis. 2016;16(3):357–65. doi: 10.1016/S1473-3099(15)00487-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imwong M, Dhorda M, Myo Tun K, Thu AM, Phyo AP, Proux S, et al. Molecular epidemiology of resistance to antimalarial drugs in the Greater Mekong subregion: an observational study. Lancet Infect Dis. 2020;20(12):1470–80. doi: 10.1016/S1473-3099(20)30228-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Small-Saunders JL, Hagenah LM, Wicht KJ, Dhingra SK, Deni I, Kim J, et al. Evidence for the early emergence of piperaquine-resistant Plasmodium falciparum malaria and modeling strategies to mitigate resistance. PLoS Pathog. 2022;18(2):e1010278. doi: 10.1371/journal.ppat.1010278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bopp S, Magistrado P, Wong W, Schaffner SF, Mukherjee A, Lim P, et al. Plasmepsin II-III copy number accounts for bimodal piperaquine resistance among Cambodian Plasmodium falciparum. Nat Commun. 2018;9(1):1769. doi: 10.1038/s41467-018-04104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee R, Liu J, Beatty W, Pelosof L, Klemba M, Goldberg DE. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc Natl Acad Sci U S A. 2002;99(2):990–5. Epub 2002 Jan 10. 10.1073/pnas.022630099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gluzman IY, Francis SE, Oksman A, Smith CE, Duffin KL, Goldberg DE. Order and specificity of the Plasmodium falciparum hemoglobin degradation pathway. J Clin Invest. 1994;93(4):1602–8. Epub 1994 April 1. 10.1172/jci117140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg DE, Slater AF, Beavis R, Chait B, Cerami A, Henderson GB. Hemoglobin degradation in the human malaria pathogen Plasmodium falciparum: a catabolic pathway initiated by a specific aspartic protease. J Exp Med. 1991;173(4):961–9. doi: 10.1084/jem.173.4.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francis SE, Banerjee R, Goldberg DE. Biosynthesis and maturation of the malaria aspartic hemoglobinases plasmepsins I and II. J Biol Chem. 1997;272(23):14961–8. doi: 10.1074/jbc.272.23.14961 [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Gluzman IY, Drew ME, Goldberg DE. The role of Plasmodium falciparum food vacuole plasmepsins. J Biol Chem. 2005;280(2):1432–7. [DOI] [PubMed] [Google Scholar]
- 35.Kucharski M, Tripathi J, Nayak S, Zhu L, Wirjanata G, van der Pluijm RW, et al. A comprehensive RNA handling and transcriptomics guide for high-throughput processing of Plasmodium blood-stage samples. Malar J. 2020;19(1):363. doi: 10.1186/s12936-020-03436-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moura PA, Dame JB, Fidock DA. Role of Plasmodium falciparum digestive vacuole plasmepsins in the specificity and antimalarial mode of action of cysteine and aspartic protease inhibitors. Antimicrob Agents Chemother. 2009;53(12):4968–78. Epub 2009 September 16. 10.1128/aac.00882-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Istvan ES, Gluzman IY, Gross J, Goldberg DE. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc Natl Acad Sci U S A. 2006;103(23):8840–5. doi: 10.1073/pnas.0601876103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egan TJ. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. J Inorg Biochem. 2008;102(5–6):1288–99. doi: 10.1016/j.jinorgbio.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 39.Olafson KN, Ketchum MA, Rimer JD, Vekilov PG. Mechanisms of hematin crystallization and inhibition by the antimalarial drug chloroquine. Proc Natl Acad Sci USA. 2015;112(16):4946–51. Epub 2015 April 2. 10.1073/pnas.1501023112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ansbro MR, Jacob CG, Amato R, Kekre M, Amaratunga C, Sreng S, et al. Development of copy number assays for detection and surveillance of piperaquine resistance associated plasmepsin 2/3 copy number variation in Plasmodium falciparum. Malar J. 2020;19(1):181. doi: 10.1186/s12936-020-03249-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shenai BR, Sijwali PS, Singh A, Rosenthal PJ. Characterization of native and recombinant falcipain-2, a principal trophozoite cysteine protease and essential hemoglobinase of Plasmodium falciparum. J Biol Chem. 2000;275(37):29000–10. doi: 10.1074/jbc.M004459200 [DOI] [PubMed] [Google Scholar]
