Abstract
Diabetes mellitus is an endocrine disorder characterized by prolonged hyperglycemia. It results from either insulin deficiency (type 1 diabetes mellitus, T1DM) or insulin resistance (type 2 diabetes mellitus, T2DM). This condition has emerged as a significant health concern in recent years. Hyperglycemia induces the overproduction of reactive oxygen species (ROS), which can modulate multiple pathways, including AGEs-RAGE, PKC stimulation, NF-κB and PI3K/AKT. These pathways contribute to diabetes-related complications such as inflammation, oxidative stress, insulin resistance, and reduced glucose uptake. The interplay of these metabolic disturbances can lead to demyelination and peripheral nerve damage, resulting in diabetic neuropathy. This is a challenging complication of diabetes for which there are limited effective treatments. Despite its low bioavailability, curcumin, a natural component extracted from turmeric, despite its low bioavailability, affects and modulates several intracellular pathways underlying neuropathic damage. Curcumin is considered a potential treatment for diabetic neuropathy (DN) because it measurably reduces markers of oxidative stress and inflammatory cytokines, while significantly alleviating neuropathic pain and improving nerve function. MicroRNAs (miRNAs or miR), which are small non-coding RNAs consisting of 19–25 nucleotides, are stable in circulation and can regulate multiple target genes. This makes them promising biomarkers for both diagnostic and therapeutic applications. Curcumin has been shown to regulate the dysregulation of relevant miRNAs associated with neuropathy by suppressing the inflammatory miR-21 while enhancing the expression of the anti-inflammatory miR-146a. Current formulations of curcumin face bioavailability challenges; however, advancements in delivery systems and structural modifications, such as nanoformulations, have significantly improved its bioavailability. These improvements overcome previous pharmacokinetic limitations and enhance the therapeutic effects of curcumin. With continued research, curcumin could ultimately become a cornerstone in managing diabetic complications and improving the quality of life for affected patients.
Keywords: Curcumin, miR-21, miR-146a, miR-155, Diabetic neuropathy
Introduction
Diabetes mellitus, is a prevalent endocrine disorder characterized by chronic hyperglycemia, is one of the fastest-growing global health concerns [1]. Prolonged hyperglycemia leads to multi-organ damage, with diabetic neuropathy (DN) being particularly significant. It affects approximately 30% of diabetic patients overall and over 50% of patients over 50 years of age [2].
The pathophysiology of diabetes involves oxidative stress resulting from hyperglycemia-induced reactive oxygen species (ROS) overproduction, metabolic disturbances, and chronic inflammation [3]. Key mechanisms include the activation of glycolytic pathways, the formation of advanced glycation end products (AGEs), the stimulation of protein kinase C (PKC), and the impairment of insulin signaling through the inhibition of the Nuclear Factor Kappa B (NF-κB) and phosphoinositide-3-kinase/ Akt (PI3K/Akt) pathways [4]. The PI3K/Akt pathway plays a crucial role in glucose metabolism; dysregulation of this pathway contributes to reduced Glucose transporter type 4 (GLUT4) and insulin resistance [5, 6]. Impaired Akt-mediated suppression of Forkhead box O1 (FOXO1) transcription factors also disrupts cellular homeostasis [7], and Jun N-terminal kinase (JNK) activation promotes insulin resistance and Beta cells (β-cell) dysfunction [8]. Both genetic and environmental factors contribute to the development of diabetes.
Curcumin, a natural compound, demonstrates therapeutic potential due to its antioxidant and anti-inflammatory properties [9]. Its antioxidant activity is mediated by the direct scavenging of ROS and reactive nitrogen species, as well as a significant reduction in oxidative stress markers and lipid peroxidation [10]. Curcumin’s anti-inflammatory effects are equally robust. They are achieved by inhibiting key inflammatory enzymes, such as cyclooxygenase-2 (COX-2) and lipoxygenase. Curcumin also suppresses of NF-κB signaling and downstream pro-inflammatory cytokines [11]. These dual mechanisms underlie curcumin’s effectiveness in managing various chronic inflammatory conditions, such as arthritis and inflammatory bowel diseases [12]. Curcumin’s therapeutic applications extend significantly into metabolic disorders, especially diabetes mellitus. Curcumin enhances insulin sensitivity through multiple pathways, upregulates endogenous antioxidant enzymes such as superoxide dismutase, and protects pancreatic β-cells [13]. These properties not only aid in glucose regulation and help prevent diabetic complications, including retinopathy, by modulating oxidative stress pathways [14]. Regarding cardiovascular health, curcumin has been shown to protect against the progression of heart failure, reduce hypertension, and prevent myocardial infarction through its pleiotropic actions on vascular function and cardiac remodeling [15]. Curcumin exhibits remarkable antimicrobial properties and has been shown to be effective against diverse pathogens [16]. It exhibits antibacterial activity includes action against Helicobacter pylori and methicillin-resistant Staphylococcus aureus (MRSA), and antiviral effects against HSV-1. Curcumin also exhibits antiparasitic activity against Plasmodium and Giardia lamblia [17]. Curcumin promotes gastric mucosal defense mechanisms in gastrointestinal health by regulating matrix metalloproteinases (MMPs) and enhancing mucin secretion, thereby reducing ulcer formation [18]. Curcumin’s ability to cross the blood-brain barrier (BBB) allows it to have significant neuroprotective effects, especially in neurodegenerative disorders such as Alzheimer’s disease. In these disorders, curcumin reduces oxidative damage and amyloid-β toxicity [19]. Curcumin’s anticancer properties are equally impressive. They encompass the inhibition of tumor cell proliferation, the induction of apoptosis, and the suppression of angiogenesis. This occurs through the modulation of vascular endothelial growth factor (VEGF) and Notch-1 signaling pathways [20]. Additional therapeutic benefits include hepatoprotective and nephroprotective effects, as well as modulation of adipocyte function in obesity-related metabolic disorders [21]. Curcumin has also shown promise as a radiosensitizing agent in cancer therapy [22]. Its ability to suppress NF-κB, TNF-α, and IL-6 [23] while simultaneously enhancing antioxidant enzyme activity is particularly relevant for managing complications such as diabetic neuropathy, which is caused by chronic inflammation and oxidative stress [24].
Emerging evidence implicates microRNAs (miRNAs) in diabetic complications. Specifically, miR-155, miR-21, and miR-146a are particularly relevant to neuropathy [25]. Interestingly, curcumin modulates these miRNAs, suggesting a novel therapeutic mechanism [26, 27]. This study investigates curcumin’s neuroprotective effects in diabetic neuropathy, with a focus on how it regulates of miR-21, miR-146a, and miR-155 to mitigate oxidative stress and inflammation.
Pathophysiology of diabetic neuropathy
DN is one of the most common chronic complications of diabetes mellitus, along with retinopathy, nephropathy, and cardiovascular disease. DN affects both the central and peripheral nervous systems, with distal symmetric polyneuropathy being the most common clinical presentation [28]. Epidemiological studies show that around 30% of people with diabetes develop peripheral neuropathy. However, more sensitive diagnostic techniques suggest that the actual prevalence may be much higher. The global diabetes epidemic has made Diabetic peripheral neuropathy) DPN (into a significant public health concern, with the annual treatment costs of painful DPN and its complications estimated at $4–13 billion in the United States alone. DPN accounts for up to 27% of direct medical expenditures in diabetes care, primarily due to complications such as foot ulcers and amputations [29].
