Abstract
Neurodermatitis and chronic eczema are characterized by severe itching and can lead to complications such as skin infections and folliculitis. Current treatment primarily involves glucocorticoid analogs which may be associated with side effects and lack of effective delivery strategies. The suspended ointment developed in this study aims to address these issues, offering improved therapeutic outcomes. The present study aimed to investigate the relationship between formulation/process variables versus the content uniformity and viscosity of neomycin sulfate and triamcinolone acetonide ointments and to explore the feasibility of using an in vitro approach to assess product sameness. Monofactor analysis was used to evaluate the impact of formulation and process variables. The new formulation was evaluated in terms of the prescription process, quality, stability, in vitro drug release behavior, and distribution of skin. The optimal prescription composition was 0.54% neomycin sulfate, 0.10% triamcinolone acetonide, 5.08% substrate A and 94.28% liquid paraffin. Quality and stability assessments confirmed that the formulation met the required standards for appearance and composition. Mathematical modeling of the drug release profile indicated that the release kinetics evaluated using the vertical diffusion cell method, closely aligned with first-order kinetics. Raman spectroscopy confirmed the successful penetration of triamcinolone acetonide into the skin, triamcinolone acetonide works primarily in the skin. Furthermore, skin irritation tests demonstrated that the formulation caused no detectable irritation. These results demonstrate that it can be a promising drug in treating neurodermatitis and chronic eczema.
Graphic Abstract
Keywords: Transdermal delivery, Suspended ointment, Neomycin sulfate, Triamcinolone acetonide, In vitro release profile, Raman spectroscopy
Introduction
In dermatology, an increasing number of semi-solid formulations for topical application are being marketed for the treatment of certain inflammatory skin diseases(Herbig et al. 2023). Neurodermatitis and chronic eczema are prevalent, long-term inflammatory skin diseases characterized by severe itching(Shahin et al. 2023), recurrent eczematous lesions, and often thickened(Silverberg 2017), rough skin with pigmentation changes(Sm et al. 2020). Both conditions exhibit high prevalence globally, substantially impacting patients'quality of life and presenting significant healthcare challenges(Tran et al. 2023; Walling and Swick 2010). Without effective treatment or management, these diseases may lead to complications such as skin infections and folliculitis(Borg et al. 2024; Zeng et al. 2023), further compromising patients'physical and mental well-being(Yew et al. 2024).
Currently, clinical treatment for this type of disease primarily involves glucocorticoid drugs(Islam et al. 2025; Prado et al. 2025; J. Zhang et al. 2025a, b; Zheng et al. 2025). However, prolonged use may lead to adverse effects, including skin atrophy and hyperpigmentation, especially in sensitive areas of the face and body(Holstroma et al. 2025; Nawan et al. 2025; Park et al. 2025; Tanaka 2025). In cases of severe atopic dermatitis, systemic immunosuppressant therapies are often used, yet long-term use can impair liver and kidney function and increase infection risk(Bautista Sanchez et al. 2025; Bhatt et al. 2025; Dabour et al. 2025). Neomycin has been widely recognized internationally in recent years, and it has been shown to have bacteriostatic activity against some drug-resistant strains at a certain minimum inhibitory concentration(Al-Jebouri 2024; Obszynski et al. 2022). Neomycin sulfate is a highly polar, hydrophilic aminoglycoside antibiotic with a molecular weight of approximately 712.72 g/mol. It is readily soluble in water (100 mg/mL) but poorly absorbed through the intact skin, which necessitates optimized formulation strategies to enhance local retention and effectiveness. Therefore, neomycin sulfate is still one of the most important antibiotics in clinical treatment(Pagkalis et al. 2011; Ye et al. 2018). Triamcinolone acetonide, a synthetic glucocorticoid, exhibits both anti-inflammatory and immunomodulatory properties(Formica et al. 2020). It is a moderately lipophilic compound with a molecular weight of 434.50 g/mol and a LogP value of approximately 2.42, favoring dermal absorption. Its crystalline nature and limited aqueous solubility (21 mg/L) necessitate its dispersion in appropriate semisolid bases for effective topical delivery. Its anti-inflammatory effects include reducing capillary permeability to alleviate congestion, inhibiting complement activation in inflammatory responses, stabilizing lysosomal membranes by suppressing phagocyte activity and blocking the release of inflammatory mediators such as kinins and histamine(Duguid et al. 1992; Heidenkummer and Kampik 1995). In addition to these anti-inflammatory actions, corticosteroids also induce vasoconstriction(Sommer et al. 1998). Clinically, triamcinolone acetonide is well-tolerated, with a low incidence of adverse effects, most of which manifest as mild local irritations that are resolved upon discontinuation(Balazic et al. 2025; Maity et al. 2025; Mardhiah et al. 2025; Yuan et al. 2025). No serious adverse events have been reported, underscoring its favorable safety profile(Serpieri et al. 2024; Shi et al. 2024). The combination of neomycin sulfate and triamcinolone acetonide can effectively prevent the factors that trigger and exacerbate the disease, and it can also compulsorily control the scratching behavior that causes lesion hyperplasia and hypertrophy(Liang et al. 2023a). The synergistic application of neomycin sulfate and triamcinolone acetonide is of high value and has some therapeutic effects as shown by the combination of existing products(Liang et al. 2023b). It is a promising undertaking to develop a semi-solid topical combined formulation of neomycin sulfate and triamcinolone acetonide for better quality.
A notable limitation of current treatment methods is the lack of effective drug delivery strategies. Following oral administration, drugs must be absorbed into the bloodstream after passing through the gastrointestinal tract, often undergoing extensive metabolism in the liver during the first-pass effect(Huang et al. 2025; Luo et al. 2025; Martínez et al. 2025; Yang et al. 2025; L. Zhang et al. 2025a, b). This process significantly reduces drug concentration, compromising efficacy. For drugs intended to target localized tissues (e.g., skin or specific lesions), the oral route may result in poor delivery efficiency. While injections can achieve rapid systemic circulation, they generally lack sustained release or precise targeting, leading to widespread distribution and potential side effects. In the case of localized lesions, such as skin lesions, injectable administration may result in suboptimal drug concentration at the target site, further limiting therapeutic efficacy(Dent et al. 2025; Feng et al. 2025; Hong et al. 2025; Prokopov et al. 2025). Neurodermatitis and chronic eczema are both inflammatory diseases with exudation, and most of them are now treated with topical preparations(Davis et al. 2024; Riva et al. 2024). Compared with other routes of administration, transdermal administration of ointments has the following advantages: (1) avoidance of first-pass effect and drug degradation in the gastrointestinal tract; (2) reduction of side effects due to systemic toxicity; (3) large absorption area; (4) slow-release or controlled-release; (5) improvement of the patient's compliance with the medication; (6) better stability of the medication; (7) better uniformity of the content of the preparation. A suspended ointment is a semisolid dosage form in which insoluble drug particles are dispersed within an ointment base. The preparation of such formulations presents several challenges, including the need for precise control of particle size to ensure uniform dispersion and consistent drug release. Stability concerns, such as particle sedimentation and aggregation, must be addressed through the incorporation of appropriate suspending agents or viscosity modifiers(Eichenauer et al. 2023). Additionally, ensuring compatibility between the drug and the ointment base is crucial to prevent drug degradation or precipitation. Optimizing the manufacturing process, particularly mixing and homogenization techniques, is essential for maintaining formulation uniformity and therapeutic efficacy. However, for semi-solid topical formulations, whether the main drug can pass through the skin smoothly and at what rate is directly related to the quality of the preparation(García-Arieta et al. 2023). It was found that the amorphous neomycin sulfate active pharmaceutical ingredient undergoes a crystalline morphology shift, partially from amorphous to crystalline(Masoud et al. 2018). This happens during the production or storage and placement of the formulation, which affects the content uniformity of the ointment(Chow et al. 2023). Therefore, it is important to determine the content uniformity of the ointment.
In this study, concerning the commercially available neomycin sulfate ointment, a new compound preparation was prepared by adding triamcinolone acetonide first. Additionally, a more comprehensive preparation quality evaluation criteria was established, and a comprehensive study was carried out on the prescription process and in vitro release of the drug in the preparation and other properties. Furthermore, the stability and content uniformity of the preparation proved to be better compared to the existing reference products.