- 42.Glushakova S, Mazar J, Hohmann-Marriott MF, Hama E, Zimmerberg J. Irreversible effect of cysteine protease inhibitors on the release of malaria parasites from infected erythrocytes. Cell Microbiol. 2009;11(1):95–105. Epub 2008 November 20. 10.1111/j.1462-5822.2008.01242.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva AM, Lee AY, Gulnik SV, Maier P, Collins J, Bhat TN, et al. Structure and inhibition of plasmepsin II, a hemoglobin-degrading enzyme from Plasmodium falciparum. Proc Natl Acad Sci U S A. 1996;93(19):10034–9. Epub 1996 September 17. 10.1073/pnas.93.19.10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Combrinck JM, Fong KY, Gibhard L, Smith PJ, Wright DW, Egan TJ. Optimization of a multi-well colorimetric assay to determine haem species in Plasmodium falciparum in the presence of anti-malarials. Malar J. 2015;14:253. doi: 10.1186/s12936-015-0729-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Combrinck JM, Mabotha TE, Ncokazi KK, Ambele MA, Taylor D, Smith PJ, et al. Insights into the role of heme in the mechanism of action of antimalarials. ACS Chem Biol. 2013;8(1):133–7. doi: 10.1021/cb300454t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allman EL, Painter HJ, Samra J, Carrasquilla M, Llinás M. Metabolomic profiling of the malaria box reveals antimalarial target pathways. Antimicrob Agents Chemother. 2016;60(11):6635–49. Epub 2016 October 21. 10.1128/AAC.01224-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geary TG, Divo AD, Jensen JB, Zangwill M, Ginsburg H. Kinetic modelling of the response of Plasmodium falciparum to chloroquine and its experimental testing in vitro. Implications for mechanism of action of and resistance to the drug. Biochem Pharmacol. 1990;40(4):685–91. doi: 10.1016/0006-2952(90)90302-2 [DOI] [PubMed] [Google Scholar]
- 48.Yayon A, Cabantchik ZI, Ginsburg H. Susceptibility of human malaria parasites to chloroquine is pH dependent. Proc Natl Acad Sci U S A. 1985;82(9):2784–8. doi: 10.1073/pnas.82.9.2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrari V, Cutler DJ. Simulation of kinetic data on the influx and efflux of chloroquine by erythrocytes infected with Plasmodium falciparum. Evidence for a drug-importer in chloroquine-sensitive strains. Biochem Pharmacol. 1991;42:S167–79. doi: 10.1016/0006-2952(91)90407-v [DOI] [PubMed] [Google Scholar]
- 50.Bray PG, Mungthin M, Hastings IM, Biagini GA, Saidu DK, Lakshmanan V, et al. PfCRT and the trans-vacuolar proton electrochemical gradient: regulating the access of chloroquine to ferriprotoporphyrin IX. Mol Microbiol. 2006;62(1):238–51. doi: 10.1111/j.1365-2958.2006.05368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldberg DE, Slater AF, Cerami A, Henderson GB. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: an ordered process in a unique organelle. Proc Natl Acad Sci U S A. 1990;87(8):2931–5. doi: 10.1073/pnas.87.8.2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayward R, Saliba KJ, Kirk K. The pH of the digestive vacuole of Plasmodium falciparum is not associated with chloroquine resistance. J Cell Sci. 2006;119(Pt 6):1016–25. doi: 10.1242/jcs.02795 [DOI] [PubMed] [Google Scholar]
- 53.Klonis N, Tan O, Jackson K, Goldberg D, Klemba M, Tilley L. Evaluation of pH during cytostomal endocytosis and vacuolar catabolism of haemoglobin in Plasmodium falciparum. Biochem J. 2007;407(3):343–54. doi: 10.1042/BJ20070934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuhn Y, Rohrbach P, Lanzer M. Quantitative pH measurements in Plasmodium falciparum-infected erythrocytes using pHluorin. Cell Microbiol. 2007;9(4):1004–13. doi: 10.1111/j.1462-5822.2006.00847.x [DOI] [PubMed] [Google Scholar]
- 55.Lehane AM, Hayward R, Saliba KJ, Kirk K. A verapamil-sensitive chloroquine-associated H+ leak from the digestive vacuole in chloroquine-resistant malaria parasites. J Cell Sci. 2008;121(Pt 10):1624–32. doi: 10.1242/jcs.016758 [DOI] [PubMed] [Google Scholar]
