Abstract
Background
Asthma demonstrates a robust daily rhythm, with airflow obstruction and airway inflammation peaking overnight. Aligning the timing of drug administration with rhythms in disease (chronotherapy) may improve therapeutic efficacy. We aimed to evaluate the impact of dosage timing for inhaled corticosteroids in asthma.
Methods
This is a randomised three-way crossover trial. Participants with mild to moderate atopic asthma were randomised to beclometasone dipropionate: (1) 400 µg once daily between 08:00 and 09:00 (ODAM); (2) 400 µg once daily between 15:00 and 16:00 (ODPM); and (3) 200 µg twice daily between 08:00 and 09:00 and between 20:00 and 21:00 (BD) for 28 days, with a 2 week washout period in between treatment periods. Six-hourly spirometry and biomarkers were measured over 24 hours following the run-in period and at the end of each treatment period.
Results
Of 25 participants, 21 completed all regimens. ODPM was superior in improving 22:00 FEV1 (median (IQR): +160 (+70, +270) ml) compared with ODAM (−20 (−80, +230) ml) and BD (+80 (−20, +200) ml). ODPM resulted in better overnight (22:00 and 04:00) suppression in blood eosinophil counts compared with BD and ODAM. All regimens improved asthma control and reduced fractional exhaled nitric oxide and serum cortisol levels with no difference among dosing regimens.
Conclusion
ODPM better suppresses the nocturnal dip in lung function and peak of blood eosinophil counts compared with BD and ODAM; this was without an increase in adverse events. Future trials are warranted to validate these findings in real-life settings and to determine which population may best benefit from chronotherapy.
Keywords: Asthma, Inhaler devices, Lung Physiology
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
Mid-afternoon (once a day) dosing of inhaled corticosteroid (beclomethasone) improved nocturnal lung function and reduced blood eosinophil counts compared with alternative dosage timings (including the usual, twice daily dosing regimen) without additional adrenal suppression.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Mid-afternoon dosing of inhaled beclomethasone may lead to better clinical outcomes without increasing steroid-related morbidity or costs. Our findings warrant further validation in a larger clinical trial to establish clinical feasibility in a real-life setting and to evaluate the health-economics impact.
Introduction
Asthma demonstrates a robust rhythm in both symptoms and pathophysiology1: up to three quarters of patients experience worsening symptoms and up to 80% of fatal asthma attacks occur overnight.2 3 Coincident with nocturnal symptoms, there is an overnight peak in airflow obstruction, airway inflammation and bronchial hyperresponsiveness.4 5 The excessive diurnal variation and nocturnal dip in lung function is also a marker for disease severity, poor control and mortality.3 4 6 Notably, glucocorticoid receptor functionality fluctuates over 24 hours in individuals exhibiting nocturnal symptoms and airflow obstruction, with impaired steroid responsiveness at night compared with mid-afternoon.7 8 We recently demonstrated enhanced steroid sensitivity in immune cells at 16:00 compared with 04:oo,9 further supporting the notion that the optimal timing for corticosteroids administration may be at this time.
Aligning dosage timing to biological rhythms of disease (chronotherapy) is a strategy to improve drug efficacy and/or reduce associated side effects. To date, results from clinical trials of inhaled corticosteroid (ICS) chronotherapy in asthma have been inconclusive. Pincus et al demonstrated once-daily mid-afternoon dosing of inhaled triamcinolone acetonide (an uncommonly used ICS in asthma) was better for improving morning peak flow compared with once-daily morning dosing and non-inferior to the standard four-times-a-day dosing regimen.10 11 Gagnon showed no difference between once-daily (at 17:00 or 22:00) or twice-daily dosing of high-dose beclomethasone dipropionate (BDP, 1000 µg) in moderate asthma.12 However, these studies focused on daytime measurements of lung function and did not investigate the effects of dosage timing on the nocturnal dip in lung function and associated changes in T2 inflammatory biomarkers.
