Abstract
Fibroblast growth factor-3 (FGF-3) is a crucial developmental regulator. Aberrant activation of this gene by mouse mammary tumor virus insertion results in pregnancy-responsive mammary tumorigenesis. To characterize better FGF-3 function in postnatal mammary gland development and cancer initiation/progression, we used a mifepristone (RU486)-inducible regulatory system to express conditionally FGF-3 in the mammary epithelium of transgenic mice. Ectopic overexpression of FGF-3 in pubescent mammary glands elicited severe perturbations in early mammary gland development leading to mammary hyperplasia. Ductal elongation was retarded, multiple cysts persisted in the virgin ducts, and ductal epithelium was expanded and multilayered. The altered ductal architecture and the persistence of hyperplastic multilayered epithelium reflect a defect in growth regulation, which resulted from an imbalance between mitogenic and apoptotic signals. By altering the duration of RU486 treatment, we showed that the persistence of mitogenic signal elicited by FGF-3 is crucial for the initiation, progression, and maintenance of the hyperplastic characteristic of the mammary epithelium. The manifestations elicited by FGF-3 could be reversed by RU486 withdrawal. In addition, synergism between the stimulus from estrogen and FGF-3 mitogenic pathways was evident and likely contributes to the pregnancy-dependent tumorigenesis of FGF-3. Taken together, the mifepristone-inducible regulatory system provides a powerful means for understanding the diverse roles of FGF-3 and its interactions with hormones in mammary gland tumorigenesis.
Keywords: mifepristone-inducible expression, int-2, mammary gland hyperplasia
The majority of mammary gland development takes place postnatally. Primary mammary epithelial ducts are established before birth but remain quiescent until puberty when ductal morphogenesis and patterning occurs in response to the systemic effects of estrogen and growth hormones. Through a process balanced by rapid proliferation of the cap cells and massive apoptosis of the body cells, coupled with the action of proteases, the ducts elongate, become progressively branched, and fill the fat pad at ≈12 weeks of age (1). The gland then remains dormant until the hormonal stimulus of pregnancy; a cyclical phase of development is initiated in synchrony with reproductive status of the animals. The cyclic phase includes extensive growth and differentiation of the ducts for the development of lobuloalveolar structures during pregnancy, milk proteins synthesis during lactation, and massive apoptosis of the alveolar epithelium during involution. After involution, the mammary gland resembles the mature ductal tree of the virgin mouse but with increase of lateral branches.
The interplay between numerous cell-cycle regulators, ovarian hormones, growth factors, and their receptors governs pre- and postnatal mammary gland development. Ablation or misexpression of these network components causes dramatic disruption of mammary gland morphogenesis and patterning, and may also result in tumor development. In addition, the stage in development when the gene is first expressed often influences the phenotypic manifestations, suggesting that temporal regulation of gene expression is a prerequisite for proper mammary gland development. Thus, an inducible system, which is able to control the temporal expression of a gene in the mammary gland, will provide a better understanding of the role of target genes in mammary development and tumorigenesis.
Fibroblast growth factor-3 (FGF-3) exhibits complex expression patterns and plays a role in the proliferation and differentiation of a diverse array of developing embryonic tissues (2). In vivo expression analysis and derivation of homozygous FGF-3 null mouse mutant have suggested roles in cell migration, tissue induction, and neuronal cell differentiation (3, 4). Insertional activation of this gene by mouse mammary tumor virus (MMTV) has been correlated with the appearance of mammary tumors (5). In addition, ectopic overexpression of FGF-3 in transgenic mice resulted in pregnancy-responsive mammary hyperplasia (6, 7). Subsequently, Ornitz et al. (8) reported that the expression of FGF-3 in the early virgin mammary gland elicited abnormal development. However, the lack of temporal and reversible transgene expression in these systems nullifies attempts to assess the temporal role that FGF-3 plays in early mammary gland development and the initiation/progression events of FGF-3 tumorigenesis. Hence, to circumvent these problems, a mifepristone (RU486)-inducible system (9) recently established in our laboratory was used to facilitate this study.
The mammary-specific inducible binary system comprises two components: (i) A chimeric transactivator, GLp65, which is responsive only to progesterone antagonists, contains the Gal4 DNA-binding domain, a mutated progesterone receptor ligand-binding domain, and an activation domain of NFκB subunit p65. (ii) The target is placed under the control of Gal4-binding sites, the upstream activating sequence (UASG). To obtain tissue-specific expression of the target gene in the mammary gland, the transactivator was placed under the control of MMTV promoter. In these binary transgenic mice, a spatiotemporal-specific expression could be demonstrated upon administration of the progestin antagonist, RU486.
Ectopic expression of FGF-3 in the mammary glands of bigenic pubescent virgin resulted in dramatic mammary gland abnormalities. The bigenic mice displayed enlargement in duct size, retardation of ductal extension, and atypical mammary gland hyperplasia in regions highly expressing FGF-3. The extent of the induced hyperplasia depended on the length and levels of FGF-3 expression. In addition, the phenotypic alterations in mammary gland could be reversed upon withdrawal of the ligand. Finally, estrogen and progesterone further enhanced the extensive mammary epithelium proliferation elicited by FGF-3, implying the contributions of these hormones to tumor progression.