- 56.Geary TG, Jensen JB, Ginsburg H. Uptake of [3H]chloroquine by drug-sensitive and -resistant strains of the human malaria parasite Plasmodium falciparum. Biochem Pharmacol. 1986;35(21):3805–12. doi: 10.1016/0006-2952(86)90668-4 [DOI] [PubMed] [Google Scholar]
- 57.Ginsburg H, Nissani E, Krugliak M. Alkalinization of the food vacuole of malaria parasites by quinoline drugs and alkylamines is not correlated with their antimalarial activity. Biochem Pharmacol. 1989;38(16):2645–54. doi: 10.1016/0006-2952(89)90550-9 [DOI] [PubMed] [Google Scholar]
- 58.Saliba KJ, Allen RJW, Zissis S, Bray PG, Ward SA, Kirk K. Acidification of the malaria parasite’s digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J Biol Chem. 2003;278(8):5605–12. doi: 10.1074/jbc.M208648200 [DOI] [PubMed] [Google Scholar]
- 59.Omara-Opyene AL, Moura PA, Sulsona CR, Bonilla JA, Yowell CA, Fujioka H, et al. Genetic disruption of the Plasmodium falciparum digestive vacuole plasmepsins demonstrates their functional redundancy. J Biol Chem. 2004;279(52):54088–96. doi: 10.1074/jbc.M409605200 [DOI] [PubMed] [Google Scholar]
- 60.Bonilla JA, Bonilla TD, Yowell CA, Fujioka H, Dame JB. Critical roles for the digestive vacuole plasmepsins of Plasmodium falciparum in vacuolar function. Mol Microbiol. 2007;65(1):64–75. doi: 10.1111/j.1365-2958.2007.05768.x [DOI] [PubMed] [Google Scholar]
- 61.Dame JB, Yowell CA, Omara-Opyene L, Carlton JM, Cooper RA, Li T. Plasmepsin 4, the food vacuole aspartic proteinase found in all Plasmodium spp. infecting man. Mol Biochem Parasitol. 2003;130(1):1–12. 10.1016/s0166-6851(03)00137-3 [DOI] [PubMed] [Google Scholar]
- 62.Bray PG, Janneh O, Raynes KJ, Mungthin M, Ginsburg H, Ward SA. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J Cell Biol. 1999;145(2):363–76. 10.1083/jcb.145.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warhurst DC, Craig JC, Raheem KS. Influence of LAR and VAR on para-aminopyridine antimalarials targetting haematin in chloroquine-resistance. PLoS One. 2016;11(8):e0160091. doi: 10.1371/journal.pone.0160091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warhurst DC, Craig JC, Adagu IS, Guy RK, Madrid PB, Fivelman QL. Activity of piperaquine and other 4-aminoquinoline antiplasmodial drugs against chloroquine-sensitive and resistant blood-stages of Plasmodium falciparum. Role of beta-haematin inhibition and drug concentration in vacuolar water- and lipid-phases. Biochem Pharmacol. 2007;73(12):1910–26. doi: 10.1016/j.bcp.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 65.Ambele MA, Sewell BT, Cummings FR, Smith PJ, Egan TJ. Synthetic Hemozoin (β-Hematin) Crystals Nucleate at the Surface of Neutral Lipid Droplets that Control Their Sizes. Cryst Growth Des. 2013;13(10):10.1021/cg4009416. doi: 10.1021/cg4009416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoang AN, Sandlin RD, Omar A, Egan TJ, Wright DW. The neutral lipid composition present in the digestive vacuole of Plasmodium falciparum concentrates heme and mediates β-hematin formation with an unusually low activation energy. Biochemistry. 2010;49(47):10107–16. doi: 10.1021/bi101397u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Birnbaum J, Scharf S, Schmidt S, Jonscher E, Hoeijmakers WAM, Flemming S, et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science. 2020;367(6473):51–9. Epub 2020 January 4. 10.1126/science.aax4735 [DOI] [PubMed] [Google Scholar]
- 68.Mesén-Ramírez P, Bergmann B, Elhabiri M, Zhu L, von Thien H, Castro-Peña C, et al. The parasitophorous vacuole nutrient channel is critical for drug access in malaria parasites and modulates the artemisinin resistance fitness cost. Cell Host Microbe. 2021;29(12):1774–87.e9. doi: 10.1016/j.chom.2021.11.002 [DOI] [PubMed] [Google Scholar]