The objective of this randomised controlled trial was to test the hypothesis that administering inhaled BDP mid-afternoon will result in superior suppression of the nocturnal peak in asthma pathophysiology (lung function and airway inflammation) compared with morning dosing and standard twice-daily dosing regimens.13 14
Methods
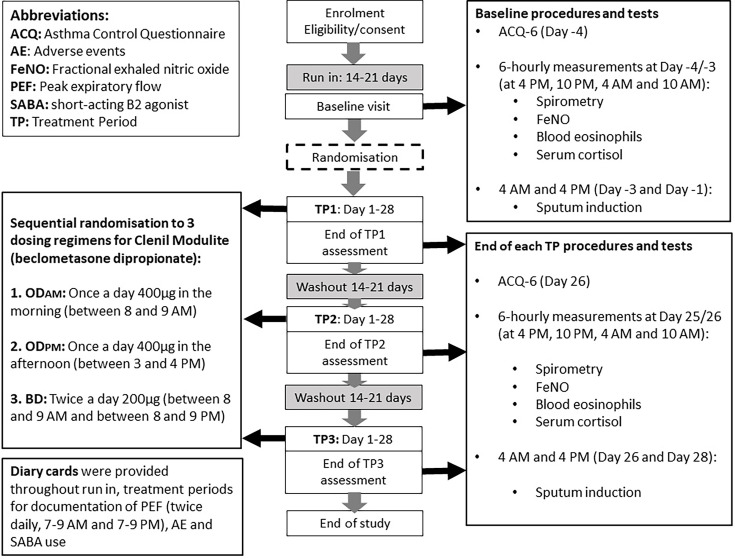
We conducted a randomised, open-label, three-way crossover trial of BDP (Clenil Modulite, delivered via pressurised metered dose inhaler) 400 µg daily dose (200 µg per inhalation) in the morning (between 08:00 and 09:oo (ODAM)) versus mid-afternoon (between 15:00 and 16:00 (ODPM)), versus 200 µg twice a day (between 08:00 and 09:00, and between 20:00 and 21:00 (BD)), in participants with mild to moderate atopic asthma. Briefly, adults aged 18–65 years with a history of physician-diagnosed asthma and with symptoms consistent with asthma for at least 1 year prior to screening were recruited.
All participants were treated with low to medium doses of ICS with or without long-acting β agonists at screening and had documented allergy to at least one common inhaled allergen (ie, cat dander, house dust mite or grass pollen) as confirmed on skin prick tests; a positive skin prick test was defined as a weal size of ≥3 mm in diameter, over the negative control. All participants had evidence of at least one of the following prior to consent: (1) bronchial hyperresponsiveness to methacholine (defined as a ≥20% fall in forced expiratory volume within 1 s (FEV1) of ≤1 mg in dose or ≤16 mg/mL in concentration)15 ; or (2) ≥12% or ≥200 mL improvement in FEV1 in bronchodilator reversibility test within 2 years prior to the study. The main exclusion criteria were smoking within the past 12 months or those with a smoking history of more than five pack years; uncontrolled asthma (6-item Asthma Control Questionnaire (ACQ-6) score of 1.5 points or more); asthma exacerbations or chest infection requiring oral corticosteroids or antibiotics within 3 months prior to screening and randomisation; any clinically significant uncontrolled diseases other than asthma. Detailed inclusion and exclusion criteria are outlined in the online supplemental figure section E1.
Non-permitted concomitant medications included systemic corticosteroids within 3 months, ICS other than study medications, nebulised β2 agonist/anticholinergics, inhaled long-acting β2 agonists, inhaled short-acting β2 agonists other than study supply, inhaled short and long-acting muscarinic antagonist within 7 days and leukotriene modifiers within 72 hours prior to screening visit and during the study period. This study was approved by the Research Ethics Committee (North West Greater Manchester South, 20/NW/0011) and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines (registered clinicaltrialsregister.eu; EudraCT: 2019-004309-28, https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-004309-28/GB). All participants provided informed written consent.
Study design
Baseline assessment
Demographic data and medical history were recorded during screening. All eligible participants stopped asthma maintenance therapies during the run-in period for 14–21 days prior to the baseline assessment. At baseline visit (day −4 and day −3), participants stayed overnight, during which six hourly (04:oo, 10:00, 16:00, 22:00) spirometry, fractional exhaled nitric oxide (FeNO), blood eosinophils and serum cortisol levels were measured, and ACQ-6 and Munich Chronotype Questionnaire administered.6 Sputum induction was carried out at 04:oo (day −3) and 16:00 (day −1), >48 hours apart to ensure the second sputum sample was not affected by the first. Randomisation numbers were assigned, which confirmed treatment sequences (figure 1).
Figure 1. Study flow chart.
Treatment periods
The baseline visit was followed by three 28-day treatment periods in random order with a washout period between each treatment period of 14–21 days. The ACQ-6, 6 hourly spirometry, FeNO and blood samples were taken on day 25 and day 26 into each treatment period. Morning and afternoon sputum induction were carried out 48 hours apart, on day 26 at 04:00 and day 28 at 16:00.
Peak flow metres and diary cards, which recorded morning and evening peak expiratory flow (PEF), salbutamol use, adverse events (AEs) and ICS adherence, were provided during each washout and treatment period. Full verbal and written instructions were given on inhaler technique. All participants were contacted weekly by telephone during the study period during which AEs, changes to concomitant medications and drug adherence were checked.
Study procedures are detailed in the online supplemental section E1.
End points
Prespecified primary end points included the treatment differences for FEV1, PEF and FeNO changes from baseline. The key secondary end points were the treatment differences in the changes in blood eosinophil counts, serum cortisol levels and ACQ-6, reliever medication use and AEs. The daily rhythms in lung function parameters, FeNO, blood eosinophil counts and serum cortisol levels were examined.
