Abstract
Marine triterpene glycosides are known to exhibit significant anticancer activity. We investigated pacificusoside C and cucumariosides C1 and C2 isolated from a sea star Solaster pacificus in prostate cancer models with varying drug resistance and in non-cancerous cells in vitro. Cucumarioside C1 showed selectivity comparable to cisplatin, whereas the other compounds were less selective. Cucumarioside C1 induced apoptosis and enhanced cytotoxic effects of cisplatin, carboplatin, docetaxel, and cabazitaxel, making it a potential candidate for combination therapy. All three glycosides were active in docetaxel-resistant cells and were neither inhibitors nor substrates of P-glycoprotein, indicating P-glycoprotein-independent activity. To explore the mechanism of anticancer activity of cucumarioside C1, we performed functional kinome profiling of treated 22Rv1 cells, predicting activation of kinases involved in stress response and survival (IKKα, IKKβ, IKKε), necroptosis (MLKL), metabolism (GCN2, PDK1), cytoskeletal dynamics (RHOK), mitophagy (PINK1), apoptosis and cell cycle regulation (PITSLRE), and immune modulation (COT). Notably, only MAP kinases p38 and ERK1/2 were predicted to be specifically inhibited, that was further validated by Western blotting. These findings may potentially explain previously reported anticancer effects of cucumarioside C1 and related marine glycosides. To our knowledge, this is the first study to report triterpene glycosides’ effects on the kinome of cancer cells.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-12914-7.
Keywords: Triterpene glycosides, Prostate cancer, Solaster pacificus, Marine natural compounds, P-glycoprotein, MAPK, Drug resistance
Subject terms: Prostate cancer, Biochemistry, Cancer, Drug discovery
Introduction
Prostate cancer is the most frequently diagnosed malignancy in men, with an annual incidence exceeding 1.3 million cases worldwide. It ranks among the top five causes of cancer-related mortality globally1. While advanced prostate cancer in the hormone-sensitive setting can often be controlled with androgen deprivation therapy (ADT) in combination with androgen receptor (AR) signaling pathway inhibitors (ARPI), with or without the addition of chemotherapy for several years, disease progression to castration resistance eventually occurs in the majority of patients2. Here, live prolonging therapies including ARPIs (enzalutamide or abiraterone), PARP inhibitors (olaparib), taxane-based chemotherapy (docetaxel or cabazitaxel), and radioligandtherapy can be offered3,4. With an increasing number of therapy lines, resistance develops more rapidly, often leading to a shorter treatment duration. The primary mechanisms of therapy resistance include AR alterations (AR splice variants, mutations, amplifications), a shift to AR-independent growth, overexpression of efflux transporters such as P-glycoprotein, alterations in tubulin dynamics, epithelial-to-mesenchymal transition (EMT), modifications to the tumor microenvironment, as well as activation of pro-survival pathways, including PI3K/AKT/mTOR, JAK/STAT, and TGF-β5. Some patients develop a particularly aggressive form called aggressive variant of prostate cancer (AVPC). AVPCs are highly metastatic and resistant to most conventional therapies6. Therapeutic options for AVPC include platinum-based agents (e.g. cisplatin and carboplatin); however, due to the therapy’s limited efficacy, the disease ultimately progresses, leading to the patient’s demise. Therefore, despite recent progress in prostate cancer therapy, there is an urgent need for novel therapeutic strategies for managing drug-resistant prostate cancer.
Marine-derived bioactive compounds represent a promising reservoir for novel anticancer agents7,8. Many naturally occurring substances, even those with well-characterized structures, continue to reveal novel biological activities and targets in both in vitro and in vivo models9. Marine triterpene glycosides are a class of secondary metabolites found mainly in sea cucumbers (holothurians) and marine sponges10. These molecules are characterized by a broad spectrum of biological activities, including cytotoxic, ichthyotoxic, antimicrobial, antiviral, radioprotective, and immunomodulatory properties. Triterpene glycosides are currently used in various pharmaceutical formulations (e.g. vaccine adjuvant QS-21), functional food products, dietary supplements, and cosmetic applications11,12. Triterpene glycosides are occasionally isolated from sea stars13,14. These secondary metabolites are rarely produced by sea stars themselves and are likely acquired through their diet. The sea star Solaster pacificus was identified as a rich source of various triterpene glycosides, as this echinoderm species primarily feeds on holothurians15–17. It is also worth noting that another group of oligoglycosides, steroidal saponins, commonly called asterosaponins, was also found in sea stars18.
The antitumor potential of marine-derived triterpene glycosides is of particular interest. The anticancer activity of these natural compounds is primarily mediated via two major mechanisms. First, their membranotropic properties enable the disruption of cancer cell membranes, which further triggers apoptosis executed via both intrinsic and extrinsic pathways19,20. Second, these compounds can modulate several oncogenic signaling pathways, including NF-κB, PI3K/Akt/mTOR, MAPK, and VEGF/VEGFR, thereby inducing cell cycle arrest, inhibition of proliferation, and suppression of angiogenesis and metastasis20–22. Structure–activity relationship (SAR) studies have revealed that both the glycone (sugar moieties) and aglycone side chains are critical determinants of biological activity. In particular, the pattern and degree of sulfation significantly influence cytotoxic potency, selectivity, and the ability to interact with cellular membranes10,23.
Previous studies have demonstrated that these compounds inhibit the proliferation of various cancer types, with some showing activity in prostate cancer models. Thus, triterpene saponins cercodemasoides A–E (sea cucumber Cercodemas anceps), stichorrenosides A–D (sea cucumber Stichopus horrens), cucumarioside A2-2 (sea cucumber Cucumaria japonica), and echinoside A (sea cucumber Actinopyga echinites) exhibited cytotoxic activity in prostate cancer models both in vitro and in vivo24–26. Additionally, frondoside A (sea cucumber Cucumaria frondosa) was found to suppress prostate cancer tumor growth, to inhibit metastatic spread to the lungs, as well as to decrease the number of circulating tumor cells in the blood via modulation of cell cycle, apoptosis, and autophagy, as well as by stimulation of anticancer immunity27. Saponins are known to exert their cytotoxic effects primarily by targeting the cholesterol of the cellular membrane, forming complexes, and therefore physically disrupting the membrane permeability and integrity28. However, evidence suggests that saponins target additional biological pathways relevant to their anticancer activity29.Triterpene glycosides from the sea star Solaster pacificus have demonstrated anticancer and cancer-preventive properties. For example, pacificusoside M effectively suppressed the colony formation of colorectal carcinoma HCT 116 and breast cancer MDA-MB-231 cells at a non-toxic concentrations. Additionally, pacificusoside M showed a significant anti-invasive potential in an in vitro model17. Pacificusoside D and cucumarioside D (sea star S. pacificus) exhibited potent cytotoxicity against human melanoma SK-MEL-2, SK-MEL-28, and RPMI-7951 cell lines, and could inhibit a colony formation of cancer cells at non-toxic concentrations. Both compounds at their non-toxic concentrations inhibited neoplastic cell transformation of JB6 Cl41 cells induced by epidermal growth factor (EGF), 12-O-tetradecanoilphorbol-13-acetate (TPA), and ionizing radiation (X-rays and UVB)16. Finally, pacificusoside C, cucumariosides C1 and C2 from the sea star S. pacificus strongly suppressed the colony formation of the HT-29, RPMI-7951, and MDA-MB-231 cells at nontoxic concentrations15. However, the mechanism of anticancer activity of the triterpene glycosides from sea star S. pacificus is still unclear. Therefore, investigating the molecular mechanisms underlying the anticancer activity of pacificusoside C and cucumariosides C1 and C2 is of high interest.