- 69.Kane J, Li X, Kumar S, Button-Simons KA, Vendrely Brenneman KM, Dahlhoff H, et al. A Plasmodium falciparum genetic cross reveals the contributions of pfcrt and plasmepsin II/III to piperaquine drug resistance. mBio. 2024;15(7):e0080524. doi: 10.1128/mbio.00805-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amato R, Pearson RD, Almagro-Garcia J, Amaratunga C, Lim P, Suon S, et al. Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: a retrospective genetic study. Lancet Infect Dis. 2018;18(3):337–45. doi: 10.1016/S1473-3099(18)30068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shafik SH, Cobbold SA, Barkat K, Richards SN, Lancaster NS, Llinás M, et al. The natural function of the malaria parasite’s chloroquine resistance transporter. Nat Commun. 2020;11(1):3922. doi: 10.1038/s41467-020-17781-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee AH, Dhingra SK, Lewis IA, Singh MK, Siriwardana A, Dalal S, et al. Evidence for regulation of hemoglobin metabolism and intracellular ionic flux by the Plasmodium falciparum chloroquine resistance transporter. Sci Rep. 2018;8(1):13578. doi: 10.1038/s41598-018-31715-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanchez CP, Manson EDT, Moliner Cubel S, Mandel L, Weidt SK, Barrett MP, et al. The knock-down of the chloroquine resistance transporter PfCRT ss linked to oligopeptide handling in Plasmodium falciparum. Microbiol Spectr. 2022;10(4):e0110122. doi: 10.1128/spectrum.01101-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science. 2009;325(5948):1680–2. doi: 10.1126/science.1175667 [DOI] [PubMed] [Google Scholar]
- 75.Bellanca S, Summers RL, Meyrath M, Dave A, Nash MN, Dittmer M, et al. Multiple drugs compete for transport via the Plasmodium falciparum chloroquine resistance transporter at distinct but interdependent sites. J Biol Chem. 2014;289(52):36336–51. doi: 10.1074/jbc.M114.614206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim J, Tan YZ, Wicht KJ, Erramilli SK, Dhingra SK, Okombo J, et al. Structure and drug resistance of the Plasmodium falciparum transporter PfCRT. Nature. 2019;576(7786):315–20. 10.1038/s41586-019-1795-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomez GM, D’Arrigo G, Sanchez CP, Berger F, Wade RC, Lanzer M. PfCRT mutations conferring piperaquine resistance in falciparum malaria shape the kinetics of quinoline drug binding and transport. PLoS Pathog. 2023;19(6):e1011436. doi: 10.1371/journal.ppat.1011436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kubota R, Ishino T, Iwanaga S, Shinzawa N. Evaluation of the effect of gene duplication by genome editing on drug resistance in Plasmodium falciparum. Front Cell Infect Microbiol. 2022;12:915656. doi: 10.3389/fcimb.2022.915656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhingra SK, Small-Saunders JL, Ménard D, Fidock DA. Plasmodium falciparum resistance to piperaquine driven by PfCRT. Lancet Infect Dis. 2019;19(11):1168–9. doi: 10.1016/S1473-3099(19)30543-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mok S, Yeo T, Hong D, Shears MJ, Ross LS, Ward KE, et al. Mapping the genomic landscape of multidrug resistance in. Sci Adv. 2023;9(45):eadi2364. Epub 2023 December 7. 10.1128/mbio.01832-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hagenah LM, Dhingra SK, Small-Saunders JL, Qahash T, Willems A, Schindler KA, et al. Additional PfCRT mutations driven by selective pressure for improved fitness can result in the loss of piperaquine resistance and altered Plasmodium falciparum physiology. mBio. 2024;15(1):e0183223. doi: 10.1128/mbio.01832-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hagenah LM, Yeo T, Schindler KA, Jeon JH, Bloxham TS, Small-Saunders JL, et al. Plasmodium falciparum African PfCRT mutant isoforms conducive to piperaquine resistance are infrequent and impart a major fitness cost. J Infect Dis. 2024. Epub 2024 December 11. 10.1093/infdis/jiae617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shrestha B, Shah Z, Morgan AP, Saingam P, Chaisatit C, Chaorattanakawee S, et al. Distribution and temporal dynamics of Plasmodium falciparum chloroquine resistance transporter mutations associated with piperaquine resistance in Northern Cambodia. J Infect Dis. 2021;224(6):1077–85. doi: 10.1093/infdis/jiab055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vanhove M, Schwabl P, Clementson C, Early AM, Laws M, Anthony F, et al. Temporal and spatial dynamics of Plasmodium falciparum clonal lineages in Guyana. PLoS Pathog. 