Statistical analysis
The sample size calculation was based on our previously published data.5 With an estimated dropout rate of 20%, we anticipated that to reach a total of 20 completed and evaluable subjects, we would need to randomise a total of 25 subjects. With n=20 completing all dosing regimens, with a type I error rate of 5% and a within group SD of 9.3% in percent predicted FEV1, there was an estimated 80% power of detecting a difference of 10% between groups in the mean group changes from baseline.5 11
The within-day variability among all time points was tested using the Friedman test in the intention-to-treat population; pairwise comparisons between time points were subsequently carried out if the overall test had a p-value<0.05. Treatment effects were described as the differences between the end of each treatment period and baseline (defined as post run-in period) values. Comparisons of treatment effects among the three treatment regimens were performed using the Freidman test in patients who had completed all treatment periods. Pairwise comparisons (Wilcoxon signed-rank test) were performed if the overall test had p<0.05. The p-values of all pairwise comparisons were also adjusted using the Benjamini-Hochberg false discovery rate correction method (FDR, <0.1 defined as significant) (rstatix R package); both raw p-values and FDR adjusted values were presented.
A mixed-effects (adjusted for repeated measures) exploratory cosinor model (cosinoRmixedeffects R package16) was used to examine the changes in the daily rhythmicity in lung function, FeNO, blood eosinophil counts and serum cortisol levels following treatments from baseline and among treatment regimens in individuals who completed all treatment regimens; data that were not normally distributed were log transformed; 95% CI of the Midline Statistic Of Rhythm (MESOR, a rhythm-adjusted mean), amplitude (a measure of half the extent of predictable variation within a cycle) and acrophase (the timing of peak within a circadian cycle)17 (figure 2) were estimated using 1000 iterations of bootstrapping. Any potential carry over effects were examined by adjusting treatment sequence using multivariate mixed-effects cosinor modelling. Frequency of reliever use and AEs were reported using descriptive statistics where the number of participants and events were summarised. The peak flow diurnal variability during the last week of the run-in period and 1 week prior to the end of each treatment period were calculated as: (difference between maximum of 03:00 and maximum of 15:00)/mean per day and averaged over the number of days; tests performed outside the predefined time windows were excluded. Missing data were excluded from the analysis. All statistical analyses were performed using R version 4.2.2 (Rstudio 2022.12.0).
Figure 2. Parameters assessed in mixed-effect cosinor modelling.
Results
Baseline characteristics
Of 25 participants who consented to participate in the study (between 18 June 2021 and 5 May 2022), 22 completed ODAM, 21 ODPM and 23 BD dosing regimens (online supplemental figure E1). Approximately equal numbers were allocated to each sequence of treatment (online supplemental figure E2). Twenty-one participants (84%) completed all treatment regimens (table 1) and had similar chronotypes (timing for sleep/wake cycle) (online supplemental figure E3). Adherence rates (BD 98.4% adherence (97.8% within instructed time window); ODAM 99.9% (96.3%) and ODPM 99.9% (98.1%)) for each of the treatment regimens were excellent.
Table 1. Baseline characteristics.
| Baseline characteristics | n=25 |
|---|---|
| Age (years)* | 42 (34–52) |
| Sex (male), n (%) | 16 (64.0) |
| Ethnicity (white), n (%) | 19 (76.0) |
| Height (cm)* | 172 (166–180) |
| Weight (kg)* | 73.8 (69.9–83.6) |
| BMI (kg/m2)* | 26.1 (24.5–28.6) |
| ACQ-6 (points)* | 1.0 (0.3–1.3) |
| FEV1 (L)* | 2.8 (2.4–3.5) |
| FEV1 % predicted (%)* | 86 (71–89) |
| FEV1/FVC (%)* | 70.6 (63.4–76.8) |
| FeNO (ppb)* | 50 (26–97) |
| Blood eosinophil counts (x109 cells/L)* | 0.23 (0.18–0.35) |
Median (IQR).
ACQ-6, 6-item Asthma Control Questionnaire; BMI, body mass index; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
At baseline, lung function demonstrated significant variation throughout 24 hours, with increased airflow obstruction overnight compared with during the daytime. This coincided with blood eosinophil levels peaking overnight. In contrast, FeNO and serum cortisol levels peaked at 10:00 and with a nadir overnight (online supplemental table E1, online supplemental figures 4; 5).
