Abstract
Busulphan (Bu) is used as a part of the conditioning regimen prior to HSCT. Neurotoxicity is one of Bu major adverse-effects. We investigated the kinetics of busulphan and its metabolites (tetrahydothiophene, tetrahydrothiophene-1-oxide, sulfolane, 3-OH-sulfolane) in patients and mice as well as the mechanisms underlying CNS-toxicity in mice. Busulphan metabolites were detectable in plasma and urine up to 72-h after the last Bu-dose. Sulfolane levels were high and reached maximum concentration at the time-point reported for the convulsions’ occurrence. Mice were treated with either busulphan or one of its metabolites, separately. Sulfolane treated-mice showed the highest brain exposure (AUCbrain/AUCplasma). Seizures and hypothermia were observed after sulfolane administration, accompanied with a significant decrease in calbindin-28k concentrations in the brain. Behavior changes but no signs of convulsions were seen in mice treated with lower sulfolane doses. Moreover, a reduction of spontaneous events during whole-cell patch clamp recordings from pyramidal neurons was observed following bath application of sulfolane. In conclusion, these are the first results showing that sulfolane is the major cause of seizures and hypothermia. Sulfolane concentration in plasma mirrors its concentration in the brain. The role of calbindin-D28K in CNS-toxicity and susceptibility to future neurodegenerative diseases should be investigated.
Subject terms: Chemotherapy, Haematopoietic stem cells, Translational research
Introduction
Hematopoietic stem cell transplantation (HSCT) is a curative treatment for several hematological and solid malignancies. The alkylating agent busulphan (Bu) is used in high doses as a part of conditioning prior to HSCT. Busulphan is metabolized mainly in the liver through conjugation with glutathione (GSH) via glutathione transferase A1 (GSTA1) [1]. The conjugation produces an unstable sulfonium ion that is rapidly degraded to tetrahydrothiophene (THT) [2]. THT is reabsorbed from bile to be oxidized to tetrahydrothiophene 1-oxide (THT 1-oxide), which is further oxidized to sulfolane (Tetrahydrothiophene 1,1-dioxide), that is finally oxidized to 3-hydroxysulfolane (3-OH sulfolane) [2, 3]. We and others have reported the involvement of flavin-containing monooxygenase 3 (FMO3) and cytochrome P450 (CYPs) Bu metabolic pathway via the oxidation of THT and probably THT 1-oxide that can affect Bu kinetics [4, 5].
Studies in monkeys and man have shown that Bu can easily cross the blood-brain barrier (BBB) [3, 6, 7]. Only 1–2% of Bu is excreted in the urine unchanged, while about 70% of the dose is excreted in rats urine within 72 h as 3-OH-sulfolane (39%), THT 1-oxide (20%), and sulfolane (13%) [2, 6].
Several old occupational studies have reported the neurotoxicity of sulfolane [8, 9]. Rats treated with an intraperitoneal (i.p.) injection of sulfolane are less active than those receiving saline [8]. Other studies have reported that sulfolane induced convulsions and hypothermia in animals [8–10].
Previous studies reported that about 10% of patients receiving high doses of Bu experience seizures [11, 12]; therefore, anticonvulsant prophylaxis such as phenytoin or diazepam [11, 13–17] are included in the conditioning regimen. However, the mechanisms underlying these seizures are not fully understood.
In the present investigation, we studied the kinetics of Bu and its metabolites in patient’s plasma and urine and in mouse plasma and organs. We further investigated the effect of Bu and its metabolites on mouse behavior, neurotoxicity markers, and changes in body temperature in order to understand the mechanism underlying the CNS-toxicity.
Materials and methods
Material used in the pharmacokinetics and ELSIA studies are listed in the complementary data.
Patients
Patients receiving Bu prior to HSCT (n = 18) from the Center for Allogeneic Stem Cell Transplantation (CAST) at Karolinska University Hospital-Huddinge-Sweden were recruited to the study. Patients’ characteristics are listed in Table 1. The study was approved by the Regional Ethics Committee (DNR 425/97, 619-17) in accordance with the Helsinki Declaration, and informed consent was obtained from all patients or their guardians. The sample size was calculated considering a 10% margin of error and a 95% confidence interval.
Table 1.
Patients’ characteristics.
| Patients characteristics | |
|---|---|
| Median patient age | 33.5 (12–66) |
| Number of children | 1 |
| Number of adults | 17 |
| Females | 9 |
| Males | 9 |
| Diagnoses | |
| ALD | 1 |
| AML | 15 |
| CML | 2 |
| Conditioning regimen | |
| Bu + Cy | 18 |
ALD adrenoleukodystrophy, AML acute myeloid leukemia, CML chronic myelogenous leukemia, Bu busulphan, Cy cyclophosphamide.
Patients received oral Bu (b.i.d., 2 mg/kg/day for 4 days) [18]. Blood samples were collected in EDTA vacutainer tubes for therapeutic drug monitoring (TDM) and dose adjustment. Extra samples were collected at 24, 48, and 72 h after Bu last-dose. Plasma was separated by centrifugation (3000 g, 5 min) and stored at −20 °C. Urine samples were collected from 11 patients regularly and stored at −20 °C.
Bu concentrations were measured by gas chromatography with an electron capture detector (GC-ECD) (SCION 436-GC; Bruker) [19], while Bu four metabolites, were measured using gas chromatography-mass spectrometry (GC-MS) (Agilent 6890N series GC equipped with Agilent 7683 auto-injector and Agilent 5973N mass selective detector, Santa Clara, CA, USA) [20].