Results
Cytotoxic activity of isolated triterpene glycosides in human prostate cancer cells
The triterpene glycosides pacificusoside C (PaC), cucumarioside C1 (CuC1), and cucumarioside C2 (CuC2) were isolated from the sea star S. pacificus and purified as previously described (Fig. 1)15. The anticancer properties of the isolated compounds were evaluated as their cytotoxic activity in a series of human prostate cancer and non-cancer cell lines using an MTT assay. Specifically, we tested their effects on prostate cancer cell lines PC3, PC3-DR, DU145, VCaP, 22Rv1, and LNCaP, as well as on non-malignant human cell lines PNT2, RWPE-1, MRC-9, and HEK293T15. Among these, LNCaP represents a hormone-sensitive cell line, whereas the other cancer cell lines exhibit resistance to androgen synthesis inhibitor abiraterone and androgen receptor (AR)-inhibitors such as enzalutamide. In 22Rv1 and VCaP cells, this resistance is mediated by the expression of AR-V7 (AR splice variant 7), which lacks an androgen-binding site, leading to constitutive activation of the AR pathway30,31. In contrast, PC3, PC3-DR, and DU145 cells do not express AR and proliferate independently of androgens30. The PC3-DR subline represents docetaxel-resistant cells derived by gradually increasing exposure of PC3 cells to this docetaxel32.
Fig. 1.
Chemical structures of pacificusoside C (PaC), cucumariosides C1 (CuC1), and C2 (CuC2) isolated from sea star S. pacificus.
The investigated compounds demonstrated cytotoxic effects in all evaluated cancer cell lines at low micro- and nanomolar concentrations, with the most pronounced activity observed in 22Rv1 and VCaP cells (Table 1). Interestingly, hormone-refractory PC3 and DU145 cells exhibited sensitivity comparable to hormone-sensitive LNCaP cells (Table 1). Docetaxel is a chemotherapeutic agent often used for treatment of castration-resistant prostate cancer3,4. Remarkably, PC3-DR cells, that demonstrated 35-fold reduced sensitivity to docetaxel compared to parental PC3 cells, displayed only a minor (2–to–threefold higher compared to PC3 cells) resistance to the triterpene glycosides (Table 1). This suggests a minimal cross-resistance between the tested compounds and docetaxel (Table 1). Furthermore, the cytotoxic activity of the natural compounds was comparable to the activity of cisplatin, a drug that is commonly used for the treatmen of AVPC33 (Table 1). We further analyzed activity of the isolated marine triterpene glycosides cancer cells versus non-cancer cells. In our in vitro experiments, the investigated triterpene glycosides were slightly more cytotoxic to non-cancer cells and therefore did not exhibit any pronounced selectivity to cancer cells. The selectivity index of CuC1 was comparable to that of cisplatin (0.7 and 0.88, respectively), whereas PaC and CuC2 had lower selectivity indexes (Table 1).
Table 1.
Cytotoxicity of PaC, CuC1, and CuC2 observed in human prostate cancer and human non-cancer cell lines.
| IC50 ([μM]* or [nM]**) | SI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer cells | Non-cancer cells | ||||||||||
| PC3 | PC3-DR | DU145 | 22Rv1 | VCaP | LNCaP | PNT2 | RWPE-1 | HEK293T | MRC-9 | ||
| PaC* | 5.68 ± 0.32 | 13.60 ± 4.22 | 15.50 ± 2.13 | 1.16 ± 0.07 | 1.86 ± 0.12 | 8.24 ± 1.07 | 3.03 ± 0.24 | 4.50 ± 0.23 | 2.85 ± 0.13 | 2.11 ± 0.27 | 0.43 |
| CuC1* | 1.59 ± 0.12 | 5.69 ± 0.78 | 3.24 ± 0.34 | 0.246 ± 0.017 | 0.472 ± 0.065 | 3.0 ± 0.54 | 0.754 ± 0.058 | 0.554 ± 0.105 | 0.66 ± 0.05 | 0.959 ± 0.248 | 0.7 |
| CuC2* | 2.21 ± 0.14 | 6.2 ± 1.47 | 2.69 ± 0.19 | 0.623 ± 0.035 | 0.487 ± 0.042 | 2.18 ± 0.19 | 1.270 ± 0.054 | 0.897 ± 0.066 | 1.59 ± 0.10 | 0.799 ± 0.062 | 0.48 |
| Doce** | 11.40 ± 3.71 | 388 ± 49.5 | 2.20 ± 0.61 | 0.604 ± 0.122 | 0.225 ± 0.215 | 5.06 ± 0.58 | > 500 | 0.423 ± 0.225 | 11.49 ± 3.22 | > 500 | 10.3 |
| Cis* | 34.60 ± 6.62 | 6.89 ± 2.29 | 1.32 ± 0.31 | 0.993 ± 0.2 | 5.02 ± 2.73 | 2.75 ± 0.39 | 9.41 ± 1.82 | 9.02 ± 1.52 | 6.44 ± 0.65 | 6.75 ± 2.15 | 0.88 |
Cells were treated with the investigated compounds for 48 h, viability was evaluated using MTT assay, and IC50 values were calculated using GraphPad Prism software. Docetaxel (Doce) and cisplatin (Cis) were used as positive controls. The values are presented as mean ± standard deviation in μM (PaC, CuC1, CuC2, and Cis) or (**) in nM (Doce). Selectivity index (SI) was calculated as [mean IC50 in non-cancer PNT2, RWPE-1, HEK293T, and MRC-9 cells] / [mean IC50 in cancer PC3, 22Rv1, DU145, VCaP, LNCaP cells]. The experiment was performed in triplicates (number of biological replicates n = 3). R2 values (goodness-of-fit) for the dose–response curves used to calculate IC₅₀ values are presented in the Supplementary Table S1.