DPN’s clinical manifestations of DPN typically begin with symmetrical distal limb numbness and sensory loss, progressing to neuropathic pain in approximately 20% of cases. Patients often describe this pain as burning, stabbing, or electric shock-like, and it is frequently accompanied by allodynia and hyperalgesia [30]. Chronic hyperglycemia and associated metabolic disturbances further compromise immune function, creating a predisposition to infections in neuropathic wounds that often progress to severe tissue damage [31]. This pathological cascade establishes DPN as the leading cause of non-traumatic lower-limb amputations in developed nations. Amputation rates are 10–20 times higher in diabetic populations than in non-diabetic populations [32].
Although the exact cause is not fully understood, current research suggests that chronic hyperglycemia is the main driver of DPN pathogenesis through multiple interconnected mechanisms. Elevated blood glucose levels persistently, trigger mitochondrial dysfunction, oxidative stress, and inflammatory cascades that collectively damage neuronal and glial cells [33, 34]. These metabolic disturbances also promote the formation of AGEs and PKC pathways, which further exacerbate neural microvascular compromise [35]. The resultant neurodegeneration manifests as characteristic histological changes, including demyelination, axonal atrophy, and impaired nerve conduction velocity the hallmark pathological features of DPN [36, 37].
The global burden of DPN continues to escalate in parallel with the diabetes pandemic. DPN develops in approximately 50% of diabetic patients, often with onset early in the disease course [38, 39]. Progression rates correlate strongly with diabetes duration, glycemic control, and patient age. Nerve conduction studies suggest that the actual prevalence may exceed clinical estimates [40]. Chinese multicenter studies report DPN prevalence of 21.92% in patients with type 1 diabetes (T1DM) and 35.34% in patients with type 2 diabetes (T2DM) patients, with distal symmetric polyneuropathy representing the predominant subtype. Distal symmetric polyneuropathy is present in approximately 28% of patients with diabetes [41]. Cardiac autonomic neuropathy demonstrates a particularly high prevalence, affecting up to 63% of diabetic patients in some cohorts [42].
The unique anatomical organization of the peripheral nervous system explains the characteristic stocking-glove distribution pattern of DPN [43, 44]. Myelinated nerve fibers depend on Schwann cells for ensheathment and metabolic support, and unmyelinated axons form Remak bundles [45, 46]. This sophisticated architecture enables rapid saltatory conduction via precisely regulated ion channels and adhesion molecules [47, 48]. However, chronic hyperglycemia disrupts Schwann cell autophagy and metabolic homeostasis, thereby impairing their critical neuroprotective functions [49, 50]. Consequently, small-diameter sensory neurons, particularly those in the dorsal root ganglia, become exceptionally vulnerable to injury due to their limited myelination [51, 52]. The resulting neurodegeneration involves demyelination of large fibers and degeneration of small unmyelinated fibers simultaneously, which is compounded by microvascular insufficiency and impaired axonal transport [53].
At the molecular level, diabetic metabolic disturbances activate multiple parallel pathways of neural injury [54]. Oxidative stress from mitochondrial dysfunction generates excessive ROS that damage cellular structures [55]. AGEs cross-link structural proteins and impair normal cellular function, while PKC activation alters vascular permeability and reduces neural perfusion. Together, these mechanisms collectively contribute to ion channel dysfunction, abnormal nociceptive signaling, and impaired nerve regeneration capacity [56].
The clinical consequences of DPN progress through predictable stages, beginning with subclinical nerve conduction abnormalities and advancing to overt sensory loss and motor impairment [57]. The loss of protective sensation creates a vicious cycle in which minor trauma progresses to ulceration and potential amputation. Mortality rates exceed 50% within five years post-amputation [58]. Current management strategies remain primarily, focus on symptom relief, emphasizing glycemic control and pain management. This highlights the critical need for disease-modifying therapies. Emerging research is exploring the use of mitochondrial-targeted antioxidants, glial cell modulators, and neurotrophic factor delivery systems as potential interventions that could alter course of the disease [59].
Key signaling pathways in DPN
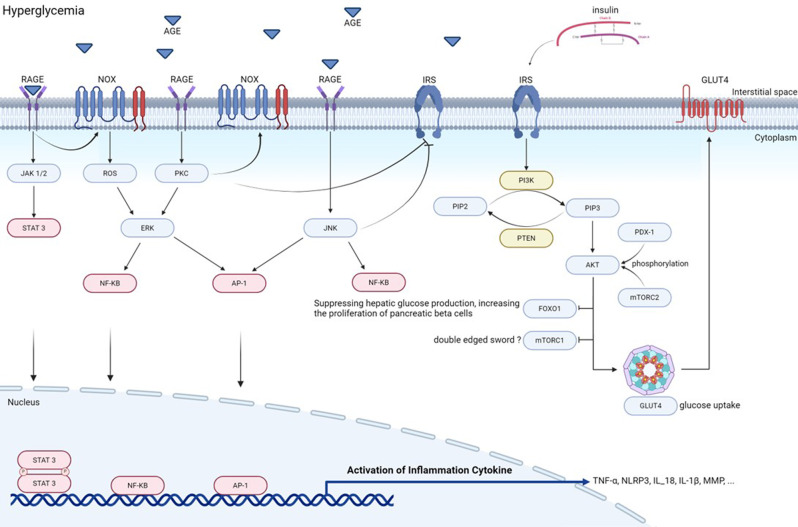
DPN develops through the interplay of multiple metabolic disturbances, including chronic hyperglycemia, insulin resistance, and dyslipidemia. These factors collectively promote oxidative stress and neural damage by activating several pathological signaling pathways (Fig. 1). The major pathways involved are insulin signaling defects, AGE-RAGE axis activation, PI3K/AKT/FOXO1 dysregulation, JNK pathway stimulation, and Peroxisome proliferator-activated receptor gamma (PPARγ) dysfunction [60]. These pathways contribute to nervous system damage through inflammatory and metabolic mechanisms (Fig. 1).
Fig. 1.
Mechanisms involved in the pathogenesis of diabetes. Hyperglycemia increases the production of AGE, which bind to receptors for advanced glycation end products (RAGEs) and trigger inflammation. This inhibits insulin receptor (IRS) activation. This activates JAK1/2, NOX, JNK, and PKC, leading to the production of STAT3, AP-1, and NF-κB. These proteins then induce the production of inflammatory cytokines such as TNF-α, NLRP3, and IL-1β. The AGE pathway also impairs IRS activity, inactivates PI3K/AKT, and prevents GLUT4 translocation and FOXO1/mTORC1 inactivation. This further disrupts insulin signaling
The role of insulin signaling in the pathogenesis of DPN
Insulin resistance which is characterized by reduced tissue sensitivity to insulin and impaired glucose transporter function, is a core defect of diabetes [61]. This condition is exacerbated by inflammatory processes that increase the infiltration of macrophages into hepatocytes, which blocks insulin signaling pathways [62]. The transcription factor NF-κB plays a central role in the inflammatory cascade by regulating interleukin-6 (IL-6) production. IL-6 directly contributes to the development of insulin resistance [63].