Mice
Male C57BL/6N mice (6–8 weeks) were purchased from Charles River (Koln, Germany). The study was approved by Stockholm South ethical committee (Dnr. S119-12 and (S53-13). Details regarding the mice housing are reported in the supplementary data.
Pharmacokinetic study
Busulphan dose was calculated to correspond to its dose in patients prior to HSCT and its equimolar dose for the metabolites. Mice received single doses (i.p) of Bu, THT, or THT-1-oxide at doses of 25, 8.8, and 10.4 mg/kg, respectively. All three compounds were dissolved in DMSO and diluted using NaCl (9 mg/mL) prior to administration (200 µL, 5% DMSO). Sulfolane and 3-OH sulfolane were dissolved in phosphate-buffered saline (PBS) and administered separately i.p. at doses of 12 and 13.6 mg/kg, respectively (200 µL). No blinding was carried out.
Blood was collected by cardiac puncture at different time-points for the kinetics of Bu and its metabolites (N = 3 per time point). Plasma was separated and stored at −20 °C. After blood collection, mice were euthanized, perfused, then organs were collected and snap-frozen.
Prior to homogenization, frozen organs were thawed at room temperature, and PBS was added (4 and 3 mL/g for liver/kidney and brain samples, respectively). The homogenates were centrifuged at 6000 × g for 18 min, and the supernatant was collected. Bu and its metabolites were quantified in organ homogenates and plasma using GC-MS [20].
Behavioral and neurological studies
Mice were transported from their room to a behavioral lab within the same facility and were divided into 5 groups. Each group (n = 4) was injected (i.p.) with only one of the compounds. Mice from one cage were subsequently placed in an MIII empty cage containing wood chips and a red house as a shelter. Mice were closely monitored from a distance of less than 1 m for one to two hours. The same experienced observer performed consecutive observations of each mouse for 1 min, spaced at 10–15-min intervals.
The same experiment was repeated to video-record any changes in mice for 1 h. Temperature changes were regularly recorded using a thermos camera. In seizure occurrence, mice were sacrificed when the episode exceeded 10 s.
Brains were collected, stored, and prepared as previously described. The supernatants were measured by ELISA for different neurotransmitters: dopamine, 5-hydroxytryptamine (5-HT), glutamate, GABA, and calbindin-28k. An extra group of mice, receiving Bu i.p. once daily for four days, was added to this experiment. Two control groups were run in parallel. One group received DMSO (5%), while the other received PBS in total volume of 200 µL.
Ex vivo electrophysiology
Slice preparation and whole-cell patch clamp are mentioned in details in the supplementary data. The following compounds were applied during ex vivo electrophysiology: Bu (100 µM) was prepared as 100 mM stock in DMSO and diluted in oxygenated ACSF. Sulfolane (1 mM) and THT 1-Oxide (2 mM) were diluted directly in oxygenated ACSF to the appropriate concentrations. Other compounds were not tested since they showed no significant effect in the previous experiments.
Responses to various current pulses were analyzed using custom scripts written for Igor Pro-6.3. Spontaneous events were detected by examining the smoothed wave’s first- and second derivatives. The minimum detection threshold for post-synaptic events was set to 6 standard deviations above the baseline. Membrane time constants were determined from a brief (5 ms) hyperpolarizing current pulse.
Data analysis
Enhanced ChemStation software G1401BA version E.02.02.1431 (Agilent, Santa Clara, CA, USA) was utilized for the collection of chromatograms and quantitative data, while kinetics estimations were performed using WinNonLin software (standard edition, version 2.0). All statistical analyses and graphs were performed using GraphPad Prism (version 4.0, GraphPad Software, Inc.). Values were considered significant when the P value < 0.05.
Results
Patients
Pharmacokinetics in plasma
Patients mean AUC/dose over the 4-day conditioning was 11542 ± 452 ng.h/mL (2815.1 ± 110.2 μmol.min/L). Busulphan maximum concentrations were detected 1–2 h post-administration, and it was undetectable 24 h after Bu last dose (Fig. 1a). THT concentrations were hardly detectable, indicating that THT is rapidly oxidized to THT 1-oxide. THT 1-oxide started to emerge in plasma after the second day of conditioning to reach a maximum concentration of 99 ± 30 ng/mL (0.95 ± 0.3 μM) 6 h after Bu 5th dose. Sulfolane plasma concentrations were the highest among the metabolites as it detectable 1 h after Bu 1st dose and reached maximum plasma levels (424 ± 159 ng/mL, 3.5 ± 1.3 μM) 8 h after the 5th dose. Sulfolane reached a steady-state level that maintained throughout the Bu- and was detectable 60 h post Bu last dose (68.5 ± 13 ng/mL, 0.6 ± 0.1 μM). 3-OH sulfolane had a similar pattern as sulfolane; however, the plasma levels were lower. 3-OH sulfolane was emerging in plasma after Bu 1st dose; the maximum concentrations were reached 6–8 h after the 5th dose (mean concentration 313 ± 150 ng/mL, 2.3 ± 1.1 μM) and it was detectable 24 h after Bu last dose (Fig. 1b).
Fig. 1. The concentrations of busulphan and its four metabolites in plasma and urine of patients during the period of conditioning with Bu and up to 3 days after the last dose administration.
a Bu concentrations in plasma. b The 4 metabolites concentrations in plasma. c Bu concentrations in urine. d The 4 metabolites concentrations in urine. Mean ± standard error of the mean (SEM).