2024;20(6):e1012013. doi: 10.1371/journal.ppat.1012013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leroy D, Macintyre F, Adoke Y, Ouoba S, Barry A, Mombo-Ngoma G, et al. African isolates show a high proportion of multiple copies of the Plasmodium falciparum plasmepsin-2 gene, a piperaquine resistance marker. Malar J. 2019;18(1):126. doi: 10.1186/s12936-019-2756-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chugh M, Sundararaman V, Kumar S, Reddy VS, Siddiqui WA, Stuart KD, et al. Protein complex directs hemoglobin-to-hemozoin formation in Plasmodium falciparum. Proc Natl Acad Sci U S A. 2013;110(14):5392–7. doi: 10.1073/pnas.1218412110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghorbal M, Gorman M, Macpherson CR, Martins RM, Scherf A, Lopez-Rubio J-J. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol. 2014;32(8):819–21. doi: 10.1038/nbt.2925 [DOI] [PubMed] [Google Scholar]
- 88.Ng CL, Siciliano G, Lee MC, de Almeida MJ, Corey VC, Bopp SE, et al. CRISPR-Cas9-modified pfmdr1 protects Plasmodium falciparum asexual blood stages and gametocytes against a class of piperazine-containing compounds but potentiates artemisinin-based combination therapy partner drugs. Mol Microbiol. 2016. 10.1111/mmi.13397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci U S A. 1997;94(20):10931–6. doi: 10.1073/pnas.94.20.10931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Price RN, Uhlemann A-C, Brockman A, McGready R, Ashley E, Phaipun L, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364(9432):438–47. doi: 10.1016/S0140-6736(04)16767-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ribacke U, Mok BW, Wirta V, Normark J, Lundeberg J, Kironde F, et al. Genome wide gene amplifications and deletions in Plasmodium falciparum. Mol Biochem Parasitol. 2007;155(1):33–44. doi: 10.1016/j.molbiopara.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 92.Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother. 2007;51(6):1926–33. doi: 10.1128/AAC.01607-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sud M, Fahy E, Cotter D, Azam K, Vadivelu I, Burant C, et al. Metabolomics Workbench: An international repository for metabolomics data and metadata, metabolite standards, protocols, tutorials and training, and analysis tools. Nucleic Acids Res. 2016;44(D1):D463-70. doi: 10.1093/nar/gkv1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saliba KJ, Kirk K. pH regulation in the intracellular malaria parasite, Plasmodium falciparum. H(+) extrusion via a V-type H(+)-ATPase. J Biol Chem. 1999;274(47):33213–9. doi: 10.1074/jbc.274.47.33213 [DOI] [PubMed] [Google Scholar]
- 95.Spillman NJ, Allen RJW, Kirk K. Acid extrusion from the intraerythrocytic malaria parasite is not via a Na(+)/H(+) exchanger. Mol Biochem Parasitol. 2008;162(1):96–9. doi: 10.1016/j.molbiopara.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 96.Allen RJ, Kirk K. The membrane potential of the intraerythrocytic malaria parasite Plasmodium falciparum. J Biol Chem. 2004;279(12):11264–72. Epub 2003 November 20. 10.1074/jbc.M311110200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schema of original loci, homology plasmids used for integration into the locus and resulting edited loci. Restriction enzyme sites and expected band sizes for Southern blots are indicated in the schema in orange for plasmepsin II and in blue for plasmepsin III KOs. (B) Southern blots with different probes, expected band sizes are indicated by arrows. Clones indicated with a star were used for phenotyping. Plasmids were either transfected in circular (cir) or linearized (lin) form. (C) WR99210 primarily targets the plasmodial dhfr and an increase in EC50 is correlated with the presence of one or several hdhfr cassettes present. Shown is the average EC50 and standard deviations of three biological replicates for each clone (one-way ANOVA with Dunnett’s post-test compared to 1D with a single integration of the hdhfr cassette. ****p < 0.0001).