Thus, CuC1 was the most active compound among the tested triterpene glycosides. Therefore, it was chosen for further experiments as the most promising molecule. To tackle the mode of action of CuC1 we chose highly aggressive hormone- and docetaxel-refractory PC3-DR cells, as well as 22Rv1 cells, which represent a hormone-refractory AR-V7-positive prostate cancer subtype.
CuC1 affects cancer cells independently of their P-glycoprotein status
Next, we investigated the low-grade cross-resistance to CuC1, observed in the docetaxel-resistant PC3-DR cell model (Table 1). A major contributor to docetaxel resistance is P-glycoprotein (also known as P-gp, MDR1), known to be overexpressed in PC3-DR cells34. P-glycoprotein is a molecular efflux pump that actively expels various xenobiotic agents, including docetaxel, from cancer cells35. Increased P-gp expression correlates with an increased IC50 for docetaxel, signifying enhanced drug resistance36. The observation that PC3 and PC3-DR cells exhibit similar sensitivity to the investigated compounds suggests that these drugs are not P-gp substrates (Table 1). To further validate this, we evaluated the effect of the isolated triterpene glycosides on P-gp activity using a calcein-AM exclusion assay. In this assay, the non-fluorescent calcein-AM passively diffuses into cells, where it is hydrolyzed by cellular esterases into fluorescent calcein. Both calcein and its precursor are P-gp substrates and are actively transported out of P-gp-highly expressing cells (e.g. PC3-DR cells), which results in reduced intracellular fluorescence. P-gp inhibitors, such as tariquidar, block P-gp function, leading to calcein accumulation and an increase in fluorescence, which therefore can be used as a reliable indicator of reduced P-gp activity37. A similar fluorescence increase is observed in the presence of docetaxel due to its competitive binding to the P-gp transporter alongside calcein38.
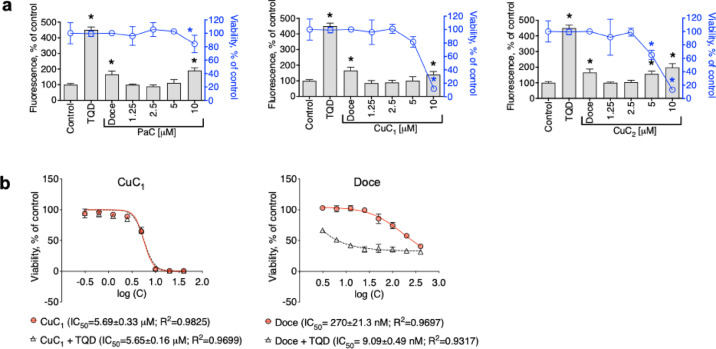
In our experiments, incubation of PC3-DR cells with CuC1 at non-cytotoxic concentrations did not result in any significant changes in calcein-AM efflux, whereas docetaxel- or tariquidar-treated cells expectedly affected P-gp activity (Fig. 2a) via the mechanisms discussed above. At the same time, an increase of intracellular fluorescence was observed at high cytotoxic concentrations of CuC1, which normally indicates an interrupted calcein excretion out of the cells due to the disruption of cellular membrane integrity by a cytotoxic agent. Notably, similar effects were also observed for PaC and CuC2 (Fig. 2a). This data suggests the investigated glycosides to be neither P-gp substrates nor P-gp inhibitors. In line with this observation, co-treatment of cells with tariquidar did not alter cytotoxicity of CuC1 in PC3-DR cells, whereas the activity of docetaxel was expectedly dramatically affected resulting in 30-fold decrease of IC50 (Fig. 2b)39. Taken together, cytotoxicity of CuC1 and related compounds was exerted independently of P-gp status, and therefore the compound was active in P-gp-overexpressing cancer cells.
Fig. 2.
Effect of the triterpene glycosides on P-gp activity in PC3-DR cells. (a), The effect of the investigated compounds on P-gp activity (grey bars) and cell viability (blue graph). PC3-DR cells were treated with the drugs for 30 min, and P-gp activity was evaluated using the calcein-AM assay, while cellular viability was determined via the MTT test in the same experimental conditions. P-gp inhibitor tariquidar (TQD, 50 nM) and a P-gp substrate docetaxel (Doce, 100 nM) were used as positive controls. Statistically significant difference from control was determined using a one-way ANOVA test, *p < 0.05. Number of biological replicates n = 3. (b), The effect of TQD on cytotoxic activity of CuC1 and Doce. PC3-DR cells were pre-treated with TQD (50 nM) for 1 h and then co-treated with CuC1 or Doce for another 48 h. Viability was evaluated using MTT assay, and IC50s were calculated using GraphPad Prism software. Number of biological replicates n = 3.
Effect of CuC1 on serine/threonine kinases in prostate cancer cells
Protein kinases regulate cellular processes through phosphorylation, with serine/threonine kinases (STKs) playing critical roles in cell death and survival. Several FDA-approved anticancer agents, including palbociclib, trametinib, and selumetinib target STKs40. Therefore, we employed functional kinome profiling using the PamTechnology platform (http://www.pamgene.com) to identify the STKs affected in prostate cancer cells treated with CuC1. This approach utilizes exposure of a set of immobilized peptide STK substrates to cell lysates, and changes in peptide phosphorylation patterns indicate alterations in the activity of respective kinases.
We intended to identify the primary/early effects of the drug. Therefore, to minimize secondary events resulting from cell death, short-term drug exposure was applied. For this assay, we used 22Rv1 cancer cells as a model, as this cell line was most sensitive to CuC1 (Table 1). We demonstrated that treatment with CuC1 for 2 h at twice the IC50 concentration (0.5 μM, selected to maximize the effect on kinome at a short exposure time) did not induce any signs of cell death in 22Rv1 cells. However, after 24 h of treatment, apoptotic markers such as cleaved PARP and caspase-3 were detected (Fig. 3a). Thus, short-term treatment regime (0.5 μM, 2 h) was further used to identify the affected STKs. The results of kinome profiling analysis were represented as log2-transformed peptide signal intensities of treated samples compared to control (Fig. 3b). Further upstream kinase analysis suggested alteration of the activity of multiple kinases following CuC1 treatment. This included activation of kinases involved in stress response and cell survival pathways (IKKα, IKKβ, IKKε), necroptosis (MLKL), metabolic regulation (GCN2, PDK1), cytoskeletal dynamics and migration (RHOK), mitophagy (PINK1), apoptosis and cell cycle regulation (PITSLRE), inflammatory and immune modulation (COT) (Fig. 3c). Notably, few STKs identified as MAP kinases, namely, p38, ERK1/2, and JNK1/2 were predicted to be significantly inhibited by the drug (Fig. 3c).
Fig. 3.
