Abstract
Uncharged tRNA acts as the effector for transcription antitermination of genes in the T box family in Bacillus subtilis and other Gram-positive bacteria. Genetic studies suggested that expression of these genes is induced by stabilization of an antiterminator element in the leader RNA of each target gene by the cognate uncharged tRNA. The specificity of the tRNA response is dependent on a single codon in the leader, which was postulated to pair with the anticodon of the corresponding tRNA. It was not known whether the leader RNA–tRNA interaction requires additional factors. We show here that tRNA-dependent antitermination occurs in vitro in a purified transcription system, in the absence of ribosomes or accessory factors, demonstrating that the RNA–RNA interaction is sufficient to control gene expression by antitermination. The tRNA response exhibits similar specificity in vivo and in vitro, and the antitermination reaction in vitro is independent of NusA and functions with either B. subtilis or Escherichia coli RNA polymerase.
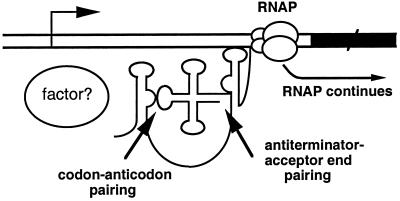
The T box transcription termination control system is widely used in Gram-positive bacteria to regulate expression of aminoacyl-tRNA synthetase, amino acid biosynthesis, and transporter genes (1). The transcripts of genes regulated by this mechanism contain a 200- to 300-nt untranslated leader that includes a factor-independent (intrinsic) transcription termination signal and a competing antiterminator structure (2). Read-through of the leader region terminator occurs when the charged to uncharged ratio of the corresponding tRNA isoacceptor is decreased, signaling a deficiency in that aminoacyl-tRNA synthetase or amino acid. Sequence comparisons and mutational studies identified a single codon, displayed at a precise position within the leader RNA structure, that determines the specificity of the amino acid response, presumably by pairing with the anticodon of the cognate tRNA (2). The acceptor end of uncharged tRNA apparently makes a second interaction with a bulged region in the antiterminator, based on genetic analyses as well as gel-shift assays using a model 39-nt antiterminator RNA (3, 4); this interaction is proposed to stabilize the antiterminator, preventing the formation of a competing terminator helix (Fig. 1). Mutational studies of the Bacillus subtilis tyrS leader and tRNATyr indicated that read-through requires additional conserved features of both RNAs (5–7).
Fig 1.
Proposed T box antitermination mechanism. The arrow indicates the transcription initiation site. The black rectangle represents the coding region of the regulated gene. Uncharged tRNA is postulated to interact with the nascent transcript at both the specifier sequence and the antiterminator bulge, stabilizing the antiterminator and preventing formation of the competing terminator. RNA polymerase (RNAP) then continues past the terminator region, and the full-length transcript is synthesized. “Factor?” indicates putative factor(s) that could modulate the leader RNA–tRNA interaction in vivo.
Although it has been established that a tRNA is essential for antitermination, it was not known whether other factors are also required to mediate the tRNA–leader interaction. There was no evidence of translation of the leader RNA (2, 3, 8), but in vivo assays could not rule out the possibility that the tRNA–leader interaction occurs within the context of a ribosome. In this study, we demonstrate specific tRNAGly-dependent antitermination of the Bacillus subtilis glyQS leader in a purified in vitro transcription system.
Materials and Methods
Bacterial Strains and Growth Conditions.
B. subtilis strain BR151MA (lys-3 trpC2) was used as the source of chromosomal DNA for amplification by PCR. Strains 1A5 (glyB133 metC3 tre-12 trpC2; Bacillus Genetic Stock Center) and KS115 (cysA14 hisA1 leuA8 metC3 trpC2; K. Sandman, Ohio State Univ., Columbus) were used for amino acid limitation experiments for glycine and cysteine, respectively. Cells were propagated in 2× YT medium (9) or in Spizizen minimal medium (10) for measurements of lacZ fusion expression. Cells containing lacZ fusions were grown in the presence of chloramphenicol at 5 μg/ml.
