Abstract
Emotional disturbances such as anxiety and depression are increasingly recognized to be closely associated with individual chronotype and levels of daytime sleepiness. A growing body of research is now exploring the complex interplay between daytime sleepiness, circadian rhythms, and emotional disturbances from diverse methodological and theoretical perspectives. Building on this foundation, the present study employed network analysis to examine the associations among chronotype, daytime sleepiness, and emotional disturbances. A total of 559 young adults (224 males, 335 females; mean age = 21.14 years) were recruited and completed the Morningness-Eveningness Questionnaire—Self-Assessment version (MEQ-SA), the Epworth Sleepiness Scale (ESS), and the Depression Anxiety Stress Scale (DASS-21). Descriptive analyses indicated that individuals who took longer and earlier naps tended to exhibit more evening-oriented chronotypes. Network analysis revealed three stable network structures, with anxiety and depressive symptoms serving as bridging nodes that connect the dimensions of daytime sleepiness, chronotype, and emotional disturbances. Across the three networks, shared core symptoms included “restlessness,” “depression,” and “anxiety,” with “restlessness” and “depression” playing pivotal roles in inter-symptom connectivity. These findings suggest that both chronotype and daytime sleepiness are highly associated with emotional symptoms. Future interventions targeting emotional disorders may benefit from simultaneously addressing both factors to enhance treatment efficacy. Additionally, attention should be given to the role of social and environmental factors in shaping emotional well-being.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-13490-6.
Keywords: Chronotype, Daytime sleepiness, Anxiety, Depression, Stress, Network analysis
Subject terms: Psychology, Health policy
Introduction
Sleep is an indispensable aspect of human life, with the capacity to rejuvenate energy and endurance, and for certain individuals, it serves as a vital mechanism for managing daily emotional stressors. Tononi and Cirelli (2003) proposed the synaptic homeostasis hypothesis, which states that sleep helps to regulate the strength of synapses in the brain, preventing them from being over-enhanced, and that this adjustment helps to make room for new learning1. Thus, sleep plays a crucial role throughout life, not only affecting emotion, but also being closely related to cognitive function, attention, working memory, and mental health2,3. In previous studies, it has been found that the quality and duration of sleep are also related to emotional processing and consolidation of emotional memories, and that sleep facilitates the lasting consolidation and processing of emotional memories in the population4–6. And when sleep is impaired, it leads to an increase in negative affects, inhibiting emotional arousal while decreasing positive emotional states and elevating overall negative affective outcomes7.
Emotions are an essential part of an individual’s experience, and both humans and animals experience emotions differently in different situations and environments, and Trampe et al. (2015) found in their study that humans experience at least one emotion at least 90% of the time8. We have found that in the early days researchers were more interested in studying the effects of emotions on our behaviour, health and so on, but in the last decade or so researchers have been focusing more on the combination of emotions and sleep, and have been more interested in investigating whether or not emotions affect sleep and the mechanisms by which they do so.
The relationship between sleep and emotion distress
In one study, participants were asked to rate their level of stress and worry before bedtime, and subsequent sleep quality data showed that individuals who experienced worry and stress before bedtime led to sleep disturbances9. In addition, the triggering of negative emotions or the exhaustion of an individual’s emotional experience can contribute to sleep disorders to varying degrees10,11. Studies have shown that adequate sleep can significantly improve emotional states, while sleep deprivation or symptoms of sleep disorders are strongly associated with increased negative emotions such as depression and anxiety. For example, people with depression often suffer from poor sleep quality, difficulty falling asleep, or early awakening, and past research has identified depression as the primary cause of these sleep disorders. However, recent research suggests that sleep disorders may also be a precursor to depression. Morphy et al. (2007) found that sleep disturbances can predict the onset of depression. Similarly, research by Chang supports this view, further highlighting a bidirectional relationship between sleep problems and depressive symptoms12. Alvaro et al. (2013), through a synthesis of studies across various ethnic and age groups, also confirmed the reciprocal association between insomnia, anxiety, and depression13. These findings collectively underscore the complexity of the relationship between sleep disturbances and emotional disorders. Therefore, some researchers have suggested that depressive symptoms can be alleviated by improving sleep14,15. Sleep disorders are defined as having a range of problems that affect the quality, duration, and timing of sleep, which may affect an individual’s daily functioning and overall health. Taking this a step further, research has found that sleep duration is also strongly associated with depression risk. When sleeping more than eight hours or less than six hours, people are more likely to suffer from depression than those whose sleep duration is controlled at six to eight hours16. In addition, one study induced a negative emotion by having 64 participants hear their own out-of-tune singing four times over three consecutive days, and found that sleep for normal sleepers gave relief from emotional distress, while the opposite occurred for insomnia sufferers, which also demonstrates that healthy sleep moderates emotions and can be effective in alleviating negative emotions17. In conjunction with the numerous studies that have found that both REM and non-REM sleep deprivation affect emotional processing areas, a study on the effects of sleep on the acquisition of fearfulness found that sleep deprivation affects brain regions capable of generating fearfulness, resulting in increased activation of the amygdala and suppression of the activity of the ventromedial prefrontal cortex (vmPFC), ultimately leading to enhanced fear learning18,19. This study also reaffirms that sleep deprivation produces negative emotions. On the other hand, emotions can in turn affect sleep. The results of one study on the relationship between unhealthy hours of sleep and emotional distress found that individuals experiencing emotional distress had a 55% chance of reporting unhealthy sleep, demonstrating that emotional distress is a significant predictor of unhealthy sleep20. In Krizan’s study of the effects of induced emotions on sleep, the data collected in this study demonstrated that negative emotions did not have a significant effect on sleep duration, but resulted in delayed sleep onset with significant results21. In conclusion, it can also be surmised that different stages of sleep deprivation affect the relevant emotional brain regions to produce different emotions, which may in turn affect sleep onset and sleep quality, and affect the daily routine and sleep habits of the population.
Relationship between daytime sleepiness, chronotype and emotional distress
Sleep is vital to physical and mental health, not only does it help the body to recover and regenerate, but it also plays a key role in cognitive function and emotion regulation. When sleep quality is inadequate, individuals may experience daytime sleepiness, which can interfere with daily life and working efficiency, so there are more and more researchers who want to explore the relationship between daytime sleep and mood in addition to nighttime sleep, and are doing so in different ways. Daytime sleepiness is usually a direct reflection of insufficient or poor quality sleep at night, causing individuals to feel tired and even unable to concentrate during the day. In addition, daytime sleepiness is closely related to chronotype, BorbeIy and Neuhaus and Wever have found that the sleep–wake cycle exhibits a regular rhythm by studying rats and humans, and thus it is known that the sleep–wake cycle constitutes a true chronotype22. Circadian rhythm refers to the natural physiological and behavioral cycles that occur approximately every 24 h within living organisms23. Chronotype have been linked to emotional distress such as anxiety, depression and stress, and studies based on the co-morbidity model have found a common symptom between them, whereby those who tend to be nocturnal are more prone to anxiety and depression, whereas those who are morning-typed have a better emotion state24,25. Leerssen et al. in a study exploring how insomnia can change emotions in the context of cognitive, behavioural, and circadian interventions, found that The combination of chronotype and cognitive behavioural therapy for insomnia can be beneficial in enhancing emotion regulation, as well as intervening and preventing the development of depression26. Therefore, maintaining good sleep habits and regular chronotype are important strategies for promoting emotion stability.