Effect of chronotherapy
Lung function
All treatment regimens improved lung function compared with baseline, but the timing of lung function improvement was different depending on dosing regimens (onlinesupplemental figures 610). There was a larger improvement in FEV1 following ODPM than ODAM (by median (IQR) of +170 (+30, +220) ml, p<0.01) and BD dosing (by +60 (−10, +240) ml, p=0.02; 10/21 participants had >+100 mL difference) at 22:00. A similar but smaller effect was also observed in the differences of improvement in FEV1 % predicted (ODPM–ODAM: by +4% (+1%, +9%), p<0.01; ODPM–BD: by +2% (−1%, +9%), p=0.02) and FEV1/FVC ratio (ODPM–ODAM: p=0.02; ODPM–BD: p=0.03) at 22:00 (table 2, online supplemental figure 11). A modest improvement in FVC % predicted was also found following ODPM compared with ODAM dosing (p=0.01, table 2, online supplemental figure 11). There was no difference in PEFR among treatment regimens (online supplemental table E2, online supplemental figure 12).
Table 2. Changes in lung function parameters at end of each treatment period compared with baseline.
| Time of measurements | ODAM | BD | ODPM | p-value† |
|---|---|---|---|---|
| Improvement in FEV1 (mL), median (IQR) | ||||
| 04:00 (n=21) | 60 (10, 200) | 110 (0, 240) | 120 (40, 210) | 0.33 |
| 10:00 (n=21) | 40 (−10, 100) | 30 (−20, 190) | 110 (−20, 200) | 0.33 |
| 16:00 (n=21) | 110 (−30, 200) | 100 (50, 260) | 80 (−40, 210) | 0.72 |
| 22:00 (n=21) | −20 (−80, 230) | 80 (−20, 200) | 160 (70, 270) | <0.01 |
| Improvement in FEV1 % predicted, median (IQR) | ||||
| 04:00 (n=21) | 2 (0, 5) | 4 (0, 6) | 4 (1, 8 ) | 0.22 |
| 10:00 (n=21) | 1 (0, 3) | 1 (−1, 7) | 3 (−1, 5) | 0.44 |
| 16:00 (n=21) | 3 (−1, 8) | 2 (2, 7 ) | 3 (−1, 6) | 0.85 |
| 22:00 (n=21) | 0 (−3, 6) | 4 (0, 5) | 5 (2, 8 ) | <0.01 |
| Improvement in FVC (mL), median (IQR) | ||||
| 04:00 (n=21) | 50 (20, 150) | 50 (−50, 270) | 100 (0, 230) | 0.44 |
| 10:00 (n=21) | 40 (0, 130) | 80 (−20, 190) | 150 (−30, 190) | 0.50 |
| 16:00 (n=21) | 20 (−180, 180) | 90 (−190, 220) | 20 (−70, 130) | 0.83 |
| 22:00 (n=20) | −35 (−208, 62) | 10 (−165, 130) | 55 (−80, 198) | 0.08 |
| Improvement in FVC % predicted, median (IQR) | ||||
| 04:00 (n=21) | 1 (0, 3) | 1 (−1, 5) | 3 (0, 5) | 0.49 |
| 10:00 (n=21) | 2 (0, 3) | 2 (0, 4) | 3 (0, 5) | 0.33 |
| 16:00 (n=21) | 1 (−5, 4) | 2 (−4, 5) | 1 (−1, 3) | 0.85 |
| 22:00 (n=20) | −1.5 (−5, 2) | 0 (−4, 3) | 2 (−2, 5) | 0.03 |
| Changes in FEV1/FVC ratio (%), median (IQR) | ||||
| 04:00 (n=21) | 0.8 (−0.5, 2.4) | 1.5 (−1.4, 5.6) | 1.9 (0.8, 2.9) | 0.07 |
| 10:00 (n=21) | 0.1 (−1.1, 1.8) | −0.8 (−2.2, 1.7) | 0.1 (−1.3, 3.6) | 0.47 |
| 16:00 (n=21) | 2.1 (0.4, 4.9) | 2.0 (0.2, 4.4) | 1.2 (−0.2, 3.2) | 0.54 |
| 22:00 (n=20) | 1.4 (−0.7, 4.2) | 1.3 (−0.7, 3.9) | 3.1 (1.2, 5.1) | <0.05 |
Bold indicates statistical significance and subsequent pairwise comparison carried out and demonstrated in online supplemental figure 11.
BD, twice a day; FEV1, forced expiratory volume ins 1 s; FVC, forced vital capacity; ODAM, once a day in the morning; ODPM, once a day in the afternoon.
Using cosinor modelling, the mean (95% CI) MESOR for FEV1 was 2.65 (2.29 to 3.02) L at baseline; a higher MESOR for FEV1 was found following the ODPM dosing regimen (2.80 (2.43 to 3.16) L) compared with ODAM (2.72 (2.35 to 3.08) L, p=0.01) and BD (2.73 (2.36 to 3.10) L, p=0.04) (figure 3). Similarly, with the FVC MESOR of 4.07 (3.59 to 4.56) L at baseline, a higher FVC MESOR was observed following ODPM (4.18 (3.70 to 4.66) L) compared with ODAM (4.09 (3.61 to 4.57) L, p<0.01) (figure 3).