In Vitro Transcription Assays.
The template for glyQS transcription was a 440-bp PCR fragment that included sequences from 135 bp upstream of the glyQS transcription start site to position 305 of the transcript; the termination site is predicted to be around position 220 (ref. 11; Fig. 2). The template for tyrS transcription was a 420-bp PCR fragment including sequences from 85 bp upstream of the transcription start site to position 335 of the transcript; the termination site is predicted to be around position 270 (12). PCR products were purified by a Qiagen PCR cleanup kit. Template DNA (10 nM) was incubated in 1× transcription buffer (13) with His-tagged B. subtilis RNA polymerase (RNAP) (6 nM) purified as described by Qi and Hulett (14). Halted complex transcription assays were carried out essentially as described by Landick et al. (15). The dinucleotide ApU (150 μM, Sigma) was used to initiate glyQS transcription. ATP and GTP were added to 2.5 μM, UTP was added to 0.75 μM, and [α-32P]UTP (800 Ci/mmol; 1 Ci = 37 GBq) was added to 0.25 μM. Transcription of glyQS was initiated in the absence of CTP; for the glyQS gene, the first C is at position +17 so that the transcription elongation complex halts after synthesis of 16 nt under these conditions. The dinucleotide ApG was used for initiation of tyrS transcription, and GTP was omitted from the initiation reaction, resulting in a halt at position +11. The initiation reaction mixtures were incubated at 37°C for 15 min and were then placed on ice. Heparin (20 μg/ml, Sigma) was added to block reinitiation, and elongation was triggered by the addition of NTPs to 10 μM final and other reagents as indicated. B. subtilis NusA protein (25 nM), purified as described previously (13), was included in the elongation reaction as indicated. Escherichia coli RNAP, prepared as described (16), was provided by I. Artsimovitch (Ohio State Univ.) and was used at 10 nM. Transcription reactions were terminated by extraction with phenol, and the products were resolved by denaturing 6% polyacrylamide gel electrophoresis and visualized by PhosphorImager analysis.
Fig 2.
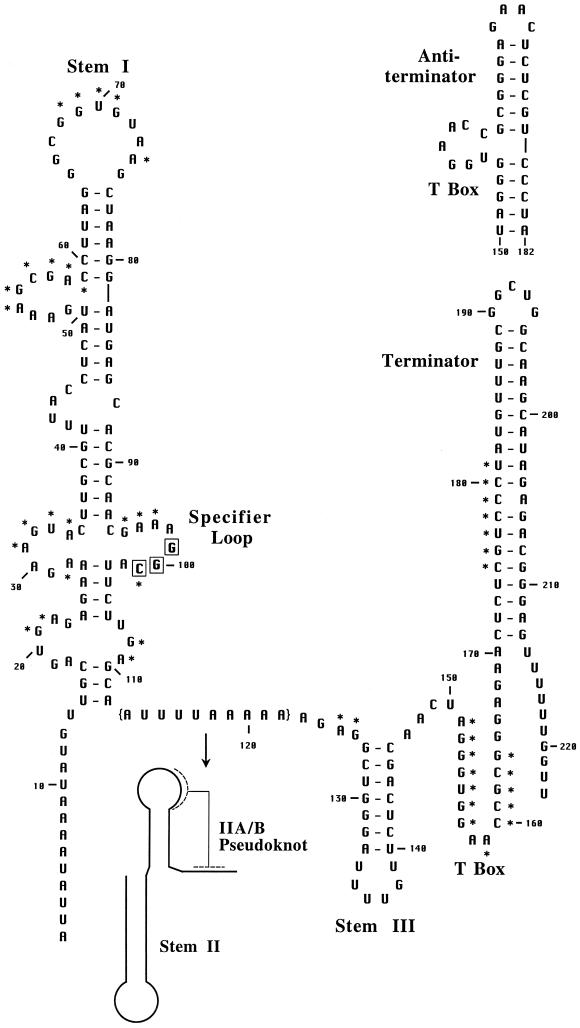
Secondary structure model of the B. subtilis glyQS leader RNA. Sequence is shown from the transcription start site (+1) through the end of the leader region terminator; the alternate antiterminator is shown above the terminator. The structure is based on the covariation model of T box family leaders (2, 5). Major conserved features are labeled, and conserved primary sequence elements are denoted with asterisks. The specifier sequence residues are boxed. The glyQS sequence was obtained from the B. subtilis genome sequence (11); DNA sequencing of this region revealed a substitution of A for U at position +6. The residues in brackets (113–122) are replaced by the stem II and IIA/B elements in most T box family leaders, including B. subtilis tyrS.