Previous research has predominantly focused on the various stages of nighttime sleep and their impacts on different aspects of human psychology, including emotions, cognition, and executive functions. However, some studies have also suggested that napping during the day can play a certain regulatory role in emotions27,28. and in the China Sleep Research Report (2024), it was shown that more than half (63.78%) of the respondents had the habit of napping, with 65.52% of them having an average daily nap length of less than one hour. From this, it can be surmised that most people may compensate for their lack of nighttime sleep by taking a nap or a siesta during the day, even though napping is usually considered a normal part of daily life for infants and toddlers. There are many different reasons why people choose to nap, with some people napping to cope with sleep deprivation (i.e., replacement naps) or to prepare for imminent sleep deprivation (i.e., preventive naps), while others nap for pleasure (i.e., appetitive naps)29. Krizan et al. noted that in studies of daytime sleep (i.e., naps), delayed sleep onset was more strongly influenced by negative emotions. In terms of sleep duration, daytime sleep pressure is lower, and naps may be more susceptible to emotion, due to the effects of homeostasis and chronotype, and thus daytime sleep may be particularly susceptible to negative emotions, although this has rarely been checked in established studies21. Additionally, for people who typically get the sleep they need every night, daytime naps may also provide considerable benefits to emotion, alertness, and cognitive performance.
An approach based on network analysis
This study aims to better reveal the relationship between chronotype and daytime sleepiness with emotional distress in the population, and network theory can be used to explain the phenomenon of co-morbidities, so it is desired to use network theory to analyse and reveal the relationship between them. According to network theory, mental disorders do not originate from an underlying single etiology, but rather are dynamic network systems consisting of multiple interrelated and interacting symptoms. In this theoretical framework, each symptom is regarded as a node, and different symptoms are connected by edges to form an overall network structure30,31. In a disorder, some symptoms that have a strong influence lead to the appearance of other symptoms (e.g., the symptom that is most strongly associated with other symptoms is called the central symptom)32–34. In addition, symptoms in one disorder may activate symptoms in another, creating a negative cycle between the two disorders that can lead to the emergence and persistence of co-morbidities35. Symptoms that connect different disorders are referred to as bridge symptoms, in psychological network models, bridge symptoms play a central role, serving as conduits through which different mental disorders influence and reinforce each other. Their presence helps explain the frequent comorbidity observed among disorders such as depression and anxiety, and offers a novel theoretical perspective for understanding the dynamic interplay between symptoms. From a theoretical standpoint, bridge symptoms shed light on the underlying mechanisms linking distinct symptom clusters. From a clinical perspective, identifying and targeting these bridge nodes may help disrupt maladaptive pathways of symptom transmission, thereby alleviating overall psychological distress36. Therefore, identifying the central and bridging symptoms in a symptom network can help to understand the mechanisms of co-morbidity and contribute to the development of more cost-effective interventions37,38. In addition, network analysis is also seen as a tool that can effectively examine the complex relationships between individual symptoms of psychopathology and specific life stressors39,40, and in research, this approach facilitates a more refined understanding of the potential causal impact of individual symptoms.
The current study
Among the few previous studies on the relationship between napping and individuals’ chronotype, napping was found to be a key factor in chronotype, with a strong relationship between the two41.The duration of the nap is related to whether the individual is a morning or night type, with night types taking longer naps and experiencing multiple drowsiness episodes during the daytime hours, whereas the opposite is true for morning types42,43. In addition, the analytical method of network theory has been used mostly in the field of social psychology, and gradually this method has been applied to other fields of psychology, such as neurology and clinical psychology and so on. In previous research, network analysis has also been introduced by scholars into the study of sleep and emotions. Through network theory, it has been revealed that there are central nodes between sleep and emotional states, or the reciprocal influences between daytime napping, circadian rhythms, and emotional states have been explored44. However, our study aims to integrate daytime sleepiness, chronotype, and emotional distress into a single network model. By employing this approach, we seek to investigate the associations among emotional distress, daytime sleepiness, and chronotype.
In summary, the aim of this study is to investigate the relationship between daily routines such as chronotype and daytime sleepiness and emotional distress of young people through the use of a network model approach, and to understand and analyse these relationships in order to better give sound advice and make effective interventions. Combined with previous research, we hypothesise that there are common symptom between stress, depression and anxiety; that the type of chronotype affects how well individuals sleep during the day; and that there are common symptom between emotional distress and daytime sleepiness, chronotype. The present study had two main aims: firstly, to investigate the relationship between daily routine and emotional distress; and secondly, to explore the common symptom between daytime sleepiness and emotional distress such as anxiety and depression.
Methods
Participants
Using a convenience sampling method, we completed the recruitment of 730 higher education students during the spring semester of 2024. The recruitment was conducted through online advertisements and flyers at two public universities. Participants anonymously completed the questionnaire survey through a link on the WJX platform (https://www.wjx.cn/). To obtain our target group for the study, we screened participants based on four criteria: whether they took naps, the frequency of naps per week, the duration of naps, and the start time of naps. A total of 647 higher education students who meet all requirements and whose responses did not contradict each other were included in the subsequent analysis. After excluding incomplete and invalid data, the final sample for further analysis included 559 participants. Our research subjects included 224 male (40.07%) and 335 female (59.93%), aged between 18 and 35 (M = 21.14, SD = 2.25). Participants completed the online survey within 10 min and received a monetary compensation of 2 yuan (approximately 0.14$). Our survey obtained written informed consent from the participants, which was also approved by the Sichuan Normal University ethics committee (SICNU-231201). The research was conducted in accordance with the Declaration of Helsinki45.
Procedure
Morningness-Eveningness Questionnaire-Self Assessment (MEQ-SA)
The Morningness-Eveningness Questionnaire-Self Assessment (MEQ-SA) is used to assess the type of chronotype in individuals46. The MEQ-SA consists of 19 entries that assess an individual’s morningness or nightness tendencies by answering questions about morning and evening preferences, wake-up and bedtime, and daytime energy levels. The abbreviations and content of the MEQ-SA items can be found in Appendix S1. The scoring range for each item varies depending on the question; for example, some questions have a scoring range from 1 (very difficult) to 4 (very easy), while others may have different scoring criteria. The MEQ-SA score ranges from 16 to 86, with higher scores indicating a morning type tendency and lower scores indicating an evening type tendency. The cut-off point is set at 41, with below 41 being evening type, 42–58 being intermediate type, and above 59 being morning type. It has been confirmed that the MEQ-SA has good retest reliability, structural validity, and sensitivity47,48. The Chinese version of the MEQ-SA used in this study was introduced and validated by Zhang Bin and colleagues. Their findings showed that the scale demonstrated acceptable psychometric properties, with Cronbach’s alpha coefficients ranging from 0.701 to 0.738, Spearman-Brown split-half reliability coefficients from 0.584 to 0.697, and test–retest reliability coefficients from 0.638 to 0.83149. In this study, the Cronbach’s α coefficient of the MEQ-SA is 0.782, indicating strong internal consistency. The fit indices of the measurement model χ2/df = 3.957, CFI = 0.780, IFI = 0.782, RMSEA = 0.073, SRMR < 0.07, indicate that the MEQ-SA has good structural validity.