(TIF)
(A) Schema of original parental loci, homology plasmid used for integration into the loci, and resulting edited locus which is identical for both parents. Restriction enzyme sites and expected band sizes for Southern blots are indicated in the schema in orange for plasmepsin II and in blue for plasmepsin III KOs. (B) Southern blots with plasmepsin III probe, expected band sizes are indicated by arrows. Clones indicated with a star were used for phenotyping. (C) Average EC50 and standard deviations of three biological replicates of WR99210 for each clone except 4E (n = 1). nd: not determined.
(TIF)
(A) Schema of original parental loci, homology plasmid used for integration into the loci and resulting edited locus which is identical for both parents. Restriction enzyme sites outside the locus were selected to confirm complete deletion of the regions between the homology regions and expected band sizes for Southern blots are indicated in the schema. Clones indicated with a star were used for phenotyping. (B) The same Southern blots was hybridized three times with the plasmepsin II, plasmepsin III, or hdhfr probe. Expected band size for each probe is indicated with arrows. The loss of hybridization for the plasmepsin II probe confirms the deletion and fusion of plasmepsin II and plasmepsin III in the KO clones.
(TIF)
Ring-stage parasites were either grown in complete media or in complete media with the addition of CCCP (15 or 5 μM), E64 (3.9 or 2.6 μM), pepA (7.5 or 5 μM), ConA (0.1 or 0.2 nM), 10 μM DHA (dead) or 0.5% DMSO for 72 h. Growth was measured by the incorporation of SYBRGreen into DNA, read by a spectrometer and normalized to parasites cultured in media only.
(TIF)
Parasites were exposed to increasing levels of PPQ in the presence of DMSO or (A) E64 at a concentration of either 2.6 μM or 3.9 μM or (B) pepstatin A (pepA) at a concentration of either 5 μM or 7.5 μM. Shown is one example of three biologically independent experiments run in triplicates. (C) Average and SD of the area under the curve (AUC) between the local minima for three biological replicates. No statistically significant difference was detected between PPQ alone and PPQ in combination with either E64 or pepA by ordinary one-way ANOVA with Tukey post-test.
(TIF)
A and B: Two clones from the Cambodian RF7 parasite line [22] with either one copy (B9) or three copies (D4) of plasmepsin II and III were used for small molecule metabolomic analysis, which includes relative quantitation of short peptides. Metabolomic analysis was run in both positive and negative mode and a total of 35 putative endogenous hemoglobin-derived peptides (i.e., dipeptides to 13-mers) were detected based on their m/z match that could be mapped to either the alpha (A) or beta (B) chains of hemoglobin. Shown are the volcano plots combining statistical significance and fold change observed in metabolites from RF7 clones D4 compared to B9. C to F: Effects of PPQ or ATQ treatment on the parasite’s metabolism. Purified P. falciparum 3D7 trophozoites were treated for 2.5 h with 140 nM PPQ or 10 nM ATQ (as a control) and volcano plots comparing metabolites from untreated vs PPQ-treated parasites for targeted metabolite analysis from PPQ (C) and ATQ (D) treated parasites compared to untreated are shown. Volcano plots comparing metabolites from untargeted analysis of all putative hemoglobin-derived peptides of amino acid length 13 or less are shown in (E) (alpha chain) and (F) (beta chain). The dotted lines depict the significance cutoff of p = 0.01 and a two-fold change in metabolite abundance. Only in N-carbamoyl-L-aspartate and dihydroorotate under ATQ treatment were significantly increased in abundance [46].