Figure 3. Dynamic circadian changes in lung function, FeNO, blood eosinophil counts and serum cortisol levels at baseline and following each dosing regimen using cosinor modelling. Top panel shows cosinor analysis over time (x-axis); the dotted line indicates MESOR, vertical dotted arrows indicate amplitude and the horizontal dotted arrows indicate acrophase (as demonstrated in figure 2); bottom panels demonstrate the overall mean (95% CI) of MESOR, amplitude and acrophase, estimated using 1000 iterations of bootstrapping. For pairwise comparison among three dosing regimens, p-value and FDR are presented: §FDR<0.1, *FDR<0.05, **FDR<0.01, ***FDR<0.001. Based on 24 hour period; acrophase is a measure of the timing of overall high values occurring in the cycle, expressed in negative radians. BD, twice a day; FDR, false discovery rate; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MESOR, Midline Statistic Of Rhythm; ODAM, once a day in the morning; ODPM, once a day in the afternoon.
Biomarkers for airway inflammation
All dosing regimens suppressed FeNO levels with no significant differences among dosing regimens (table 3, figure 3, online supplemental figure 13).
Table 3. Changes in biomarkers at the end of each treatment period compared with baseline.
| Time of measurements | ODAM | BD | ODPM | p-value |
|---|---|---|---|---|
| Changes in FeNO (ppb), median (IQR) | ||||
| 04:00 (n=21) |
−8.5 (−12.0, –1.0) |
−4.5 (−14.0, 4.5) |
−2.0 (−23.0, 2.0) |
0.85 |
| 10:00 (n=21) |
−10.0 (−23.0, –5.5) |
−10.5 (−29.5, –1.0) | −9.5 (−33.0, –4.0) |
0.87 |
| 16:00 (n=21) |
−6.5 (−20.5, –2.0) |
−7.5 (−21.0, 0.5) |
−12.5 (−27.0, –0.5) |
0.69 |
| 10:00 (n=21) |
−8.0 (−18.5, –2.0) |
−8.5 (−23.5, –2.0) |
−9.5 (−28.5, 1.0) |
0.74 |
| Changes in blood eosinophil counts (x109 cells/L), median (IQR) | ||||
| 04:00 (n=19) |
−0.10 (−0.22, –0.03) |
−0.08 (−0.20, –0.03) |
−0.12 (−0.24, –0.10) |
0.04 |
| 10:00 (n=16) |
−0.04 (−0.19, –0.01) |
−0.04 (−0.12, 0) |
−0.09 (−0.20, –0.03) |
0.65 |
| 16:00 (n=21) |
−0.13 (−0.25, –0.03) |
−0.10 (−0.17, –0.01) |
−0.12 (−0.19, –0.05) |
0.07 |
| 22:00 (n=19) |
−0.13 (−0.15, 0) |
−0.06 (−0.16, –0.02) |
−0.18 (−0.21, –0.07) |
<0.01 |
Bold indicates statistical significance and subsequent pairwise comparison carried out and demonstrated in online supplemental figure 15.
BD, twice a day; FeNO, fractional exhaled nitric oxide; ODAM, once a day in the morning; ODPM, once a day in the afternoon.
All regimens reduced blood eosinophil counts compared with baseline at all time points (online supplemental figure 14). The reduction in blood eosinophil counts overnight following ODPM dosing was significantly larger than those following BD dosing (by median (IQR) of −0.07 (−0.13, –0.05)×109 cells/L at 22:00, p<0.01; −0.04 (−0.08, –0.02)×109 cells/L at 04:00, p=0.03); this was also observed compared with ODAM dosing, but to a smaller effect (by −0.05 (−0.08, –0.01)×109 cells/L at 22:00, p=0.03; −0.04 (−0.08, +0.01)×109 cells/L at 04:00, p=0.04) (table 3, online supplemental figure 15).
With a baseline mean (95% CI) MESOR in blood eosinophil counts of 0.31 (0.25 to 0.39)×109 cells/L, both ODPM (0.17 (0.14 to 0.22)×109 cells/L) and ODAM (0.18 (0.14 to 0.22)×109 cells/L) resulted in lower MESOR following the treatment period compared with the standard BD dosing regimen (0.23 (0.18 to 0.29)×109 cells/L, p<0.01) (figure 3).
The number of participants who were able to produce sufficient sputum were limited for statistical analysis (online supplemental figure 16, online supplemental section E4).
Serum cortisol levels
All treatment regimens suppressed serum cortisol levels compared with baseline, but the degree of suppression differed among treatment regimens at different time points; there was no difference in the 10:00 cortisol suppression among treatment regimens (online supplemental table E3, online supplemental figures 17; 18). While all dosing regimens reduced MESOR for serum cortisol levels compared with baseline (median (IQR) of 149 (127, 174) nmol/L), there were no differences among three dosing regimens (figure 3).