Epworth Sleepiness Scale (ESS)
The Epworth Sleepiness Scale (ESS) is used to assess an individual’s level of daytime sleepiness in daily life50. The ESS consists of 8 items, each describing a common situation in which respondents are asked to rate their likelihood of dozing off or napping. The abbreviations and content of each item of the ESS are shown in Appendix S1. On a 4-point scale from 0 to 3, with 0 indicating "would never doze," and 3 indicating "high chance of dozing." The total score ranges from 0 to 24, with higher scores indicating a higher level of sleepiness. A score greater than 10 on the ESS is considered to indicate significant daytime sleepiness. Studies have confirmed that the ESS has good test–retest reliability and validity, moreover, the ESS has been translated into Chinese and its reliability and validity have been assessed in Chinese populations. The results indicated that the Chinese version of the ESS demonstrated good test–retest reliability, with a Cronbach’s alpha of 0.81, suggesting strong reliability and internal consistency51–53. In this study, the Cronbach’s α coefficient of the ESS is 0.759, indicating strong internal consistency. The fit indices of the measurement model χ2/df = 6.140, CFI = 0.876, IFI = 0.877, RMSEA = 0.096, SRMR < 0.06, indicate that the ESS has good structural validity.
Depression Anxiety Stress Scales (DASS-21)
The Depression- Anxiety- Stress Scales (DASS-21) is used to assess an individual’s symptoms of depression, anxiety, and stress over the past week54. The DASS-21 consists of 21 items, with each item assessing symptoms related to depression, anxiety, and stress, and each dimension contains 7 items. The abbreviations and content of each item of the DASS-21 can be found in Appendix S1. On a 4-point scale from 0 to 3, with 0 indicating “does not apply to me,” and 3 indicating "applies to me most of the time." The total score ranges from 0 to 63, with higher scores indicating more severe symptoms. The cutoff points for the DASS-21 are 9 for depression, 7 for anxiety, and 14 for stress. Studies have confirmed that the DASS-21 has good test–retest reliability, structural validity, and sensitivity, furthermore, the Chinese version of the DASS-21 has been widely used among both adolescents and adults in China, and has been shown to possess good reliability and validity55–57. In this study, the Cronbach’s α coefficient of the DASS-21 is 0.955, indicating strong internal consistency. The fit indices of the measurement model χ2/df = 4.182, CFI = 0.918, IFI = 0.919, RMSEA = 0.075, SRMR < 0.05, indicate that the DASS-21 has good structural validity.
Analysis procedure
Data analysis consisted of two steps: first, descriptive statistics and Pearson correlation analysis were conducted in SPSS, followed by visualization network analysis in R software (version 4.3.1). The graph package was used to estimate the network structure and perform visualization. Our network structure was based on the Extended Bayesian Information Criterion58 (EBIC) and constructed using Graphical Least Absolute Shrinkage and Selection Operator59 (LASSO) as the EBICglasso model. The EBICglasso model can penalize spurious associations, thereby addressing the issue of overestimating associations between components, and generates a sparse network that only shows significant associations. Setting the adjustment parameter to 0.5 results in a more concise and interpretable network60,61. Additionally, the qgraph package also reports centrality measures, including betweenness, closeness, strength, and expected influence62,63. Regarding node centrality, strength is considered the most stable centrality measure, reflecting the degree of direct relevance of a node to other nodes in the network by calculating the sum of the absolute weights of all edges connected to a node64–67. Furthermore, the expected influence (EI) can also consider potential negative edges. The thickness of the edges reflects the strength of the relationship between two nodes, and the color represents different directions of correlation, with blue indicating positive correlation and red indicating negative correlation. The stability of edges and centrality can also be estimated using the bootstrap method 95% confidence interval (N = 1000) through the bootnet package. The correlation stability (CS) coefficient describes the centrality of the nodes. Previous studies have suggested that the coefficient is best above 0.5 for interpretability and stability60. Additionally, we used R packages such as qgraph, mgm, and bootnet for additional analyses of bridging symptoms and their stability. Furthermore, we used the NetworkComparisonTest package for gender comparison between two networks, with related results found in the supplementary materials.
Result
Descriptive statistics
Descriptive statistics and Spearman correlation analysis results are shown in Table 1. Nap frequency was significantly negatively correlated with depressive symptoms (r = -0.10, p < 0.05), while nap duration was significantly negatively correlated with MEQ scores (r = -0.16, p < 0.01). Since higher scores on the MEQ-SA indicate a greater tendency toward morningness and lower scores reflect eveningness, this result suggests that individuals who nap for longer durations are more likely to exhibit evening-type circadian preferences. Nap start time was also significantly negatively correlated with MEQ scores (r = -0.20, p < 0.01). The total score of the Epworth Sleepiness Scale (ESS) was significantly positively correlated with stress (r = 0.41, p < 0.01), anxiety (r = 0.41, p < 0.01), and depressive symptoms (r = 0.39, p < 0.01). Additionally, there was a significant negative correlation between ESS scores and MEQ scores (r = -0.30, p < 0.01). Given that higher scores on the ESS indicate greater levels of daytime sleepiness, this finding suggests that individuals who experience higher levels of daytime sleepiness are more likely to exhibit evening-type circadian preferences. MEQ scores were significantly negatively correlated with stress (r = -0.20, p < 0.01), anxiety (r = -0.20, p < 0.01), and depressive symptoms (r = -0.19, p < 0.01).
Table 1.
Descriptive statistics and Spearman correlation matrix for stress, anxiety, depressive symptoms, ESS, and MEQ in the napping population (N = 559).
| M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Nap frequency | - | - | 1 | -0.08 | -0.06 | -0.02 | 0.00 | -0.06 | -0.07 | -0.10* |
| 2 Nap duration | - | - | -0.08 | 1 | 0.02 | -0.03 | -0.16** | 0.00 | 0.05 | 0.02 |
| 3 Nap start time | - | - | -0.06 | 0.02 | 1 | 0.06 | -0.20** | 0.07 | 0.07 | 0.04 |
| 4 ESS | 10.03 | 4.31 | -0.02 | -0.03 | 0.06 | 1 | -0.30** | 0.41** | 0.41** | 0.39** |
| 5 MEQ | 49.77 | 8.97 | 0.00 | -0.16** | -0.20** | -0.30** | 1 | -0.20** | -0.20** | -0.19** |
| 6 Stress | 11.70 | 9.48 | -0.06 | 0.00 | 0.07 | 0.41** | -0.20** | 1 | 0.85** | 0.86** |
| 7 Anxiety | 9.32 | 8.73 | -0.08 | 0.05 | 0.07 | 0.41** | -0.20** | 0.85** | 1 | 0.84** |
| 8 Depression | 8.22 | 8.41 | -0.10* | 0.02 | 0.04 | 0.40** | -0.19** | 0.86** | 0.84** | 1 |
p* < 0.05, p** < 0.01.
Furthermore, stress, anxiety, and depressive symptoms were all highly significantly positively correlated with each other (r > 0.84, p < 0.01), indicating a strong mutual influence among these mental health variables.
The results also validate our hypothesis that circadian type affects how individuals sleep during the day.