Unmodified B. subtilis tRNAGly and tRNATyr were made by T7 transcription using a PCR fragment generated with a 5′ oligonucleotide primer that included a T7 promoter sequence, positioned so that the first base of the transcript is the first position of the tRNA, and the final position of the PCR product corresponds to 3′ position of the tRNA. T7 transcription was carried out by using an Ampliscribe T7 transcription kit (Epicentre Technologies, Madison, WI). The tRNA transcripts were purified on a 6% denaturing polyacrylamide gel, visualized by using UV shadowing, and eluted into 300 mM NaOAc, pH 4.5/1 mM EDTA. The tRNA was purified by extraction with phenol, precipitated with ethanol, and suspended in water. The resulting tRNA was refolded by incubation at 80°C for 2 min and slow cooling to room temperature before use in the transcription assays at 70 nM. Modified E. coli tRNATyr was purchased from Sigma.
Mutations in the glyQS template DNA and tRNAGly were introduced by PCR, using oligonucleotide primers containing the desired alterations.
β-Galactosidase Measurements.
The glyQS DNA fragment used for in vitro transcription was inserted into the lacZ fusion vector pFG328 (17) and integrated in single copy into the B. subtilis chromosome by recombination into a bacteriophage SPβ prophage. Cells were grown in minimal medium containing all required amino acids at 50 μg/ml until mid-exponential growth phase and were then collected and divided into two cultures, containing all required amino acids or with one amino acid at 5 μg/ml. Growth was continued for 4 h, and cells were harvested and assayed for β-galactosidase activity, expressed as Miller units (9). Glycine starvation experiments were carried out in strain 1A5 (Gly−), and cysteine starvation experiments were carried out in strain KS115 (Cys−). All samples were assayed in duplicate, and growth experiments were carried out at least twice; variation was <10%.
Results
tRNA-Directed Antitermination in Vitro.
Previous attempts to replicate tRNATyr-directed antitermination of the B. subtilis tyrS leader in vitro by using multiround transcription assays under a variety of conditions with purified B. subtilis RNAP were unsuccessful (13). We observed efficient termination at the leader region terminator and no response to tRNATyr. Luo et al. (18) similarly failed to demonstrate tRNAThr-directed antitermination of the B. subtilis thrS gene in vitro. To increase the range of experimental conditions that could be tested, we moved to halted complex assays (15) so that transcription initiation and elongation could be carried out separately under different sets of conditions. Because correct folding of the nascent transcript was likely to be essential to antitermination, we tested the B. subtilis glyQS leader, which (like all glycyl leaders) lacks the complex stem II and stem IIA/B pseudoknot elements present in most other leaders, including B. subtilis tyrS (Fig. 2; unpublished results).
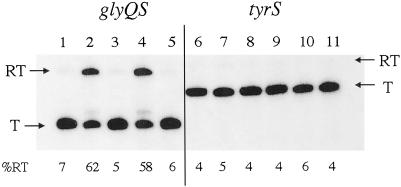
In the presence of 30 mM MgCl2 and low NTP concentrations (10 μM) during the elongation reaction, the glyQS leader region terminator was highly efficient (Fig. 3). Addition of a T7 RNAP-generated transcript of B. subtilis tRNAGly dramatically increased read-through from 5% (lane 1 or 3) to approximately 60% (lane 2 or 4), with a corresponding decrease in the amount of the terminated transcript. No tRNA-dependent read-through was observed at lower MgCl2 concentrations or at higher NTP concentrations (data not shown). Mg2+ plays a crucial role in RNA folding reactions (19) and may facilitate folding of the nascent transcript into the correct conformation for interaction with the tRNA and antitermination. The rate of transcription elongation is decreased at low NTP concentrations (20), which may favor formation of the correct RNA structure or facilitate pausing by RNAP.