The same results were obtained following adjustment of treatment sequence within the model, suggesting there was no carry over effect (online supplemental figure 19).
Asthma control, reliever medication use and adverse events
All dosing regimens improved ACQ-6 scores with no difference seen among dosing regimens (online supplemental figure 20). There was no difference in reliever medication use and AE profiles among dosing regimens (online supplemental table E4, online supplemental figure 21)
Discussion
We have shown that once-daily mid-afternoon dosing of BDP resulted in greater improvement in FEV1 and reduction in blood eosinophil counts compared with once-daily morning and standard twice-daily dosing regimens. Mid-afternoon dosing demonstrated the greatest effect on improving nocturnal airflow obstruction, reaching significance compared with other dosing regimes at 22:00, and blood eosinophilia which reached significance compared with other dosing regimes at 10:00 and 04:00. Mid-afternoon dosing did not demonstrate additional overall cortisol suppression (measured in MESOR) or AEs compared with the alternative dosage timings. Our findings further support the hypothesis that the optimal timing of ICS administration is at 16:00, coincident with enhanced glucocorticoid sensitivity at that time. The notion that the onset of the inflammatory cascade begins mid-afternoon may also explain the findings we observed, and the attenuation of the predictable rhythmic recruitment of airway inflammatory cells at this time point may abolish the subsequent excessive nocturnal dip in lung function in asthma.18 19
We used cosinor modelling to evaluate the effect of ICS chronotherapy on the dynamic fluctuation in asthma. This analytical method uses multiple measurements to model the continuous diurnal variation observed in asthma, including the rhythmic peak and trough changes which may be easily missed (resulting in an underestimation of amplitude) unless continuous or sufficiently frequent measurements occur over a 24-hour period. Not only would this be impractical, but the frequent test procedures would profoundly disrupt sleep and circadian rhythm of study participants.
We have confirmed that maximal airflow obstruction and inflammation occur overnight in asthma; FeNO peaks late morning, with a nadir overnight.1 5 20 21 The magnitude of within-day variations in lung function and inflammatory biomarkers were large, highlighting the importance of timing of tests when results are interpreted.1
Previously, Beam and colleagues found that a single dose of oral prednisolone at 15:00 resulted in a significant improvement in the nocturnal dip in lung function, blood eosinophil counts and a pan-cellular reduction in bronchoalveolar lavage cytology at 04:00 in uncontrolled asthma, but these were not observed in participants dosed at 08:00 or 20:00.18 Frezza et al also demonstrated that a single dose of inhaled BDP or fluticasone propionate given at 16:00 was sufficient to reduce the nocturnal dip in FEV1 at 04:00 in patients with steroid-naïve asthma compared with placebo, but no comparison was made with other times of dosing.19 Similarly, Postma et al found that evening (between 16:00 and 20:00) administration of inhaled ciclesonide resulted in more pronounced improvement in the morning peak expiratory flow compared with a once-daily morning dosing regimen.22 Although several randomised controlled trials have previously investigated mid-afternoon dosing of ICS in asthma,10,12 our study is the first to show the effect of chronotherapy on the daily fluctuations in both physiological and immunological domains over 24 hours and compared these with widely used dosing regimens.
The current study is limited by small sample size, short therapeutic and follow-up duration. Nevertheless, the three-way cross-over design allows repeated measurements to be performed within the same individual, increasing study power for parameters of interest. While the small effect size may increase the risk of type II error (false negative results), we have demonstrated statistical significance in the prespecified key end points in MESOR and 22:00 time point; the negative results may be due to the limited power. Nevertheless, the data presented in our study form the basis for the design of future larger trials to establish the efficacy of ICS chronotherapy.
Opinions regarding what constitutes the minimum clinically important difference (MCID) in FEV1 varies considerably (100–200 mL).23 24 While we have found a 170 mL difference in the improvement of FEV1 at 22:00 following mid-afternoon compared with the morning dosing regimen, the benefit over the standard twice-daily regimen was smaller (median +60 mL). However, it is also important to note that almost half of participants had >100 mL of FEV1 improvement at 22:00 following ODPM than BD dosing. We did not demonstrate any difference in ACQ-6 among dosing regimens, likely due to mild symptoms at baseline and short treatment duration. Although the MCID in the reduction of blood eosinophil counts is unclear, a level of >0.2×109 cells/L has been associated with increased asthma exacerbations.25 We observed that mid-afternoon dosing improved the MESOR of blood eosinophil counts from over 0.2×109 cells/L following a twice-daily dosing regimen to under this threshold, suggesting mid-afternoon dosing may result in a reduction in asthma exacerbation risk compared with twice-daily dosing.26 27 However, this could not be confirmed due to the limited follow-up period. An efficacy trial with an extended dosing duration is needed to validate our findings, and to establish the longer-term impact of chronotherapy in asthma control, exacerbation rates, adherence and side effects. Our study population included individuals with mild-to-moderate allergic asthma and therefore the results cannot be extrapolated to other phenotypes.