(TIF)
We determined the minimal amino acid needs of parasites to allow for enough DNA replication to perform drug susceptibility assays by SYBR Green I staining. KH001_053G10, KH001_053G8, G8PMII/III_KO and KH004_057 were synchronized and set up at 1% parasitemia and 2% hematocrit in regular RPMI media or RPMI media with isoleucine, methionine, and glutamine as the only amino acid sources. The two conditions were then mixed in 10% increments (90% regular media plus 10% amino acid-free (except isoleucine, methionine, and glutamine) media, 80% and 20% etc.) in 96 well plates and incubated at 37°C for 72 h. Growth was analyzed by adding SYBR Green to the plates, measuring the fluorescence, and normalizing the signal to parasites grown in regular media. The final assays conditions for drug susceptibility assays were set at 25% full amino acid RPMI and termed amino acid-limited media.
(TIF)
KH001_053G10, KH001_053G8, G8PMII/III_KO, and KH004_057 parasites were synchronized and set up at 0.5% parasitemia in either regular media or amino acid-limited media. Parasite replication was measured by estimating the parasitemia in the second cycle by flow cytometry of SYBR Green-stained parasite samples and dividing it by the initial parasitemia. Shown are the average replication rates for three biological replicates with SD. There were no statistically significant differences detected between the strain grown in either regular or amino acid-limited media by one-way ANOVA followed by Dunnett’s post-test, ns = no significance. The replication rate for all strains was significantly less in amino acid-limited media compared to regular media by Student’s t-test: *p < 0.05.
(TIF)
A-F) Parasites were exposed to increasing levels of CQ in acidic (pH = 6.74), normal (pH = 7.5) or basic (pH = 8.24) media. Shown is one example for each tested line of three biologically independent experiments run in triplicates. G-L) Parasites were exposed to increasing levels of CQ in the presence of DMSO or CCCP at a concentration of either 5 μM or 15 μM. The EC50 was calculated where possible (an # indicates when parasites were not killed completely at the highest concentration), and the average and SD are shown in (F and L). Statistics show one-way ANOVA with Tukey post-test for each strain tested in the presence of CCCP or two tailed paired Student’s t-test for the external pH changes: *p < 0.05; **p < 0.01; ***p < 0.001.
(TIF)
Plasmids were either transfected in circular form (cir) or were linearized with BglI before transfection (lin). Shown are all the clones analyzed for every transfection that was recovered. Integration was confirmed by PCR of the 5’ and 3’ region and appearance of a band at the right size was considered a positive result (green check mark). The absence of the transfection plasmid was screened for with primers targeting the backbone of the plasmid which is lost after correct integration (red cross). Quantitative PCR was also used to confirm copy numbers of plasmepsin II and III as well as the hdhfr gene inserted into the locus. Primers are listed in S11 Table.
(XLSX)
Parasite lines were exposed to various concentration of drug and the EC50 was calculated for each drug. Each experiment was run in triplicate and at least three biological replicates were performed for each parasite line/clone. Shown are the EC50, SD and sample size for each line.
(XLSX)
(A) Combination assays of parasites in the presence of increasing concentrations of PPQ and a constant concentration of E64, pepA, ConA, or CCCP measured as AUC. (B) Parasites were cultured either in regular or amino acid-limited media and exposed to increasing concentrations of PPQ and the AUC was measured. (C) EC50 for ConA and CCCP. (D) Combination assays in the presence of increasing concentrations of CQ and a constant concentration of CCCP measured as EC50.
(XLSX)
Different heme species were extracted from parasites by subsequent cellular fractionation steps. Tightly synchronized KH001_053G10 and KH001_053G8 ring-stage parasites were exposed to various PPQ concentrations for 32 h. The amount of iron species (Fe) per sample was estimated based on the standard curve run for each biological replicate and the percentages for each species per sample were calculated. Shown is each value from three biological replicates run in quadruplicate with the average and SD of percentage of hemozoin Fe, hemoglobin, and free heme Fe. Statistical comparisons of the drug-treated lines to their untreated controls were performed using two-tailed unpaired Student’s t-tests *p < 0.05; **p < 0.01.