Pharmacokinetics in urine
Bu concentrations were constantly high upon the start of the conditioning. The highest concentrations were detected 12 h after the first dose (1.5 µg/mL, 6.1 μM). Bu was detectable in urine up-to 3 days post Bu last dose (Fig. 1c). THT and sulfolane urine levels were relatively low in all samples (mean concentration: 47.3 ng/mL [0.5 μM] and 145.3 ng/mL [1.2 μM], respectively). THT 1-oxide was high in urine, particularly in samples taken 4–6 h post-dose (mean maximal concentration 5.9 µg/mL, 56.6 μM). The highest concentrations of Bu-metabolites were detected for 3-OH sulfolane (mean maximal concentration: 35.1 µg/mL, 257.8 μM 4–6 h post-dose) (Fig. 1d).
Mice
Pharmacokinetics in plasma
Both Bu and THT levels were low (<8 μM and <0.46 μM respectively) throughout all timepoints. THT 1-oxide peak plasma concentration was 83.6 μM, measured 2 h after injection. Sulfolane peaked at 43.3 μM 10 min post-injection and was completely eliminated from plasma 6 h post-administration. Finally, 3-OH sulfolane peak plasma concentration was 142.8 μM after 10 min (Fig. 2a). Accordingly, the largest calculated maximum concentration (Cmax) was found for 3-OH sulfolane followed by THT-1-oxide. Busulphan showed the longest elimination half-life followed by THT and THT-1 oxide. The shortest half-life was observed for sulfolane. THT-1-oxide had the highest AUC followed by 3-OH sulfolane (Table 2).
Fig. 2. The concentrations busulphan and its metabolites in mice 10 min - 8 h post-injection.
a plasma, (b) liver, (c) brain and (d) kidneys; Mean ± SEM.
Table 2.
Pharmacokinetic parameters for Bu and its metabolites in mice plasma and organs; Mean ± SD.
| Compounds | AUC [μmol.min/L] | HL [h] | Cmax [μmol/L] |
|---|---|---|---|
| Plasma | |||
| Bu | 3329.4 ± 521.4 | 4.48 ± 0.96 | 7.67 ± 0.51 |
| THT | 112.2 ± 5.4 | 3.19 ± 0.23 | 0.34 ± 0.01 |
| THT 1-oxide | 17405.4 ± 5548.2 | 1.98 ± 0.86 | 92.10 ± 13.47 |
| Sulfolane | 2707.8 ± 208.8 | 0.57 ± 0.07 | 54.45 ± 3.38 |
| 3-OH sulfolane | 12550.2 ± 3011.4 | 1.18 ± 0.36 | 100.68 ± 39.20 |
| Liver | |||
| Bu | 8826 ± 3820.8 | 19.36 ± 9.35 | 5.18 ± 0.31 |
| THT | 3955.8 ± 548.4 | 2.41 ± 0.45 | 17.24 ± 1.11 |
| THT-1-Oxide | – | – | – |
| Sulfolane | 6010.2 ± 264.6 | 0.32 ± 0.04 | 217.38 ± 29.79 |
| 3-OH Sulfolane | 112035.6 ± 196254 | 4.84 ± 186.99 | 94.73 ± 4.95 |
| Brain | |||
| Bu | 4579.8 ± 1366.2 | 3.85 ± 1.58 | 12.21 ± 1.64 |
| THT | – | – | – |
| THT-1-Oxide | 12539.4 ± 2170.8 | 1.17 ± 1.01 | 81.54 ± 5.61 |
| Sulfolane | 3925.8 ± 1255.8 | 1.06 ± 0.46 | 42.84 ± 8.43 |
| 3-OH Sulfolane | 3295.8 ± 709.8 | 0.65 ± 32.74 | 21.63 ± 2.34 |
| Kidneys | |||
| Bu | 1039.8 ± 352.8 | 1.22 ± 0.62 | 8.26 ± 1.31 |
| THT | – | – | – |
| THT-1-Oxide | 16959.6 ± 8016.6 | 2.12 ± 1.39 | 80.45 ± 17.97 |
| Sulfolane | 6063 ± 1397.4 | 0.96 ± 0.30 | 73.20 ± 10.79 |
| 3-OH Sulfolane | 3197.4 ± 832.8 | 1.11 ± 0.44 | 29.29 ± 3.44 |
AUC area under the curve, Bu busulphan, Cmax maximum plasma concentration, HL half-life, THT tetrahydrothiophene.
Pharmacokinetics in organs
The concentrations of Bu and its metabolites in mice livers, brains, and kidneys were measured at different time points (Fig. 2b–d, respectively, Table 2). Busulphan levels were low throughout all organs and time points. THT was detected only in the liver at low concentrations. THT-1-oxide reached peak organ concentrations at 2 h for the brain and kidneys. The mean concentration of THT-1-oxide for the brain and kidneys were 80.39 μM and 73.78 μM, respectively. Mice injected with sulfolane displayed maximum concentration peaks 30 min post-injection for all organs. The highest mean concentration of sulfolane was 73.6 μM in the liver. The highest concentration of the metabolites in the liver was 3-OH sulfolane with a mean concentration of 92.6 μM 30 min post-injection.
The largest AUC calculated was for 3-OH sulfolane in liver. In brain and kidney, the largest AUC calculated was for THT-1-oxide. Busulphan had the highest half-life in the liver and brain, while THT-1-oxide had the highest half-life in the kidneys. Cmax varied among organs, with the highest value being sulfolane in the liver, while THT-1-oxide had the highest Cmax for both brain and kidney tissues.
An important factor in assessing neurotoxicity was the distribution of Bu and its metabolites in mouse brains and plasma. Bu and sulfolane displayed the highest AUCbrain/AUCplasma ratio (Table 3).