While mid-afternoon dosing may be complicated by the challenges around non-adherence in some patients,28 the advantages of taking ICS at the optimal timing may overcome some of the identified barriers (eg, achieving efficacy with lower cost).29 30 Our findings also provide key opportunities for novel chronopharmacological and chronotherapeutics development,31 32 leading to the possibilities of tailored therapy based on individuals’ preference in timing of drug administration and their biological rhythm in disease.
Conclusion
Mid-afternoon dosing of inhaled BDP better improved nocturnal lung function and inflammatory biomarkers compared with the alternative dosage timings without additional adrenal suppression, hence may lead to better clinical outcomes without increasing steroid-related morbidity or costs. A future large, real-world chronotherapy study framed within the new asthma guidelines,33 using formoterol/ICS will determine if afternoon dosing leads to a reduction in exacerbations, better overall symptom control, health-economic benefits, and crucially, determine patient preference for afternoon dosing.
Supplementary material
Acknowledgements
We would like to thank the study participants for their time and commitment to the study and the Medicines Evaluation Unit (MEU) study team for help with the completion of the study. RW, AS, DS and HJD are supported by the Manchester ‘NIHR Biomedical Research Centre’ (BRC). DWR NIHR Oxford Health Biomedical Research Centre grant reference number: NIHR203316. MRC MR/W019000/1 and MR/V034049/1. This study is funded/supported by the National Institute for Health and Care Research (NIHR) Oxford Health Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. NIHR Oxford Health Biomedical Research Centre grant reference number: NIHR203316.
Footnotes
Funding: This work was supported by the JP Moulton Charitable Foundation. The funder has no role in the study design, data collection, analysis, and design of the manuscript. HJD is funded by MRC-CSF-MR/V029460/1 and received support from Asthma and Lung UK AUK-WAPG22/100005. RM is funded by Asthma and Lung UK WAPG22/100005.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by North West Greater Manchester South, 20/NW/0011 Participants gave informed consent to participate in the study before taking part.
Data availability free text: The study protocol is available on trial registration website. Individual participant data that underlie the results reported in this article after deidentification will be shared 3 years following article publication with investigators whose proposed use of data has been approved by the study sponsor. Proposal will be directed to pmoore@crosolutions.co.uk. To gain access, data requestors will need to sign a data access agreement.
Data availability statement
Data are available upon reasonable request.
References
- 1.Wang R, Murray CS, Fowler SJ, et al. Asthma diagnosis: into the fourth dimension. Thorax. 2021;76:624–31. doi: 10.1136/thoraxjnl-2020-216421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner-Warwick M. Nocturnal asthma: a study in general practice. J R Coll Gen Pract. 1989;39:239–43. [PMC free article] [PubMed] [Google Scholar]
- 3.Hetzel MR, Clark TJ, Branthwaite MA. Asthma: analysis of sudden deaths and ventilatory arrests in hospital. Br Med J. 1977;1:808–11. doi: 10.1136/bmj.1.6064.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin RJ, Cicutto LC, Ballard RD. Factors related to the nocturnal worsening of asthma. Am Rev Respir Dis. 1990;141:33–8. doi: 10.1164/ajrccm/141.1.33. [DOI] [PubMed] [Google Scholar]
- 5.Durrington HJ, Gioan-Tavernier GO, Maidstone RJ, et al. Time of Day Affects Eosinophil Biomarkers in Asthma: Implications for Diagnosis and Treatment. Am J Respir Crit Care Med. 2018;198:1578–81. doi: 10.1164/rccm.201807-1289LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juniper EF, O’Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 7.Kraft M, Vianna EO, Martin RJM, et al. Nocturnal asthma is associated with reduced glucocorticoid receptor binding affinity and decreased steroid responsiveness at night. J Allergy Clin Immunol. 1999;103:66–71. doi: 10.1016/s0091-6749(99)70527-0. [DOI] [PubMed] [Google Scholar]
- 8.Kraft M, Hamid Q, Chrousos GP, et al. Decreased Steroid Responsiveness at Night in Nocturnal Asthma. Am J Respir Crit Care Med . 2001;163:1219–25. doi: 10.1164/ajrccm.163.5.2002058. [DOI] [PubMed] [Google Scholar]
- 9.Krakowiak K, Maidstone RJ, Chakraborty A, et al. Identification of diurnal rhythmic blood markers in bronchial asthma. ERJ Open Res. 2023;9:00161-2023. doi: 10.1183/23120541.00161-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pincus DJ, Humeston TR, Martin RJ. Further studies on the chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J Allergy Clin Immunol. 1997;100:771–4. doi: 10.1016/s0091-6749(97)70272-0. [DOI] [PubMed] [Google Scholar]
- 11.Pincus DJ, Szefler SJ, Ackerson LM, et al. Chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J Allergy Clin Immunol. 1995;95:1172–8. doi: 10.1016/s0091-6749(95)70073-0. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon M, Côte J, Milot J, et al. Comparative safety and efficacy of single or twice daily administration of inhaled beclomethasone in moderate asthma. Chest. 1994;105:1732–7. doi: 10.1378/chest.105.6.1732. [DOI] [PubMed] [Google Scholar]
- 13.British National Formulary. [31-Jan-2024]. https://bnf.nice.org.uk Available. Accessed.