Under persistent hyperglycemic conditions, proteins and lipids undergo non-enzymatic glycation to form AGEs which bind to their receptors and activate multiple damaging pathways including PARP-mediated oxidative stress, glutamate accumulation, and tissue injury [64]. Although the direct effects of insulin on neurons are somewhat controversial, it clearly influences DPN pathogenesis through both glucose-dependent and -independent mechanisms [65]. Notably, insulin acts as a neurotrophic factor, supporting sensory nerve function and promoting axonal repair via PI3K-Akt signaling [66–68]. The differential effects of T1DM versus T2DM diabetes are particularly noteworthy. In T1DM, insulin therapy can improve DPN by activating axonal repair pathways [69]. However, in T2DM, altered phosphorylation of insulin receptor substrates impairs these reparative mechanisms, limiting the therapeutic potential of insulin [70]. The PI3K-Akt pathway’s role in maintaining the synthesis of key neuromodulatory proteins further underscores its importance in DPN pathogenesis [64].
Elevated free fatty acids (FFAs) are a consequence and driver of insulin resistance. Reduced inhibition of hormone-sensitive lipase in adipocytes leads to the continuous release of FFAs [71]. These FFAs impair insulin signaling through multiple mechanisms, including the accumulation of intracellular ceramide and DAG, PKC-mediated IRS phosphorylation and inactivation [72], suppression of glycolysis and glucose oxidation, and TNF-α-mediated stimulation of lipolysis [73]. In T2DM, FFAs activate the NLRP3 inflammasome, promoting IL-1β production [74, 75] and subsequent oxidative stress, which that drives DPN progression [76]. NF-κB activation exacerbates insulin resistance further through JNK stimulation [77], while macrophage-derived cytokines (TNF-α, IL-1β and IL-6) and MCP-1-mediated macrophage recruitment sustain chronic inflammation [78–80]. Additional contributors to this inflammatory state include proinflammatory eicosanoids released in response to environmental factors and oxidative stress [81].
AGE-RAGE axis in diabetic complications
The formation of AGEs through non-enzymatic glycation is a key molecular consequence of chronic hyperglycemia [82]. The interaction between AGEs and their receptor, RAGE, triggers ROS-mediated inflammation, which significantly contributes to insulin resistance [83] through the activation of multiple signaling cascades, including the PI3K/Akt/mTOR pathway [84], JAK2/STAT signaling [85], the Nox/ROS/NF-κB cascade [86], and MAPK-mediated NF-κB activation [87]. This inflammatory response is amplified by synergistic interactions among AGE-RAGE binding, hyperglycemia, and PKC activation. These interactions collectively stimulate Nox-mediated ROS generation [88, 89]. Key mediators of this process include the Nox/ROS/NF-κB axis and MCP-1 [90], along with various proinflammatory factors, such as NF-κB, TNF-α, and interleukins [91].
In pancreatic β-cells, metabolic dysregulation and PKC pathway activation disrupt normal function. This occurs by increasing ROS production and decreasing neuronal and glial cell viability [92]. PKC activation in diabetes primarily results from elevated diacylglycerol (DAG) and ROS levels [93], which promote IRS-1 phosphorylation and impair insulin signaling [94]. The AGE pathway contributes to diabetic complications through multiple mechanisms, including reduced insulin synthesis in β-cells [95], neural damage via oxidative stress and inflammation [96], and direct involvement in DPN pathogenesis. These diverse effects underscore the significance of the AGE-RAGE axis in diabetic neuropathy [97].
PI3K/AKT/FOXO1 pathway dysregulation
The insulin signaling cascade begins with the binding of the hormone to its receptor, which activates IRS. This leads to the generation of PIP3 by PI3K and the recruitment of AKT to the membrane [98]. Complete AKT activation requires phosphorylation by both Phosphoinositide-dependent protein kinase 1 (PDK-1) and mechanistic target of rapamycin complex 2 (mTORC2) [99], with PTEN providing negative regulation through PIP3 dephosphorylation [100]. AKT exerts multiple metabolic effects, including the promotion of GLUT4 translocation and glucose uptake [101], regulation of mTORC1 activity [102], and the phosphorylation of FOXO transcription factors. In pancreatic β-cells, mTORC1 plays dual roles in regulating both cellular function and survival in response to metabolic stress [103]. Among the FOXO family members, FOXO1 is particularly important for glucose homeostasis. It influences hepatic gluconeogenic enzyme expression, pancreatic α-cell function, and β-cell proliferation by regulating pancreatic and duodenal homeobox 1 (Pdx-1) [104–106]. Impairment of the PI3K/AKT pathway in obesity and T2DM leads to reduced insulin secretion and exacerbates insulin resistance, creating a vicious cycle of metabolic dysfunction that contributes to DPN development [107, 108].
The JNK pathway activation in metabolic disease
Chronic low-grade inflammation and elevated FFAs related to obesity stimulate JNK-mediated phosphorylation of insulin receptor substrates [109, 110].This blocks PI3K/Akt/GLUT4 signaling, reducing insulin sensitivity, and promoting insulin resistance [111]. Genetic or pharmacological inhibition of JNK decreases inflammatory gene expression and improves insulin sensitivity [112]. In obese individuals, oxidative stress activates both the JNK and NF-κB pathways concurrently [113], leading to the elevated production of TNFα and IL-6 which further exacerbates insulin resistance [114, 115]. TNF-α also inhibits PPARγ function via ERK/JNK-mediated phosphorylation, creating another mechanism that sustains metabolic dysfunction [116, 117].
The PPARγ pathway in metabolic regulation
PPARγ, a member of the nuclear receptor superfamily, regulates numerous metabolic processes [118]. It interacts with JNK signaling to influence adipocyte differentiation, glucose and lipid metabolism, inflammatory responses, and insulin sensitivity [119]. In diabetes, reduced PPARγ activity coincides with increased expression of AP-1 and NF-κB expression [120]. Activation of PPARγ can counteract these changes by reducing the expression of inflammatory factors expression, blocking JNK signaling, and improving insulin sensitivity [121]. This suggests that PPARγ has therapeutic potential for DPN.
Curcumin as an antioxidant: mechanisms of action and therapeutic potential in diabetic neuropathy
Curcumin, the primary bioactive polyphenol found in turmeric (Curcuma longa L.), has shown great promise in managing diabetes due to its powerful antioxidant and anti-inflammatory properties [122, 123]. First isolated in 1815, this bright yellow compound (C₂₁H₂₀O₆; MW: 368.38 g/mol) has a unique molecular structure that gives its diverse therapeutic effects [124]. The molecule features three critical functional groups: a β-diketone moiety that enables free radical scavenging; methoxy phenol groups that facilitate electron transfer reactions; and phenolic hydroxyl groups that provide metal chelation capabilities. These structural elements enable curcumin to exhibit antioxidant activity through various complementary mechanisms. These mechanisms include the direct neutralization of ROS and nitrogen species via hydrogen donation from the phenolic groups; the interruption of lipid peroxidation chain reactions through peroxyl radical scavenging; and the prevention of metal-induced free radical generation via transition metal chelation, particularly with iron and copper [124, 125].