Table 3.
Distributions between brain and plasma for busulphan and its metabolites.
| Compound | Ratio AUC Brain/AUC Plasma |
|---|---|
| Bu | 1.38 |
| THT | – |
| THT-1-Oxide | 1.07 |
| Sulfolane | 1.45 |
| 3-OH Sulfolane | 0.26 |
AUC area under the curve, Bu busulphan, THT tetrahydrothiophene.
Behavioral and neurological changes
Mice injected with Bu, THT and sulfolane showed several behavioral changes that started 10 10 min post-injection. These changes included digging, rearing, burying their head into bedding, nibbling at pieces of bedding, licking, grooming, pushing bedding and sniffing. All mice were awake in the respective corners/nests 1–1.5 h post-injection. No significant observations were recorded when mice were injected with THT-1-oxide, 3-OH sulfolane, or in both control groups. Detailed behavioral changes are listed in the supplementary data.
Myoclonic seizures were observed in 40% of the mice injected with sulfolane 20–30 min post-injection (Supplementary Video 1). One mouse injected with Bu had myoclonic seizures (partial attack) after 45 min.
In sulfolane-treated mice, a significant (P < 0.0001) decrease in calbindin-28k concentrations was observed in the brains compared to the control group. Calbindin-28k was also significantly decreased after Bu-administration for 4-days. No other significant changes were observed (Fig. 3a–e). Hypothermia was significant (P < 0.005) in mice injected with sulfolane, as well as THT-1-oxide, compared to the control group (Fig. 3f).
Fig. 3. Changes of neurotransmitters and temperature in mice.
Neurotransmitters were measured in mouse brain after i.p injection of Bu or one of its metabolites: a Dopamine, b 5-hydroxytryptamine (5-HT), c Glutamate, d GABA and e Calbindin 28 K. Temperatures were measured in mice at several time points after i.p injection of Bu or one of its metabolites; Mean ± SEM (f).
Ex vivo electrophysiology
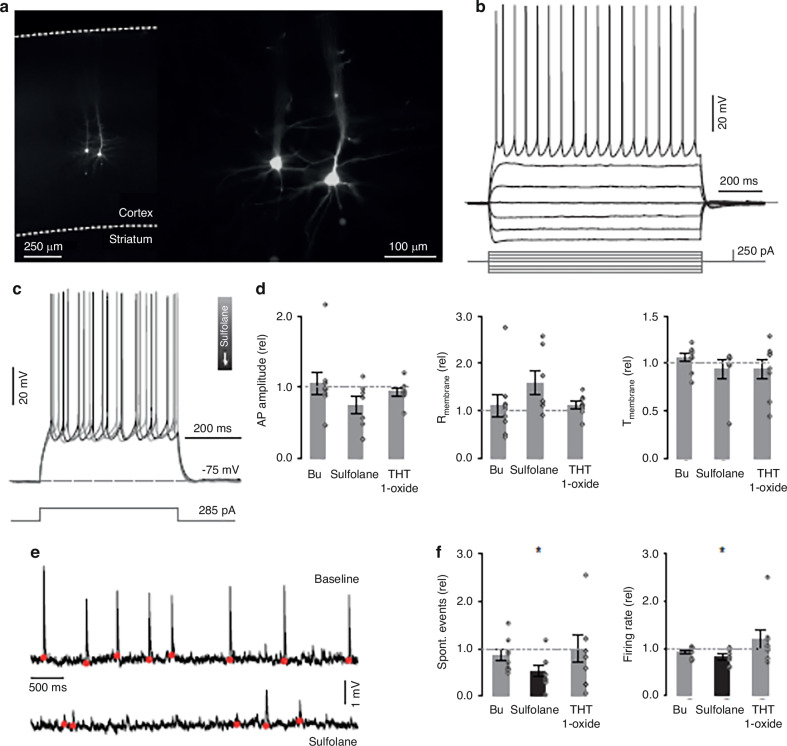
Whole-cell patch clamp recordings from pyramidal neurons in layer 5 of the somatomotor cortex were performed. Pyramidal neuron identity was confirmed by their typical I-V response (Fig. 4a) and characteristic morphology following post-hoc immunohistochemistry staining (Fig. 4b). A range of current steps and ramps was injected to probe the intrinsic electrophysiological parameters of each neuron prior to and following bath application of Bu, sulfolane and THT 1-oxide. During wash-in, a brief depolarizing current pulse was applied to monitor potential gradual changes in electrophysiological parameters (Fig. 4c).
Fig. 4. Effects of busulphan, sulfolane, and THT-1-oxide application on cortical neurons.
a Location and morphology of stained pyramidal neurons that were visualized post-hoc. b Electrophysiological parameters such as I-V relationships for each pyramidal neuron were recorded pre- and post-compound application. c The response to suprathreshold depolarizing current steps was recorded during application. d Action potential amplitude, membrane resistance, and membrane time constant of pyramidal neurons relative to baseline, following bath application of Bu, sulfolane, and THT-1-Oxide. e spontaneous input on a pyramidal neuron prior to and following the application of sulfolane. f The maximum firing rate in pyramidal neurons decreased following the application of sulfolane. Spontaneous events decreased in frequency following the application of sulfolane but not Bu or THT-1-Oxide. Error bars indicate ±SEM.