Material and methods
Materials
The materials and reagents used in this study were as follows: Neomycin sulfate was obtained from Yichang Sanxia Pharmaceutical Co., Ltd., China, and triamcinolone acetonide was purchased from Jiangsu Ruidian Pharmaceutical Co., Ltd., China. Liquid paraffin was supplied by Xi’an Jinxiang Pharmaceutical Excipients Co., Ltd., China. Reference standards for HPLC analysis were provided by the National Institutes for Food and Drug Control, China. Healthy male ICR mice (20 ± 2 g) were supplied by Nanjing Anokang Biological Technology Co., Ltd., China. All animal care and experimental protocols strictly adhered to established ethical guidelines and received prior approval from the China Pharmaceutical University Animal Ethical Experimentation Committee.
Design of experiments and preparation of compound neomycin sulfate ointment
This study referenced commercially available formulations combining neomycin sulfate and triamcinolone acetonide, with modifications made to the original prescription. The final formulation included a single dose of 35,000 units of neomycin sulfate and 10 mg of triamcinolone acetonide. The batch size was set at 50 g, and the detailed composition included neomycin sulfate, triamcinolone acetonide, liquid paraffin, and substrate A. Substrate A, a polyethylene composite, was incorporated as an additive or modifier to enhance the ointment’s rheological properties and bioadhesion(Nikseresht et al. 2024). The optimal concentration of Substrate A was determined through screening experiments.
To prepare the formulation, the prescribed amount of liquid paraffin and Substrate A was weighed and heated until completely melted. The mixture was maintained at a constant temperature for further use. Triamcinolone acetonide was then added to the molten substrate and stirred thoroughly, maintaining the temperature throughout. Separately, the prescribed amount of neomycin sulfate was weighed and mixed with 60% liquid paraffin, first by shearing the solution at room temperature, followed by heating. The neomycin sulfate solution was combined with the triamcinolone acetonide solution, after which heating was stopped, and the mixture was cooled using circulating tap water. As the viscosity increased, the preparation was processed using a high-speed homogenizer. After homogenization, the mixture was stirred for a specified period and left to stand for 2–3 h, yielding the final product. The flowchart is shown in Fig. 1.
Fig. 1.
Preparation process of compound neomycin sulfate ointment
The formulation and process parameters were optimized through mono-factor analysis. For the formulation, key variables such as the particle size of the active pharmaceutical ingredients (APIs) and the proportion of oily substrate were systematically varied. For the process, mixing temperature, mixing time, and cooling temperature were individually adjusted. At each condition, the resulting preparation was evaluated based on its appearance and content uniformity, which served as the primary criteria for optimization. The optimal formulation was selected as the one exhibiting the best combination of uniform drug distribution and desirable physical characteristics. These evaluation criteria were chosen because content uniformity directly reflects dispersion efficiency, while appearance indicates formulation stability and patient acceptability. Specific operations are as follows. 1) Particle Size Investigation of Raw Materials: The original API was processed into different particle sizes using air-flow pulverization, and ultra-micronization. API particle size was determined three times in succession using a laser particle sizer. These APIs with varying particle sizes were incorporated into ointments to examine the impact of particle size on the ointment's appearance, viscosity, and content uniformity. 2) Optimization of Oily Substrate Quantity: To determine the optimal amount of the oily matrix, three formulation gradients were designed. 4.0616%, 5.0770%, and 6.0924% for substrate A, respectively. 3) Mixing Temperature Investigation: With other variables unchanged, temperatures of 80 °C, 90 °C, and 100 °C were tested to determine their impact on the formulation’s appearance, viscosity, and content uniformity. 4) Mixing Time Investigation: Emulsification and mixing times of 1, 2, and 3 h were tested to evaluate their impact on the appearance, viscosity, and content uniformity of the final product. 5) Cooling Temperature Investigation: Cooling temperatures of 0 °C, −10 °C, and −20 °C were examined to determine their effects on appearance, viscosity, and content uniformity.
Determination of drug and content uniformity testing
Within one week after ointment preparation and packaging, individual units of packed aluminum tubes were selected for determination and content uniformity(Dong et al. 2018; Thakkar et al. 2020). For each unit, the bottom seal was cut off and a vertical cut was made from the bottom to the top of the tube to expose the ointment drug product. The product was inspected for any changes in its physical appearance. Divide the contents into 3 equal parts (upper, middle, and lower), accurately weigh the appropriate amount, add petroleum ether, ultrasonic shake, make it completely dissolved, put it in the separation funnel, extract with pure water, and measure the content of API in each section of the ointment by HPLC.
High-performance liquid chromatography (HPLC)
The HPLC system consisted of an Agilent 1260 Series equipped with a degasser, binary solvent pump, autosampler, temperature-controlled column compartment, and a diode array detector. The current method of neomycin sulfate employed a chromatographic column with octadecylsilane-bonded silica gel as the filler. The column temperature was maintained at 25 °C. The mobile phase consisting of a 2% trifluoroacetic acid solution (0.01% pentafluoropropionic acid + 0.6% sodium hydroxide) was pumped at a flow rate of 0.8 mL/min. A sample volume of 25 μL was injected into the column. Detection was carried out by an integrating pulse amperometric electrochemical detector with a gold electrode (3 mm diameter recommended) as the detection electrode, an Ag/AgCl composite electrode as the reference electrode, a titanium alloy counter electrode, and post-column alkaline solution; 50% NaOH solution with a recommended flow rate of alkaline solution of 0.3 mL/min. The current method of triamcinolone acetonide employed chromatographic columns packed with octadecylsilane-bonded silica gel (Agilent Eclipse Plus C18, 4.6 × 150 mm, 5 μm). The column temperature was maintained at 30 °C. The mobile phase consisting of methanol: water (60:40, v/v) was pumped at a flow rate of 1 mL/min. A sample volume of 20 μL was injected into the column and the triamcinolone acetonide was detected at 240 nm.
Stability of compound neomycin sulfate ointment
Influencing Factor Test: Light and moisture significantly affect the quality of the preparation over time, especially because each preparation is exposed to these factors after being opened(Ubhe et al. 2024). To address this, samples were filled into high-density aluminum tubes to simulate practical conditions. The samples were stored under high-temperature conditions (40 °C), high-humidity conditions (75% RH), and light exposure (4500 lx ± 500 lx) for 30 days to evaluate the potential impacts on product quality.
Accelerated Stability Test: Three batches of the preparation were sealed in aluminum tubes and stored in a constant temperature and humidity chamber at 30 °C ± 2 °C/65% RH ± 5% RH. Samples were taken after one, two, and three months(Lacassia et al. 2024). Key properties, including appearance, viscosity, and content were analyzed to assess stability over time.
Long-term Stability Test: The same three batches were also sealed in aluminum tubes and stored under long-term conditions at 25 °C ± 2 °C/60% RH ± 5% RH. Samples were collected in March to evaluate appearance and content, ensuring the formulation's stability under typical storage conditions(Padamwar and Pokharkar 2006).
Rheological characterization
Rheological behaviors of ointments were evaluated using a hybrid rheometer (Discovery HR-10) equipped with a Peltier stage (25 °C) and a fixture (40 mm). For each test, approximately 2 mL of ointment sample was placed on the lower plate before slowly lowering the upper plate to the preset trimming gap. After trimming excessive material, the following procedures were performed in sequence on each sample to characterize ointment rheological behavior: 1) The stress and viscosity change with shear rate (0.1–1000 s⁻1) was recorded at 25 °C using a 40 mm fixture. 2) The energy storage modulus (G') and loss modulus (G") are evaluated at a fixed frequency of 10 rad/s and a strain range of 0.1% to 1000%. 3) The shear strain was fixed at 2.0%, the angular frequency range was set to 0.1 rad/S-100 rad/s, and the curve of energy storage modulus and loss modulus with frequency was recorded. 4) The sample was scanned between 25 °C and 45 °C at a fixed strain of 2% and angular frequency of 10 rad/s. Changes in the energy storage modulus (G') and loss modulus (G'') were monitored to determine the fluid's temperature sensitivity(Andjic et al. 2024).
In vitro release testing (IVRT)
The vertical diffusion cell (VDC) used in this study had a volume of 8 mL and a release area of 2.2 cm2, and the formulation was uniformly applied to the artificial membrane using a spatula. The temperature was maintained at (32.0 ± 0.5) °C with 600 rpm magnetic stirring during the experiment, and 2 mL of samples were taken at 1, 2, 4, 6, 10, 12, 24, 36, and 48 h. 2 mL of the blank acceptor medium was added. The sample solution was filtered through a 0.45 μm organic filter membrane, and 1 mL of the filtrate was discarded as the test solution. Control solution: dry neomycin sulfate/triamcinolone acetonide 105℃ for 3 h, take about 7 mg, weigh precisely, put in a 50 mL volumetric flask, add the appropriate amount of acceptance medium to dissolve, then dilute to the scale, shake well as a control solution. According to Liquid chromatographic conditions used before, inject the control solution and test solution into HPLC, calculate the concentration of the drug by peak area (A) according to the external standard method, and calculate the cumulative release of the drug. The amount of drug released per unit area can also be calculated according to the following formula(Siepmann and Peppas 2011).