Despite its well-documented therapeutic potential, curcumin faces significant pharmacokinetic challenges that limit its clinical utility. The compound has poor aqueous solubility and is chemically unstable at neutral and alkaline pH levels. It is also sensitive to light exposure in both solid and dissolved forms [126]. Following administration, curcumin undergoes extensive metabolism through two primary pathways [127–129]. The first pathway consists of phase II conjugation reactions, including glucuronidation to form curcumin glucuronide and sulfation to produce curcumin sulfate. These reactions primarily occurr in intestinal and hepatic cytosolic fractions. The second pathway consists of phase I reductive metabolism, in which curcumin is enzymatically converted into dihydrocurcumin, tetrahydrocurcumin (THC), hexahydrocurcumin, and hexahydrocurcuminol in enterocytes and hepatocytes. Subsequent secondary metabolism generates additional compounds, such as dihydro-ferulic acid, and ferulic acid through biliary transformation [122]. Notably, reduced curcumin metabolites also undergo glucuronidation, yielding compounds such as dihydrocurcumin glucuronide and THC glucuronide. While these metabolic processes contribute to curcumin’s rapid systemic clearance, they may produce bioactive metabolites with distinct pharmacological properties that contribute to its therapeutic effects [129].
Beyond its antioxidant capacity, curcumin exhibits significant anti-inflammatory properties by modulating key cellular pathways. Curcumin inhibits NF-κB signaling by preventing IκB kinase activation. This reduces the thereby reducing expression of pro-inflammatory cytokines. Curcumin also regulates the JAK/STAT pathway. This decreases levels of inflammatory mediators, including IL-1, IL-1β, IL-8, TNF-α, and iNOS [130, 131]. Curcumin also suppresses NLRP3 inflammasome activation, reducing IL-1β and IL-18 secretion. Additionally, it activates PPAR-γ, contributing to its broad anti-inflammatory effects [132, 133] (Fig. 2). Clinical studies have validated curcumin’s therapeutic potential in diabetes management through multiple measurable outcomes. Research demonstrates its ability to reduce oxidative stress markers, such as MDA, while enhancing antioxidant defenses by increasing superoxide dismutase activity and total antioxidant capacity [134]. Curcumin improves key metabolic parameters, including fasting blood glucose and glycated hemoglobin levels [135, 136].
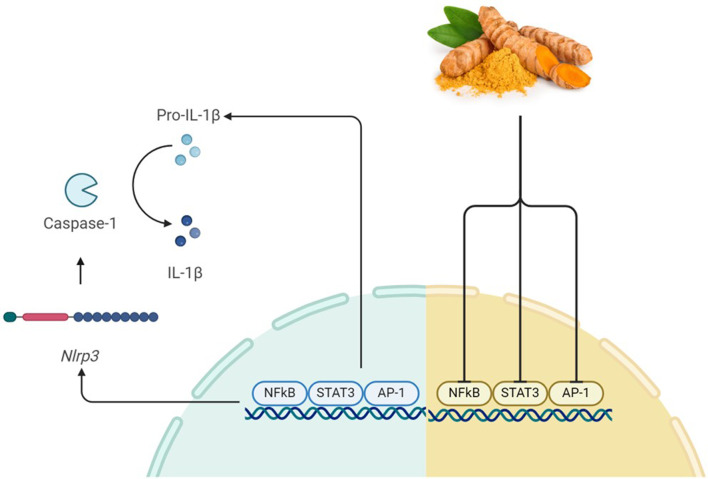
Fig. 2.
The effects of Curcumin on NLRP3 signaling. The binding of STAT3, AP-1, and NF-κB to DNA induces the production of pro-inflammatory cytokines and NLRP3. This activates caspase1 which and converts pro-IL-1β to active IL-1β. Curcumin prevents the production of these inflammatory cytokines by inhibiting the initiation of this mechanism
In the context of diabetic neuropathy, curcumin addresses multiple pathological mechanisms through its diverse pharmacological actions. Regarding the modulation of oxidative stress, curcumin effectively scavenges the free radicals generated by chronic hyperglycemia while also enhancing the body’s endogenous antioxidant systems, such as glutathione peroxidase and catalase [137]. Curcumin also inhibits two major contributors to oxidative damage in diabetic nerves: advanced glycation end-product formation and protein kinase C activation [138]. Curcumin’s anti-inflammatory properties are valuable for treating DN because it significantly reduces levels of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, which are elevated in DN [136]. Curcumin suppresses NF-κB and MAPK pathways, which are key signaling routes involved in neuroinflammation. It also and modulates inflammasome activity, particularly the NLRP3 inflammasome. This reduces IL-1β production and subsequent inflammatory responses [132, 139] (Fig. 3).
Fig. 3.
The effects of curcumin on the molecular mechanisms involved in diabetic neuropathy. Curcumin has various effects on diabetes and diabetic neuropathy. It inhibits the MAPK, NF-κB, and AP-1 inflammatory pathways via direct and indirect mechanisms. Curcumin reduces malondialdehyde (MDA) and increases SOD to inactivate ROS, and prevent inflammation. It also inhibits the production and secretion of inflammatory cytokines, such as NLRP3, IL-1β, and IL-1
The metabolic benefits of curcumin are also relevant for managing diabetic neuropathy, as it enhances insulin sensitivity by improving insulin receptor signaling and preserving β-cell function, which may slow the progression of diabetes [135, 140]. Curcumin regulates adipokine production, increasing beneficial adiponectin while decreasing pro-inflammatory leptin and resistin. This contributes to improved overall metabolic control [136]. Emerging research highlights curcumin’s epigenetic effects on diabetic complications. The compound alters the expression of microRNAs associated with oxidative stress and inflammation, including miR-21, miR-146a, and miR-155. These miRNAs regulate various aspects of DN pathogenesis [26, 27]. Curcumin influences histone modifications and DNA methylation patterns, which could provide long-term benefits in neuropathy prevention and treatment through epigenetic regulation [141].
The clinical utility of curcumin has long been limited by its poor bioavailability and limited permeability of the BBB [142]. To address these challenges, researchers have pursued two complementary strategies: developing synthetic analogues and advanced nanoformulations. Among these delivery systems, the self-nanoemulsifying drug delivery system (SNEDDS) has shown particular promise. SNEDDS have demonstrated a 7411% increase in area under the curve (AUC) and a significantly extended plasma half-life compared to native curcumin in preclinical studies [143]. Concurrent efforts in medicinal chemistry have produced novel analogues such as THC and feruloyl-curcumin, which are designed to optimize pharmacokinetic properties [144]. THC, a hydrogenated derivative of curcumin, has demonstrated significant efficacy in alleviating DN by reducing oxidative stress and inflammation. Studies show that THC (40–80 mg/kg) improves thermal and mechanical hyperalgesia, enhances motor nerve conduction velocity (MNCV), and restores sciatic functional index (SFI) in rodent models [145, 146]. Its mechanisms include inhibition of TNF-α, IL-1β, and lipid peroxidation, alongside boosting endogenous antioxidants. Feruloyl curcumin, another analog, exhibits similar neuroprotective effects by modulating pain pathways and suppressing pro-inflammatory cytokines [147]. Both compounds enhance nerve regeneration and functional recovery, suggesting promise as adjuvants in DN management. However, clinical trials are needed to validate these preclinical findings [148].