Materials and Methods
Generation of MMTV-GLp65 Transgenic Mice.
The transgenic construct was created by cloning a 2.7-kb blunt-ended Asp-718–BamHI fragment of the GLp65 transactivator into the blunt-ended EcoRI site of the MMTV-KCR vector (10) to yield the MMTV-KCR-GLp65 vector. The KCR fragment was derived from the rabbit β-globin gene. To create GLp65 transgenic mice, a 5.41-kb Acc65I-BamHI fragment was released from MMTV-KCR-GLp65 and isolated using Geneclean. The fragment (2 ng/ml) was injected into FvB/N-fertilized eggs to generate transgenic mice by using standard protocols (11). Founder mice were analyzed by PCR and Southern hybridization (12). Heterozygous MMTV-GLp65 mice were bred with homozygous UASG-FGF-3 monogenic mice [obtained from Philip Leder (Harvard University, Cambridge, MA) and described by Ornitz et al. (8)] to generate bigenic mice.
Administration of RU486 to Mice.
RU486 purchased from Biomol (Plymouth Meeting, PA) was used to make pellets (Innovative Research of America) for a constant daily release of RU486 at 150, 300, and 450 μg/kg body weight.
RNase Protection Assays.
Total RNA was isolated from mouse tissues by using the Trizol reagent (GIBCO). Expression of transgenes was detected by RNase protection assay with the RPAIII kit (Ambion, Austin, TX). Five to ten micrograms of total RNA was hybridized with 32P-labeled FGF-3 (12), GLp65, Cyclin D1 (PharMingen) and control cyclophilin or L32 antisense riboprobes and assayed as described (Ambion).
Whole Mount, Histology, and Immunohistochemistry.
Whole-mount staining of the left fourth inguinal mammary gland was performed as described (10). For histological analyses, glands were fixed in cold 4% paraformaldehyde and embedded in paraffin. The 7-μm sections were rehydrated, microwaved for 20 min in 10 mM sodium citrate, and incubated with anti-E-Cadherin (Zymed) or p42/p44 mitogen-activated protein kinase (MAPK) (Cell Signaling, Beverly, MA) antibodies, followed by secondary antibodies (Molecular Probes) and mounting with aqueous mounting media (Vector Laboratories). For BrdUrd incorporation studies, the mice were injected i.p. with 100 μg/g body weight of BrdUrd 2 h before sacrifice to label cells in the S phase. The BrdUrd-labeled cells were detected by immunohistochemistry with monoclonal anti-BrdUrd antibody (Dako) as described (10). Analyses of 300–400 epithelial cell nuclei per section from four monogenic and bigenic glands were performed.
Results
Ligand-Inducible Expression of FGF-3 in Transgenic Mice.
To achieve mammary gland-specific expression of FGF-3 in a mifepristone-dependent manner, we first generated the MMTV-GLp65 line by using the transgenic construct depicted in Fig. 1A (MMTV-KCR-GLp65). Four transactivator mouse lines expressing GLp65 in the mammary glands were identified by ribonuclease protection assay (RPA) (data not shown). The transgenic line 5277Fo used in this study predominantly expressed GLp65 mRNA in the mammary gland and not in other tissues (Fig. 1B). By crossing this transactivator line with the UASG-FGF-3 target, we obtained bigenic mice (GLp65/UASG-FGF-3). To characterize our regulatory system, we implanted the RU486 pellets s.c. with a dosage of 150 μg/kg body weight for 2 weeks. Placebo pellets lacking RU486 served as a vehicle control. As expected, FGF-3 RNA was detected only in bigenic mice given RU486, and no detectable leakage was seen in monogenic mice given RU486 or in bigenic mice given placebo (Fig. 1C). Hence, we show that the FGF-3 target is tightly regulated in an RU486-dependent fashion in our transgenic model. Kinetic studies indicated that FGF-3 RNA became detectable level one day after administration of RU486 pellet and was maintained at a high level for weeks (Fig. 1D). In addition, a dose-dependent increase of FGF-3 RNA was detected when RU486 concentration was increased from 150 to 450 μg/kg body weight (Fig. 1E). As expected, no detectable FGF-3 was observed in the control monogenic mice given RU486 (UASG-FGF-3).
Fig 1.