Qn (µg·cm−2) is the cumulative amount of drug released per unit area at time (n); Cn (µg·cm−3) is the concentration of drug in the receiving medium at different sampling times (n); Vs is the sample volume (cm3); Vc is the receiving chamber volume; and Ac is the effective diffusion area (cm2).
The amount of drug released per unit area was plotted against the square root of time, and the slope of the straight line obtained by linear fitting was the release rate.
When neomycin sulfate was released in vitro by the vertical diffusion cell method, a phosphate buffer of pH 5.8 was chosen as the acceptor medium, and 3.0 μm polyethersulfone membranes were selected for subsequent in vitro release studies.
When triamcinolone acetonide was released in vitro by vertical diffusion cell method, ethanol–water (30:70) was chosen as the acceptor medium, and 3 μm polyethersulfone membranes were selected for subsequent in vitro release assay studies.
Distribution of skin
Raman spectroscopic determination of APIs and blank excipients was carried out to determine the assay conditions, followed by Raman spectroscopic studies on skin (mouse skin).
Neomycin Sulfate Raman Spectroscopy: An appropriate amount of Neomycin Sulfate API was placed on a slide and evenly spread. The experimental conditions were as follows: laser wavelength of 532 nm, exposure time of 0.1 s (10 Hz), 200 cumulative scans, laser power of 8.0 mv, 50X objective lens, step size of 3.0 μm, and depth of 12 μm. The Raman spectrum of Neomycin Sulfate API was measured six times(Lu et al. 2024).
Triamcinolone Acetonide Raman Spectroscopy: A suitable amount of Triamcinolone Acetonide API was placed on a slide and evenly spread. The experimental parameters included a laser wavelength of 532 nm, exposure time of 0.1 s (10 Hz), 200 cumulative scans, laser power of 8.0 mv, 50X objective lens, step size of 3.0 μm, and depth of 12 μm. Each sample was measured six times to obtain the Raman spectrum of Triamcinolone Acetonide API.
Raman Spectroscopy of Blank Excipients: An appropriate amount of the blank excipient mixture was placed on a slide and spread evenly. The instrumental conditions were identical to the API scans: laser wavelength of 532 nm, exposure time of 0.1 s (10 Hz), 200 cumulative scans, laser power of 8.0 mv, 50X objective lens, step size of 3.0 μm, and depth of 12 μm. Six measurements were taken for each sample.
Raman Spectroscopic Study of Mouse Skin: A confocal Raman imaging microscope (Thermo DXR3xi, U.S.A.) was used for skin measurements. Experimental parameters included a laser wavelength of 532 nm, exposure time of 0.1 s, 100 cumulative scans, laser power of 8.0 mv, and 50X magnification. Two-dimensional scanning was performed along the depth of the XZ plane with a step size of 12 μm, while the scanning depth along the XY plane was 90 μm(Ohashi et al. 2022).
Mouse skin samples were collected from the abdominal area, stored at −20 °C, and thawed in saline for 10 min before use. Skin slices measuring 2 cm × 2 cm were prepared and cleaned to remove dirt and subcutaneous fat. The samples were placed in petri dishes, washed with saline, and subjected to three in vitro transdermal experiments conducted simultaneously(Fujii et al. 2019). Skin samples were removed at 6, 12, and 24 h, respectively, for Raman spectroscopic analysis.
Healthy rats were randomly assigned to three experimental groups: the compound neomycin sulfate ointment group, the blank excipient group, and the blank saline group. Hair on both sides of the spine was removed 24 h before the experiment, ensuring the skin remained intact. For self-control, the depilated area was divided into three sections. In one section, the epidermal layer was intentionally disrupted using a sterilized sharp needle, followed by the application of compound neomycin sulfate ointment. The other two sections received equal amounts of blank excipient and saline, respectively, each covering an area of 3 cm × 3 cm. The applied materials were secured using non-irritating medical adhesive tape. After 72 h, skin reactions were assessed, including erythema, edema, hyperpigmentation, hemorrhage, and skin roughness, along with the time of onset and resolution(Bajgai et al. 2021). At the end of the observation period, skin samples were excised and fixed in 10% neutral buffered formalin. Paraffin sections were then prepared, stained with hematoxylin and eosin (H&E), and examined under a light microscope. The skin irritation response was scored according to Table 1 and Table 2.
Table 1.
Skin irritation scale
| Stimulus response | Score |
|---|---|
| No erythema, no edema | 0 |
| Erythema and edema are barely visible | 2 |
| Erythema is visible, with an edematous margin higher than the surrounding skin | 4 |
| Erythema is moderate to severe, edema is elevated by about 1 mm and well-defined | 6 |
| Purple-red spots with crust formation, edema elevated 1 mm or more and expanded | 8 |
Table 2.
Evaluation Criteria for Skin Irritation Intensity
| Evaluation of irritation | Score |
|---|---|
| Non-irritation | 0.00–0.49 |
| Mildly irritation | 0.50–2.99 |
| Moderate irritation | 3.00–5.99 |
| Intense stimulation | 6.00–8.00 |
Statistical analysis
The data collected for studies were analyzed using Origin software determining the impact of formulation and process variables. The data were also analyzed for significant differences. One asterisk: p-value less than 0.05, a statistically significant difference. Two asterisks: p-value less than 0.01, a very significant difference. Three asterisks: p-value less than 0.001, the difference is very, very significant.
Results
Determination of drug and content uniformity testing
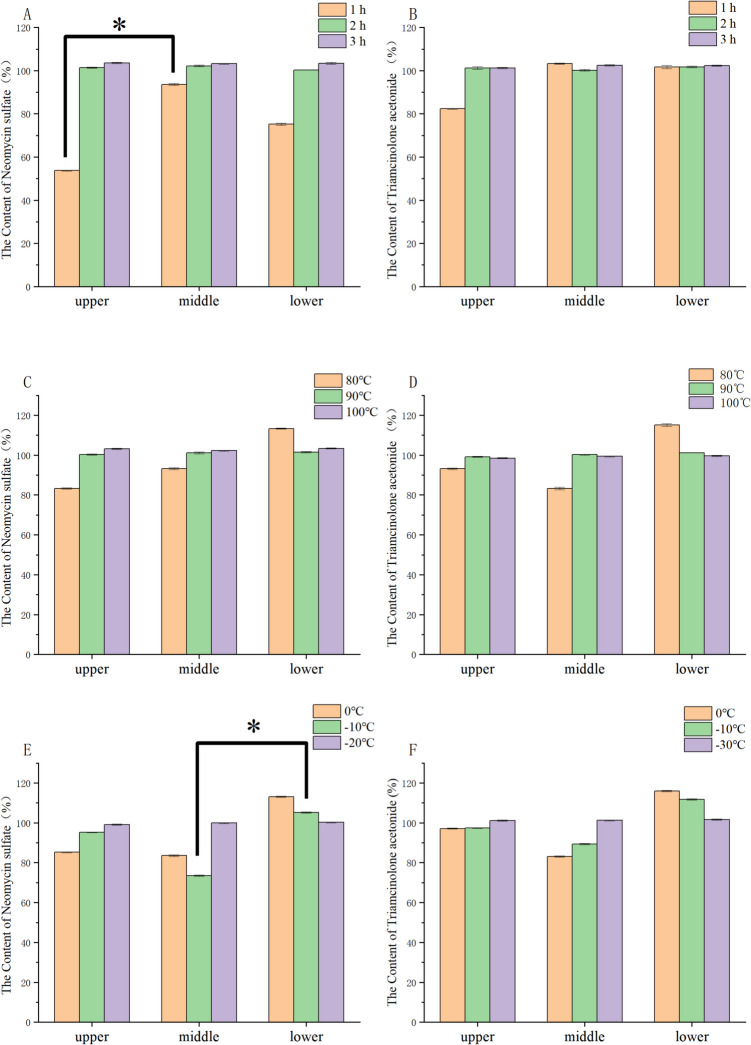
The results of three API particle size determinations are shown in Table 3. The preparation using the original API exhibited a poor appearance, with the larger particle-sized API (D50 = 31.29 μm) and the oily substrate remaining dispersed independently within the ointment. The content uniformity results indicated that the original API did not adequately combine with the oily substrate to form a uniform suspension. As shown in Fig. 2A, the micronized and airflow-pulverized neomycin sulfate met the requirements for homogeneous suspension, resulting in a more uniform and refined formulation with improved content uniformity. As shown in Fig. 2B, for triamcinolone acetonide, due to its low concentration in the formulation, only the ultra-fine powdered API (D50 = 4.496 μm) ensured content uniformity that met the required standards.