The network structure of stress, anxiety, and depression
Figure 1 depicts the network structure of stress, anxiety, and depression. Out of all 210 edges, 135 are non-zero edges. The edge and centrality stability in this symptom network are considered moderate (see Appendices S2 and S3). Specifically, the strongest connections are between collapse and depression (A15-D13; r = 0.180), uneasiness and despair (S11-D10; r = 0.179), and mental fatigue and initiation difficulty (S8-D5; r = 0.175). As shown in Fig. 2, the symptoms with the highest strength are uneasiness (S11), followed by depression (D13), fear (A20), difficulty relaxing (S12), and overreaction (S6). Figure 3 shows that worry (A9), uneasiness (S11), depression (D13), and fear (A20) are central bridging symptoms in the network of stress, anxiety, and depression, and these bridging nodes have strong stability in strength, these symptoms act as crucial links, connecting and transmitting influence to other symptoms within the network (see Appendix S4). In addition, the results of the network comparison test for stress, anxiety, and depression between male and female groups are presented in Appendix S5. The results confirm our hypothesis that there is a common symptom relationship between stress, anxiety and depression.
Fig. 1.
Network structure of stress, anxiety, and depression symptoms among higher education students (N = 549). The left panel presents the visualized symptom network, while the right panel lists the node identifiers along with the abbreviations corresponding to each subscale dimension.
Fig. 2.
The centrality indices of the network between stress, anxiety, and depression.
Fig. 3.
The bridge centrality indices of the network between stress, anxiety, and depression.
The network structure of stress, anxiety, depression, and daytime sleepiness
Figure 4 presents the network structure of stress, anxiety, depression, and daytime sleepiness among higher education students. Out of 406 edges, 199 non-zero edges were revealed. The stability analysis of the edges and centrality indices also showed good network stability (see Appendices S6 and S7). Specifically, the strongest connections are uneasiness with despair (S11-D10; r = 0.177), collapse with depression (A15-D13; r = 0.176), and mental fatigue with initiation difficulty (S8-D5; r = 0.169). Additionally, the symptoms with the highest strength are uneasiness (S11), depression (D13), fear (A20), shortness of breath (A4), and overreaction (S6), as shown in Fig. 5. The strong association between anxiety and hopelessness may indicate a high overlap between persistent hypervigilance and a perceived loss of control over the future—two core features of anxiety. The robust link between feelings of breakdown and depression may reflect a decline in psychological resilience commonly observed in depressive states, ultimately leading to emotional collapse. Additionally, the connection between mental exhaustion and difficulty initiating activities may point to a comorbid relationship between depleted motivational energy and impaired executive functioning. Figure 6 demonstrates that in the network of stress, anxiety, depression, and daytime sleepiness, the central bridging symptoms are uneasiness (S11), depression (D13), worry (A9), fear (A20), initiation difficulty (D5), and collapse (A15), with the bridging nodes’ strength exhibiting strong stability (see Appendix S8). This finding suggests that these high-strength bridging nodes may serve as critical links between daytime sleepiness and the three types of emotional distress, allowing these domains to influence each other through shared symptom pathways. Furthermore, the results of the network comparison test for stress, anxiety, depression, and daytime sleepiness between male and female groups are presented in Appendix S9.
Fig. 4.
Network structure of stress, anxiety, depression, and daytime sleepiness symptoms among higher education students (N = 549). The left panel displays the visualized network structure, while the right panel provides the node identifiers along with abbreviations indicating their corresponding symptom dimensions.
Fig. 5.
The centrality indices of the network between daytime sleepiness, stress, anxiety, and depression.
Fig. 6.
The bridge centrality indices of the network between daytime sleepiness, stress, anxiety, and depression.
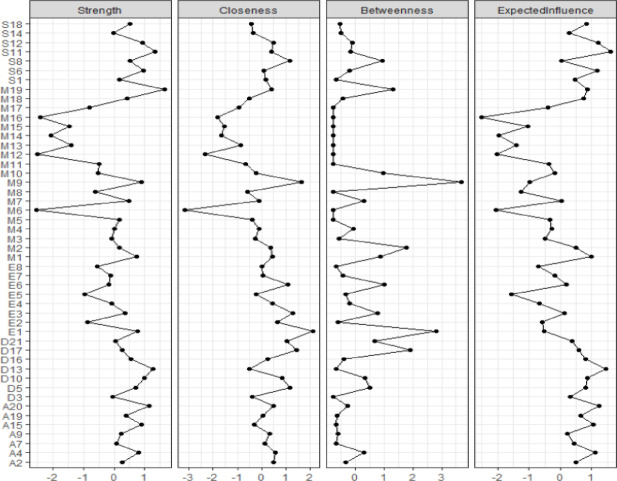
The network structure of chronotype, daytime sleepiness, and emotional distress
Figure 7 illustrates the network structure of sleep preference, daytime sleepiness, and mental health among higher education students. Out of 1128 edges, 325 non-zero edges were identified. The stability analysis of the edges and centrality indices also indicated good network stability (see Appendices S10 and S11). Specifically, the strongest connections are uneasiness with despair (S11-D10; r = 0.164), collapse with depression (A15-D13; r = 0.161), and mental fatigue with initiation difficulty (S8-D5; r = 0.157). Additionally, the symptoms with the highest strength are self-assessed chronotype (M19), uneasiness (S11), depression (D13), fear (A20), and despair (D10), as shown in Fig. 8. Figure 9 demonstrates that in the network of sleep preference, daytime sleepiness, and mental health, the central bridging symptoms are uneasiness (S11), depression (D13), worry (A9), fear (A20), initiation difficulty (D5), collapse (A15), restlessness (S1), shortness of breath (A4), overreaction (S6), and lack of self-worth (D17), with the bridging nodes’ strength showing strong stability (see Appendix S12). “Anxiety” and “depression” emerged as key nodes within this network, functioning not only as central symptoms but also as bridging symptoms. As such, the interactions among chronotype, daytime sleepiness, and emotional distress are closely linked to these two critical nodes. Furthermore, the results of the network comparison test for chronotype, daytime sleepiness, and emotional distress between male and female groups are presented in Appendix S13. The network data supports our hypothesis that there is a common symptom between emotional distress and daytime sleepiness and chronotype.
Fig. 7.
Network structure of chronotype, daytime sleepiness, and emotional distress among higher education students (N = 549). The left panel presents the visualized network structure, while the right panel lists the node identifiers and the abbreviations representing their respective symptom dimensions.
Fig. 8.
The centrality indices of the network between chronotype, daytime sleepiness, and emotional distress.
Fig. 9.

The bridge centrality indices of the network between chronotype, daytime sleepiness, and emotional distress.
Discussion
From the perspective of network analysis, the present study revealed the structural relationships among chronotype, daytime sleepiness, and emotional distress based on large-scale questionnaire data. We constructed three stable network models and identified shared symptoms across these networks. In the third and most comprehensive network model-chronotype, daytime sleepiness, and emotional distress-we identified tree central nodes including “Self-assessed chronotype”, “anxiety”, and “depression”. Among them, “Self-assessed chronotype” and “anxiety” emerged as the most strongly connected core symptoms, while “anxiety” and “depression” functioned as key bridge nodes linking distinct symptom clusters. In addition, descriptive statistical analyses revealed close associations between individuals’ chronotype and both daytime sleepiness and emotional distress. These findings contribute to a deeper understanding of the complex interplay among chronotype, daytime sleepiness, and emotional distress, and may inform early identification and intervention strategies for young individuals experiencing emotional difficulties or disorders.