Functional kinome profiling of STKs (serine/threonine kinases) in 22Rv1 cells and validation of results. (a) Expression of apoptotic markers. The cells were treated with CuC1 for the indicated time, and the expression of proteins was analyzed by Western blotting. (b, c) Functional kinome profiling. A heatmap displaying log2-transformed phosphorylation levels of 99 analyzed STK substrate peptides (b) and upstream kinase analysis, which predicted kinases affected by CuC1 (c). Values of the normalized kinase statistics represented by bars, where log2 < 0 indicates decreased kinase activity and log2 > 0 denotes increased activity. Statistically significant changes having a specificity score log2 > 1.3 are highlighted by red bars. Number of biological replicates n = 3. (d), Western blot analysis of MAP kinases. 22Rv1 cells were treated with CuC1 for 2 h and the MAPK expression was analyzed by Western blotting. β-actin was used as a loading control. The band intensity of the protein of interest was quantified using Image Lab software and normalized to the band intensity β-actin (a), or to the band intensity of non-phospho MAPK (d), and is indicated by red numbers. Full-size blots are presented in Supplementary Fig. S2.
The vast majority of modern anticancer drugs used in the clinic are kinase inhibitors41. Hence, we focused on STKs predicted to be inhibited by CuC1. To validate the findings of functional kinome profiling, Western blot analysis was employed. Thus, we could confirm an inhibition of active (phosphorylated) forms of ERK1/2 and p38, but not JNK1/2, in cells exposed to the drug for 2 h (Fig. 3d). This was confirmed by analysis of phosphor-MAPK / MAPK ratio (Fig. 3d). Both ERK1/2 and p38 kinases were previously associated with tumor growth, invasion as well as drug resistance42,43. Notably, saponin from bark of the Quillaja saponaria tree, which was used as a reference compound, had a distinct effect on the kinome of cells and induced an activation of ERK1/2 kinase in similar experimental conditions, whereas no significant effect on p38 kinase was registered (Supplementary Fig. S1).
CuC1 potentiates cytotoxicity of taxanes and platinum agents in prostate cancer cells
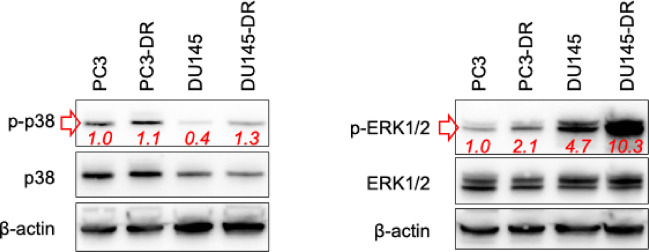
Next, we evaluated the potential clinical relevance of CuC1 in combination with several approved chemotherapeutics. Taxanes are commonly used in the treatment of advanced prostate cancer, whereas platinum-based therapies demonstrate significant efficacy in patients with DNA repair deficiencies or AVPC33. Moreover, cisplatin (Cis) and carboplatin (Carbo) were reported to activate all three MAPKs, whereas docetaxel (Doce) and cabazitaxel (Caba) activated p38 and JNK1/2 while inhibiting ERK1/2 in prostate cancer cells44. Additionally, persistent ERK1/2 signaling was identified to mediate taxane-resistance in prostate cancer45. Thus, we first showed that p38 and ERK1/2 kinases are overactivated in the taxane-resistant PC3-DR and DU145-DR prostate cancer cells compared to their drug-sensitive counterparts (Fig. 4). Then, we investigated the effects of CuC1 in combination with Cis, Carbo, Doce, and Caba in prostate cancer cells. Accordingly, we used docetaxel- and hormone-resistant PC3-DR cells as well as hormone-resistant 22Rv1 cells.
Fig. 4.
Expression of p38 and ERK1/2 in the taxane-resistant and taxane-sensitive prostate cancer cells. The protein expression was analyzed by Western blotting. β-actin was used as a loading control. The band intensity of the protein of interest was quantified using Image Lab software and normalized to the band intensity of non-phospho MAPK, and is indicated by red numbers. Full-size blots are presented in Supplementary Fig. S2.
The effects of CuC1 in combination with platinum agents and taxanes were similar in both PC3-DR and 22Rv1 cells. Specifically, a synergistic effect was detected when higher doses of Cis, Carbo, Doce, and Caba were combined with CuC1, whereas an additive effect was observed at lower concentrations of these chemotherapeutics in their combination with CuC1 (Fig. 5). In 22Rv1 cells the overall effects of these combinations were additive, which was indicated by a δ-score being in the range of − 10 < δ < 10, while for PC3-DR cells the areas with δ > 10 were detected indicating a true synergism (Fig. 5). However, it should be noted that for combination CuC1 plus Caba in 22Rv1 cells, the lowest detected δ value was − 10.7, indicating a minimal antagonistic effect at the defined concentration range (Fig. 5).
Fig. 5.
Effects of CuC1 in combination with taxanes and platinum agents. PC3-DR and 22Rv1 cells were co-treated with cisplatin (Cis), carboplatin (Carbo), docetaxel (Doce), or cabazitaxel (Caba) in combination with CuC1 at the indicated concentrations for 48 h. Cellular viability was evaluated using the MTT assay (number of biological replicates n = 3.) and is represented in the Supplementary Table S2. The cytotoxic effects were further analyzed, and 3D heat maps were constructed using SynergyFinder 3.0 software. The drug effects in combination (synergistic, additive, or antagonistic) were analyzed and visualized using the ZIP algorithm and are indicated by δ-score. δ > 10: Synergistic interactions (dark red area); − 10 > δ > 10: additive effect; δ < − 10: Antagonistic interactions (dark green area). A ZIP synergy score represents a mean δ overall the concentration range used.
Discussion
Marine triterpene glycosides possess various biological activities, including anticancer activity. In this study, we evaluated the effects of three marine triterpene glycosides pacificusoside C, cucumariosides C1 and C2 (PaC, CuC1, CuC2) in prostate cancer models in vitro. While the compounds possessed a selectivity comparable to the approved anticancer medication cisplatin, its activity in cancer cells was not affected by an overexpression of P-gp—a major mechanism of multidrug resistance of various cancer entities. Therefore, the minor cross-resistance observed in the docetaxel-resistant PC3-DR cells is related to factors other than P-gp status of the cells. Among possible factors, there are modulations of cellular metabolism, tubulin dynamics, altered cellular membrane composition, different proliferative rates of the cells, or activation of general pro-survival and antiapoptotic pathways5. Moreover, taxane-resistant cells were shown to have an altered metabolism of cholesterol, one of the primary targets of saponins, resulting in an altered cholesterol content in the cellular membrane46. Such alterations may result in reduced sensitivity to saponin-like compounds28.