Table 3.
Particle size distribution results for two APIs
| Drug | D(μm) | Original API | Air-flow pulverization | Ultra-micronization |
|---|---|---|---|---|
| Neomycin sulfate | D10 | 6.530 ± 0.031 | 5.580 ± 0.025 | 5.110 ± 0.021 |
| D50 | 31.29 ± 0.042 | 16.04 ± 0.027 | 11.90 ± 0.028 | |
| D90 | 84.68 ± 0.025 | 42.74 ± 0.045 | 26.38 ± 0.032 | |
| Triamcinolone acetonide | D10 | 5.421 ± 0.024 | 2.192 ± 0.032 | 1.269 ± 0.034 |
| D50 | 6.342 ± 0.015 | 5.455 ± 0.036 | 4.496 ± 0.019 | |
| D90 | 11.379 ± 0.02 | 10.590 ± 0.032 | 7.079 ± 0.026 |
Fig. 2.
Results of content uniformity: (A) Effect of particle size on content uniformity of neomycin sulfate; (B) Effect of particle size on content uniformity of triamcinolone acetonide; (C) Effect of the amount of substrate A on neomycin sulfate content uniformity; (D) Effect of the amount of substrate A on triamcinolone acetonide content uniformity. Note: * p < 0.05; **p < 0.01; ***p < 0.001
The evaluation results were summarized in Fig. 2C and 2D. There was a significant effect on content uniformity when the substrate A content was 4.0616%. Additionally, overly thin formulations failed to maintain uniform API suspension, resulting in particle sedimentation. The quality standard was best met when the content of substrate A was 5.0770%.
The duration of mixing affects particle dispersion and viscosity. When the mixing time was 0.5 h, large particles and low viscosity were visible indicating incomplete drug dispersion. As shown in Figs. 3A and 3B, the particle size became uniform and content uniformity met the required standards at 2 h. Thus, 2 h was selected as the optimal mixing time.
Fig. 3.
Results of content uniformity: (A) Effect of mixing time on content uniformity of neomycin sulfate; (B) Effect of mixing time on content uniformity of triamcinolone acetonide; (C) Effect of mixing temperature on content uniformity of neomycin sulfate; (D) Effect of mixing temperature on content uniformity of triamcinolone acetonide; (E) Effect of cooling temperature on content uniformity of neomycin sulfate; (F) Effect of cooling temperature on content uniformity of triamcinolone acetonide. Note: * p < 0.05; **p < 0.01; ***p < 0.001
The temperature required to emulsify Substrate A impacts the quality of the ointment. When the emulsification temperature was below 80 °C, the substrate did not fully melt, resulting in an uneven appearance and large agglomerates. At 90 °C, the mixing state improved, yielding better appearance and content uniformity, similar to results at 100 °C. However, considering energy consumption and equipment constraints, a temperature of 90 °C was selected for the emulsification process. Content uniformity results are shown in Fig. 3C and 3D.
Cooling temperature also affects content uniformity. At −20 °C, the viscosity reached 72,500 cP, meeting formulation requirements. The content uniformity results are shown in Figs. 3E and 3F. Therefore, −20 °C was chosen as the optimal cooling temperature.
Under the same preparation process, formulations with smaller API particle sizes exhibited higher viscosity as shown in Table 3. As shown in Fig. 4A, the quantity of the oily matrix significantly influenced the viscosity of the ointment. Excessive viscosity compromised extrudability, while insufficient viscosity increased fluidity, reducing the product’s ability to adhere to the skin(Chen et al. 2019). As shown in Fig. 4D, mixing time has little effect on viscosity. As shown in Fig. 4E, the higher the mixing temperature, the higher the viscosity. The results of viscosity are provided in Fig. 4F. At 0 °C and −10 °C, the viscosity increase was minimal, resulting in high fluidity and poor consistency.
Fig. 4.
Results of viscosity: (A) Effect of neomycin sulfate particle size on viscosity; (B) Effect of triamcinolone acetonide particle size on viscosity; (C) Effect of substrate A content on viscosity; (D) Effect of mixing time content on viscosity; (E) Effect of mixing temperature content on viscosity; (F) Effect of cooling temperature content on viscosity. Note: * p < 0.05; **p < 0.01; ***p < 0.001
After experimental optimization, the final formulation was determined to contain 0.54% neomycin sulfate, 0.10% triamcinolone acetonide, 5.08% substrate A, and 94.28% liquid paraffin.
Stability of compound neomycin sulfate ointment
The results of the influencing factor test, accelerated stability test and long-term stability test are shown in Tables 4, 5, and 6. Under long-term experimental conditions, the preparation was stable for 3 months under the condition of 25℃ ± 2℃/60%RH ± 5%RH.
Table 4.
Results of influencing factor test
| Conditions | The content of neomycin sulfate (%) | The content of triamcinolone acetonide (%) | ||
|---|---|---|---|---|
| 10 days | 30 days | 10 days | 30 days | |
| High temperature 40℃ | 101.75 | 100.18 | 98.05 | 98.18 |
| High humidity 75%RH | 101.03 | 100.30 | 99.83 | 99.30 |
| Illumination(4500 lx ± 500 lx) | 101.84 | 101.60 | 100.54 | 100.21 |
Table 5.
Results of the acceleration test
| Sample | Time | The content of neomycin sulfate (%) | The content of triamcinolone acetonide (%) |
|---|---|---|---|
| Sample 1 | 0 day | 102.18 | 99.58 |
| 1 month | 101.58 | 99.18 | |
| 2 months | 101.77 | 98.87 | |
| 3 months | 105.12 | 98.52 | |
| Sample 2 | 0 day | 102.16 | 101.16 |
| 1 month | 102.03 | 99.53 | |
| 2 months | 100.57 | 100.97 | |
| 3 months | 100.95 | 99.95 | |
| Sample 3 | 0 day | 104.50 | 99.50 |
| 1 month | 104.07 | 99.07 | |
| 2 months | 104.06 | 98.86 | |
| 3 months | 103.39 | 98.79 |
Table 6.
Results of long-term stability test
| Sample | Time | The content of neomycin sulfate (%) | The content of triamcinolone acetonide (%) |
|---|---|---|---|
| Sample 1 | 0 day | 103.18 | 99.18 |
| 3 months | 102.75 | 98.75 | |
| Sample 2 | 0 day | 101.16 | 100.16 |
| 3 months | 101.79 | 99.79 | |
| Sample 3 | 0 day | 104.50 | 101.21 |
| 3 months | 103.34 | 101.54 |
Rheological characterization
The results of the rheological evaluation are as follows. The fluid properties of the formulations were analyzed in the study, with the relationships between viscosity and shear stress as a function of shear rate illustrated in Fig. 5A and 5B. At 25 °C, the viscosity of the ointments decreased with increasing shear rate, while shear stress increased proportionally, exhibiting typical pseudoplastic (shear-thinning) behavior. This shear-thinning characteristic facilitates easy extrusion of the ointment from aluminum tube packaging. Additionally, the friction generated during manual application enhances spreading across mucosal surfaces, increases the contact area with the skin, and improves adhesion(Alawdi and Solanki 2021). As shown in Fig. 5C, with a fixed shear strain of 2.0% and an angular frequency range of 0.1–100 rad/s, the storage modulus (G') remained higher than the loss modulus (G'') at 25 °C, confirming the solid-like behavior of the formulations. The moduli showed minimal variation with increasing frequency, suggesting that the combination of the active pharmaceutical ingredient (API) and the oily matrix yields a highly stable system. The linear viscoelastic region results are shown in Fig. 5D, within a specific strain range, the storage modulus (G') and loss modulus (G'') remained stable, with G'consistently higher than G''. This indicates that the sample is in a stable viscoelastic state, defining the linear viscoelastic region of the product. As shown in Fig. 5E, when subjected to 2% strain at an angular frequency of 10 rad/s over 60 s at 25 °C, both the storage modulus (G') and loss modulus (G'') remained constant, indicating structural stability over time. Furthermore, when the temperature was gradually increased from 5 °C to 60 °C, no significant changes were observed in the moduli, with G'consistently greater than G''. The results of the oscillation time scan are shown in Fig. 5F. This confirms that the formulation is not temperature-sensitive, maintaining stable performance across the tested temperature range.