Evening-type individuals are generally more prone to experiencing negative emotional states such as anxiety and depression. We found that chronotype scores were significantly negatively correlated with levels of stress, anxiety, and depression, indicating that individuals with a stronger tendency toward eveningness are more likely to experience negative emotional states. Our findings were consistent with previous studies. For instance, Silva (2020) and colleagues conducted a study among undergraduate students using the Morningness-Eveningness Questionnaire (MEQ) and the State-Trait Anxiety Inventory (STAI), and found that evening-type students exhibited significantly higher levels of state anxiety compared to their morning-type counterparts68. Moreover, previous research has consistently demonstrated that chronotype can contribute to physiological system dysregulation, which in turn increases vulnerability to emotional disturbances69–71. This finding further supports the link between chronotype and emotional distress. Furthermore, our results showed that both the duration and onset time of daytime napping were negatively associated with chronotype, suggesting that individuals who nap for longer periods or begin their naps earlier are more likely to exhibit evening-type tendencies. Our results are consistent with previous findings42. And our finding may be attributed to the fact that evening-type individuals, compared to morning types, often struggle to obtain sufficient sleep during the night. As a result, they may rely on longer daytime naps to compensate for sleep loss and restore energy. Additionally, this pattern could be influenced by social and environmental factors. Specifically, evening-type individuals tend to fall asleep later at night, but are required to wake up early on workdays due to fixed social schedules. This misalignment causes the midpoint of sleep on workdays to occur earlier than that on free days (e.g., weekends). The discrepancy between these two midpoints is referred to as “social jetlag”- a concept introduced by Roenneberg and colleagues, who defined it as the difference between biological time and socially imposed schedules across workdays and free days72. This misalignment, known as social jetlag, exacerbates the discrepancy between the internal biological clock and external social time, leading to dysregulation in the secretion of circadian hormones such as melatonin and cortisol. Such hormonal disruptions can impair emotional stability and contribute to the development of negative emotional states73,74. Mansour and colleagues also suggested that social jetlag, along with accumulated sleep debt on workdays, contributes to an increased frequency of daytime napping. There was a strong correlation between anxiety, depression, and stress, and network analyses revealed common symptoms across these conditions. In the network analysis of stress, anxiety and depression symptoms, core symptoms such as “uneasiness” in stress symptoms, “depression” in depression symptoms and “fear” in anxiety symptoms were identified. In addition, bridging symptoms in the network were identified, such as “uneasiness”, “worry” and “depression”.
In the network model comprising daytime sleepiness and three emotional states, anxiety, depression, and fear demonstrated the highest strength centrality. Moreover, anxiety, depression, and worry emerged as key bridge symptoms linking daytime sleepiness and emotional distress. These central nodes may play a pivotal role in shaping the overall structure and function of the network. Notably, anxiety exhibited both the highest centrality and served as a primary bridge between emotional symptoms and daytime sleepiness, suggesting a strong association between anxiety and excessive daytime sleepiness. This finding aligns with results reported by Demir, who observed that individuals experiencing heightened anxiety before bedtime were more likely to exhibit increased levels of daytime sleepiness75. Similar findings have also been observed in studies examining sleep quality and its influencing factors among university students76. We therefore speculate that anxiety may increase individuals’ vigilance during wakefulness, which adversely affects the quality of nocturnal sleep. Consequently, this disruption in nighttime rest may lead individuals to engage in daytime napping or dozing, even under conditions of relatively low daytime stress. Similar to anxiety, depressive symptoms also exhibit high strength centrality and serve as prominent bridge symptoms, indicating that depression plays a critical role in linking daytime sleepiness with emotional symptoms. Specifically, it connects stress, anxiety, and daytime sleepiness, a pattern consistent with the findings reported by Gonsalvez77. Both the current study and previous research provide growing evidence for a consistent and robust association between depressive symptoms and excessive daytime sleepiness78–80. The emergence of depressive symptoms is likely to trigger excessive daytime sleepiness. In addition, fear also exhibits high bridge centrality, suggesting that it serves as a key mediator linking daytime sleepiness with other psychological problems, such as anxiety and stress.
In the network model examining relationships among chronotype, daytime sleepiness, and emotional disturbances such as stress, depression, and anxiety, three core symptoms emerged: self-reported chronotype, anxiety, and depression. Among these, anxiety and depression served as key bridge symptoms, linking chronotype and daytime sleepiness with emotional disturbances and facilitating the propagation of influence across the network. Prior research has shown that misalignment between an individual’s actual sleep–wake schedule and their biological chronotype—such as when a naturally evening-type person is forced to adhere to an early morning work schedule—can result in sleep deprivation and chronotype disruption, ultimately contributing to the onset of negative emotional states. The identification of chronotype as a central symptom in the present study provides further support for this mechanism. Anxiety and depression, in turn, may represent the psychological manifestations of this temporal misalignment81. Moreover, uneasiness can be viewed as a subcomponent of stress-related emotional responses. Koch et al. (2017) investigated the interaction between chronotype and the stress regulatory system, revealing that their interplay has profound implications for overall health82. Lazarus et al. (1993) proposed that when individuals are confronted with uncertain or complex situations—conditions often associated with feelings of “uneasiness”—this mild unease may serve as the seed of psychological stress83. When stress intensifies, it can further amplify uneasiness across cognitive, physiological, and emotional domains84,85, creating a vicious cycle that may ultimately impair sleep quality or disrupt sleep patterns. Based on these findings, we hypothesize that uneasiness, as a derivative of stress, may function as a bridging factor between stress and chronotype. In addition, depression emerged in our network as a highly central and stable bridge symptom, suggesting that it plays a crucial role in linking chronotype, daytime sleepiness, and the three emotional disturbances, thereby facilitating their mutual influence.
The findings obtained through descriptive statistical analysis and network analysis in this study are consistent with the hypothesis of circadian rhythm disruption, which posits that persistent misalignment or disruption between an individual’s endogenous biological clock and external environmental cues (e.g., light exposure, work schedules, social rhythms), or self-regulated behaviors (e.g., sleep–wake patterns, diet, physical activity), can disturb neurophysiological homeostasis. Such disruptions may contribute to a range of health problems, particularly emotional disturbances and impairments in cognitive functioning86,87. As Roenneberg has noted, “modern humans are not sleeping too little, but at the wrong time.” This reflects his broader concept of “living against the clock”, which describes the imposition of societal schedules that override the natural rhythms of individual biological clocks88. “Living against the clock” is considered one of the core mechanisms underlying various modern health and emotional problems. Circadian misalignment is not merely a descriptive phenomenon; it may act as a mechanistic driver of emotional disorders such as depression and anxiety. Therefore, regulating chronotype and better aligning them with societal demands may be essential for mitigating emotional disturbances. Furthermore, patterns of daytime sleepiness—particularly the timing and duration of daytime naps—can serve as important indicators of an individual’s vulnerability to emotional dysregulation.