The synthetic curcumin derivative J147 is an example of the success of this approach. It exhibits improved bioavailability while retaining the multifaceted therapeutic potential of its parent compound [142]. J147 has demonstrated particular efficacy in DN models due to its ability to target multiple pathogenic pathways simultaneously [22]. These molecular effects translate to clinically relevant outcomes, including the prevention of diabetes-induced slowing of MNCV and the rapid alleviation of tactile allodynia following a single dose [22]. Preclinical comparisons highlight the superior performance of optimized curcumin formulations. For instance, the hybrid molecule CNB-001,, has demonstrated enhanced neuroprotective effects especially in alleviating neuropathic pain and inflammation [149]. Similarly, various analogues demonstrate improved modulation of neurotrophic factors and apoptotic pathways, resulting in improved neuronal survival and nerve regeneration [19]. Additionally, some derivatives offer metabolic benefits, such as upregulation of insulin-like growth factor-1 (IGF-1) and improved glycemic control in diabetic models [19].
While these advances are promising, significant gaps remain in translating preclinical findings to clinical practice. The mild glycemic effects observed with J147 treatment, such as reductions in blood glucose and HbA1c levels, suggest potential benefits for residual beta-cell function but require validation in human trials [22]. Current research efforts are exploring next-generation formulations, including nanoparticles and liposomes, to enhance therapeutic efficacy. The field now faces the critical challenge of moving from compelling preclinical results to rigorous clinical validation. validation. Particular emphasis is placed on establishing optimal dosing regimens and long-term safety profiles in human populations with diabetic neuropathy [144].
MiRNAs in diabetic neuropathy: mechanisms and therapeutic potential
miRNAs are a class of small, non-coding RNAs that play a fundamental role in post-transcriptional gene regulation by interacting with target mRNA sequences in a sequence-specific manner [150]. These 19–25 nucleotide molecules function through two primary mechanisms: complete complementarity, which leads to mRNA degradation, and partial complementarity, which resultsin translational repression [151]. Due to their remarkable stability in circulation and ability to regulate multiple target genes simultaneously, miRNAs are considered promising diagnostic biomarkers and potential therapeutic targets for various diseases [152, 153]. Regarding diabetes and its complications, there is emerging evidence that highlights the critical involvement of specific miRNA networks in modulating key pathological processes, including chronic inflammation, β-cell dysfunction, and insulin resistance [154, 155]. The complex regulatory capacity of miRNAs, whereby a single species can influence entire signaling pathways while being influenced by metabolic alterations, provides a unique opportunity to develop novel intervention strategies for DN that address multiple aspects of its pathogenesis simultaneously [156, 157]. This multi-target approach maybe especially valuable, considering the heterogeneous nature of diabetic complications and the limitations of current single-target therapies.
The fundamental properties of MiRNAs in diabetes
In the context of diabetes pathophysiology, miRNAs have emerged as key modulators of several fundamental biological pathways, including insulin signaling cascades, immune-mediated inflammatory responses, lipid metabolism regulation, and oxidative stress homeostasis [158]. The diagnostic potential of miRNAs has become increasingly apparent, and circulating miRNA profiles in bodily fluids and plasma show promise as sensitive biomarkers for predicting metabolic diseases, such as diabetes [158]. However, it is important to note that oxidative stress conditions characteristic of diabetes can significantly disrupt normal miRNA expression profiles [159]. These alterations contribute to several diabetic complications through multiple mechanisms, including the induction of chronic inflammation, β-cell loss or dysfunction, impaired insulin secretion, and the development of insulin resistance [160].
Therapeutic potential in DPN linked to dysregulation of MiRNA
Recent studies have identified miRNAs as key regulators in the development of diabetic neuropathy, affecting neuroinflammatory processes and neuronal integrity. The activation of NF-κB signaling and the subsequent release of pro-inflammatory cytokines (IL-1β and TNF-α) in diabetic neuropathic tissues demonstrate the substantial involvement of miRNA-mediated regulatory mechanisms in disease progression [160, 161]. These molecular alterations are exacerbated by chronic hyperglycemia, which promotes the infiltration of macrophages (particularly M1-polarized subsets) and sustains a pro-inflammatory microenvironment [162]. Beyond inflammation, miRNAs are essential for neuronal development and maintenance. Experimental experimental evidence shows that disrupting miRNA processing through Dicer knockdown leads to widespread neurodegeneration [163, 164]. Their involvement in various neurological disorders suggests the existence of conserved mechanisms that could be targeted therapeutically [165, 166]. Notably,, the overlapping miRNA signatures observed in diabetic microvascular complications (e.g., retinopathy and nephropathy) and neuropathy suggest common pathways for intervention [161, 162]. The dual capacity of miRNAs to regulate inflammatory cascades and neuronal survival mechanisms, coupled with their stability and detectability in biological fluids, positions them as promising diagnostic biomarkers and therapeutic targets for diabetic neuropathy. Pharmacological modulation of specific miRNA networks, particularly those that influence NF-κB signaling and macrophage polarization, may provide a multifaceted approach to attenuating neuropathic progression while addressing the underlying inflammatory and degenerative processes [160, 161].
miR-21: A multifaceted regulator of diabetic complications
Recent studies have identified miR-21 as a key regulator of insulin/IGF signaling pathways, establishing it as a potential diagnostic marker and therapeutic target for T2DM [167]. In diabetic neuropathy, IL-1β-induced upregulation of miR-21 in pancreatic islets significantly contributes to the development of neuropathic pain [168]. This occurs through the modulation of key pain-related pathways by miR-21, including MMPs and extracellular signal-regulated kinase (ERK) signaling components [169–171].
The mechanisms by which miR-21 influences neuropathic pain involve several interconnected processes. First, miR-21 enhances MMP activity, which is characteristically elevated in neuropathic pain conditions [172]. Second, miR-21 directly regulates MMP activity in neuropathic states [173–176]. Third, it promotes ERK phosphorylation in injured neurons and glial cells. Fourth, it reduces natural inhibitors of the ERK signaling pathway [177]. Together, these actions contribute to the development and maintenance of neuropathic pain in diabetes.
Furthermore, miR-21 interacts with NF-κB pathways in ways that are particularly relevant to T1DM pathology [178]. This interaction suggests that modulating miR-21 could be an effective way to treat chronic DPN. Experimental studies have shown that pharmacological inhibition of miR-21 alleviates neuropathic pain symptoms, supporting this notion. This has been achieved through the intrathecal delivery of specially designed inhibitors that bind to and neutralize the -activity of miR-21 [168].