No changes in action potential amplitude, resting membrane resistance, or membrane time constant were observed following the bath application of these compounds (Fig. 4d). Only following bath application of sulfolane, a decrease in spontaneous post-synaptic events (n = 8, P = 0.036, Z = 2.1, Wilcoxon signed ranks test) (Fig. 4e, f) as well as a in maximum pyramidal neurons firing rate (n = 7, P = 0.028, Wilcoxon signed ranks test) (Fig. 4f) were observed. Neurons exposed to sulfolane exhibited altered intrinsic properties and a change in external input. This suggests sulfolane enacts physiologically observable changes in neural circuits that are exposed to this compound.
Discussion
Busulphan administration prior to HSCT is associated with various adverse effects, including seizures [21–26]. Approximately 10% of the patients without seizure prophylaxis experienced seizures that mostly occur on the second day (after 6–7 doses when Bu is administered q.i.d) [27, 28]. Convulsions were also reported to occur 18–24 h after Bu last dose [29], indicating that Bu itself might not be the cause of seizures due to its short half-life.
Historically, sulfolane, as an industrial product, was reported to induce neurotoxicity in animals, including convulsions and hypothermia [8–10, 30]. However, there are no reports regarding its toxic effect as a Bu-metabolite. Additionally, no underlying mechanism has been reported.
In the current investigation, sulfolane showed the highest concentrations in patients’ plasma, reaching the peak at the time previously reported for seizures. Bu metabolites were detectable in plasma and urine up to 72 h post last-dose. In mice, sulfolane displayed the highest AUCbrain/AUCplasma. Seizures and hypothermia were observed after sulfolane injection with a significant decrease in calbindin-28k concentrations in brain. Moreover, a decrease in spontaneous events during whole-cell patch clamp recordings from pyramidal neurons was observed following bath application of sulfolane.
Sulfolane appeared early in patients’ plasma (1 h after 1st Bu-dose). In contrast to Bu, the plasma concentrations of sulfolane increased steadily and reached maximal steady-state levels around 6–8 h after the 5th dose Bu administration, which is in the same time frame for the previously reported convulsions in patients [27, 28]. Moreover, sulfolane was detected in patients’ plasma up to 60 h after Bu last dose, which can also be correlated to the late convulsions previously reported [29]. In agreement with our findings, sulfolane, in addition to other Bu metabolites, was detected in pre-graft plasma from allogeneic HCT recipients [31].
Accordingly, sulfolane quantification can be valuable in patients, especially those at high risk for convulsions. Patients with high sulfolane levels are at high risk for seizures and those are the ones who need prophylactic treatment. In this case, there will be no need for prophylaxis administration for all patients or, at least, it can be provided as a shorter course (personalized prophylaxis). This will certainly reduce the treatment load, potential drug-drug interaction, seizure prophylaxis adverse effects in addition to the economic impact.
However, further investigation in patients was not possible, so we performed animal experiments to confirm our findings and explore the underlying mechanisms.
In mice, both THT-1-oxide and sulfolane concentrations were high in plasma and organs at early time points with relatively high AUC in both plasma and organs. Additionally, sulfolane had the highest AUCbrain/AUCplasma ratio, which might explain the convulsions’ episodes reported in animals and seen in patients.
During the behavior study, an overall decrease in movement was observed in mice injected with sulfolane during the open field tests, and burrowing was significantly decreased. These results coincide with a previous study, where neurotoxicity was observed in mice treated with sulfolane in the form of hypoactivity and convulsions [10]. Sulfolane reaches a peak concentration in the brain at the same time we observed both seizures and hypothermia (30 min), which is in agreement with the previous reports [8–10, 30]. In addition to sulfolane, THT-1-oxide had high concentrations and AUC in brain and induced hypothermia.
We and others have previously reported the involvement of CYPs in the Bu metabolic pathway [4, 5]. CYPs are expressed in the brain which can induce THT-1-oxide oxidation to sulfolane that can increase sulfolane concentrations in the brain and hence, more neurotoxicity [32, 33].
Mice injected with sulfolane had significant decrease in calbindin-28k in brain. Calbindin-28k is a major calcium-binding protein that is vital in maintaining calcium homeostasis and its decrease was previously reported to induce convulsions [34]. Interestingly, mice injected with 4-d Bu also showed a significant reduction in calbindin-28k levels which strongly suggests that sulfolane is the main cause of convulsions and indeed, several Bu-doses are required to reach steady state concentrations and induce its effect. Long-term decrease in Calbindin-28k was reported to be associated with CNS toxicity and increased susceptibility to neurodegenerative diseases. Given its crucial neuroprotective role, Calbindin-28k may serve as a biomarker for long-term neurodegeneration in patients who went HSCT as children [35, 36].
On the other hand, hypothermia can alter calcium accumulation and handling [37, 38], as well as, calbindin-28k concentrations [39]. However, further investigations are required to explore this correlation and the underlying mechanisms between calbindin-28k, convulsions, and hypothermia.
Finally, the whole-cell patch clamp test has explained the neurotoxic effect of sulfolane. Exposure to sulfolane has decreased spontaneous post-synaptic events, reduced the maximum firing rate for pyramidal neurons, and altered the neurons both intrinsic properties and external input.