According to network theory, bridge symptoms play a pivotal role in the transmission of pathology across symptom domains. In our final network model, anxiety and depression emerged as key bridge symptoms, potentially reflecting a cascade of physiological-to-psychological effects triggered by circadian misalignment. Depressive symptoms often manifest as difficulties in falling asleep or early morning awakening—patterns closely associated with circadian disruption. Research has shown that individuals with depression frequently exhibit a phase advance of their biological clock, characterized by earlier melatonin secretion and sleep onset, yet with impaired sleep maintenance, leading to disrupted sleep architecture. Moreover, depression can alter hormonal rhythms, such as an abnormally early morning peak in cortisol secretion, which further exacerbates negative affect. These rhythm disturbances may contribute to a self-sustaining “emotion–physiology cycle”89,90. The role of anxiety and depression as bridges connecting chronotype, daytime sleepiness, and emotional distress suggests that targeting these emotional symptoms may indirectly alleviate rhythm-related dysfunctions. This insight highlights potential targets for early intervention and transdiagnostic treatment strategies. Future interventions for mood disorders may benefit from chronotype-based personalization. For instance, cognitive behavioral therapy tailored for evening-type individuals could incorporate light therapy, sleep phase shifting, and delayed activity scheduling. Similarly, psychological interventions such as CBT-I or mindfulness-based therapy may be effective in simultaneously improving circadian regulation and emotional well-being91,92.
This study still has some limitations, firstly, in this study, we recruited participants without screening for mental health conditions. Moreover, the sample consisted exclusively of individuals aged 18 to 35 who were receiving higher education, without inclusion of a broader range of ages or educational backgrounds. These factors limit the external validity of our findings and constrain the generalizability of the results. Future research could expand the sample to include more diverse populations in terms of age, educational background, and mental health status, in order to enhance the generalizability and applicability of the findings. Secondly, this study employed a cross-sectional design and did not conduct longitudinal tracking of participants’ sleep patterns and emotional distress. As a result, it is not possible to determine the causal direction among the variables—namely, daytime sleepiness, chronotype, and emotional distress. Therefore, future studies could adopt a longitudinal design to more robustly examine the dynamic interplay between sleep rhythms and emotional distress. Moreover, the data for this study were collected within a specific cultural context (China), without accounting for cross-cultural differences—such as social expectations, emotional expression and regulation, and sleep patterns—that may influence the results. Additionally, the study did not account for certain potential confounding variables, such as diet, physical activity, mental health history, and environmental factors, which could also affect the interpretation of the findings. Future research should aim to replicate and extend this work across different cultural contexts and systematically incorporate and control for key potential confounders. This would allow for a more thorough examination of how cultural differences and underlying variables influence the relationships among chronotype, daytime sleepiness, and emotional distress, thereby enhancing the credibility of the conclusions. Finally, our study relied on data collected through three different standardized questionnaires and involved extensive quantitative analysis. However, it lacked qualitative data collection methods—such as open-ended surveys or interviews—which limited our ability to gain deeper insights into participants’ experiences and perspectives on sleep and emotional well-being. Future research could incorporate qualitative methods, to obtain richer qualitative data and provide a more comprehensive understanding of how participants construct meaning around sleep and emotional well-being.
Future research could incorporate cultural factors into the investigation of daytime sleepiness, chronotype, and emotional distress, exploring whether variations in national or sociocultural environments influence these relationships. Additionally, it would be valuable to examine the impact of social rhythm pressures—such as academic demands—on circadian functioning, as well as the potential amplifying effect of cognitive biases on both sleep and emotional disturbances. Building on the current findings, future studies may also focus on developing and evaluating targeted interventions for emotion-related problems arising from sleep disturbances. Such interventions could include sleep hygiene education, cognitive-behavioral therapy, or chronotype training, with the goal of enhancing the effectiveness of treatments for emotional distress93,94.
Conclusion
In the present study, we analyzed the relationship and interaction involving sleep preference, daytime sleepiness, and emotional distress (stress, depression, and anxiety), through a network theory that can reveal the phenomenon of common symptoms. In the network between chronotype, daytime sleepiness, and the emotions of stress, depression, and anxiety, “biological clock type” and “restlessness” are the main symptoms in this network, and “restlessness” and “depression” are the strong bridging symptoms in the network. The network analyses in the present study provide good insights into the relationship between sleep preference, daytime sleepiness and emotional distress, and provide some evidence for intervention and prevention of negative emotion, which implies that we need to pay attention to “restlessness” and “depression” and intervene when necessary.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
W.Z.: Data curation, Methodology, Software, Writing- Review & Editing. Y.Z.: Writing- Original draft, Writing- Review & Editing. H.H.: Writing- Review & Editing. X.S.: Data curation. Z.W.: Data curation. M.L.: Methodology, Software, Writing- Review & Editing. H.D.: Methodology, Software, Writing- Review & Editing.
Funding
This work was supported by National Natural Science Foundation of China [grant number, 32300928]; The Ministry of Education of Humanities and Social Science project [grant number, 23YJC190003, 24YJC710029]; Natural Science Foundation of Sichuan Province [grant number, 2025ZNSFSC1023].
Data availability
All data, models, or codes used during the study are available from the corresponding author by request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenzhao Zhang and Yingqi Zhu contributed equally to this work.
Contributor Information
Min Li, Email: limin_evalee@163.com.
Haoran Dou, Email: haorandou@sicnu.edu.cn.