Fig. 5.
Results of in vitro evaluation: (A) stress-shear rate curve; (B) viscosity-shear rate curve; (C) Modulus-Frequency Curve; (D) Linear viscoelastic region; (E) Temperature sweep; (F) Oscillation time scan
In vitro release testing (IVRT)
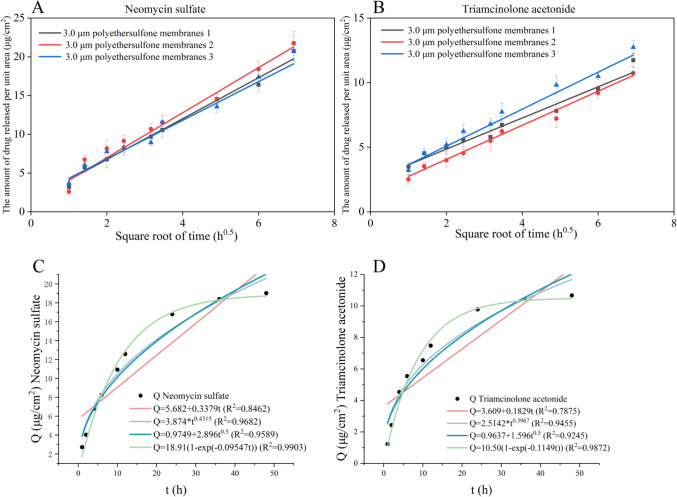
Through the establishment of the in vitro release method, the screened membrane material as well as the membrane pore size were used for the in vitro release test of the formulation (n = 3), and the results are shown in Figs. 6A and 6B.
Fig. 6.
(A) Linear regression of in vitro release of neomycin sulfate; (B) Linear regression of in vitro release of triamcinolone acetonide; (C) The fitting results of neomycin sulfate; (D) The fitting results of triamcinolone acetonide
The cumulative release profiles of the formulations were put to zero, one, Higuchi and Peppas release equations. The fitting results are shown in Figs. 6C and 6D. The preparation has a first-order release rule, indicating that the drug delivery system is formed by substrate A and liquid paraffin. The active drugs, neomycin sulfate, and triamcinolone sulfate are released from the mechanism mainly in the form of diffusion, and the regression equation has a good correlation.
The release kinetics of formulations under different IVRT methods were studied using the four release models with the following equations.
Zero-order equation:
| 1 |
First-order equation:
| 2 |
Higuchi equation:
| 3 |
Ritger-Peppas equation:
| 4 |
Distribution of skin
The Raman profiles of neomycin sulfate API, triamcinolone acetonide, and blank excipient are shown in Figs. 7A, 7B, and 7C. The signal-to-noise ratio (S/N) of the Raman spectrum for neomycin sulfate was 45.3, which did not meet the qualitative requirements of Raman spectroscopy(Orlando et al. 2021). The S/N ratio of triamcinolone acetonide was 249.3, indicating strong Raman absorption that meets the requirements for Raman characterization. The absorption peak at 1654 nm was selected as the characteristic peak for triamcinolone acetonide in subsequent skin permeation studies using Raman spectroscopy. The S/N ratio of the blank excipient spectrum was also 45.3, with poor Raman absorption intensity. However, its absorption did not interfere with the characteristic peaks of triamcinolone acetonide(Roozeboom et al. 1983).
Fig. 7.
Results of distribution of skin: (A) Raman profiles of neomycin sulfate API; (B) Raman profiles of triamcinolone acetonide; (C) Raman profiles of blank excipient; (D): Raman profiles of triamcinolone acetonide in vitro transdermal 6 h, 12 h and 24 h (from left to right) (E) Staining results of the preparation group; (F) Staining results of the blank excipient group; (G) Staining results of the saline group
In summary, due to weak Raman absorption, neomycin sulfate is not suitable for studying intradermal drug penetration and retention via Raman spectroscopy, necessitating the use of the tape-stripping method for further investigation(Rowe et al. 2024). In contrast, triamcinolone acetonide exhibits a strong absorption peak at 1654 nm, which is not affected by the blank excipients, making it suitable for analysis via confocal Raman spectroscopy.
The penetration of triamcinolone into mouse skin at different time points, as observed under a Raman microscope, was shown in Fig. 7D. The results indicated that triamcinolone penetration progresses deeper into the skin over time, following a time-dependent pattern. Measurements taken at 6, 12, and 24 h, along with the retention time of the topical preparation in the skin, suggested that the drug primarily exerts its therapeutic effect within the skin(Suzuki et al. 2014).
Skin irritation intensity assessment scores are as follows, the score of the preparation group is 0.21, the score of the blank excipient group is 0.17, and the score of the saline group is 0.31, the results of the scores showed none of them to be irritating. The results of hematoxylin and eosin (H&E) staining were presented in Figs. 7E, 7 F, and 7G. The images revealed that the skin microstructure in the preparation group was comparable to that of the blank excipient group and the saline group, with all samples exhibiting intact structures. No damage to skin appendages was observed, and the epithelium showed no signs of edema or hyperplasia(Rodríguez-Luna et al. 2018). The basal layer cells were neatly arranged, maintaining intact cellular structures, and there was no evidence of inflammatory cell infiltration in the epithelium or subcutaneous tissues. These findings indicated that the preparation does not induce skin irritation.
Discussion
The drug content analysis demonstrated that API particle size, substrate concentration, and processing conditions collectively influenced the ointment's uniformity and rheological properties. Larger neomycin sulfate particles (D50 = 31.29 μm) resulted in poor dispersion and phase separation, whereas micronized APIs produced homogeneous suspensions. In particular, ultra-fine triamcinolone acetonide (D50 = 4.496 μm) met uniformity requirements due to its low concentration. The optimal substrate A concentration was identified as 5.0770%, as lower levels led to sedimentation. A mixing time of 2 h ensured uniform particle dispersion, while shorter times resulted in incomplete mixing. An emulsification temperature of 90 °C enabled effective substrate melting without excessive energy input, and subsequent cooling at −20 °C improved both viscosity and uniformity. Moreover, smaller API particle sizes were associated with increased formulation viscosity, which was also modulated by substrate concentration and cooling temperature, whereas mixing time had minimal impact. Overall, appropriate control of API particle size and processing parameters ensured the formulation’s stability, uniformity, and desirable application characteristics. The results of the stability test indicate that the preparations are stable under high temperatures, high humidity, and light conditions. The formulation did not show any significant change in all the items examined during the accelerated test for 3 months, and the formulation using an aluminum tube package had good stability during the accelerated test for 3 months.
In ointment formulations, the rheological properties of the product must be carefully evaluated concerning their interaction with human skin(Bao and Burgess 2018). Key factors such as the active ingredient, drug concentration, dispersion state, droplet size, and distribution significantly influence the product’s extrusion behavior, application feel, and absorption efficacy(Das et al. 2016). In pharmaceutical applications, the rheological characteristics of topical creams are essential for quality control(Davies and Amin 2020). The formulations exhibited typical pseudoplastic (shear-thinning) behavior, characterized by decreasing viscosity and increasing shear stress with rising shear rates, facilitating ease of application and enhanced skin adhesion. Oscillatory rheological analysis revealed solid-like behavior, as evidenced by storage modulus (G') consistently exceeding loss modulus (G'') across all tested frequencies, indicating strong structural stability. The linear viscoelastic region further confirmed that both G'and G''remained stable under applied strain. Additionally, time and temperature sweep tests demonstrated that the formulations maintained stable rheological properties over time and across a temperature range of 5 °C to 60 °C, confirming excellent thermal resistance and suitability for topical application.
Drug release from semi-solid formulations is typically governed by a combination of factors, including the properties of the API—such as crystallinity, solubility, and particle size—along with the rheological characteristics of the semi-solid matrix and the conditions under which release occurs(Patere et al. 2020). Consequently, release kinetics may vary under different conditions. In the case of the formulations, the release profiles of neomycin sulfate and trimethoprim, measured using the VDC method were consistent with a first-order release model. The suspension ointment, a slow-release semi-solid formulation likely exhibits drug release and is primarily controlled by the absorption of the solid drug which is directly proportional to the amount of drug remaining in the substrate(Ueda et al. 2010).