From another clinical perspective, we have noticed the accumulation of Bu metabolites for several days in patients’ plasma and urine after Bu last dose. Busulphan/cyclophosphamide (Cy) are commonly used in the clinical setting for conditioning prior to HSCT, as Cy is given to the patients 24 h after the end of Bu treatment. Cyclophosphamide is a prodrug that is bioactivated via CYP2B6 and several other CYPs [40–42]. Since CYPs are also involved in the Bu metabolism [4, 5], the presence of Bu metabolites in patients’ blood during Cy treatment can alter Cy bioactivation and increase its toxicity. Additionally, the 24 h interval between Bu and Cy was reported to decrease the incidence of hepatotoxicity [43, 44] while alteration of the administration order to Cy-Bu gave the same outcome but reduced adverse effects [45–48]. Cy bioactivation is a rate-limited step that is controlled mainly by cytochrome P450 oxidoreductase (POR). It is well-known that this bioactivation is rather fast for Cy and its active metabolite (4-OH-Cy) half-life in biological fluids is 4 min only [49]. Giving Cy upfront will give the CYPs the chance to recover before Bu administration and should not alter its metabolism. Accordingly, the Bu/Cy conditioning protocols should be revised.
In conclusion, this is the first study presenting the kinetic of busulphan and its metabolites in patients’ plasma as well as in mice organs and identifying sulfolane as the main cause of neurotoxicity. Monitoring sulfolane kinetics is clinically essential to minimize its neurotoxicity considering the long-term effect of Calbindin-28k decrease. Moreover, the accumulation of the metabolites in plasma after Bu last dose might alter the kinetics of the subsequently administered drugs, which should be considered in order to increase the treatment efficacy and improve the clinical outcome.
Supplementary information
Acknowledgements
This study was supported by grants from the Cancer Research Funds of Radiumhemmet (No: 161082), the Swedish Childhood Cancer Foundation (Barncancerfonden, No: PR2017-0083), and KI Research Foundation Grants (No: 2020-01402 and 2018-02377).
Author contributions
I El-Serafi designed the experiments, participated in the investigations and data analysis, supervised some of the co-authors and wrote the original draft and the final manuscript. S Berglund designed the experiments, participated in the investigations and data analysis and wrote the original draft. F BenKessou designed the experiments, participated in the investigations and data analysis and wrote the original draft. A Codita designed the experiments, participated in the investigations and data analysis and wrote the original draft. M Saghafian designed the experiments and participated in the investigations. D Lindskog participated in the investigations and data analysis and wrote the original draft. M Dorst designed the experiments, participated in the investigations and data analysis and wrote the original draft. G Silberberg designed the experiments and supervised one of the co-authors. M Abedi-Valugerdi participated in the data analysis, supervised one of the co-authors and wrote the original draft. W Zheng participated in the investigations and data analysis and wrote the original draft. R He participated in the investigations and data analysis and wrote the original draft. M Renault participated in the investigations and data analysis. W Zhou designed the experiments, participated in the data analysis and supervised some of the co-authors. C Yu designed the experiments, participated in the data analysis and supervised some of the co-authors. M Vosough, participated in the data analysis and wrote the final manuscript. S Oerther designed the experiments, participated in the data analysis and supervised one of the co-authors. Y Zhao participated in the investigations and data analysis and wrote the original draft. J Mattsson designed the experiments, participated in the data analysis and supervised one of the co-authors. M Hassan designed the experiments, participated in the data analysis, supervised some of the co-authors and wrote the original draft and the final version.
Funding
Open access funding provided by Karolinska Institute.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ibrahim El-Serafi, Email: i.elserafi@ajman.ac.ae.
Moustapha Hassan, Email: moustapha.hassan@ki.se.
Supplementary information
The online version contains supplementary material available at 10.1038/s41409-025-02608-z.
References
- 1.Hassan M, Ehrsson H. Metabolism of 14C-busulfan in isolated perfused rat liver. Eur J Drug Metab Pharmacokinet. 1987;12:71–76. [DOI] [PubMed] [Google Scholar]
- 2.Hassan M, Ehrsson H. Urinary metabolites of busulfan in the rat. Drug Metab Dispos. 1987;15:399–402. [PubMed] [Google Scholar]
- 3.Hassan M, Ehrsson H, Wallin I, Eksborg S. Pharmacokinetic and metabolic studies of busulfan in rat plasma and brain. Eur J Drug Metab Pharmacokinet. 1988;13:301–5. [DOI] [PubMed] [Google Scholar]
- 4.Uppugunduri CR, Rezgui MA, Diaz PH, Tyagi AK, Rousseau J, Daali Y, et al. The association of cytochrome P450 genetic polymorphisms with sulfolane formation and the efficacy of a busulfan-based conditioning regimen in pediatric patients undergoing hematopoietic stem cell transplantation. Pharmacogenomics J. 2014;14:263–71. 10.1038/tpj.2013.38. [DOI] [PubMed] [Google Scholar]