Fig 3.
In vitro transcription of the glyQS and tyrS leader regions. Lanes 1–5, glyQS DNA; lanes 6–11, tyrS DNA. Lanes 1, 3, and 6, no tRNA added; lanes 2, 4, and 7, tRNAGly (T7 transcript); lanes 5, 8, and 11, tRNATyr (T7 transcript); lane 9, E. coli tRNATyr (modified, purchased from Sigma); lanes 3–9, NusA added; lanes 1, 2, 10, and 11, no NusA. T, terminated transcript; RT, read-through transcript. Percent read-through is indicated at the bottom of each lane.
Antitermination of the glyQS leader responded specifically to tRNAGly; addition of tRNATyr had no effect (Fig. 3, lane 5). Neither tRNAGly nor tRNATyr addition increased read-through of the B. subtilis tyrS leader region terminator (Fig. 3, lanes 6–11). Therefore, either the tyrS and glyQS leaders have different requirements for the tRNA–leader interaction or the tyrS leader fails to fold properly under the in vitro conditions used. The stem II and stem IIA/B pseudoknot elements present in tyrS but absent in glyQS may be responsible for this difference. Single nucleotide substitutions that disrupt conserved elements of the stem IIA/B region in the tyrS leader result in loss of read-through in vivo, indicating that this region is functionally required in the tyrS context (5). A variant of the tyrS leader in which the specifier sequence and antiterminator were changed to match the anticodon and acceptor end of tRNAGly has been constructed, and expression was shown to be induced in vivo in response to limitation for glycine (21). This leader variant failed to respond to tRNAGly in vitro (data not shown), indicating that the codon–anticodon interaction (GGC·GCC for glyQS vs. UAC·GUA for tyrS) is not sufficient to explain the lack of tRNATyr-directed antitermination of the tyrS leader in vitro.
glyQS Antitermination in Vitro Is Independent of NusA and Functions with E. coli RNAP.
The NusA protein affects transcription elongation rates and sensitivity of RNAP to pause and termination sites, and participates in a number of transcription termination control systems, including phage λ N and Q antitermination (22). Addition of B. subtilis NusA to the glyQS antitermination reaction resulted in a small increase in termination in the absence of tRNA, especially at high NTP concentrations (ref. 13; data not shown). However, the tRNAGly-dependent increase in read-through occurred both in the presence and absence of NusA (compare Fig. 3, lanes 1 and 2 with lanes 3 and 4), indicating that NusA is not required for glyQS antitermination under these conditions.
The ability of E. coli RNAP to replace B. subtilis RNAP in the tRNA-dependent transcription antitermination assay was tested to determine whether this activity was sensitive to the source of RNAP. RNAP from E. coli and B. subtilis has been shown to exhibit different patterns of recognition of pausing and termination signals (ref. 23; unpublished results). E. coli RNAP exhibited tRNAGly-dependent read-through similar to that observed with B. subtilis RNAP (data not shown), indicating that the antitermination event is dependent on features of the transcript, but not on the enzyme that generates the transcript. Introduction of T box leaders, including glyQS, into E. coli, from which this antitermination system is absent, generally resulted in a high level of read-through of the leader region terminator independent of amino acid limitation (data not shown), suggesting that the leader region terminators function poorly in the heterologous host and that the proper leader–tRNA interaction does not occur.
Specificity Determinants for Antitermination in Vitro and in Vivo.