The Raman spectroscopy results indicated that neomycin sulfate, due to its low signal-to-noise ratio (S/N = 45.3), is unsuitable for evaluating skin penetration using this technique, necessitating the use of tape-stripping for further assessment(Jeong et al. 2021; Jonsdottir et al. 2022). In contrast, triamcinolone acetonide exhibited a strong Raman signal (S/N = 249.3) with a distinct characteristic peak at 1654 nm, which was not affected by the blank excipient. Therefore, Raman spectroscopy is appropriate for investigating the skin permeation of triamcinolone acetonide. Raman imaging further revealed that triamcinolone acetonide penetrated deeper into mouse skin in a time-dependent manner, suggesting that its primary therapeutic action is localized within the skin layers. Additionally, skin irritation tests demonstrated low irritation scores in all groups, with no observable signs of erythema or edema. Histological analysis confirmed the integrity of the skin structure, with no evidence of edema, hyperplasia, or inflammation. Collectively, these findings indicate that the formulation is non-irritating and safe for topical application.
Conclusions
This study developed a novel compounded formulation by incorporating the API into an oily substrate. The formulation was systematically evaluated for its prescription screening process, quality, stability, in vitro drug release, and skin irritation potential. The results demonstrated significant improvements in content uniformity and API stability. Transdermal administration enabled a systemic therapeutic effect while reducing toxic side effects and enhancing the drug's bioavailability. Drug penetration was assessed using Raman spectroscopy, focusing on triamcinolone acetonide permeation at different time points. Most importantly, the results of this study have confirmed that formulation-associated changes in product performance can be evaluated by using a battery of in vitro methods. Hence, by formulating a neomycin sulfate and triamcinolone acetonide suspended ointment and developing an in vitro evaluation method, this study demonstrated the ointment's potential as a therapeutic option for restrictive neurodermatitis and chronic eczema through assessments of in vitro release and skin distribution.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Conceptualization, Jiong Wu, Qian Tang and Hongxiu Zhang; methodology, Jiong Wu and Qian Tang; formal analysis, Jiong Wu, Ruixi Liao and Xufei Zhao; investigation, Jiong Wu, Ruixi Liao and Hongxiu Zhang; validation, Xufei Zhao and Aiping Shi; writing-original draft, Jiong Wu and Qian Tang; writing-review and editing, Yan Shen, and Aiping Shi; supervision, Hongxiu Zhang and Qian Tang; funding acquisition, Yan Shen, and Aiping Shi; project administration, Jiong Wu, Qian Tang, Hongxiu Zhang and Yan Shen. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Innovation and Entrepreneurship Training Program of China Pharmaceutical University(202410316179).
Data Availability
Data available on request from the authors.
Declarations
Ethics
Not applicable.
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Aiping Shi is the first corresponding author on the article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiong Wu, Qian Tang these authors are contributed equally.
Contributor Information
Hongxiu Zhang, Email: zhang_hongxiu@163.com.
XiuJuan Feng, Email: kingdunfxj@126.com.
Aiping Shi, Email: shiaiping@txsrmyy.com.
References
- Alawdi, S., Solanki, A.B., 2021. Mucoadhesive Drug Delivery Systems: A Review of Recent Developments. JSRMBS 2, 50–64. 10.47631/jsrmbs.v2i1.213
- Al-Jebouri, M.M., 2024. Impact of Sublethal Disinfectant Exposure on Antibiotic Resistance Patterns of Pseudomonas aeruginosa. Med Princ Pract 1–15. 10.1159/000542322 [DOI] [PMC free article] [PubMed]
- Andjic M, Bradic J, Kocovic A, Simic M, Krstonosic V, Capo I, Jakovljevic V, Lazarevic N (2024) Immortelle Essential-Oil-Enriched Hydrogel for Diabetic Wound Repair: Development, Characterization, and In Vivo Efficacy Assessment. Pharmaceutics 16:1309. 10.3390/pharmaceutics16101309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgai J, Xingyu J, Fadriquela A, Begum R, Kim DH, Kim C-S, Kim S-K, Lee K-J (2021) Effects of mineral complex material treatment on 2,4- dinitrochlorobenzene-induced atopic dermatitis like-skin lesions in mice model. BMC Complement Med Ther 21:82. 10.1186/s12906-021-03259-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazic, E., Konisky, H., Axler, E., Kobets, K., 2025. Concurrent Lichen Planopilaris and Female Androgenic Alopecia in Skin of Color: A Case Series. J Drugs Dermatol 24, 320–322. 10.36849/JDD.7318 [DOI] [PubMed]
- Bao Q, Burgess DJ (2018) Perspectives on Physicochemical and In Vitro Profiling of Ophthalmic Ointments. Pharm Res 35:234. 10.1007/s11095-018-2513-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista Sanchez R, Khader Y, Khanna D (2025) Management of cutaneous manifestations of systemic sclerosis: current approaches and emerging therapies. Curr Opin Rheumatol. 10.1097/BOR.0000000000001082 [DOI] [PubMed] [Google Scholar]
- Bhatt A, Gupta P, Furie R, Vashistha H (2025) A focused report on IFN-1 targeted therapies for lupus erythematosus. Expert Opin Investig Drugs. 10.1080/13543784.2025.2473060 [DOI] [PubMed] [Google Scholar]
- Borg E, Munro D, Thoning H (2024) The management of Chronic Hand Eczema: A retrospective patient record review. Contact Dermatitis 90:365–371. 10.1111/cod.14477 [DOI] [PubMed] [Google Scholar]
- Chen Y, Chaves Figueiredo S, Yalçinkaya Ç, Çopuroğlu O, Veer F, Schlangen E (2019) The Effect of Viscosity-Modifying Admixture on the Extrudability of Limestone and Calcined Clay-Based Cementitious Material for Extrusion-Based 3D Concrete Printing. Materials 12:1374. 10.3390/ma12091374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow PS, Lim RTY, Cyriac F, Shah JC, Badruddoza AZM, Yeoh T, Yagnik CK, Tee XY, Wong ABH, Chia VD, Wang G (2023) Influence of Manufacturing Process on the Microstructure, Stability, and Sensorial Properties of a Topical Ointment Formulation. Pharmaceutics 15:2219. 10.3390/pharmaceutics15092219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabour S-E, Elbenni A, Abdel-Megid AM, Alorfali W (2025) Systemic Lupus Erythematosus and Dermatomyositis Overlap Syndrome: Diagnostic Challenges and Management Insights. Cureus 17:e78424. 10.7759/cureus.78424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U, Behera SS, Singh S, Rizvi SI, Singh AK (2016) Progress in the Development and Applicability of Potential Medicinal Plant Extract-Conjugated Polymeric Constructs for Wound Healing and Tissue Regeneration. Phytother Res 30:1895–1904. 10.1002/ptr.5700 [DOI] [PubMed] [Google Scholar]
- Davies A, Amin S (2020) Rheology of Cosmetic Products: Surfactant Mesophases, Foams and Emulsions. J Cosmet Sci 71:481–496 [PubMed] [Google Scholar]