Characterization of the RU486-inducible system. (A) The MMTV-KCR-GLp65 construct contains a 1.54-kb MMTV-LTR and GLp65 transactivator linked to KCR as described in Material and Methods. (B) RNase protection assay of 10 μg of RNA from different transgenic mice tissues showed that GLp65 was specifically expressed in the mammary glands (M.Gl). (C) RU486 induction of FGF-3 was seen only in bigenic mammary glands (Bi-Tg: GLp65/UASG-FGF-3) and not in monogenic target (UASG-FGF-3) or transactivator (MMTV-GLp65) glands given 150 μg/kg of body weight of RU486 or bigenic given placebo treatment for 2 weeks. Expression of GLp65 was seen only in monogenic transactivator and bigenic glands. (D) FGF-3 expression in bigenic mice was induced after 1 day of 150 μg/kg of body weight of RU486 and persisted for the duration of the treatment. (E) Dose-dependent increase in FGF-3 expression was seen only in bigenic mice. FGF-3 expression was not detected in monogenic mice given RU486.
Phenotypic Consequences of FGF-3 Induction in Pubescent Mammary Gland.
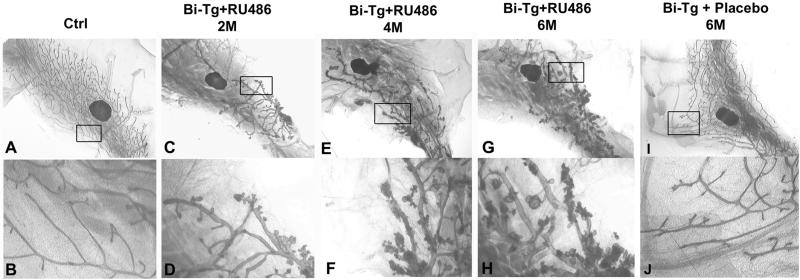
To elucidate the role of FGF-3 in early mammary gland development, FGF-3 expression was induced in 3-week-old bigenic mice with 450 μg/kg RU486 for 2 months. Monogenic UASG-FGF-3 and wild-type controls were also given a similar dosage of RU486. The control (Fig. 2 A and B) and bigenic mice treated with placebo (Fig. 2 I and J) consistently displayed normal mammary ductal development with the fat pad completely filled with branched ducts (Fig. 2 A and I). At higher magnifications, the epithelial ducts displayed a smooth surface (Fig. 2 B and J). However, ectopic expression of the FGF-3 in bigenic mice at early puberty resulted in abnormal mammary gland development (Fig. 2 C–H). The bigenic glands showed prominent retardation in ductal elongation with the ducts displaying less side-branching and failing to penetrate beyond the lymph node proximal to the nipple (Fig. 2C). Profound differences were also visible because the bigenic ducts seemed more dilated and were punctuated with small aberrant lobular protuberances (Fig. 2D). After 4 months of induction, the bigenic gland showed extensive but aberrant lobuloalveolar development (Fig. 2 E and F). The extent of the induced lobular hyperplasia also depended on the duration of FGF-3 expression. A longer FGF-3 expression elicited a more severe form of aberrant lobular hyperplasia (compare Fig. 2 F and H for 4- and 6-month FGF-3 expression, respectively). The progression also depended on the expression level of FGF-3 and could be accelerated by increasing the concentrations of RU486 (data not shown). Therefore, this result suggests that expression of FGF-3 can induce perturbations in the ductal elongation as well as morphogenesis. More importantly, we show that FGF-3 is not only able to initiate but is also crucial for the progression of mammary gland hyperplasia.
Fig 2.
Induced FGF-3 expression initiates and promotes progression of mammary hyperplasia. Whole-mount analyses of mammary glands from bigenic mice treated with 450 μg/kg of RU486 for 2 (C, D), 4 (E, F), and 6 (G, H) months show that prolonged induction of FGF-3 increased the severity of hyperplasia. Monogenics (Ctrl) treated with RU486 (A, B) and bigenics treated with placebo for 6 months (I, J) manifest normal ductal phenotype. (B, D, F, H, and J are higher magnifications of A, C, E, G, and I, respectively.)
These dramatic morphological changes implied that prominent changes occur in the properties of the tissues. Histological analyses revealed precocious mammary hyperplasia in 100% of bigenic mice treated with RU486. Whereas wild-type ducts had a clear lumen within a monolayer of luminal epithelial cells (Fig. 3 A and D), a majority of ducts in the bigenic gland were multilayered and partially filled with loosely associated epithelial cells that presumably arose by alterations in cell–cell adhesion within the ductal wall (Fig. 3B). Some ducts displayed a more severe histological abnormalities and were surrounded by dense connective tissue with an increased number of fibroblasts. In addition, the epithelial cells became completely disorganized and exhibited a nonuniform nuclear morphology (Fig. 3C). The hyperplasia at 6 months of RU486 treatment was equivalent to a moderate grade mammary intraepithelial neoplasia (13). Colocalization of FGF-3 mRNA with the hyperplastic mammary epithelium (Fig. 3 E and F) was demonstrated by in situ hybridization with an FGF-3 antisense riboprobe (12). Thus, this finding is consistent with FGF-3 acting primarily as an autocrine or ultrashort range paracrine growth factor in the mammary epithelium (14).
Fig 3.