At the molecular level, the effects of miR-21 in DPN involve several key mechanisms. First, it regulates PTEN, a natural inhibitor of PI3K [179], which is downregulated after peripheral nerve damage [180]. This PTEN modulation contributes to the development of neuropathic pain [181] and influences pancreatic β-cell function and insulin secretion [182]. Together, these findings underscore the multifaceted role of miR-21 in diabetic complications and its potential as a therapeutic target (Fig. 4).
Fig. 4.

The role of miRNA-21 in diabetes and inflammation. MiRNA-21 has diverse effects on diabetes-induced inflammation. It inhibits PTEN and promotes GLUT4 translocation and glucose uptake. It also inhibits FOXO1 to prevent hepatic glucose production. However, it increases ERK activity, leading to greater production of inflammatory cytokines, such as NF-κB and AP-1, thereby exacerbating inflammation. Curcumin reduces the negative effects of miRNA-21 on diabetes and diabetic neuropathy
miR-146a: an anti-Inflammatory regulator in diabetic neuropathy
The human miR-146 family consists of two members with distinct genomic locations: miR-146a on chromosome 5q33 and miR-146b on chromosome 10q24 [183]. Emerging clinical evidence suggests that the expression levels of miR-146a could serve as a specific biomarker for T1DM. Studies have consistently shown decreased levels of miR-146a in the whole blood of patients with T2DM compared to healthy individuals [184].
Under inflammatory conditions, NF-κB activation triggers a cascade of proinflammatory mediators, including IL-1, IL-6, TNF-α, and IFN-γ [185]. Interestingly, the same NF-κB activation simultaneously stimulates miR-146a expression. This creates a negative feedback loop that targets and downregulates components of IL-1 and TNF-α receptor signaling pathways [186]. The clinical importance of reduced miR-146a expression is well documented across various autoimmune and inflammatory diseases affecting multiple organ systems, particularly the cardiovascular, respiratory, neurological, and dermatological systems [187].
In the context of diabetic neuropathy, miR-146a exhibits several neuroprotective effects. Experimental studies have revealed its ability to enhance nerve conduction velocity and reduce neuronal damage in animal models of the condition [188]. At the cellular level, miR-146a modulates microglial polarization by suppressing pro-inflammatory M1 activation and promoting anti-inflammatory M2 activation. This immunomodulatory function significantly reduces key inflammatory cytokines, particularly TNF-α and IL-1β [189]. The relationship between hyperglycemia and miR-146a expression reveals a complex regulatory mechanism. Acute hyperglycemic conditions initially suppress miR-146a expression; however the presence of NF-κB binding sites in the miR-146a gene subsequently leads to upregulation during prolonged hyperglycemia [186]. However, chronic hyperglycemia lasting more than two months ultimately results in decreased levels of miR-146a in models of DPN. This sustained reduction of miR-146a contributes to uncontrolled NF-κB activation, resulting in progressive tissue damage and elevated secretion of inflammatory cytokines [190].
Therapeutic approaches that focus on upregulating miR-146a have shown considerable potential for treating diabetic neuropathy. Current research suggests that increasing miR-146a expression can prevent the onset of DN [191] and improve diabetes-related cognitive impairments by inhibiting the AGE/NF-κB signaling pathway [192] (Fig. 5). Furthermore, when combined with specific therapeutic agents, increased miR-146a expression can promote beneficial cellular apoptosis, offering a multifaceted approach to managing diabetic complications [193].
Fig. 5.
The role of miRNA-146a in inflammation: Hyperglycemia exerts a dual influence on the regulation of miR-146a. Increased AGE and activation of the inflammatory pathway lead to elevated NF-κB production and increased expressionof this miRNA. However, hyperglycemia also decreases the expression of this miRNA. Curcumin increases miRNA-146a the expression of miR-146a showing potential to inhibit inflammation
miR-155: differential roles in T1DM versus T2DM
Clinical investigations have revealed distinct expression patterns of miR-155 across different diabetes subtypes. Notably, patients with DN have significantly lower plasma levels of miR-155-5p than diabetic individuals without neurological complications [194]. Additionally, studies of adipose tissue reveal an intriguing pattern where obese non-diabetic individuals demonstrate elevated miR-155 expression [195], while T1DM patients exhibit increased levels relative to healthy controls [196].
These contrasting expression patterns strongly suggest that miR-155 plays fundamentally different roles in the pathogenesis of T1DM versus T2DM. In T2DM, diminished miR-155 expression correlates with pancreatic dysfunction. Preclinical models demonstrate that miR-155 deficiency leads to multiple β-cell impairments, including reduced expansion capacity during hypercholesterolemia, decreased insulin secretion, increased glucagon expression, and altered islet cell composition [197]. These findings establish miR-155 as a critical regulator of β-cell homeostasis; its upregulation serves as an adaptive response to nutritional excess. The breakdown of this adaptive mechanism likely represents a pivotal event in the progression from prediabetes to overt T2DM [198].
Conversely, in T1DM, miR-155 appears to play a detrimental role through distinct mechanisms. Current evidence suggests that increased expression of miR-155 in immune cells contributes to the destruction of β-cell [199]. This pathogenic effect may be driven by STAT3 activation, which promotes miR-155 overexpression [200] and has been associated with various autoimmune disorders [201]. Thus, the STAT3-miR-155 axis emerges as a potential therapeutic target for modulating autoimmune responses in T1DM) (Fig. 6).
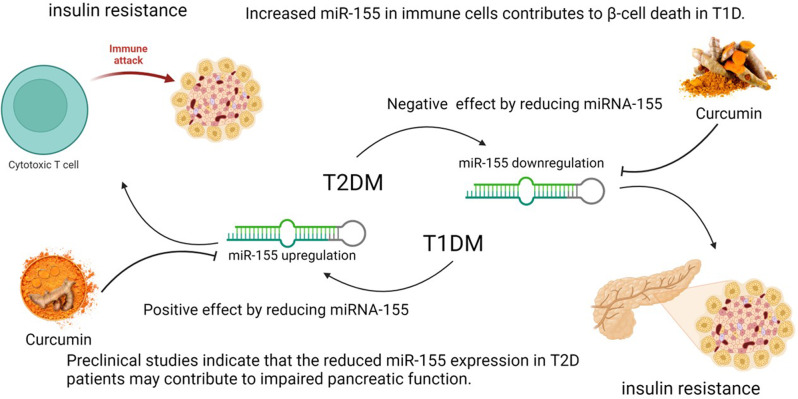
Fig. 6.