- 14.Global Initiative for Asthma (GINA) Main Report. 2023. https://ginasthma.org/gina-reports Available.
- 15.Schulze J, Rosewich M, Riemer C, et al. Methacholine challenge--comparison of an ATS protocol to a new rapid single concentration technique. Respir Med. 2009;103:1898–903. doi: 10.1016/j.rmed.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Hou R, Tomalin LE, Suárez-Fariñas M. cosinoRmixedeffects: an R package for mixed-effects cosinor models. BMC Bioinformatics. 2021;22:553. doi: 10.1186/s12859-021-04463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11:16. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beam WR, Weiner DE, Martin RJ. Timing of prednisone and alterations of airways inflammation in nocturnal asthma. Am Rev Respir Dis. 1992;146:1524–30. doi: 10.1164/ajrccm/146.6.1524. [DOI] [PubMed] [Google Scholar]
- 19.Frezza G, Terra-Filho J, Martinez JAB, et al. Rapid effect of inhaled steroids on nocturnal worsening of asthma. Thorax. 2003;58:632–3. doi: 10.1136/thorax.58.7.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheer FAJL, Hilton MF, Evoniuk HL, et al. The endogenous circadian system worsens asthma at night independent of sleep and other daily behavioral or environmental cycles. Proc Natl Acad Sci U S A. 2021;118:e2018486118. doi: 10.1073/pnas.2018486118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson M, Maidstone R, Loudon A, et al. Circadian rhythm of exhaled biomarkers in health and asthma. Eur Respir J. 2019;54:1901068. doi: 10.1183/13993003.01068-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postma DS, Sevette C, Martinat Y, et al. Treatment of asthma by the inhaled corticosteroid ciclesonide given either in the morning or evening. Eur Respir J. 2001;17:1083–8. doi: 10.1183/09031936.01.00099701. [DOI] [PubMed] [Google Scholar]
- 23.Bonini M, Di Paolo M, Bagnasco D, et al. Minimal clinically important difference for asthma endpoints: an expert consensus report. Eur Respir Rev. 2020;29:190137. doi: 10.1183/16000617.0137-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129:S65–87. doi: 10.1016/j.jaci.2011.12.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallah N, Rodriguez-Segade S, Gonzalez-Barcala FJ, et al. Blood eosinophil count as predictor of asthma exacerbation. A meta-analysis. Pediatr Allergy Immunol. 2021;32:465–78. doi: 10.1111/pai.13403. [DOI] [PubMed] [Google Scholar]
- 26.Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3:849–58. doi: 10.1016/S2213-2600(15)00367-7. [DOI] [PubMed] [Google Scholar]
- 27.Kerkhof M, Tran TN, van den Berge M, et al. Association between blood eosinophil count and risk of readmission for patients with asthma: Historical cohort study. PLoS One. 2018;13:e0201143. doi: 10.1371/journal.pone.0201143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rand CS, Wise RA. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med. 1994;149:S69–76. doi: 10.1164/ajrccm/149.2_Pt_2.S69. [DOI] [PubMed] [Google Scholar]
- 29.Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnaires. Immunol Allergy Clin North Am. 2005;25:107–30. doi: 10.1016/j.iac.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 30.McQuaid EL. Barriers to medication adherence in asthma: The importance of culture and context. Ann Allergy Asthma Immunol. 2018;121:37–42. doi: 10.1016/j.anai.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Ohdo S, Koyanagi S, Matsunaga N. Chronopharmacology of immune-related diseases. Allergol Int. 2022;71:437–47. doi: 10.1016/j.alit.2022.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Buttgereit F, Spies CM, Bijlsma JWJ. Novel glucocorticoids: where are we now and where do we want to go? Clin Exp Rheumatol. 2015;33:S29–33. [PubMed] [Google Scholar]
- 33.Asthma: diagnosis, monitoring and chronic asthma management (BTS, NICE, SIGN) https://www.nice.org.uk/guidance/NG245 n.d. Available. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.