To the best of our knowledge, the current research is the very first study of the effect of triterpene glycosides on the kinome of cancer cells. Kinome profiling revealed a possible induction of multiple kinases in the cells treated with CuC1. Thus, predicted IKKα, IKKβ, and IKKε kinases are a part of the NF-κB signaling pathway, which regulates cell survival, inflammation, and stress responses. Activation of this pathway promotes either cell survival (in the vast majority of cases) or apoptosis, depending on the biological context47. Moreover, in prostate cancer, IKKβ is known to mediate cancer cell stemness and epithelial-to-mesenchymal transition, and therefore, potentially contributes to tumor progression and drug resistance48. On the contrary, activation of MLKL, an important mediator of necroptosis, proposes this type of programmed cell death is also involved in the cytotoxic action of CuC1 and related drugs49. Activation of PITSLRE kinase suggests promotion of apoptotic pathways or disruption cell cycle progression in the treated cancer cells50, which was in line with the detected upregulation of other apoptotic markers (Fig. 3a).
The predicted activation of GCN2 and PDK1 further proposes that CuC1 affects cellular metabolism51,52. Additionally, PDK1 is a mediator of the PI3K/AKT pathway, and the activation of this axis may contribute to the resistance of the cancer cells52. Rho kinase is known to regulate cytoskeletal dynamics and increase or suppress motility, depending on the cellular context53, while PINK1 is involved in mitophagy and is a part of a mitochondria quality control system54. COT (MAP3K8) kinase is involved in inflammatory signaling, and its modulation could affect the tumor microenvironment or immune responses55. However, the activation of the above-mentioned kinases should further be validated using alternative methods.
Taken together, the results of the functional kinome profiling suggest CuC1 to simultaneously modulate several processes, including metabolism, survival, mitochondrial quality control, and cytoskeletal dynamics. The cells also seem to activate prosurvival pathways, including NF-κB and PI3K/AKT signaling, that may partially mitigate the effect of the drug. This hypothesis awaits further validation in upcoming studies.
A very interesting finding was CuC1-mediated inhibition of ERK1/2 and p38 MAP kinases, that was the first predicted by functional kinome profiling and further validated by Western blotting. Recently, we reported a chemopreventive activity pacificusoside D and cucumarioside D, triterpene glycosides structurally closely related to CuC1, in the JB6 Cl41 cells model16. In this model, the JB6 Cl41 cells were exposed to epidermal growth factor (EGF) or a chemical carcinogen 12-O-tetradecanoilphorbol-13-acetate (TPA) to induce the malignant transformation of the cells56. Previously, it was shown that activation of MAPKs is a critical step of the malignant transformation event in JB6 Cl41 model and that MAPK inhibition suppresses this process56. Thus, the inhibition of ERK1/2 and p38 MAPKs reported in the current research may explain the cancer-preventive activity of CuC1 and related compounds. As the activity of multiple kinases was predicted to be affected in CuC1-treated cells (Fig. 3c), it is unlikely that CuC1 acts as a specific molecular inhibitor of p38 and ERK1/2 kinases. Instead, we propose that the observed inhibition of p38 and ERK1/2 activity in prostate cancer cells is a secondary effect of CuC1 treatment, potentially resulting from various biological mechanisms that warrant further investigation.
Finally, we showed that CuC1 potentiates the cytotoxic effects of cisplatin, carboplatin, docetaxel, and cabazitaxel in PC3-DR and 22Rv1 cells. This observation can be potentially explained by the ability of CuC1 to inhibit ERK1/2 and p38 MAPK pathways, which are known to be over-activated in the docetaxel-resistant prostate cancer cells and contribute to their drug resistance57. This makes CuC1 a potential combinational partner for the above-mentioned chemotherapeutics, however, further research is required here to analyze the combinational treatment options in more detail, including an in vivo safety and efficacy profiles. Additionally, our data contributes to the understanding of the anti-cancer properties of some triterpene glycosides produced by marine invertebrates that are used in Traditional Chinese Medicine for cancer treatment29.
Conclusion
In summary, the marine triterpene glycosides pacificusoside C, cucumariosides C1 and C2 were shown to be cytotoxic in human prostate cancer cells bearing various levels of drug resistance. The compounds were active in docetaxel-resistant prostate cancer cells, were neither P-glycoprotein inhibitors nor substrates, and exerted their cytotoxicity independently of the P-glycoprotein status. Additionally, cucumarioside C1 potentiates cytotoxic effects cisplatin, carboplatin, docetaxel or cabazitaxel in the docetaxel-resistant cells. Cucumarioside C1 was revealed to activate kinases involved in various cellular processes, such as metabolic regulation, cytoskeletal dynamics and migration, cell cycle regulation, apoptosis, necroptosis, survival, mitophagy, inflammatory and immune modulation. Moreover, we revealed and validated a CuC1-mediated inhibition of p38 and ERK1/2 MAP kinases in prostate cancer cells. This phenomenon may explain some anticancer effects previously reported for cucumarioside C1 and related compounds. To the best of our knowledge, this is the very first study reporting the effect of triterpene glycosides on the kinome of cancer cells.
Materials and methods
Marine natural compounds
Pacificusoside C, cucumariosides C1 and C2 were isolated from the sea star Solaster pacificus as previously described15. Specimens of S. pacificus were collected near Iturup Island (Sea of Okhotsk) by scuba diving in August 201215. The purity of compounds was confirmed by 1H NMR, TLC, and ESI mass-spectrometry (Supplementary Fig. S3-S15). For the biological experiments, the stock solutions of compounds in 100% DMSO (vehicle) were used.
Reagents and antibodies
The following reagents were used: MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma, Taufkirchen, Germany); tariquidar (P-glycoprotein inhibitor) (MedChemExpress, Monmouth Junction, NJ, USA); calcein-AM (BIOZOL, Eching, Germany); MTS viability assay kit (CellTiter 96 Aqueous One Solution Reagent, Promega, Madison, WI, USA); cisplatin, carboplatin, docetaxel, and cabazitaxel (Pharmacy of the University Hospital Hamburg-Eppendorf, Hamburg, Germany).
The following primary and secondary antibodies were used: Rabbit anti-cleaved Caspase-3 (mAb, #9664, 1:1000, Cell Signaling), mouse anti-ERK1/2 (mAb, #9107, 1:2000, Cell Signaling), rabbit anti-JNK1/2 (mAb, #9258, 1:1000, Cell Signaling), rabbit anti-p38 (mAb, #9212, 1:1000, Cell Signaling), rabbit anti-PARP (pAb, #9542, 1:1000, Cell Signaling), rabbit anti-phospho-ERK1/2 (mAb, #4377, 1:1000, Cell Signaling), rabbit anti-phospho-JNK1/2 (mAb, #4668, 1:1000, Cell Signaling), rabbit anti-phospho-p38 (mAb, #4511, 1:1000, Cell Signaling), goat anti-rabbit IgG-HRP (#7074, 1:5000, Cell Signaling), mouse anti-α-tubulin (mAb, T5168, 1:5000, Sigma-Aldrich), goat anti-β-actin-HRP (pAb, sc-1616, 1:10,000, Santa Cruz), sheep anti-mouse IgG-HRP (NXA931, 1:10,000, GE Healthcare).