Histological and in situ analyses of the transgenic mammary gland. (A–C) Sections were stained with hematoxylin and eosin (H&E). Comparison of controls (Ctrl: monogenics or wild types treated with RU486 or bigenics treated with placebo in A) and bigenics (Bi-Tg) given RU486 for 2 months showed that the bigenic glandular epithelium was multilayered and loosely associated (B) in contrast to a uniform singular layer of cells in the control (A). In a more hyperplastic region (C), the bigenic epithelium was completely disorganized and surrounded by dense connective tissue. (D–F) By in situ hybridization (ISH), high levels of FGF-3 expression were detected as blue-black precipitates in the bigenic (E, F) but not in control epithelium of monogenic mice given 2 months of RU486 treatment (D).
Ectopic Expression of FGF-3 Results in Elevated Epithelial Cell Proliferation.
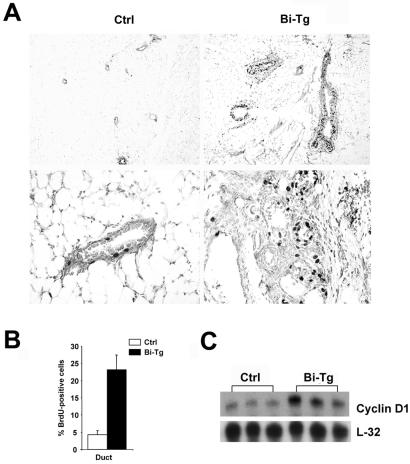
To determine whether mammary hyperplasia was the result of elevated cell proliferation, a BrdUrd incorporation assay was performed. Monogenic control and bigenic females with 2 months of RU486 treatment were administrated BrdUrd, whose incorporation into DNA was detected by immunohistochemistry (Fig. 4A). The proliferation index was calculated as a percentage of BrdUrd-positive epithelial cells out of 300–400 cells per section (Fig. 4B). The proliferation rate in the bigenic mammary ductal epithelium was consistently higher than that of the monogenic control [23.2% in the bigenic gland (Bi-Tg) vs. 4.2% in the monogenic (Ctrl) control], which therefore clearly demonstrated the mitogenic nature of FGF-3, enabling it to induce hyperproliferation of the mammary epithelium.
Fig 4.
Elevated proliferation of FGF-3-expressing epithelial cells. (A) More prominent nuclei positively staining for BrdUrd were observed in the bigenic (Bi-Tg) than control (Ctrl) sections. The lower two panels are the respective higher magnifications of the upper panels. (B) Quantitation of 300–400 mammary epithelial nuclei per section for BrdUrd staining of RU486- treated monogenic control and bigenic glands. Values are an average fraction of BrdUrd-positive nuclei per section from n = 4 animals per group. (C) RNase protection assay indicates that cyclin D1 is up-regulated in bigenic (Bi-Tg) but not control (Ctrl) glands. Ten micrograms of RNA from three individual RU486-treated monogenic and bigenic mice were hybridized with cyclin D1 and L32 antisense riboprobes.
Because cyclin D1 is the major G1 cyclin whose disruption frequently occurs in the tumor cell (15), we examined cyclin D1 expression in control and bigenic mammary glands. RPA analysis revealed that a 2- to 4-fold increase in cyclin D1 was detected in the hyperplastic bigenic glands (Fig. 4C). Monogenic mice treated with RU486 and bigenic mice treated with placebo consistently showed no significant difference from the wild-type control. Thus, ectopic expression of FGF-3 led to an elevated expression of cyclin D1, which contributes to the development of mammary neoplasia.
Altered Expression of Cell Adhesion Molecules in the Hyperplastic Mammary Epithelium.
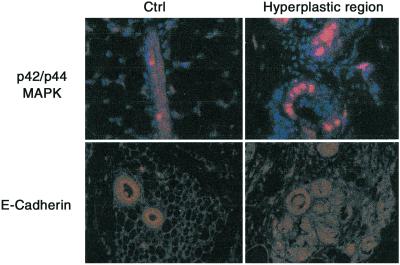
FGF-3 binding to its receptor activates the MAPK signaling pathway (16). As expected, numerous phospho-MAPK-positive cells were visible in the hyperplastic mammary epithelium as compared with the control (Fig. 5). In the hyperplastic region, the epithelium became disorganized, implying a loss of cell–cell contacts. E-cadherin, which is the adhesive component of adheren junctions, is notably absent or dysfunctional in most of the advanced, undifferentiated, and aggressive breast and other epithelial carcinomas (17, 18). Thus, it was of particular interest to examine the expression pattern of this adhesive molecule in bigenic mice. As illustrated in Fig. 5, whereas the normal epithelium exhibited a high level of luminal expression of E-cadherin, the transformed epithelium lost its polarized expression pattern with further down-regulation of E-cadherin expression in these cells. On the other hand, the bigenic mammary epithelial cells still retained a continuous expression of laminin, a marker for basement membrane, indicating that the basement membrane was still intact (data not shown). Hence, these results show that although FGF-3 overexpression could elicit mammary hyperplasia, its expression was insufficient to confer invasive and metastatic properties to the mammary epithelium.