References
- 1.Tononi, G. & Cirelli, C. Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull.62(2), 143–150 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Banks, M. I. & Smith, P. H. Intracellular recordings from neurobiotin-labeled cells in brain slices of the rat medial nucleus of the trapezoid body. J. Neurosci.12(7), 2819–2837 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks, S. & Dinges, D. F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med.3(5), 519–528 (2007). [PMC free article] [PubMed] [Google Scholar]
- 4.He, J. et al. Effect of conditioned stimulus exposure during slow wave sleep on fear memory extinction in humans. Sleep38(3), 423–431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murkar, A. L. A. & de Koninck, J. Consolidative mechanisms of emotional processing in REM sleep and PTSD. Sleep Med. Rev.41, 173–184 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Schenker, M. T. et al. Sleep and fear conditioning, extinction learning and extinction recall: A systematic review and meta-analysis of polysomnographic findings. Sleep Med. Rev.59, 101501 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Palmer, C. A. et al. Sleep loss and emotion: A systematic review and meta-analysis of over 50 years of experimental research. Psychol. Bull.150(4), 440 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Trampe, D., Quoidbach, J. & Taquet, M. Emotions in everyday life. PLoS ONE10(12), e0145450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen, T. & Levin, R. Nightmares: A new neurocognitive model. Sleep Med. Rev.11(4), 295–310 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Talamini, L. M., Bringmann, L. F., de Boer, M. & Hofman, W. F. Sleeping worries away or worrying away sleep? Physiological evidence on sleep-emotion interactions. PLoS ONE8(5), e62480 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandekerckhove, M. et al. The role of presleep negative emotion in sleep physiology. Psychophysiology48(12), 1738–1744 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Chang, W. et al. Relationship between sleep-wake circadian Chronotype and anxiety and depression of medical students in a University in Wuhu city in 2020. Wei Sheng Yan Jiu = Journal of Hygiene Research51(3), 417–422 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Alvaro, P. K., Roberts, R. M. & Harris, J. K. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep36(7), 1059–1068 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manber, R. et al. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep31(4), 489–495 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCall, W. V. et al. Treatment of insomnia in depressed insomniacs: Effects on health-related quality of life, objective and self-reported sleep, and depression. J. Clin. Sleep Med.6(4), 322–329 (2010). [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneita, Y. et al. The relationship between depression and sleep disturbances: A Japanese nationwide general population survey. J. Clin. Psychiatry67(2), 196–203 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Wassing, R., Benjamins, J. S., Talamini, L. M., Schalkwijk, F. & Van Someren, E. J. Overnight worsening of emotional distress indicates maladaptive sleep in insomnia. Sleep42(4), zsy268 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Feng, P., Becker, B., Feng, T. & Zheng, Y. Alter spontaneous activity in amygdala and vmPFC during fear consolidation following 24 h sleep deprivation. Neuroimage172, 461–469 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Jung, T. & Noh, J. Alteration of fear behaviors in sleep-deprived adolescent rats: Increased fear expression and delayed fear extinction. Anim. Cells Syst.25(2), 83–92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seixas, A. A. et al. Linking emotional distress to unhealthy sleep duration: analysis of the 2009 National Health Interview Survey. Neuropsychiatr. Dis. Treat.11, 2425–2430 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krizan, Z., Boehm, N. A. & Strauel, C. B. How emotions impact sleep: A quantitative review of experiments. Sleep Med. Rev.74, 101890 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Borbély, A. A. Sleep regulation: circadian rhythm and homeostasis. In Sleep: Clinical and Experimental Aspects 83–103 (Springer, 1982). [Google Scholar]
- 23.Halberg, F., Halberg, E., Barnum, C. P. & Bittner, J. J. Physiologic 24-hour periodicity in human beings and mice, the lighting regimen and daily routine. Photoperiod. Relat. Phenom. Plants Anim.55, 803–878 (1959). [Google Scholar]
- 24.Mathew, A. R., Pettit, J. W., Lewinsohn, P. M., Seeley, J. R. & Roberts, R. E. Co-morbidity between major depressive disorder and anxiety disorders: shared etiology or direct causation?. Psychol. Med.41(10), 2023–2034 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park, C. I. et al. Relationships between chronotypes and affective temperaments in healthy young adults. J. Affect. Disord.175, 256–259 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Leerssen, J. et al. Cognitive, behavioral, and circadian rhythm interventions for insomnia alter emotional brain responses. Biol. Psychiatry Cogn. Neurosci. Neuroimaging9(1), 60–69 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Lau, E. Y. Y. et al. Effects of REM sleep during a daytime nap on emotional perception in individuals with and without depression. J. Affect. Disord.260, 687–694 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Schäfer, S. K. et al. Sleep’s impact on emotional recognition memory: A meta-analysis of whole-night, nap, and REM sleep effects. Sleep Med. Rev.51, 101280 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Dinges, D. F. & Broughton, R. J. Sleep and alertness: Chronobiological, behavioral, and medical aspects of napping (1989).
- 30.Borsboom, D. Psychometric perspectives on diagnostic systems. J. Clin. Psychol.64(9), 1089–1108 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Borsboom, D. & Cramer, A. O. Network analysis: An integrative approach to the structure of psychopathology. Annu. Rev. Clin. Psychol.9(1), 91–121 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Borsboom, D. A network theory of mental disorders. World Psychiatry16(1), 5–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cramer, A. O. et al. Major depression as a complex dynamic system. PLoS ONE11(12), e0167490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNally, R. J. et al. Mental disorders as causal systems: A network approach to posttraumatic stress disorder. Clin. Psychol. Sci.3(6), 836–849 (2015). [Google Scholar]
- 35.Cramer, A. O., Waldorp, L. J., Van Der Maas, H. L. & Borsboom, D. Comorbidity: A network perspective. Behav. Brain Sci.33(2–3), 137–150 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Kaiser, T., Herzog, P., Voderholzer, U. & Brakemeier, E. L. Unraveling the comorbidity of depression and anxiety in a large inpatient sample: Network analysis to examine bridge symptoms. Depress. Anxiety38(3), 307–317 (2021). [DOI] [PubMed] [Google Scholar]
- 37.Fried, E. I. & Cramer, A. O. Moving forward: Challenges and directions for psychopathological network theory and methodology. Perspect. Psychol. Sci.12(6), 999–1020 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Meuret, A. E., Tunnell, N. & Roque, A. Anxiety disorders and medical comorbidity: treatment implications. In Anxiety Disorders: Rethinking and Understanding Recent Discoveries 237–261 (2020). [DOI] [PubMed]
- 39.Li, K. et al. Network analysis of the relationship between negative life events and depressive symptoms in the left-behind children. BMC Psychiatry21, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, K. et al. The Ripple Effect: Unveiling the bidirectional relationship between negative life events and depressive symptoms in medical cadets. Psychol. Res. Behav. Manag.16, 3399–3412 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aschoff, J. Naps as integral parts of the wake time within the human sleep-wake cycle. J. Biol. Rhythms9(2), 145–155 (1994). [DOI] [PubMed] [Google Scholar]
- 42.Gaina, A. et al. Morning-evening preference: Sleep pattern spectrum and lifestyle habits among Japanese junior high school pupils. Chronobiol. Int.23(3), 607–621 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Rahafar, A., Mohamadpour, S. & Randler, C. Napping and morningness-eveningness. Biol. Rhythm. Res.49(6), 948–954 (2018). [Google Scholar]
- 44.Jordan, D. G. et al. Investigating sleep, stress, and mood dynamics via temporal network analysis. Sleep Med.103, 1–11 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Org.79(4), 373 (2001). [PMC free article] [PubMed] [Google Scholar]
- 46.Horne, J. A. & Östberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol.4(2), 97–110 (1976). [PubMed] [Google Scholar]
- 47.Adan, A. & Almirall, H. Horne & Östberg morningness-eveningness questionnaire: A reduced scale. Personal. Individ. Differ.12(3), 241–253 (1991). [Google Scholar]
- 48.Roenneberg, T. & Merrow, M. Entrainment of the human circadian clock. In Cold Spring Harbor Symposia on Quantitative Biology Vol. 72 293–299 (Cold Spring Harbor Laboratory Press, 2007). [DOI] [PubMed] [Google Scholar]
- 49.Zhang, B., Hao, Y. & Rong, R. Reliability and validity of the Morningness-Eveningness Questionnaire. Chin. J. Behav. Med. Sci.15(9), 856–858 (2006). [Google Scholar]
- 50.Johns, M. W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep14(6), 540–545 (1991). [DOI] [PubMed] [Google Scholar]
- 51.Chen, N. H. et al. Validation of a Chinese version of the Epworth sleepiness scale. Qual. Life Res.11, 817–821 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Johns, M. W. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep15(4), 376–381 (1992). [DOI] [PubMed] [Google Scholar]
- 53.Wang, B.-Q., Li, X.-Y., Chuang, M.-L., Huang, Y.-S., Chen, Y.-H., Chiu, K.-L., & Chen, N.-H. A study on the reliability and validity of the Chinese version of the Epworth Sleepiness Scale (Doctoral dissertation) (2003).
- 54.Lovibond, S. H. Manual for the depression anxiety stress scales. Psychology Foundation of Australia (1995).