- Davis, M.I., Pennington, M.T., Yang, Y.W., Swanson, L.A., 2024. Vulvar lichen simplex chronicus presenting as recurrent itchy gray papules. Am J Obstet Gynecol S0002–9378(24)00841-X. 10.1016/j.ajog.2024.08.018 [DOI] [PubMed]
- Dent C, Coutelle N, Gill M, Simon P, Doarn M, Nydick J (2025) Clinical Effectiveness of an Orthosis After Collagenase Clostridium Histolyticum Injection for Dupuytren Contracture. J Hand Surg Am S0363–5023(25):00059. 10.1016/j.jhsa.2025.01.023 [DOI] [PubMed] [Google Scholar]
- Dong Y, Qu H, Pavurala N, Wang J, Sekar V, Martinez MN, Fahmy R, Ashraf M, Cruz CN, Xu X (2018) Formulation characteristics and in vitro release testing of cyclosporine ophthalmic ointments. Int J Pharm 544:254–264. 10.1016/j.ijpharm.2018.04.042 [DOI] [PubMed] [Google Scholar]
- Duguid IG, Boyd AW, Mandel TE (1992) Adhesion molecules are expressed in the human retina and choroid. Curr Eye Res 11(Suppl):153–159. 10.3109/02713689208999526 [DOI] [PubMed] [Google Scholar]
- Eichenauer E, Jozić M, Glasl S, Klang V (2023) Spruce Balm-Based Semisolid Vehicles for Wound Healing: Effect of Excipients on Rheological Properties and Ex Vivo Skin Permeation. Pharmaceutics 15:1678. 10.3390/pharmaceutics15061678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y., Tang, Y., Hu, J., Chen, C., Zhong, G., Xiang, C., Chen, N., Bian, J., Shi, S., Zhang, Y., 2025. Phaseolus lunatus L. polysaccharide microneedles loaded with sinomenine hydrochloride for synergistic rheumatoid arthritis management and analgesia. Int J Biol Macromol 141511. 10.1016/j.ijbiomac.2025.141511 [DOI] [PubMed]
- Formica ML, Ullio Gamboa GV, Tártara LI, Luna JD, Benoit JP, Palma SD (2020) Triamcinolone acetonide-loaded lipid nanocapsules for ophthalmic applications. Int J Pharm 573:118795. 10.1016/j.ijpharm.2019.118795 [DOI] [PubMed] [Google Scholar]
- Fujii MY, Yamamoto Y, Koide T, Hamaguchi M, Onuki Y, Suzuki N, Suzuki T, Fukami T (2019) Imaging Analysis Enables Differentiation of the Distribution of Pharmaceutical Ingredients in Tacrolimus Ointments. Appl Spectrosc 73:1183–1192. 10.1177/0003702819863441 [DOI] [PubMed] [Google Scholar]
- García-Arieta A, Gordon J, Gwaza L, Merino V, Mangas-Sanjuan V (2023) Regulatory Requirements for the Development of Second-Entry Semisolid Topical Products in the European Union. Pharmaceutics 15:601. 10.3390/pharmaceutics15020601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenkummer HP, Kampik A (1995) Surgical extraction of subretinal pseudotumors in age related macular degeneration. Clinical, morphologic and immunohistochemical results. Ophthalmologe 92:631–639 [PubMed] [Google Scholar]
- Herbig ME, Evers D-H, Gorissen S, Köllmer M (2023) Rational Design of Topical Semi-Solid Dosage Forms-How Far Are We? Pharmaceutics 15:1822. 10.3390/pharmaceutics15071822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstroma A, Balodis A, Brokans A, Viksna A (2025) Prolonged Corticosteroid Use in the Treatment of Tuberculous Meningoencephalitis: A Case Report. Medicina (Kaunas) 61:214. 10.3390/medicina61020214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G, Li J, Wei W, Wu Y, Li L, Chen Y, Xie D, Qu Q, Rojas OJ, Hu G, Li Y, Guo J (2025) Starfish-Inspired Synergistic Reinforced Hydrogel Wound Dressing: Dual Responsiveness and Enhanced Bioactive Compound Delivery for Advanced Skin Regeneration and Management. ACS Nano. 10.1021/acsnano.4c17291 [DOI] [PubMed] [Google Scholar]
- Huang Y-T, Chen T-L, Huang Y-L, Kuo C-H, Peng Y-F, Tang S-C, Jeng J-S, Huang C-F, Lin S-Y, Lin F-J (2025) Impact of levetiracetam on direct oral anticoagulant level and outcomes among older Asian patients with atrial fibrillation. Front Pharmacol 16:1505665. 10.3389/fphar.2025.1505665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MZ, Krajewska M, Prochaska K, Saha SC (2025) In vitro and in silico studies of the interaction between glucocorticoid drug mometasone furoate and model lung surfactant monolayer. RSC Adv 15:5951–5964. 10.1039/d5ra00004a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong WY, Kwon M, Choi HE, Kim KS (2021) Recent advances in transdermal drug delivery systems: a review. Biomater Res 25:1–15. 10.1186/s40824-021-00226-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir F, Snorradottir BS, Gunnarsson S, Georgsdottir E, Sigurdsson S (2022) Transdermal Drug Delivery: Determining Permeation Parameters Using Tape Stripping and Numerical Modeling. Pharmaceutics 14:1880. 10.3390/pharmaceutics14091880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacassia C, Spennacchio A, Lopalco A, Lopedota AA, la Forgia FM, Fontana S, Franco M, Denora N (2024) Lipophilic Ready-to-use Vehicles and Compounded Topical Medications. Int J Pharm Compd 28:146–150 [PubMed] [Google Scholar]
- Liang Y, Liang J, Huang Q, Tian X, Shao L, Xia M, Liu Y (2023a) Knuckle Pads Successfully Treated with 2% Crisaborole Ointment Combined with Triamcinolone Acetonide and Neomycin Plaster: A Case Report. Clin Cosmet Investig Dermatol 16:1893–1897. 10.2147/CCID.S414268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Liang J, Huang Q, Tian X, Shao L, Xia M, Liu Y (2023b) Knuckle Pads Successfully Treated with 2% Crisaborole Ointment Combined with Triamcinolone Acetonide and Neomycin Plaster: A Case Report. CCID 16:1893–1897. 10.2147/CCID.S414268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Wang Y, Mei R, Chong X, Chen L, Ning B, Zhang R, Zhuang X (2024) Surface enhanced Raman scattering microtips for microenvironment pH determination of semi-solid preparations. Anal Methods 16:7468–7475. 10.1039/d4ay01523a [DOI] [PubMed] [Google Scholar]
- Luo Z, Wang J, Niu Z, Hu C, Chintala M, Luo X, Lee T-I, Plotnikov AN, Zannikos P (2025) Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Milvexian in Healthy Chinese Adults. Drug des Devel Ther 19:1503–1514. 10.2147/DDDT.S488414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity, S., Vora, A., Kanhed, A.M., Misra, A., Wairkar, S., 2025. Improved pharmacokinetic parameters and reduced tissue distribution of prodrug of Triamcinolone acetonide in lipid nanospheres- A preliminary investigation. Drug Dev Ind Pharm 1–13. 10.1080/03639045.2025.2475333 [DOI] [PubMed]
- Mardhiah J, Halim AS, Heng S, Saipolamin AQ (2025) Scoping review for pain mitigation during intralesional injections of corticosteroid for hypertrophic scar and keloid treatment. BMJ Open 15:e092800. 10.1136/bmjopen-2024-092800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez E, Gamboa J, Finkielstein CV, Cañas AI, Osorio MA, Vélez Y, Llinas N, Castro CI (2025) Oral dosage forms for drug delivery to the colon: an existing gap between research and commercial applications. J Mater Sci Mater Med 36:24. 10.1007/s10856-025-06868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoud, M.S., Yacout, G.A., El-Saadany, S.K., Abd-El-Khalek, B.A., 2018. Synthesis, Characterization and Biological Activity of Neomycin Sulphate Complexes.