Fig 5.
FGF-3 down-regulates extracellular matrix factors. More bigenic hyperplastic cells stain positive for phospho-MAPK than control cells. Loss of polarized expression of E-Cadherin occurred in bigenic but not in control cells. Sections were stained with anti-phospho-MAPK and E-cadherin antibodies, and positive signals visualized with Cyanine 3 (red). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue).
The Phenotypic Effect of FGF-3 Expression Is Reversible.
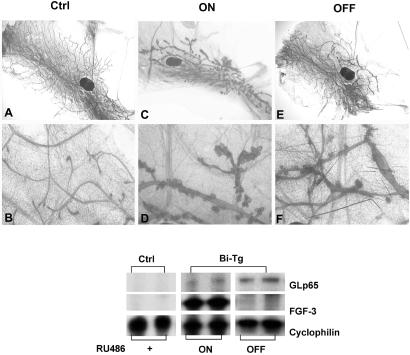
The utility of an inducible bigenic system is best illustrated by its ability to turn FGF-3 transgene expression on and off at will. To test our system, we implanted a group of 3-week-old bigenic mice with RU486 pellets at a dosage of 450 μg/kg for 2 months followed by a withdrawal of RU486 for another 2 months. One of the fourth and fifth inguinal mammary glands were removed from the mice 2 months after first induction and subjected to whole-mount and RPA analyses, respectively, to ensure the expression of the transgene. Pronounced perturbations in mammary gland development were consistently seen subsequent to the expression of FGF-3 (Fig. 2 C and D). As illustrated in Fig. 6 A and B, the control monogenic UASG-FGF-3 mice treated with RU486 for 4 months displayed normal ductal development. On the other hand, constitutive expression of FGF-3 in the bigenic glands for 4 months resulted in retarded ductal extension and precocious mammary hyperplasia (ON in Fig. 6 C and D). Nevertheless, nearly complete normal ductal morphology was seen in the mice withdrawn from RU486 treatment for 2 months. The ducts extended beyond the lymph node and had regularly spaced side branches similar to those of control mice (OFF in Fig. 6E). At a higher magnification, we observed only a small proportion of ducts being slightly wider and punctuated with a few small cystic vesicles (Fig. 6F). FGF-3 can be reinduced in these mice and prior exposure to FGF-3 does not alter the susceptibility of the mice to subsequent oncogenic stimuli (data not shown). The corresponding expression of FGF-3 transgene was evident by RPA as shown in Fig. 6 (Lower). With 4 months of RU486 treatment, the FGF-3 transgene was steadily expressed in bigenic glands (ON). In contrast, the FGF-3 transgene was turned off and no detectable FGF-3 was observed in the bigenic mice after 2 months withdrawal of RU486 (OFF). Taken together, the pronounced phenotypic alternations depended on FGF-3 expression, and this expression is crucial for the maintenance and progression of the mammary gland hyperplasia.
Fig 6.
FGF-3-elicited mammary gland hyperplasia is reversible. Whole-mount analyses showed that the hyperplastic glands of bigenic mice treated with 450 μg/kg RU486 for 4 months (ON in C and D) can be reversed after a 2-month withdrawal of RU486 (OFF in E and F). Monogenic glands from mice given RU486 displayed normal morphology. (Lower) RPA analyses indicates that the FGF-3 transgene can be turned on and off in the bigenic glands in response to the administration and withdrawal of RU486. FGF-3 was undetectable in the controls.
Interaction of Ovarian Hormones with FGF-3 in Mammary Gland Tumorigenesis.
Pregnancy responsiveness is one of the characteristic features of hyperplastic lesions elicited by FGF-3 (6–8). The hyperplasia arises as a result of pregnancy and often regresses between pregnancies. To address this hormone-responsive behavior, the hormonal effects on the initiation and the progression of FGF-3-elicited mammary hyperplasia were examined. On administration of RU486 for 2 weeks, the ovariectomized monogenic control and the bigenic mice were supplemented with the (50 μg/kg) progesterone, estrogen, or both for 3 consecutive days. Equal amounts of sesame oil were also administrated as vehicle control. Comparable expression levels of FGF-3 were detected in bigenic mice and short-term hormonal treatment did not cause any significant alteration in the FGF-3 expression level (data not shown). Although the monogenic control exhibited an entirely normal mammary tree, overexpression of FGF-3 conferred the ability to form cystic structures in the mammary ducts of ovariectomized bigenic mice in the absence of hormone supplementation. Surprisingly, the ducts were similar in size to the control (data not shown). Treatment with estrogen but not progesterone induced expansion of the ductal epithelium in the bigenic but not in monogenic mice. The bigenic ductal size was wider and resembled those shown previously. In addition, progesterone could synergize with estrogen to elicit a more severe manifestation (data not shown).