- 55.Gong, X., Xie, X., Xu, R. & Luo, Y. Psychometric properties of the Chinese version of the Depression Anxiety Stress Scale-21 (DASS-21) in Chinese college students. Chin. J. Clin. Psychol.18(4), 443–446 (2010). [Google Scholar]
- 56.Henry, J. D. & Crawford, J. R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol.44(2), 227–239 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Osman, A. et al. The Depression Anxiety Stress Scales-21 (DASS-21): Further examination of dimensions, scale reliability, and correlates. J. Clin. Psychol.68(12), 1322–1338 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Chen, J. & Chen, Z. Extended Bayesian information criteria for model selection with large model spaces. Biometrika95(3), 759–771 (2008). [Google Scholar]
- 59.Friedman, J., Hastie, T. & Tibshirani, R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics9(3), 432–441 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Epskamp, S., Borsboom, D. & Fried, E. I. Estimating psychological networks and their accuracy: A tutorial paper. Behav. Res. Methods50, 195–212 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foygel, R. & Drton, M. Extended Bayesian information criteria for Gaussian graphical models. Adv. Neural Inf. Process. Syst. 23 (2010).
- 62.Epskamp, S., Cramer, A. O., Waldorp, L. J., Schmittmann, V. D. & Borsboom, D. qgraph: Network visualizations of relationships in psychometric data. J. Stat. Softw.48, 1–18 (2012). [Google Scholar]
- 63.Robinaugh, D. J., LeBlanc, N. J., Vuletich, H. A. & McNally, R. J. Network analysis of persistent complex bereavement disorder in conjugally bereaved adults. J. Abnorm. Psychol.123(3), 510 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beard, C. et al. Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychol. Med.46(16), 3359–3369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bringmann, L. F. et al. What do centrality measures measure in psychological networks?. J. Abnorm. Psychol.128(8), 892 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Epskamp, S., Maris, G., Waldorp, L. J. & Borsboom, D. Network psychometrics. The Wiley Handbook of Psychometric Testing: A Multidisciplinary Reference on Survey, Scale and Test Development 953–986 (2018).
- 67.Robinaugh, D. J., Hoekstra, R. H., Toner, E. R. & Borsboom, D. The network approach to psychopathology: A review of the literature 2008–2018 and an agenda for future research. Psychol. Med.50(3), 353–366 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silva, V. M., Magalhaes, J. E. D. M. & Duarte, L. L. Quality of sleep and anxiety are related to circadian preference in university students. PLoS ONE15(9), e0238514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alvaro, P. K., Roberts, R. M. & Harris, J. K. The independent relationships between insomnia, depression, subtypes of anxiety, and chronotype during adolescence. Sleep Med.15(8), 934–941 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Landgraf, D., McCarthy, M. J. & Welsh, D. K. Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Curr. Psychiatry Rep.16, 1–11 (2014). [DOI] [PubMed] [Google Scholar]
- 71.Landgraf, D. et al. Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biol. Psychiat.80(11), 827–835 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wittmann, M., Dinich, J., Merrow, M. & Roenneberg, T. Social jetlag: misalignment of biological and social time. Chronobiol. Int.23(1–2), 497–509 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Henderson, S. E., Brady, E. M. & Robertson, N. Associations between social jetlag and mental health in young people: A systematic review. Chronobiol. Int.36(10), 1316–1333 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Wulff, K., Gatti, S., Wettstein, J. G. & Foster, R. G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci.11(8), 589–599 (2010). [DOI] [PubMed] [Google Scholar]
- 75.Demir, G. Daytime sleepiness and related factors in nursing students. Nurse Educ. Today59, 21–25 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Aysan, E., Karakose, S., Zaybak, A. & Ismailoglu, E. G. Sleep quality among undregraduate students and influencing factors. Deuhyo Ed7(3), 193–198 (2014). [Google Scholar]
- 77.Gonsalvez, I., Li, J. J., Stevens, C., Chen, J. A. & Liu, C. H. Preexisting depression and daytime sleepiness in women and men. Behav. Sleep Med.20(4), 380–392 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bixler, E. O. et al. Excessive daytime sleepiness in a general population sample: The role of sleep apnea, age, obesity, diabetes, and depression. J. Clin. Endocrinol. Metab.90(8), 4510–4515 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Chellappa, S. L., Schröder, C. & Cajochen, C. Chronobiology, excessive daytime sleepiness and depression: Is there a link?. Sleep Med.10(5), 505–514 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Zhang, D., Zhang, Z., Li, H. & Ding, K. Excessive daytime sleepiness in depression and obstructive sleep apnea: More than just an overlapping symptom. Front. Psych.12, 710435 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levandovski, R. et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int.28(9), 771–778 (2011). [DOI] [PubMed] [Google Scholar]
- 82.Koch, C. E., Leinweber, B., Drengberg, B. C., Blaum, C. & Oster, H. Interaction between circadian rhythms and stress. Neurobiol. Stress6, 57–67 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazarus, R. S. From psychological stress to the emotions: A history of changing outlooks. Annu. Rev. Psychol.44, 1–22 (1993). [DOI] [PubMed] [Google Scholar]
- 84.Borkovec, T. D., Robinson, E., Pruzinsky, T. & DePree, J. A. Preliminary exploration of worry: Some characteristics and processes. Behav. Res. Ther.21(1), 9–16 (1983). [DOI] [PubMed] [Google Scholar]
- 85.Boden, M. T. & Berenbaum, H. Emotional responding to emotional information: The role of perceived emotional control in stress and anxiety. Cogn. Ther. Res.31(4), 547–559 (2007). [Google Scholar]
- 86.Salgado-Delgado, R., Tapia Osorio, A., Saderi, N. & Escobar, C. Disruption of circadian rhythms: A crucial factor in the etiology of depression. Depress. Res. Treat.2011(1), 839743 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walker, W. H. II., Walton, J. C. & Nelson, R. J. Disrupted circadian rhythms and mental health. Handb. Clin. Neurol.179, 259–270 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roenneberg, T. Internal Time: Chronotypes, Social Jet Lag, and Why You’re So Tired (Harvard University Press, 2012). [Google Scholar]
- 89.Daut, R. A. & Fonken, L. K. Circadian regulation of depression: A role for serotonin. Front. Neuroendocrinol.54, 100746 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Germain, A. & Kupfer, D. J. Circadian rhythm disturbances in depression. Hum. Psychopharmacol. Clin. Exp.23(7), 571–585 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Asarnow, L. D. et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: A report from the TRIAD study. J. Clin. Sleep Med.15(4), 573–580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schulte, P. F. Evening chronotype, disordered eating behavior, bipolar disorder, and light therapy. Acta Psychiatr. Scand.143(2), 181 (2021). [DOI] [PubMed] [Google Scholar]
- 93.Bei, B., Ong, J. C., Rajaratnam, S. M. & Manber, R. Chronotype and improved sleep efficiency independently predict depressive symptom reduction after group cognitive behavioral therapy for insomnia. J. Clin. Sleep Med.11(9), 1021–1027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Okul, E. B. & Fertelli, T. K. The effects of sleep hygiene education on sleep quality, pain, and depression in individuals with fibromyalgia. Pain Manag. Nurs.26, 282–289 (2024). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, models, or codes used during the study are available from the corresponding author by request.