- 5.El-Serafi I, Terelius Y, Abedi-Valugerdi M, Naughton S, Saghafian M, Moshfegh A, et al. Flavin-containing monooxygenase 3 (FMO3) role in busulphan metabolic pathway. PLoS One. 2017;12:e0187294. 10.1371/journal.pone.0187294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassan M, Oberg G, Ehrsson H, Ehrnebo M, Wallin I, Smedmyr B, et al. Pharmacokinetic and metabolic studies of high-dose busulphan in adults. Eur J Clin Pharmacol. 1989;36:525–30. [DOI] [PubMed] [Google Scholar]
- 7.Hassan M, Oberg G, Ericson K, Ehrsson H, Eriksson L, Ingvar M, et al. In vivo distribution of [11C]-busulfan in cynomolgus monkey and in the brain of a human patient. Cancer Chemother Pharmacol. 1992;30:81–85. [DOI] [PubMed] [Google Scholar]
- 8.Ruppert PH, Dyer RS. Acute behavioral toxicity of sulfolane: influence of hypothermia. Toxicol Lett. 1985;28:111–6. [DOI] [PubMed] [Google Scholar]
- 9.Mohler FS, Gordon CJ. Thermoregulatory responses of the rabbit to subcutaneous injection of sulfolane. Arch Toxicol. 1988;62:216–9. [DOI] [PubMed] [Google Scholar]
- 10.Andersen ME, Jones RA, Kurlansik L, Mehl RG, Jenkins LJ Jr. Sulfolane-induced convulsions in rodents. Res Commun Chem Pathol Pharm. 1976;15:571–80. [PubMed] [Google Scholar]
- 11.Eberly AL, Anderson GD, Bubalo JS, McCune JS. Optimal prevention of seizures induced by high-dose busulfan. Pharmacotherapy. 2008;28:1502–10. 10.1592/phco.28.12.1502. [DOI] [PubMed] [Google Scholar]
- 12.Vassal G, Deroussent A, Hartmann O, Challine D, Benhamou E, Valteau-Couanet D, et al. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res. 1990;50:6203–7. [PubMed] [Google Scholar]
- 13.Hassan M, Oberg G, Bjorkholm M, Wallin I, Lindgren M. Influence of prophylactic anticonvulsant therapy on high-dose busulphan kinetics. Cancer Chemother Pharmacol. 1993;33:181–6. [DOI] [PubMed] [Google Scholar]
- 14.Soni S, Skeens M, Termuhlen AM, Bajwa RP, Gross TG, Pai V. Levetiracetam for busulfan‐induced seizure prophylaxis in children undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2012;59:762–4. [DOI] [PubMed] [Google Scholar]
- 15.Tavajohi R, Shahrami B, Rostami T, Kiumarsi A, Honarmand H, Hadjibabaie M. Optimal regimen of levetiracetam for prevention of busulfan-induced seizure in patients undergoing hematopoietic stem cell transplantation: A review of available evidence. J Oncol Pharm Pr. 2023;29:927–33. 10.1177/10781552231159056. [DOI] [PubMed] [Google Scholar]
- 16.Germeraad RS, Demandt AMP, Rouhl RPW. Phenytoin as seizure prophylaxis in hematopoietic stem cell transplantation with busulfan conditioning. Front Neurol. 2022;13:928550. 10.3389/fneur.2022.928550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes K, Garrity L, Nelson AS, Lane A, Teusink-Cross A. Comparison of levetiracetam versus phenytoin/fosphenytoin for busulfan seizure prophylaxis at a pediatric institution. Pediatr Transpl. 2021;25:e14026 10.1111/petr.14026. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Rehim M, Hassan Z, Blomberg L, Hassan M. On-line derivatization utilizing solid-phase microextraction (SPME) for determination of busulphan in plasma using gas chromatography-mass spectrometry (GC-MS). Ther Drug Monit. 2003;25:400–6. [DOI] [PubMed] [Google Scholar]
- 19.Hassan M, Ehrsson H. Gas chromatographic determination of busulfan in plasma with electron-capture detection. J Chromatogr. 1983;277:374–80. [DOI] [PubMed] [Google Scholar]
- 20.El-Serafi I, Terelius Y, Twelkmeyer B, Hagbjork AL, Hassan Z, Hassan M. Gas chromatographic-mass spectrometry method for the detection of busulphan and its metabolites in plasma and urine. J Chromatogr B Anal Technol Biomed Life Sci. 2013;913-914:98–105. 10.1016/j.jchromb.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 21.McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000;39:155–65. [DOI] [PubMed] [Google Scholar]
- 22.Masson E, Zamboni WC. Pharmacokinetic optimisation of cancer chemotherapy. Effect on outcomes. Clin Pharmacokinet. 1997;32:324–43. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Liu M, Wang Q, Wang S, Liu X, Li B, et al. Risk factors for hemorrhagic cystitis in children undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. BMC Pediatr. 2024;24:333. 10.1186/s12887-024-04815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu W, Xue N, Yang H, Gao P, Guo J, Han D. Treosulfan Versus Busulfan-based Conditioning in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-analysis. J Pediatr Hematol Oncol. 2023;45:370–6. 10.1097/MPH.0000000000002735. [DOI] [PubMed] [Google Scholar]
- 25.Bardi E, Mulder RL, van Dalen EC, Bhatt NS, Ruble KA, Burgis J, et al. Late hepatic toxicity surveillance for survivors of childhood, adolescent and young adult cancer: Recommendations from the international late effects of childhood cancer guideline harmonization group. Cancer Treat Rev. 2021;100:102296. 10.1016/j.ctrv.2021.102296. [DOI] [PubMed] [Google Scholar]
- 26.El-Serafi I, Remberger M, El-Serafi A, Benkessou F, Zheng W, Martell E, et al. The effect of N-acetyl-l-cysteine (NAC) on liver toxicity and clinical outcome after hematopoietic stem cell transplantation. Sci Rep. 2018;8:8293. 10.1038/s41598-018-26033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcus RE, Goldman JM. Convulsions due to high-dose busulphan. Lancet. 1984;2:1463. [DOI] [PubMed] [Google Scholar]