Subsequent histological analyses showed that the control duct (Ctrl) always remained monolayered regardless of the treatment and no significant difference was found in the bigenic glands (Bi-Tg) treated with vehicle (OIL) versus untreated (Fig. 7). Nevertheless, estrogen treatment induced the formation of multilayered ductal epithelial cells in the bigenics (E2). A more severe defect was seen in the bigenic duct upon both estrogen and progesterone treatment whereby the luminal space of the duct was completely occluded by epithelial cells (E2+P4). Taken together, FGF-3 was able to elicit the formation of cystic ducts independent of ovarian hormones, and these hormones can synergize with FGF-3 to elicit profound ductal hyperplasia, which may account for the hormone-responsive nature of FGF-3 induced hyperplastic lesions.
Fig 7.
Hormonal responsiveness of FGF-3-elicited hyperplasia. Monogenic and bigenic mice were ovariectomized and treated with RU486 (450 μg/kg) for 2 weeks. Subsequently, 50 μg/kg of estrogen or estrogen/progesterone were given for 3 consecutive days. Sesame oil was used as a vehicle control. Histological analyses showed that the bigenic (Bi-Tg) glands given estrogen manifested multilayered cells, which became more severe with estrogen and progesterone cotreatment. In contrast, a uniform layer of epithelium was shown in all monogenic controls (Ctrl). Vehicle treatment does not alter the bigenic ductal morphology.
Discussion
Ductal Morphogenesis and Patterning.
Increasing evidence indicates that ductal patterning and morphogenesis in mammary gland development are two separate genetic events (19, 20). We show that overexpression of FGF-3 in puberty can interrupt these two genetic programs resulting in dramatic alterations in the overall mammary gland architecture.
The ductal patterning is a highly regulated process that results from end-bud bifurcations and turning maneuvers in response to local environmental signals from the stroma and nearby mammary epithelium. The FGF-3 protein contains a putative signal peptide and it may act primarily as an autocrine or as a short-range growth factor in mammary epithelium (14). The local FGF-3 mitogenic signal enhanced the proliferation rate of the bigenic epithelium as illustrated in the BrdUrd incorporation study. Thus, it is unlikely that the inability of ducts to elongate is caused by reduced epithelial cell proliferation. Alternatively, disruption of the synthesis and deposition of extracellular matrix material may influence branching pattern and growth of the mammary gland. Several cell-adhesion molecules emerge as the candidates that may interact with FGF-3, and we showed that the expression of E-cadherin was down-regulated and became unpolarized in the transformed epithelium of the bigenic gland. In addition, Wnt-1 (21, 22) and transforming growth factor β (23, 24) have been shown to interact intimately with FGF. Thus, it is likely that no single alteration is solely responsible for the phenotypes observed, which may instead be the cumulative result of relatively minor alterations in multiple cellular functions.
Similarly, proper ductal morphogenesis also requires that cell–cell and cell–substrate adhesion systems be coordinated spatiotemporally with epithelial differentiation and apoptosis. In the bigenic gland, FGF-3 increased the proliferation rate of the mammary epithelium as demonstrated in the BrdUrd incorporation assay. On the other hand, terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling apoptosis assay did not show any significant difference between the control and bigenic glands (data not shown). Thus, overexpression of FGF-3 clearly interrupted the balance between apoptotic and proliferating signals in normal mammary epithelium. More importantly, FGF-3 activated the MAPK cascade, which has been shown to be a differentiation signal crucial for alveolar morphogenesis in mammary epithelium cell culture (20). Taken together, these alterations may result in the disorganization of the ductal cells and the persistence of aberrant lobular hyperplasia observed in the virgin bigenic ducts.
FGF-3 Is Essential for the Maintenance and Progression of Mammary Gland Hyperplasia.
Accumulating evidence shows that tumorigenesis is dependent on the expression of oncoproteins. In the deoxycycline-inducible H-Ras mouse melanoma model, de-induction of H-Ras resulted in regression of primary and explanted tumors (25). Recently, mammary adenocarcinomas induced by c-myc were shown to require continued expression of c-myc oncoprotein (26). Similarly, we showed that continuous overexpression of FGF- 3 promotes progression of mammary gland hyperplasia and that regression resulted after the withdrawal of RU486. Thus, a continued requirement for FGF-3 in promoting and sustaining non-cell-autonomous cell proliferation is essential for hyperplasia progression and maintenance.
Despite high levels and rapid presentation of phenotypes in the virgin bigenic females, very few females succumbed to mammary tumors in the absence of pregnancy. Most bigenic females developed pronounced hyperplasia after 6 months of RU486 treatment. This finding is consistent with the requirement of pregnancy for tumor development (6–8) and the occurrence of proviral MMTV-induced FGF-3 tumors at a higher frequency in pregnancy than in the virgin nulliparous state (27). Therefore, these studies suggest that a certain differentiated state and hormonal status of the mammary gland may be an important determinant for FGF-3 tumorigenesis. These studies also imply that high levels of FGF-3 expression are insufficient to evoke a full tumorigenic response. Thus, FGF-3, probably facilitates the retention and accumulation of cells susceptible to further somatic mutation and successive mutations in tumor suppressor genes and/or activation of dominant oncogenes are then required for full tumorigenesis.