Cell lines and culture conditions
The cell lines were purchased from ATCC (Manassas, VA, USA) or ECACC (Salisbury, UK). The following cell lines were used: Human prostate cancer cells LNCaP (ATCC; RRID:CVCL_0395), VCaP (ECACC; RRID:CVCL_2235), 22Rv1 (ATCC; RRID:CVCL_1045), PC3 (ATCC; RRID:CVCL_0035), and DU145 (ATCC; RRID:CVCL_0105); human prostate non-cancer cells PNT2 (ATCC; RRID:CVCL_2164) and RWPE-01 (ATCC; RRID:CVCL_3791). Human non-cancer embryonic kidney cells HEK293T (ECACC; RRID:CVCL_0063) and human fibroblast cells MRC-9 (ECACC; RRID:CVCL_2629). The docetaxel-resistant cells PC3-DR were generated by long-term treatment of human prostate cancer PC3 cells with sublethal concentrations of docetaxel as previously described32 and were provided by Prof. Z. Culig (Innsbruck Medical University, Austria). All cell lines were recently authenticated by a commercial service (Multiplexion GmbH, Heidelberg, Germany) and were cultured as previously described58,59. Cells were kept in culture for a maximum of three months and were routinely examined for bacterial, fungal, and mycoplasma infections.
MTT assay
Cell viability was measured using the MTT assay. Cells (6 × 103 cells/well in 100 μL/well of media) were plated in 96-well plates. The plates were incubated overnight, and the medium was replaced with fresh medium containing drugs at the indicated concentrations. The plates were further incubated for 48 h, unless otherwise stated, the solution of MTT reagent in PBS was added to each well, and cells were incubated for another 2–4 h to form formazan crystals. Then the media was removed and the plates were dried overnight. 50 μL of DMSO was added to each well to dissolve the formazan crystals. The absorbance of each well was measured using an Infinite F200PRO reader (TECAN, Männedorf, Switzerland). The cell viability was estimated as an inhibition concentration (IC50), which was calculated using GraphPad Prism software v.9.1.1 (GraphPad Software, San Diego, CA, USA). IC₅₀ values were determined by fitting the data to a nonlinear regression model (log[inhibitor] versus normalized response—variable slope). R2 values (goodness-of-fit) for the dose–response curves used to calculate IC₅₀ values are represented on the respective figures and in the Supplementary Table S1. A selectivity index (SI) was calculated as: SI = [(IC50(PNT2) + IC50(RWPE-1) + IC50(HEK293T) + IC50(MRC-9))/4]/[(IC50(PC3) + IC50(22Rv1) + IC50(DU145) + IC50(VCaP) + IC50(LNCaP))/5]. For calculation of SI the IC50 values for Doce in PNT2 and MRC-9 cells were assumed to be 500 nM.
Examination of drug effects in combination
The effect of combinational treatment with established chemotherapies was evaluated as previously reported54. In brief, cells were exposed to individual drugs of their combinations for 48 h, and the viability was measured by MTT assay and was then analyzed using SynergyFinder 3.0 software (https://synergyfinder.fimm.fi) and the ZIP score algorithm60. Statistical analysis was conducted automatically by the software using its inbuilt algorythms based on the input data type and size. The effects of drug combinations (synergistic, additive, or antagonistic) were assigned using a δ-score, where δ > 10 indicated synergistic interactions (dark red area), − 10 > δ > 10 refers to additive effect, and δ < − 10 suggests antagonistic interactions (dark green area). The raw cell viability data used for synergy analysis presented as % of effect (0 refers to 0% of dead cells compared to the control, i.e. no cytotoxicity effect; 100 refers to 100% dead cells compared to the control) can be found in the Supplementary Table S2.
P-gp activity assay
The evaluation of the activity of P-glycoprotein (P-gp) was performed using calcein-AM assay as described previously34. P-gp-overexpressing PC3-DR cells (10 × 103 cells/well in 100 µL/well of media) were seeded in a black 96-well plate with clear flat bottoms. Plates were incubated overnight, and the medium was replaced with 50 µL of PBS containing the tested drug at the indicated concentrations and incubated for 30 min. The calcein-AM solution in PBS (50 µL) was added to each well to achieve a final calcein-AM concentration of 1 µM. The plates were incubated for 15 min, and the green calcein fluorescence was recorded using an Infinite F200PRO plate reader (TECAN). The viability of cells treated in the same experimental settings was evaluated using the MTS assay as described previously34.
Functional kinome profiling
Functional kinome profiling was performed as described previously61. The PamStation®12 system and STK-PamChip® array (PamGene International, ´s-Hertogenbosch, The Netherlands) were used. The array is designed to analyze the activity of serine-/threonine kinases (STKs), each chip contains 140 peptide phospho-sites that are analogs for the substrates of respective STKs. The proteins were extracted from the 22Rv1 cells treated with CuC1 (0.5 µM, 2 h) or vehicle-treated cells using M-PER Mammalian Extraction Buffer supplemented with protease and phosphatase inhibitor cocktails (Pierce, Waltham, Massachusetts, USA). The cell lysates were mixed with ATP and applied on the chip according to the manufacturer’s protocol. The phosphorylation of specific peptide sequences was then detected using primary anti-phospho-Ser/Thr and secondary immunoglobulin-FITC antibodies (PamGene International). The signals were recorded using a dedicated camera and Evolve software v. 1.0 (PamGene International). The quality of the signals was controlled, and the signal intensities were log2-transformed and analyzed using the BioNavigator software v. 6.0 (PamGene International).
Western blotting
Western blotting was performed as previously described62. In brief, 22Rv1 cells were seeded in Petri dishes (1 × 106 cells/dish in 10 mL/dish of media), incubated overnight, and treated with the drug for a specific time. Cells were harvested by scratching, washed with cold PBS, and lysed with RIPA buffer supplemented with protease and phosphatase inhibitor cocktails. Protein lysates were separated using precast Mini-PROTEAN® TGX Stain-FreeTM gels (Bio-Rad, Hercules, CA, USA) and SDS-PAGE. Proteins were transferred on a PVDF membrane and detected using specified primary and secondary antibodies. The protein signals were visualized and detected using the ECL chemiluminescence system (Thermo Scientific, Rockford, IL, USA). The original blot images were cropped, and the figures were composed using MS PowerPoint software v. 2310, build 16924.20150 (Microsoft Inc., Redmond, WA, USA). The signal (band) intensity was quantified using Image Lab v. 6.1.0. build 7 software (Bio-Rad Laboratories, Hercules, CA, USA) and normalized to loading control (for non-phospho proteins), or normalized to signal intensity of non-phospho proteins (for phospho proteins). The original uncropped images are available in Supplementary Fig. S2.