The specificity of the tRNA effect was further tested by using glyQS leader variants and corresponding tRNAGly variants (Fig. 4A). The GGC glycine specifier sequence was changed to a UGC cysteine codon, and position A158 of the glyQS antiterminator bulge was changed to a U; this position is a secondary determinant of the specificity of the tRNA–leader interaction (3). The mutations were tested separately and in combination, both in vitro and in vivo. The wild-type glyQS template exhibited efficient antitermination in vitro only in combination with the corresponding wild-type tRNAGly (Fig. 4B, lane 2). The UGC specifier mutation in the glyQS leader resulted in decreased antitermination by wild-type tRNAGly; this effect was suppressed by a variant of tRNAGly with an anticodon complementary to UGC (Fig. 4C, lane 3). The A158→U substitution in the antiterminator also decreased antitermination directed by wild-type tRNAGly and was suppressed by a corresponding change in the tRNA discriminator base (Fig. 4D, lane 4). The specificity pattern also held for the UGC/A158→U double mutant, with the most efficient antitermination occurring in response to the matching tRNA (Fig. 4E, lane 5). The native GGC⋅GCC codon–anticodon pairing consistently resulted in more efficient antitermination than the UGC⋅GCA cysteinyl combination; this could be due to the extra G⋅C pair or to other features of the glyQS leader that are adapted to the native glycyl combination.
Fig 4.
Specificity of the glyQS–tRNAGly interaction. (A) Interaction of the glyQS leader (black) in the antiterminator conformation with tRNAGly (green). Substitutions at the specifier sequence and antiterminator regions of the leader, and at the anticodon and acceptor end of the tRNA, are shown with arrows. (B–E) In vitro transcription reactions using different combinations of variants of glyQS templates and tRNAGly. (B) Wild-type glyQS template DNA (GGC specifier sequence, A158 antiterminator). (C) glyQS-UGC template DNA (cysteine specifier sequence, A158 antiterminator). (D) glyQS-A158→U template DNA (GGC specifier, U158 antiterminator). (E) glyQS-UGC/A158→U template DNA (cysteine specifier sequence, U158 antiterminator). Lane 1, no tRNA; lane 2, wild-type tRNAGly (GCC anticodon, U73 discriminator); lane 3, tRNAGly-GCA (GCA anticodon, U73 discriminator); lane 4, tRNAGly-U73→A (GCC anticodon, A73 discriminator); lane 5, tRNAGly-GCA/U73→A (GCA anticodon, A73 discriminator). T, terminated transcript; RT, read-through transcript. Percent read-through is indicated at the bottom of each lane.
In agreement with the in vitro results, a wild-type glyQS-lacZ transcriptional fusion exhibited induction in vivo in response to limitation for glycine, but it failed to respond to limitation for cysteine (Table 1). Replacement of the GGC glycine specifier sequence with a UGC cysteine codon resulted in loss of the response to glycine and induction in response to limitation for cysteine; both tRNAGly and tRNACys contain a U at the discriminator position. The A158→U mutation, either alone or in combination with the UGC cysteine specifier sequence mutation, abolished the response to either glycine or cysteine, consistent with the loss of a match with the U discriminator position. The maximum expression observed under glycine limitation conditions was approximately one-third of that observed in a construct from which the terminator was deleted, indicating that full induction was not observed under these conditions.
Table 1.
Expression of glyQS-lacZ fusions in vivo
| Fusion
|
Glycine starvation | Cysteine starvation | ||||
|---|---|---|---|---|---|---|
| +Glycine | −Glycine | Ratio | +Cysteine | −Cysteine | Ratio | |
| GGC-A158 | 42 | 130 | 3.1 | 34 | 29 | 0.85 |
| UGC-A158 | 58 | 42 | 0.72 | 31 | 90 | 2.9 |
| GGC-U158 | 24 | 11 | 0.45 | ND | ND | ND |
| UGC-U158 | 20 | 6.0 | 0.30 | 14 | 9.1 | 0.65 |
| ΔTerm | 350 | 360 | 1.0 | ND | ND | ND |
Expression was measured by activity of β-galactosidase, in Miller units (9). ND, not determined; ΔTerm, terminator deleted.