Hormonal Dependence of FGF-3 Oncogenicity.
Ectopic overexpression of various growth factors can confer varying degrees of increased estrogen-independent in vitro and in vivo growth to the estrogen-responsive MCF-7 human breast carcinoma cell (28–30). These findings suggest that signal transduction pathways can provide an alternative or interacting mitogenic stimulus to that supplied by estrogen. Evidently, overexpression of FGF-3 in vivo increased the ability of the mammary epithelium to confer an estrogen-independent proliferation that resulted in mammary hyperplasia in both virgin and ovariectomized bigenic mice. Conversely, participation of estrogen with the FGF-3 mitogenic signal was also shown in bigenic mice. The elevation of estrogen receptor expression by immunohistochemistry with FGF-3 overexpression in the bigenic mice implies that the bigenic epithelium retained an increased estrogen responsiveness (data not shown). Hence, administration of estrogen significantly enhanced the malignant phenotype in the ovariectomized bigenic mice. In addition, progesterone can reinforce the estrogen effect, and the combined treatment consistently conferred a more severe hyperplastic manifestation. A convergence of growth factor and steroid receptor signaling mechanisms is also evident (31–33). It was shown that signaling through growth factors might enhance the activity of the estrogen receptor through MAPK phosphorylation of a serine residue within the transactivating domain of estrogen receptor. This signaling may then activate estrogen receptor in the absence of estrogen or increase the level of estrogen–dependent activation of genes mediated by estrogen receptor. Thus, synergism between FGF-3 and hormonal mitogenic stimuli is probably required to elicit full tumorigenesis and this requirement may account for the pregnancy dependence of FGF-3-elicited mammary tumorigenesis.
In summary, we have established a useful animal model in which a dominantly acting oncoprotein can be somatically regulated in vivo. The system allowed us to show that the overexpression of FGF-3 in pubescent mice interrupted the intrinsic genetic programs for mammary gland development. In addition, FGF-3 can confer hormonal-independent mitogenicity and plays a critical role in the initiation and maintenance of mammary hyperplasia. However, the combinatorial action from hormonal stimuli is likely a prerequisite for FGF-3 to confer full tumorigenesis.
Acknowledgments
We thank Dr. P Leder for the UASG-FGF-3 mice; Drs. M. J. Tsai, D. Medina, N. Takamoto, and J. P. Lydon for critical discussions; and Ms. M. J. Chu for technical help. This work is supported by National Institutes of Health and DAMD grants (to S.Y.T.). E.S.W.N. and Z.Q.M. are recipients of fellowships from the Croucher Foundation, Hong Kong Special Administrative Region, China and Department of Defense (DAMD-17–98-1–8025), respectively.
Abbreviations
FGF-3, fibroblast growth factor-3
MMTV, mouse mammary tumor virus
MAPK, mitogen-activated protein kinase
UASG, upstream activating sequence
RPA, ribonuclease protection assay
References
- 1.Humphreys R. C., Krajewska, M., Krnacik, S., Jaeger, R., Weiher, H., Krajewski, S., Reed, J. C. & Rosen, J. M. (1996) Development (Cambridge, U.K.) 122, 4013-4022. [DOI] [PubMed] [Google Scholar]
- 2.Jakobovits A., Shackleford, G. M., Varmus, H. E. & Martin, G. R. (1986) Proc. Natl. Acad. Sci. USA 83, 7806-7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansour S. L., Goddard, J. M. & Capecchi, M. R. (1993) Development (Cambridge, U.K.) 117, 13-28. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson D. G., Bhatt, S. & McMahon, A. P. (1989) Development (Cambridge, U.K.) 105, 131-136. [DOI] [PubMed] [Google Scholar]
- 5.Peters G., Brookes, S., Smith, R. & Dickson, C. (1983) Cell 33, 369-377. [DOI] [PubMed] [Google Scholar]
- 6.Muller W. J., Lee, F. S., Dickson, C., Peters, G., Pattengale, P. & Leder, P. (1990) EMBO J. 9, 907-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stamp G., Fantl, V., Poulsom, R., Jamieson, S., Smith, R., Peters, G. & Dickson, C. (1992) Cell Growth Differ. 3, 929-938. [PubMed] [Google Scholar]