Data and statistical analysis
Statistical analysis was performed using GraphPad Prism software v.9.1.1 (GraphPad Prism software Inc., La Jolla, CA, USA). The data are represented as mean ± standard deviation (SD). For the comparison of multiple groups, one-way ANOVA combined with Dunnett’s post-hoc tests was used. All experiments were carried out in triplicate (n = 3, biological replicates), unless otherwise stated. The difference between the groups was considered to be statistically significant and indicated with an asterisk (*) if p < 0.05.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to Prof. Zoran Culig (Innsbruck Medical University, Austria) for providing of the drug-resistant PC3-DR cell line
Author contributions
Conception and design: S.A.D.; Development of methodology: S.A.D.; Main biological assays and data interpretation: S.A.D., J.H., O.Y.B., N.S., M.G., C.B., G.v.A.; Kinome profiling: M.K. and K.H.; Isolation and purification of the compounds: T.V.M., A.A.K., N.V.I., V.A.S.; Data analysis: All authors; Original draft writing: S.A.D. and G.v.A.; Manuscript review and editing: All authors; Review and/or revision of the final version of the manuscript: All authors; Artwork: S.A.D.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organized by Projekt DEAL. This study received financial support from the University Medical Center Hamburg-Eppendorf.
Data availability
The original data are available in the Supplementary as well as from the corresponding author on a reasonable request.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay, J. et al. Cancer statistics for the year 2020: An overview. Int. J. Cancer149, 778–789. 10.1002/ijc.33588 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Hussain, M. et al. Metastatic hormone-sensitive prostate cancer and combination treatment outcomes: A review. JAMA Oncol.10, 807–820. 10.1001/jamaoncol.2024.0591 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Cai, M. et al. Current therapy and drug resistance in metastatic castration-resistant prostate cancer. Drug Resist. Updates68, 100962. 10.1016/j.drup.2023.100962 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Vellky, J. E. & Ricke, W. A. Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia22, 566–575. 10.1016/j.neo.2020.09.002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakur, N. et al. Therapy resistance in prostate cancer: Mechanism, signaling and reversal strategies. Explor. Target. Antitumor. Ther.5, 1110–1134. 10.37349/etat.2024.00266 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merkens, L. et al. Aggressive variants of prostate cancer: Underlying mechanisms of neuroendocrine transdifferentiation. J. Exp. Clin. Cancer Res.41, 46. 10.1186/s13046-022-02255-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stonik, V. A. Marine natural products: A way to new drugs. Acta Naturae1, 15–25 (2009). [PMC free article] [PubMed] [Google Scholar]
- 8.Dyshlovoy, S. A. & Honecker, F. Marine compounds and cancer: Updates 2022. Mar. Drugs.10.3390/md20120759 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atanasov, A. G. et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov.20, 200–216. 10.1038/s41573-020-00114-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park, J. I., Bae, H. R., Kim, C. G., Stonik, V. A. & Kwak, J. Y. Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers. Front. Chem.2, 77. 10.3389/fchem.2014.00077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacaille-Dubois, M.-A. & Wagner, H. New perspectives for natural triterpene glycosides as potential adjuvants. Phytomedicine37, 49–57. 10.1016/j.phymed.2017.10.019 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Aminin, D. L. et al. Anticancer activity of sea cucumber triterpene glycosides. Mar. Drugs13, 1202–1223 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan, Y. C. et al. A new triterpene glycoside from Asterias rollentoni. J. Asian Nat. Prod. Res.8, 631–636. 10.1080/10286020500208626 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Ivanchina, N. V., Kalinovsky, A. I., Malyarenko, T. V., Kicha, A. A. & Dmitrenok, P. S. A. Holothurian triterpene glycoside holothurin A2 (= echinoside A) isolated from the starfish Choriaster granulatus. Nat. Prod. Commun.10.1177/1934578x19858522 (2019). [Google Scholar]
- 15.Malyarenko, T. V. et al. New triterpene glycosides from the far eastern starfish Solaster pacificus and their biological activity. Biomolecules10.3390/biom11030427 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malyarenko, T. V. et al. In Vitro anticancer and cancer-preventive activity of new triterpene glycosides from the far eastern starfish Solaster pacificus. Mar. Drugs10.3390/md20030216 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malyarenko, T. V. et al. New Rare triterpene glycosides from pacific sun star, Solaster pacificus, and their anticancer activity. Mar. Drugs22, 19 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stonik, V. A., Kicha, A. A., Malyarenko, T. V. & Ivanchina, N. V. Asterosaponins: Structures, taxonomic distribution. Biog. Biol. Act. Mar. Drugs10.3390/md18120584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, J., Han, H., Chen, X., Yi, Y. & Sun, H. Cytotoxic and apoptosis-inducing activity of triterpene glycosides from Holothuria scabra and Cucumaria frondosa against HepG2 cells. Mar. Drugs12, 4274–4290. 10.3390/md12084274 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aminin, D. L. et al. Anticancer activity of sea cucumber triterpene glycosides. Mar. Drugs13, 1202–1223. 10.3390/md13031202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wargasetia, T. L. & Widodo,. Mechanisms of cancer cell killing by sea cucumber-derived compounds. Invest. New Drugs35, 820–826. 10.1007/s10637-017-0505-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondol, M. A. M., Shin, H. J., Rahman, M. A. & Islam, M. T. Sea cucumber glycosides: Chemical structures, producing species and important biological properties. Mar. Drugs.10.3390/md15100317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silchenko, A. S. et al. Sulfated triterpene glycosides from the far eastern sea cucumber Cucumaria djakonovi: Djakonoviosides C(1), D(1), E(1), and F(1); cytotoxicity against human breast cancer cell lines quantitative structure-activity relationships. Mar. Drugs10.3390/md21120602 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menchinskaya, E. S. et al. Anticancer activity of the marine triterpene glycoside cucumarioside A2-2 in human prostate cancer cells. Mar. Drugs22, 20 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, M. et al. Echinoside A, a new marine-derived anticancer saponin, targets topoisomerase2α by unique interference with its DNA binding and catalytic cycle. Ann. Oncol.21, 597–607. 10.1093/annonc/mdp335 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Vien, L. T. et al. Triterpene tetraglycosides from Stichopus herrmanni semper, 1868. Nat. Prod. Commun. 17. 10.1177/1934578x221105369 (2022). [Google Scholar]