- 28.De RLC, Tomas J, Figuera A, Berberana M, Fernandez-Ranada J. High dose busulfan and seizures. Bone Marrow Transplant. 1991;7:363–4. [PubMed] [Google Scholar]
- 29.Grigg AP, Shepherd JD, Phillips GL. Busulphan and phenytoin. Ann Intern Med. 1989;111:1049–50. [DOI] [PubMed] [Google Scholar]
- 30.Andersen ME, Jones RA, Mehl RG, Hill TA, Kurlansik L, Jenkins LJ Jr. The inhalation toxicity of sulfolane (tetrahydrothiophene-1,1-dioxide). Toxicol Appl Pharmacol. 1977;40:463–72. [DOI] [PubMed] [Google Scholar]
- 31.McCune JS, Navarro SL, Risler LJ, Phillips BR, Ren S, Schoch HG, et al. The presence of busulfan metabolites and pharmacometabolomics in plasma drawn immediately before allograft infusion in hematopoietic cell transplant recipients. Clin Transl Sci. 2023;16:2577–90. 10.1111/cts.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedlund E, Gustafsson JA, Warner M. Cytochrome P450 in the brain; a review. Curr Drug Metab. 2001;2:245–63. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson CS, Tyndale RF. Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends Pharmacol Sci. 2011;32:708–14. 10.1016/j.tips.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montpied P, Winsky L, Dailey JW, Jobe PC, Jacobowitz DM. Alteration in levels of expression of brain calbindin D-28k and calretinin mRNA in genetically epilepsy-prone rats. Epilepsia. 1995;36:911–21. 10.1111/j.1528-1157.1995.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 35.Geula C, Nagykery N, Wu CK, Bu J. Loss of calbindin-D28K from aging human cholinergic basal forebrain: relation to plaques and tangles. J Neuropathol Exp Neurol. 2003;62:605–16. 10.1093/jnen/62.6.605. [DOI] [PubMed] [Google Scholar]
- 36.Iacopino A, Christakos S, German D, Sonsalla PK, Altar CA. Calbindin-D28K-containing neurons in animal models of neurodegeneration: possible protection from excitotoxicity. Brain Res Mol Brain Res. 1992;13:251–61. 10.1016/0169-328x(92)90033-8. [DOI] [PubMed] [Google Scholar]
- 37.Bickler PE, Buck LT, Hansen BM. Effects of isoflurane and hypothermia on glutamate receptor-mediated calcium influx in brain slices. Anesthesiology. 1994;81:1461–9. 10.1097/00000542-199412000-00022. [DOI] [PubMed] [Google Scholar]
- 38.Kondratiev TV, Wold RM, Aasum E, Tveita T. Myocardial mechanical dysfunction and calcium overload following rewarming from experimental hypothermia in vivo. Cryobiology. 2008;56:15–21. 10.1016/j.cryobiol.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 39.George S, Scotter J, Dean JM, Bennet L, Waldvogel HJ, Guan J, et al. Induced cerebral hypothermia reduces post-hypoxic loss of phenotypic striatal neurons in preterm fetal sheep. Exp Neurol. 2007;203:137–47. 10.1016/j.expneurol.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Sladek NE. Metabolism of oxazaphosphorines. Pharm Ther. 1988;37:301–55. [DOI] [PubMed] [Google Scholar]
- 41.Hassan M, Andersson BS. Role of pharmacogenetics in busulfan/cyclophosphamide conditioning therapy prior to hematopoietic stem cell transplantation. Pharmacogenomics. 2013;14:75–87. 10.2217/pgs.12.185. [DOI] [PubMed] [Google Scholar]
- 42.Raccor BS, Claessens AJ, Dinh JC, Park JR, Hawkins DS, Thomas SS, et al. Potential contribution of cytochrome P450 2B6 to hepatic 4-hydroxycyclophosphamide formation in vitro and in vivo. Drug Metab Dispos. 2012;40:54–63. 10.1124/dmd.111.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassan M, Ljungman P, Ringden O, Hassan Z, Oberg G, Nilsson C, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transpl. 2000;25:915–24. 10.1038/sj.bmt.1702377. [DOI] [PubMed] [Google Scholar]
- 44.El-Serafi I, Remberger M, Ringden O, Torlen J, Sundin M, Bjorklund A, et al. Reduced Risk of Sinusoidal Obstruction Syndrome of the Liver after Busulfan-Cyclophosphamide Conditioning Prior to Allogeneic Hematopoietic Stem Cell Transplantation. Clin Transl Sci. 2020;13:293–300. 10.1111/cts.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadeghi B, Jansson M, Hassan Z, Mints M, Hagglund H, Abedi-Valugerdi M, et al. The effect of administration order of BU and CY on engraftment and toxicity in HSCT mouse model. Bone Marrow Transplant. 2008;41:895–904. 10.1038/sj.bmt.1705996. [DOI] [PubMed] [Google Scholar]
- 46.McCune JS, Batchelder A, Deeg HJ, Gooley T, Cole S, Phillips B, et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant. 2007;13:853–62. 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Rezvani AR, McCune JS, Storer BE, Batchelder A, Kida A, Deeg HJ, et al. Cyclophosphamide followed by intravenous targeted busulfan for allogeneic hematopoietic cell transplantation: pharmacokinetics and clinical outcomes. Biol Blood Marrow Transplant. 2013;19:1033–9. 10.1016/j.bbmt.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantoni N, Gerull S, Heim D, Halter J, Bucher C, Buser A, et al. Order of application and liver toxicity in patients given BU and CY containing conditioning regimens for allogeneic hematopoietic SCT. Bone Marrow Transpl. 2011;46:344–9. 10.1038/bmt.2010.137. [DOI] [PubMed] [Google Scholar]
- 49.El-Serafi I, Afsharian P, Moshfegh A, Hassan M, Terelius Y. Cytochrome P450 Oxidoreductase Influences CYP2B6 Activity in Cyclophosphamide Bioactivation. PLoS One. 2015;10:e0141979. 10.1371/journal.pone.0141979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request.