The different role of miRNA-155 in diabetes. The regulation of miRNA-155 differs between T1DM and T2DM diabetes. In T1DM, increased expression of miR-155 in immune cells can enhance the attack and destruction of pancreatic beta cells, which reduces reducing insulin secretion and increases insulin resistance. In contrast, decreased miRNA-155 expression in T2DM disrupts pancreatic cell function, leading to insulin resistance. Curcumin inhibits the expression of miRNA-155 and shows potential as a treatment for T1DM, but not T2DM
Curcumin as a MiRNA modulator in diabetic neuropathy
Current evidence demonstrates that curcumin exerts comprehensive therapeutic effects on DN by modulating multiple miRNA pathways simultaneously. As a potent epigenetic regulator, curcumin significantly downregulates the expression of miR-21 [27, 202] a key mediator of neuropathic pain while upregulating the expression of the anti-inflammatory miR-146a [193, 203] and exhibiting context-dependent modulation of the miR-155 in different types of diabetes [204, 205]. These coordinated miRNA alterations underlie curcumin’s multifaceted mechanisms of action: (1) Substantial anti-inflammatory effects through NF-κB and JAK/STAT pathway inhibition [130, 131], (2) Improved metabolic control via enhanced insulin sensitivity [135, 136], (3) Neuroprotection against oxidative damage in neuronal cells [138], and (4) Analgesic effects through nociceptive pathway modulation [206]. This multi-target approach has distinct advantages over single-target therapies because it simultaneously addresses the complex pathophysiology of diabetic neuropathy. However, the clinical translation of curcumin faces significant challenges due to its poor bioavailability [207, 208]. This has prompted the development of advanced delivery systems, including nanoparticle formulations [207], liposomal encapsulation [209], and absorption enhancers to optimize therapeutic efficacy while minimizing side effects [208]. Ongoing clinical trials are evaluating these innovative formulations, establishing curcumin as a promising candidate for comprehensive management of DN management through due to its unique ability to modulate miRNAs.
Therapeutic efficacy and safety profile of Curcumin in DN management
Clinical outcomes and mechanistic efficacy
Clinical evidence demonstrates curcumin’s significant therapeutic potential for treating DN due to its multiple pharmacological actions. In human trials, supplementation with 500 mg of turmeric capsules containing 22.1 mg of curcumin, administered three times daily for eight weeks, produced significant reductions in serum TGF-β and IL-8 levels, and proteinuria in patients with T2DM nephropathy. These results indicate potent anti-inflammatory and antioxidant effects relevant to neuropathy management [210]. These clinical findings align with curcumin’s well-characterized molecular mechanisms, particularly its inhibition of the NF-κB and COX-2 pathways. These pathways have been extensively documented in inflammatory conditions and multiple myeloma models [211]. Preclinical studies using STZ-induced diabetic rats provide important mechanistic insights. Chronic curcumin administration (50 mg/kg/day for 14 days) significantly reduced mechanical allodynia, a hallmark symptom of neuropathic pain, through mechanisms involving the opioid system (µ- and δ-opioid receptor activation) and the suppression of proinflammatory cytokines (TNF-α and IL-1β) [212, 213]. The partial reversal of curcumin’s anti-allodynic effects by naloxone pretreatment implicates endogenous opioid signaling in its analgesic action. Notably, these neuroprotective effects occurred independently of glycemic control, underscoring curcumin’s direct ability to counteract hyperglycemia-induced oxidative neuronal damage [212]. At the molecular level, curcumin demonstrates the ability to regulate microRNA networks involved in the pathogenesis of DN. It effectively suppresses NF-κB activation and downstream inflammatory mediators (TNF-α and IL-1β) by upregulating miR-146a, resulting in improved nerve conduction velocity and reduced oxidative stress [188]. Curcumin also exerts potent anti-fibrotic activity by targeting the TGF-β1/Smad3 signaling pathway and miR-21 expression. This prevents pathological neural fibrosis [214].
The clinical translation of these mechanisms has shown promise, particularly with advanced formulations. For example, nano-curcumin supplementation in patients with diabetic sensorimotor polyneuropathy produced significant improvements in metabolic parameters (reduced HbA1c and fasting glucose) and neuropathy-specific outcomes (enhanced Toronto Clinical Neuropathy Scores, reflex function, and temperature sensitivity) after eight weeks of treatment [215]. These clinical benefits are supported by curcumin’s broader metabolic effects, including improvements in insulin resistance (HOMA-IR), serum FFAs, and lipoprotein lipase activity in patients with T2DM [216]. Curcumin’s therapeutic profile is further enhanced by its effects on systemic inflammation and oxidative stress. In patients with metabolic syndrome, curcuminoid-piperine combinations (1000 mg/day) significantly reduced oxidative stress markers while increasing antioxidant capacity, as evidenced by changes in glutathione and MDA levels [217]. This multifaceted mechanism profile, which encompassesanti-inflammatory, antioxidant, antifibrotic, and metabolic-modulating activities, positions curcumin as a uniquely comprehensive therapeutic candidate for DN [216].
Accumulated evidence from clinical trials and mechanistic studies strongly supports curcumin’s therapeutic potential in managing diabetic neuropathy. Its ability to target multiple pathological pathways underlying neuropathy progression, combined with its excellent safety profile, suggests it has substantial clinical utility. However, more research is needed to optimize dosing regimens, develop formulations with enhanced bioavailability, and confirm long-term efficacy in larger patient populations [218].
Safety and interaction profiling
Curcumin has an excellent safety profileconsistently reported only mild gastrointestinal discomfort as the primary adverse effect, even at high doses (500–8000 mg/day) and during long-term administration (up to 12 months) [144]. However, pharmacokinetic studies have revealed important drug interactions, particularly with antidiabetic agents. One randomized controlled trial demonstrated that co-administering curcumin (475 mg/day) with glyburide enhanced the latter’s bioavailability through P-glycoprotein inhibition while maintaining unchanged peak plasma concentrations. This finding suggests the need for dosage modifications in combination therapies [219].
The anti-inflammatory efficacy of curcumin has been thoroughly investigated in multiple clinical settings, particularly in the management of arthritis. This compound significantly modulates critical inflammatory pathways by reducing IL-1, IL-6, TNF-α, MMP-9, COX-2, and 5-LOX activity [220–222]. Clinical assessments have shown substantial improvements in arthritis evaluation metrics (DAS, ACR, WOMAC, LPFI and VAS) and quality of life indicators after curcumin treatment [220, 222, 223]. These benefits are associated with measurable decreases in inflammatory biomarkers, such as IL-1β, COX-2, TNF-α, TGF-β, IL-6, and MCP-1 [224, 225]. In metabolic disorders, curcumin supplementation has demonstrated significant anti-inflammatory effects. Studies on patients with T2DM revealed improvements in endothelial function accompanied by reductions in MDA, endothelin-1 (ET-1), IL-6, and TNF-α levels [225]. Postoperative studies have documented that curcumin can alleviate inflammatory symptoms and tenderness as effectively as phenylbutazone [225]. Investigations in patients with metabolic syndrome have shown that curcumin can markedly decrease circulating cytokines (TNF-α, IL-6, TGF-β and MCP-1) [226, 227].