- 27.Dyshlovoy, S. A. et al. The marine triterpene glycoside frondoside a exhibits activity in vitro and in vivo in prostate cancer. Int. J. Cancer138, 2450–2465. 10.1002/ijc.29977 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Jiang, M. et al. Recent advances in the anti-tumor activities of saponins through cholesterol regulation. Front. Pharmacol.10.3389/fphar.2024.1469392 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Y.-X., Himaya, S. W. A. & Kim, S.-K. Triterpenoids of marine origin as anti-cancer agents. Molecules18, 7886–7909 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampson, N., Neuwirt, H., Puhr, M., Klocker, H. & Eder, I. E. In vitro model systems to study androgen receptor signaling in prostate cancer. Endocr. Relat. Cancer20, R49–R64. 10.1530/erc-12-0401 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Nelson, P. S. Targeting the androgen receptor in prostate cancer—A resilient foe. New Engl. J. Med.371, 1067–1069. 10.1056/NEJMe1409306 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Puhr, M. et al. Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of mir-200c and mir-205. Am. J. Pathol.181, 2188–2201. 10.1016/j.ajpath.2012.08.011 (2012). [DOI] [PubMed] [Google Scholar]
- 33.von Amsberg, G., Dyshlovoy, S. & Kaune, M. Aggressive variant prostate cancer and transdifferentiated neuroendocrine prostate cancer: From diagnosis to therapy. Urologie64, 246–255. 10.1007/s00120-024-02511-3 (2025). [DOI] [PubMed] [Google Scholar]
- 34.Dyshlovoy, S. A. et al. New diterpenes from the marine sponge Spongionella sp. overcome drug resistance in prostate cancer by inhibition of P-glycoprotein. Sci. Rep.12, 13570. 10.1038/s41598-022-17447-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callaghan, R., Luk, F. & Bebawy, M. Inhibition of the multidrug resistance P-glycoprotein: Time for a change of strategy?. Drug Metab. Dispos.42, 623–631. 10.1124/dmd.113.056176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Neill, A. J. et al. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol. Cancer10, 126–126. 10.1186/1476-4598-10-126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weidner, L. D. et al. Tariquidar is an inhibitor and not a substrate of human and mouse p-glycoprotein. Drug Metab. Dispos.44, 275. 10.1124/dmd.115.067785 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dyshlovoy, S. A. et al. Cytotoxic N-methylpretrichodermamide B reveals anticancer activity and inhibits p-glycoprotein in drug-resistant prostate cancer cells. Mar. Drugs20, 597 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly, R. J. et al. A pharmacodynamic study of docetaxel in combination with the p-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer. Clin. Cancer Res.17, 569–580. 10.1158/1078-0432.Ccr-10-1725 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhillon, A. S., Hagan, S., Rath, O. & Kolch, W. MAP kinase signalling pathways in cancer. Oncogene26, 3279–3290. 10.1038/sj.onc.1210421 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Cohen, P., Cross, D. & Jänne, P. A. Kinase drug discovery 20 years after imatinib: Progress and future directions. Nat. Rev. Drug Discov.20, 551–569. 10.1038/s41573-021-00195-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martínez-Limón, A., Joaquin, M., Caballero, M., Posas, F. & de Nadal, E. The p38 pathway: From biology to cancer therapy. Int. J. Mol. Sci.10.3390/ijms21061913 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braicu, C. et al. A comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers10.3390/cancers11101618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe, H. et al. Molecular mechanism mediating cytotoxic activity of cabazitaxel in docetaxel-resistant human prostate cancer cells. Anticancer Res.41, 3753. 10.21873/anticanres.15167 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Hongo, H., Kosaka, T. & Oya, M. Analysis of cabazitaxel-resistant mechanism in human castration-resistant prostate cancer. Cancer Sci.109, 2937–2945. 10.1111/cas.13729 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui, A. et al. Steroidal saponins: Natural compounds with the potential to reverse tumor drug resistance (review). Oncol. Lett.28, 585. 10.3892/ol.2024.14719 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoesel, B. & Schmid, J. A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer12, 86. 10.1186/1476-4598-12-86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Y. et al. Targeting IκB Kinase β/NF-κB signaling in human prostate cancer by a novel iκB kinase β inhibitor cmpdA. Mol. Cancer Ther.15, 1504–1514. 10.1158/1535-7163.Mct-15-0999 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu, X., Nagy, L. E. & Gautheron, J. Mediators of necroptosis: From cell death to metabolic regulation. EMBO Mol. Med.16, 219–237. 10.1038/s44321-023-00011-z (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, S. et al. Interaction of p58PITSLRE, a G2/M-specific protein kinase, with cyclin D3*. J. Biol. Chem.277, 35314–35322. 10.1074/jbc.M202179200 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Guo, F. & Cavener, D. R. The GCN2 eIF2α kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab.5, 103–114. 10.1016/j.cmet.2007.01.001 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Bayascas, J. R. PDK1: The major transducer of PI 3-kinase actions. Curr. Top Microbiol. Immunol.346, 9–29. 10.1007/82_2010_43 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Amano, M., Nakayama, M. & Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton67, 545–554. 10.1002/cm.20472 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narendra, D. P. & Youle, R. J. The role of PINK1-parkin in mitochondrial quality control. Nat. Cell Biol.26, 1639–1651. 10.1038/s41556-024-01513-9 (2024). [DOI] [PubMed] [Google Scholar]
- 55.Hao, J. et al. Effect of MAP3K8 on prognosis and tumor-related inflammation in renal clear cell carcinoma. Front. Genet.12, 674613. 10.3389/fgene.2021.674613 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuno, H., Cho, Y. Y., Ma, W. Y., Bode, A. M. & Dong, Z. Effects of MAP kinase inhibitors on epidermal growth factor-induced neoplastic transformation of human keratinocytes. Mol. Carcinog.45, 1–9. 10.1002/mc.20160 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatano, K. et al. Residual prostate cancer cells after docetaxel therapy increase the tumorigenic potential via constitutive signaling of CXCR4, ERK1/2 and c-Myc. Mol. Cancer Res.11, 1088–1100. 10.1158/1541-7786.Mcr-13-0029-t (2013). [DOI] [PubMed] [Google Scholar]
- 58.Dyshlovoy, S. A. et al. inspired by sea urchins: Warburg effect mediated selectivity of novel synthetic non-glycoside 1,4-naphthoquinone-6s-glucose conjugates in prostate cancer. Mar. Drugs18, 251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dyshlovoy, S. A. et al. Efficacy and mechanism of action of marine alkaloid 3,10-dibromofascaplysin in drug-resistant prostate cancer cells. Mar. Drugs.10.3390/md18120609 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ianevski, A., Giri, A. K. & Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acid. Res.50, W739–W743. 10.1093/nar/gkac382 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dyshlovoy, S. A. et al. Marine alkaloid monanchoxymycalin C: A new specific activator of JNK1/2 kinase with anticancer properties. Sci. Rep.10, 020–69751 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dyshlovoy, A. S. et al. Successful targeting of the Warburg effect in prostate cancer by glucose-conjugated 1,4-naphthoquinones. Cancers10.3390/cancers11111690 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data are available in the Supplementary as well as from the corresponding author on a reasonable request.