(XLSX)
Tightly synchronized parasites were harvested at different time-points throughout the life cycle; the average and SD of percentage of hemozoin Fe, hemoglobin, and free heme Fe are shown for three independent experiments for parasites with a single locus (KH001_053G10), duplicated locus (KH001_053G8), G8PMII_KO, G8PMIII_KO, or G8PMII/III_KO. Statistical comparisons at each time point were performed between the single copy KH001_053G10 parasites and all other lines using two-tailed unpaired Student’s t-tests *p < 0.05; **p < 0.01. Additionally, parasites were incubated with 2 μM PPQ from 24 to 36 h post synchronization and harvested at 36 h. Statistical comparisons of PPQ-treated to untreated parasites at the 36 h timepoint from three independent biological replicates were performed using two-tailed unpaired Student’s t-tests *p < 0.05; **p < 0.01; ***p < 0.001.
(XLSX)
Two clones from the Cambodian RF7 parasite line with either one copy (B9) or three copies (D4) of plasmepsin II and III were used for metabolomic analysis, which includes the relative quantitation of short peptides [22]. High performance liquid chromatography-mass spectrometry (HPLC-MS)-based metabolomic analysis was run in both positive and negative mode, and a total of 35 putative endogenous hemoglobin derived peptides (dipeptides to 13-mers) based on m/z match were detected that could be mapped to either the alpha or beta chains of hemoglobin. The log2 fold changes of D4/B9 of all detected hemoglobin peptides are shown for three biological replicates each by positive and negative mode. The combined average log2 of D4/B9 and the -log10(p-value) of all putative peptides were plotted in S6A and S6B Fig.
(XLSX)
3D7 trophozoites were treated for 2.5 h with 140 nM PPQ or 10 nM ATQ and the log2 fold changes for PPQ/no drug and ATQ/no drug are listed for three and six independent biological replicates, respectively. Data for this targeted approach is collected in negative mode and uses a retention-time-validated reference set of 115 targeted metabolites [46]. Only in N-carbamoyl-L-aspartate and dihydroorotate under ATQ treatment were significantly upregulated, as expected from previous studies [46]. The combined average log2 of PPQ/no drug and ATQ/no drug and the -log10(p-value) of all samples were plotted in S6C and S6D Fig.
(XLSX)
3D7 trophozoites were treated for 2.5 h with 140 nM PPQ and the log2 fold changes for PPQ/no drug are listed for three independent biological replicates. High performance liquid chromatography-mass spectrometry (HPLC-MS)-based metabolomic analysis was run in both positive and negative mode and a total of 220 putative endogenous hemoglobin derived peptides (dipeptides to 13-mers) were detected based on m/z match that could be mapped to either the alpha or beta chains of hemoglobin. The combined average log2 of PPQ/no drug and the -log10(p-value) of all samples were plotted in S6E and S6F Fig.
(XLSX)
Parasites were exposed to increasing levels of CQ or DHA for 72 h or PPQ for 84 h in acidic (pH = 6.74), normal (pH = 7.5) or basic (pH = 8.24) media. Included are the EC50 or AUC data for four biologically independent experiments run in triplicates for Dd2, 3D7, KH001_053G10, KH001_053G8, and KH004_057 including the average and SD of the AUC or EC50. Unpaired Student’s t-test between pH at 7.5 and lower or higher pH if more than three values could be determined: **p < 0.01, ***p < 0.001.
(XLSX)
A) DV pH traces of Dd2 parasites exposed to PPQ (50 μM, 10 μM, 5 μM and 1 μM) and control treatments Concanamycin A (ConA, 100 nM), CCCP (10 µM), CQ (10 µM) and NH4Cl (10 mM) over 90 min run in technical duplicates. B) DV pH was quantified for each treatment as an average of the measurements taken between 45 and 60 mins of compound exposure. Shown is the average pH for three to four independent experiments (performed with blood from different donors).
(XLSX)
This table includes all primers used in this study.
(XLSX)
Data Availability Statement
All metabolomics data has been made publicly available through the Metabolomics Workbench (93) (https://www.metabolomicsworkbench.org,) with the following study numbers: ST003904, ST003902, and ST003906. This work is supported by Metabolomics Workbench/National Metabolomics Data Repository (NMDR) (grant# U2C-DK119886), Common Fund Data Ecosystem (CFDE) (grant# 3OT2OD030544) and Metabolomics Consortium Coordinating Center (M3C) (grant# 1U2C-DK119889). All other data produced in this manuscript is provided in the supplementary tables and figures.