Discussion
The ability of tRNA mutants to suppress tyrS leader mutations provided strong evidence for the role of uncharged tRNA as the effector for transcription antitermination in the T box system (2, 3). However, it was unknown whether the tRNA acted alone or in conjunction with trans-acting factors required to mediate the leader RNA–tRNA interaction. The demonstration of specific tRNAGly-directed antitermination in a purified in vitro transcription system provides clear evidence that the leader–tRNA interaction is sufficient, at least in the case of the glyQS leader.
Recognition of the tRNA by the leader RNA seems to mimic recognition by an aminoacyl-tRNA synthetase, which often exploits the anticodon and discriminator positions as specificity determinants (24). As is also true for tRNA charging, the leader RNA–tRNA interaction in vivo probably involves additional determinants (6, 21). Other systems in which uncharged tRNA is monitored, such as the yeast GCN2 system (25), require a protein component; tRNA mimics such as the E. coli thrS regulatory target site are also recognized by a protein (26). In translationally coupled transcription attenuation systems such as the E. coli trp operon, tRNA charging is monitored by a translating ribosome (27). The T box system is unique in that uncharged tRNA is recognized directly by an RNA, in the absence of protein factors or a ribosome. This may represent a more ancient regulatory mechanism, a vestige of the RNA world, and provides a further example of the ability of RNA to carry out complex interactions in the absence of protein cofactors. Peptidyltransferase activity is RNA-catalyzed (28), and codon–anticodon recognition requires specific interactions between residues in the decoding site of the ribosome and the mRNA and tRNA (29). The glyQS in vitro antitermination system exhibits codon–anticodon recognition mediated by RNA alone.
The demonstration of tRNAGly-dependent glyQS antitermination in vitro does not rule out the participation of proteins in the T box system in vivo, in particular for the more structurally complex leaders, like tyrS, which are the predominant class of leaders found in nature (unpublished results). The RNA component of RNase P similarly has catalytic activity in vitro, but it requires a protein component for function in vivo (30), and certain ribozymes use facilitator proteins to promote proper folding (31). The in vitro transcription conditions used in this study may have served to replace functions provided by other factors within a cell, but these factors are clearly not essential for the RNA–RNA interaction itself.
Acknowledgments
We thank F. M. Hulett for providing the strain for production of B. subtilis RNAP; I. Artsimovitch for providing E. coli RNAP and advice on halted complex transcription conditions; T. R. Moir for technical assistance; and M. Ibba, I. Artsimovitch, and J. R. Reeve for comments on the manuscript. This work was supported by National Institutes of Health Grant GM47823.
Abbreviations
RNAP, RNA polymerase
References
- 1.Henkin T. M. (2000) Curr. Opin. Microbiol. 3, 149-153. [DOI] [PubMed] [Google Scholar]
- 2.Grundy F. J. & Henkin, T. M. (1993) Cell 74, 475-482. [DOI] [PubMed] [Google Scholar]
- 3.Grundy F. J., Rollins, S. M. & Henkin, T. M. (1994) J. Bacteriol. 176, 4518-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerdeman M. S., Henkin, T. M. & Hines, J. V. (2002) Nucleic Acids Res. 30, 1065-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rollins S. M., Grundy, F. J. & Henkin, T. M. (1997) Mol. Microbiol. 25, 411-421. [DOI] [PubMed] [Google Scholar]
- 6.Grundy F. J., Collins, J. A., Rollins, S. M. & Henkin, T. M. (2000) RNA 6, 1131-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler W. C., Grundy, F. J., Murphy, B. A. & Henkin, T. M. (2001) RNA 7, 1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandoni J. A., Fulmer, S. B., Brizzio, V., Zahler, S. A. & Calvo, J. M. (1993) J. Bacteriol. 175, 7581-7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller J. H., (1972) Experiments in Molecular Genetics (Cold Spring Harbor Lab. Press, Plainview, NY).