- Nawan A, Wu Z, Jiang B, Wang G, Zhang W, Feng Y (2025) Effect of combination of multiple anti-inflammatory drugs strategy on postoperative delirium among older patients undergoing hip fracture surgery: a pilot randomized controlled trial. BMC Med 23:108. 10.1186/s12916-025-03946-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikseresht T, Rezaei M, Sharifian E, Khatony A (2024) Lubratex eye ointment with polyethylene cover significantly reduces corneal abrasion in ICU patients: a randomized controlled trial. Sci Rep 14:20443. 10.1038/s41598-024-71601-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obszynski J, Loidon H, Blanc A, Weibel J-M, Pale P (2022) Targeted modifications of neomycin and paromomycin: Towards resistance-free antibiotics? Bioorg Chem 126:105824. 10.1016/j.bioorg.2022.105824 [DOI] [PubMed] [Google Scholar]
- Ohashi R, Fujii A, Fukui K, Koide T, Fukami T (2022) Non-destructive quantitative analysis of pharmaceutical ointment by transmission Raman spectroscopy. Eur J Pharm Sci 169:106095. 10.1016/j.ejps.2021.106095 [DOI] [PubMed] [Google Scholar]
- Orlando A, Franceschini F, Muscas C, Pidkova S, Bartoli M, Rovere M, Tagliaferro A (2021) A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 9:262. 10.3390/chemosensors9090262 [Google Scholar]
- Padamwar MN, Pokharkar VB (2006) Development of vitamin loaded topical liposomal formulation using factorial design approach: drug deposition and stability. Int J Pharm 320:37–44. 10.1016/j.ijpharm.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Pagkalis S, Mantadakis E, Mavros MN, Ammari C, Falagas ME (2011) Pharmacological Considerations for the Proper Clinical Use of Aminoglycosides. Drugs 71:2277–2294. 10.2165/11597020-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahn MB, Jeong DC (2025) Endocrine and metabolic comorbidities in juvenile-onset systemic lupus erythematosus. Front Med (Lausanne) 12:1429337. 10.3389/fmed.2025.1429337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patere S, Newman B, Wang Y, Choi S, Mekjaruskul C, Jay M, Lu X (2020) Influence of in vitro release methods on assessment of tobramycin ophthalmic ointments. Int J Pharm 590:119938. 10.1016/j.ijpharm.2020.119938 [DOI] [PubMed] [Google Scholar]
- Prado VC, Moenke K, Pegoraro NS, Saccol CP, Nogueira-Librelotto DR, Rechia GC, Oliveira SM, Cruz L (2025) Nano-Based Hydrogel for Cutaneous Sesamol Delivery in UVB-Induced Skin Injury. AAPS PharmSciTech 26:75. 10.1208/s12249-025-03071-1 [DOI] [PubMed] [Google Scholar]
- Prokopov AY, Gazitaeva ZI, Sidorina AN, Peno-Mazzarino L, Radionov N, Drobintseva AO, Kvetnoy IM (2025) Influence of Injectable Hyaluronic Gel System on Skin Microbiota, Skin Defense Mechanisms and Integrity (Ex vivo Study). Clin Cosmet Investig Dermatol 18:459–473. 10.2147/CCID.S491685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva HR, Yoon T, Hendricks AJ, Malick H, Chisholm CD, Housewright CD, Singh V, Guevara A (2024) Dupilumab for Chronic Hand Eczema: A Systematic Review and Meta-Analysis. Dermatitis. 10.1089/derm.2024.0186 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Luna A, Ávila-Román J, González-Rodríguez ML, Cózar MJ, Rabasco AM, Motilva V, Talero E (2018) Fucoxanthin-Containing Cream Prevents Epidermal Hyperplasia and UVB-Induced Skin Erythema in Mice. Mar Drugs 16:378. 10.3390/md16100378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozeboom F, Robson HE, Chan SS (1983) Laser raman study on the crystallization of zeolites A, X and Y. Zeolites 3:321–328. 10.1016/0144-2449(83)90176-8 [Google Scholar]
- Rowe D, Rowe M, Pontifex C, Stubbs D (2024) Tape Stripping Method in Dermatological and Pharmacological Research: Evaluating Fresh and Frozen-Thawed Porcine Skin. Cureus 16:e68477. 10.7759/cureus.68477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpieri M, Bonaffini G, Ottino C, Quaranta G, Manassero L, Von Degerfeld MM (2024) Conservative treatment of a synovial cyst in a golden eagle (Aquila chrysaetos) with triamcinolone acetonide. J Vet Med Sci 86:592–595. 10.1292/jvms.23-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin TB, Sreekantaswamy SA, Hawkes JE, Butler DC (2023) Treatment Strategies for Chronic Pruritus and Eczema/Dermatitis in Older Adults Under the Category of Chronic Eczematous Eruptions of Aging (CEEA). Am J Clin Dermatol 24:405–418. 10.1007/s40257-023-00767-7 [DOI] [PubMed] [Google Scholar]
- Shi M, Lu Z, Qin A, Cheng J, Chen S, Xing Y (2024) A controlled clinical study on efficacy and safety of periocular triamcinolone acetonide injection for treating ocular myasthenia gravis. BMC Ophthalmol 24:33. 10.1186/s12886-024-03313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepmann J, Peppas NA (2011) Higuchi equation: Derivation, applications, use and misuse. International Journal of Pharmaceutics, Mathematical Modeling of Drug Delivery Systems 418:6–12. 10.1016/j.ijpharm.2011.03.051 [DOI] [PubMed] [Google Scholar]
- Silverberg JI (2017) Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol Clin 35:283–289. 10.1016/j.det.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Sm, L., Ad, I., S, W., 2020. Atopic dermatitis. Lancet (London, England) 396. 10.1016/S0140-6736(20)31286-1
- Sommer A, Veraart J, Neumann M, Kessels A (1998) Evaluation of the vasoconstrictive effects of topical steroids by laser-Doppler-perfusion-imaging. Acta Derm Venereol 78:15–18. 10.1080/00015559850135751 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Uchino T, Miyazaki Y, Kagawa Y (2014) The effect of storage time on the release profile of dexamethasone dipropionate from admixtures of steroid and heparinoid ointments. Pharmazie 69:104–108 [PubMed] [Google Scholar]
- Tanaka Y (2025) Revolutionary Changes in the Management of Lupus Nephritis: Towards De-Glucocorticoid or No-Glucocorticoid. Drugs. 10.1007/s40265-025-02156-2 [DOI] [PubMed] [Google Scholar]
- Thakkar R, Ashour EA, Shukla A, Wang R, Chambliss WG, Bandari S, Murthy N, Repka MA (2020) A Comparison Between Lab-Scale and Hot-Melt-Extruder-Based Anti-inflammatory Ointment Manufacturing. AAPS PharmSciTech 21:200. 10.1208/s12249-020-01738-5 [DOI] [PubMed] [Google Scholar]
- Tran M, Lea V, Zhao C, Kristoffersen S (2023) Venous eczema and chronic venous disease. BMJ-British Medical Journal 382:e074602. 10.1136/bmj-2022-074602 [DOI] [PubMed] [Google Scholar]
- Ubhe A, Oldenkamp H, Wu K (2024) Small Molecule Topical Ophthalmic Formulation Development-Data Driven Trends & Perspectives from Commercially Available Products in the US. J Pharm Sci 113:2997–3011. 10.1016/j.xphs.2024.07.023 [DOI] [PubMed] [Google Scholar]
- Ueda, C.T., Shah, V.P., Derdzinski, K., Ewing, G., Flynn, G., Maibach, H., Marques, M., Rytting, H., Shaw, S., Thakker, K., Yacobi, A., 2010. Topical and Transdermal Drug Products. Dissolution Technol. 17, 12–25. 10.14227/DT170410P12
- Walling HW, Swick BL (2010) Update on the management of chronic eczema: new approaches and emerging treatment options. CCID 3:99–117. 10.2147/CCID.S6496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ye J, Li R, Li R, Liu X, Han J, Yang Y, Ran N, Yuan M, Zhang Z, Chong W, Ji X (2025) Nanozyme-functionalized microalgal biohybrid microrobots in inflammatory bowel disease treatment. Biomaterials 319:123231. 10.1016/j.biomaterials.2025.123231 [DOI] [PubMed] [Google Scholar]
- Ye Y, Ling N, Gao J, Zhang M, Zhang X, Tong L, Ou D, Wang Y, Zhang J, Wu Q (2018) Short communication: Roles of outer membrane protein W (OmpW) on survival and biofilm formation of Cronobacter sakazakii under neomycin sulfate stress. J Dairy Sci 101:2927–2931. 10.3168/jds.2017-13517 [DOI] [PubMed] [Google Scholar]
- Yew YW, Barbieri J, Chen SC (2024) Burden of chronic skin disease from an Asian perspective: Assessment of health state utilities and quality of life in a Singapore cohort. JAAD Int 17:86–93. 10.1016/j.jdin.2024.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Zhang S, Hu D, Wang B, Li Y (2025) Efficacy of triamcinolone acetonide combined with recombinant bovine basic fibroblast growth factor in preventing scar formation after adult circumcision using a stapler device: A randomized controlled trial. Medicine (Baltimore) 104:e41500. 10.1097/MD.0000000000041500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng K, Chen D, Yang G, Yu Y, Li T (2023) Acupuncture for neurodermatitis: a case report. Acupunct Med 41:114–115. 10.1177/09645284221146201 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhao J, Zhou X, Maiwulanjiang M (2025b) Preliminary investigation of anti-fatigue effects and potential mechanisms of meiju oral liquid in mouse and zebrafish models. PLoS ONE 20:e0316761. 10.1371/journal.pone.0316761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Read, J., Mittal, G., Poston, R.N., Reilly, J., Howling, G., Golland, B., Sukhorukov, G.B., Gould, D., 2025. Injectable biodegradable microchamber array films for long-term delivery of glucocorticoids. J Control Release 113590. 10.1016/j.jconrel.2025.113590 [DOI] [PubMed]
- Zheng, Q., Wang, T., Wang, S., Chen, Z., Jia, X., Yang, H., Chen, H., Sun, X., Wang, K., Zhang, L., Fu, F., 2025. The anti-inflammatory effects of saponins from natural herbs. Pharmacol Ther 108827. 10.1016/j.pharmthera.2025.108827 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.