- 8.Ornitz D. M., Moreadith, R. W. & Leder, P. (1991) Proc. Natl. Acad. Sci. USA 88, 698-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B., Chua, S. S., Burcin, M. M., Reynolds, S. D., Stripp, B. R., Edwards, R. A., Finegold, M. J., Tsai, S. Y. & DeMayo, F. J. (2001) Proc. Natl. Acad. Sci. USA 98, 5898-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Z. Q., Chua, S. S., DeMayo, F. J. & Tsai, S. Y. (1999) Oncogene 18, 4564-4576. [DOI] [PubMed] [Google Scholar]
- 11.Hogan B., Costantini, F. & Lacy, E., (1986) Manipulating the Mouse Embryo, A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 12.Chua S. S., Ma, Z. Q., Gong, L., Lin, S. H., DeMayo, F. J. & Tsai, S. Y. (2002) Oncogene 21, 1899-1908. [DOI] [PubMed] [Google Scholar]
- 13.Cardiff R. D., Anver, M. R., Gusterson, B. A., Hennighausen, L., Jensen, R. A., Merino, M. J., Rehm, S., Russo, J., Tavassoli, F. A., Wakefield, L. M., et al. (2000) Oncogene 19, 968-988. [DOI] [PubMed] [Google Scholar]
- 14.Ornitz D. M., Cardiff, R. D., Kuo, A. & Leder, P. (1992) J. Natl. Cancer Inst. 84, 887-892. [DOI] [PubMed] [Google Scholar]
- 15.Sicinski P., Donaher, J. L., Parker, S. B., Li, T., Fazeli, A., Gardner, H., Haslam, S. Z., Bronson, R. T., Elledge, S. J. & Weinberg, R. A. (1995) Cell 82, 621-630. [DOI] [PubMed] [Google Scholar]
- 16.Klint P. & Claesson-Welsh, L. (1999) Front Biosci. 4, D165-D177. [DOI] [PubMed] [Google Scholar]
- 17.Christofori G. & Semb, H. (1999) Trends Biochem. Sci. 24, 73-76. [DOI] [PubMed] [Google Scholar]
- 18.Perl A. K., Wilgenbus, P., Dahl, U., Semb, H. & Christofori, G. (1998) Nature (London) 392, 190-193. [DOI] [PubMed] [Google Scholar]
- 19.Lewis M. T., Ross, S., Strickland, P. A., Sugnet, C. W., Jimenez, E., Scott, M. P. & Daniel, C. W. (1999) Development (Cambridge, U.K.) 126, 5181-5193. [DOI] [PubMed] [Google Scholar]
- 20.Niemann C., Brinkmann, V., Spitzer, E., Hartmann, G., Sachs, M., Naundorf, H. & Birchmeier, W. (1998) J. Cell Biol. 143, 533-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradley R. S. & Brown, A. M. C. (1990) EMBO J. 9, 1569-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papkoff J. & Schryver, B. (1990) Mol. Cell. Biol. 10, 2723-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nugent M. A. & Edelman, E. R. (1992) J. Biol. Chem. 267, 21256-21264. [PubMed] [Google Scholar]
- 24.Coleman-Krnacik S. & Rosen, J. M. (1994) Mol. Endocrinol. 8, 218-229. [DOI] [PubMed] [Google Scholar]
- 25.Chin L., Tam, A., Pomerantz, J., Wong, M., Holash, J., Bardeesy, N., Shen, Q., O'Hagan, R., Pantginis, J., Zhou, H., et al. (1999) Nature (London) 400, 468-472. [DOI] [PubMed] [Google Scholar]
- 26.D'Cruz C. M., Gunterh, E. J., Boxer, R. B., Hartman, J. L., Sintasath, L., Moody, S. E., Cox, J. D., Ha, S. I., Belka, G. K., Golant, A., et al. (2001) Nat. Med. 7, 235-239. [DOI] [PubMed] [Google Scholar]
- 27.Clausse N., Smith, R., Calberg-Bacq, C. M., Peters, G. & Dickson, C. (1993) Int. J. Cancer 55, 157-163. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., el-Ashry, D., Chen, D., Ding, I. Y. & Kern, F. G. (1995) Breast Cancer Res. Treat. 34, 97-117. [DOI] [PubMed] [Google Scholar]
- 29.Miller D. L., el-Ashry, D., Cheville, A. L., Liu, Y., McLeskey, S. W. & Kern, F. G. (1994) Cell Growth Differ. 5, 1263-1274. [PubMed] [Google Scholar]
- 30.Pietras R. J., Arboleda, J., Reese, D. M., Wongvipat, N., Pegram, M. D., Ramos, L., Gorman, C. M., Parker, M. G., Sliwkowski, M. X. & Slamon, D. J. (1995) Oncogene 10, 2435-2446. [PubMed] [Google Scholar]
- 31.Aronica S. M. & Katzenellenbogen, B. S. (1993) Mol. Endocrinol. 7, 743-752. [DOI] [PubMed] [Google Scholar]
- 32.Ignar-Trowbridge D. M., Teng, C. T., Ross, K. A., Parker, M. G., Korach, K. S. & McLachlan, J. A. (1993) Mol. Endocrinol. 7, 992-998. [DOI] [PubMed] [Google Scholar]
- 33.Kato S., Sasaki, H., Suzawa, M., Masushige, S., Tora, L., Chambon, P. & Gronemeyer, H. (1995) Mol. Cell. Biol. 15, 5858-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]