- 10.Anagnostopoulos C. & Spizizen, J. (1961) J. Bacteriol. 81, 741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunst F., Ogasawara, N., Moszer, I., Albertini, A. M., Alloni, G., Azevedo, V., Bertero, M. G., Bessieres, P., Bolotin, A., Borchert, S., Borriss, R., et al. (1997) Nature (London) 390, 249-256. [DOI] [PubMed] [Google Scholar]
- 12.Henkin T. M., Glass, B. L. & Grundy, F. J. (1992) J. Bacteriol. 174, 1299-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy F. J., Moir, T. R., Haldeman, M. T. & Henkin, T. M. (2002) Nucleic Acids Res. 30, 1646-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi Y. & Hulett, F. M. (1998) Mol. Microbiol. 28, 1187-1197. [DOI] [PubMed] [Google Scholar]
- 15.Landick R., Wang, D. & Chan, C. L. (1996) Methods Enzymol. 274, 334-353. [DOI] [PubMed] [Google Scholar]
- 16.Hager D. A., Jin, D. J. & Burgess, R. R. (1990) Biochemistry 29, 7890-7894. [DOI] [PubMed] [Google Scholar]
- 17.Grundy F. J., Waters, D. A., Allen, S. H. G. & Henkin, T. M. (1993) J. Bacteriol. 175, 7348-7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo D., Condon, C., Grunberg-Manago, M. & Putzer, H. (1998) Nucleic Acids Res. 26, 5379-5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treiber D. K. & Williamson, J. R. (2001) Curr. Opin. Struct. Biol. 11, 309-314. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes G. & Chamberlin, M. J. (1974) J. Biol. Chem. 249, 6675-6683. [PubMed] [Google Scholar]
- 21.Grundy F. J., Hodil, S. E., Rollins, S. M. & Henkin, T. M. (1997) J. Bacteriol. 179, 2587-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman D. I. & Court, D. L. (2001) Curr. Opin. Microbiol. 4, 201-207. [DOI] [PubMed] [Google Scholar]
- 23.Artsimovitch I., Svetlov, V., Anthony, L., Burgess, R. R. & Landick, R. (2000) J. Bacteriol. 182, 6027-6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giege R., Sissler, M. & Florentz, C. (1998) Nucleic Acids Res. 26, 5017-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu H., Dong, J., Hu, C., Francklyn, C. S. & Hinnebusch, A. G. (2001) EMBO J. 20, 1425-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sankaranarayanan R., Dock-Bregeon, A.-C., Romby, P., Caillet, J., Springer, M., Rees, B., Ehresmann, C., Ehresmann, B. & Moras, D. (1999) Cell 97, 371-381. [DOI] [PubMed] [Google Scholar]
- 27.Landick R., Turnbough, C. L., Jr. & Yanofsky, C. (1996) in Escherichia coli and Salmonella: Cellular and Molecular Biology, eds. Neidhardt, F. C., Curtis, R., III, Ingraham, J. L., Lin, E. C. C., Low, K. B., Magasanik, B., Reznikoff, W. S., Riley, M., Schaecter, A. & Umbarger, H. E. (Am. Soc. Microbiol., Washington, DC), pp. 1263–1286.
- 28.Ban N., Nissen, P., Hansen, J., Moore, P. B. & Steitz, T. A. (2000) Science 289, 905-920. [DOI] [PubMed] [Google Scholar]
- 29.Ogle J. M., Brodersen, D. E., Clemons, W. M., Jr., Tarry, M. J., Carter, A. P. & Ramakrishnan, V. (2001) Science 292, 897-902. [DOI] [PubMed] [Google Scholar]
- 30.Guerrier-Takada C., Gardiner, K., Marsh, T., Pace, N. & Altman, S. (1983) Cell 35, 849-857. [DOI] [PubMed] [Google Scholar]
- 31.Weeks K. M. & Cech, T. R. (1995) Cell 82, 221-230. [DOI] [PubMed] [Google Scholar]