The compound’s interaction with opioid systems requires clinical attention. For example, its effects are partially reversed by naloxone, which suggests that it should be used with caution when combined with opioid medications. Notably, curcumin’s analgesic properties are independent of glycemic control, offering neuropathic pain relief without impacting blood glucose levels in diabetic models [212]. While initial obesity research showed a limited impact primarily on triglyceride levels [228, 229], recent studies using advanced formulations (e.g., phytosomal curcumin with piperine) have reported significant improvements in BMI, body fat distribution, and anthropometric parameters [230, 231]. Furthermore, curcumin modulates immune responses in obesity by regulating the levels of circulating IL-18, IL-4, and VEGF [232]. These comprehensive findings establish curcumin as a safe, multi-target therapeutic option for diverse inflammatory and metabolic conditions (Tables 1 and 2).
Table 1.
Clinical trials on anti-inflammatory activity of Curcumin
| Adverse effects, toxicity | Effect of curcumin: Treatment, dose and formulation | Trial Length | Reference |
|---|---|---|---|
| NO | The curcumin group showed an 84% decrease in inflammation, which as slightly better than phenylbutazone (86%) and much better than the placebo group (62%). Patients took 400 mg of curcumin, 100 mg of phenylbutazone, or 250 mg of a placebo three times daily. | 6 days | [226] |
| NO | Patients who took the herbal supplement Articulin-F experienced significant improvements in pain and disability scores. The supplement contains a blend of withania somnifera root, boswellia serrata stem, curcuma longa rhizome, and zinc. | 6 months | [220] |
| NO | Notable improvements in endothelial function and reductions in key biomarkers, including MDA, ET-1, IL-6, and TNF-α levels, were observed in the treatment groups. Patients received one of the following: NCB-02 (a standardized C3 curcuminoid preparation at 300 mg/day), atorvastatin (10 mg/day), or a placebo. | 8 weeks | [227] |
| NO | Patients reported less knee pain during daily activities, after taking 2000 mg of Curcuma domestica extract per day. | 6 weeks | [221] |
| NO | Patients who took curcuminoid supplements significant reductions in multiple inflammatory markers, including TNF-α, TGF-β, IL-6 compared to the placebo group. The treatment consisted of capsules containing 500 mg C3 Complex capsules (95% curcuminoids), enhanced with 5 mg BioPerine, taken three times daily for a total of 1500 mg per day). | 8 weeks | [225] |
| NO | Patients who took either Curcuma domestica extract (1500 mg/day) or ibuprofen (1200 mg/day) experienced similar improvements in total pain, and physical function. However, the turmeric group showed an additional benefit: significantly reduced systemic oxidative stress. | 4 weeks | [223] |
| NO | Curcuminoids (30 mg three times daily) were found to be comparable to diclofenac sodium (25 mg three times daily). Both treatments reduced COX-2 secretion. | 4 weeks | [224] |
| NO | Patients experienced improvements in pain and daily function. However, the results showed no meaningful difference between the group taking curcumin (1000 mg/day, providing 250 mg of active curcuminoids) and the control group. | 3 months | [222] |
Table 2.
Clinical trials on effect of Curcumin to metabolic & obesity disorders
| Adverse effects, toxicity | Effect of curcumin: Treatment, dose and formulation | Trial Length | Reference |
|---|---|---|---|
| NO | The C3 Complex formula (1000 mg/day) significantly reduced blood of IL-1β, IL-4, and VEGF. | 4 weeks | [232] |
| NO | The curcumin supplement lost more weight (up to 5%) and burned more fat (up to 8.4%). | 30 days | [230] |
| Rare (3/40), stomachache and nausea | The curcumin supplement (70 mg active per day) helped people lose weight and lower BMI. It also improved markers of cholesterol, blood sugar, and liver health. | 8 weeks | [231] |
| NO | In this study neither red pepper (1000 mg/day) nor turmeric (2800 mg/day) supplements had a measurable impact on oxidative stress, inflammation, or general metabolic health markers. | 10-week | [229] |
| - | The C3 Complex capsules, which contain 500 mg of curcuminoids and 5 mg of BioPerine, lowered triglyceride levels. | 30 days | [228] |
(1) NCB-02: Standardized C3 curcuminoid preparation (300 mg/day) (2) MDA: Malondialdehyde (3) ET-1: Endothelin-1 (4) C3 Complex: A standardized curcuminoid extract (95% curcuminoids) (5) BioPerine: Piperine extract from black pepper (6) Substance P: A neuropeptide (7) hs-CRP: High-sensitivity C-reactive protein (8) CGRP: Calcitonin Gene-Related Peptide (9) MCP-1: Monocyte Chemoattractant Protein-1 (10) WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index 11. COX-2: Cyclooxygenase-2 12. VEGF: Vascular Endothelial Growth Factor 13. IFN-γ: Interferon-gamma 14. EGF: Epidermal Growth Factor 15. BMI: Body Mass Index 16. Phosphatidylserine: A phospholipid
Translational challenges and innovative therapeutic strategies for Curcumin in diabetic neuropathy
Fundamental translational barriers
Curcumin has been extensively studied for its therapeutic potential; however, its clinical application faces significant pharmacokinetic barriers that limit bioavailability and efficacy. The compound has an excellent safety profile, and the Joint FAO/WHO Expert Committee on Food Additives and the European Food Safety Authority have determined an Acceptable Daily Intake of 0–3 mg/kg body weight [233]. Though clinical trials in healthy subjects generally confirm its safety, some adverse effects, including diarrhea, headache, rash, and yellow stool, have been reported at doses ranging from 500 to12,000 mg [234]. Longer-term administration (0.45–3.6 g/day for 1–4 months) has been associated with occasional cases of nausea, diarrhea, and elevated serum alkaline phosphatase and lactate dehydrogenase levels [235]. The pharmacokinetic limitations of curcumin are multifaceted, beginning with poor aqueous solubility which severely restricts gastrointestinal absorption. Studies demonstrate negligible serum concentrations following oral administration in rats and humans. Even doses as high as 2 g/kg fail to produce detectable plasma levels [236, 237]. This absorption challenge is further complicated by extensive first-pass metabolism, whereby curcumin rapidly undergoes conjugation via glucuronidation and sulfation. Over 99% of the circulating compound exists as pharmacologically inactive metabolites [129].
Systemic elimination primarily occurs through fecal excretion. Approximately 75% of the administered curcumin is unchanged and urinary elimination is minimal [238]. The compound exhibits variable elimination half-lives ranging from 1.45 to 44.5 h in rodent models. Human studies suggest even shorter durations in systemic circulation [239]. Tissue distribution studies reveal that absorbed curcumin primarily localizes in gastrointestinal tissues, with limited penetration into target organs such as the liver, kidneys, and brain [240]. A notable characteristic of curcumin’s pharmacokinetics is its dose-independent absorption ceiling: the percentage of the absorbed compound remains constant at 60%-66% regardless of the administered dose [241]. These fundamental limitations have sparked significant debate about curcumin’s clinical potential. Some researchers question whether the observed biological effects stem from the parent compound or its metabolites [242]. Concerns have also been raised about potential assay interference in drug screening tests [243], though proponents argue for more rigorous clinical evaluation of properly formulated products [244]. Current research focuses on overcoming these barriers through three primary strategies: advanced formulation technologies, such as including nanoparticle encapsulation and micellar systems [245, 246]; structural modifications to create more stable analogues; and metabolic interventions, such as co-administration with absorption enhancers. Despite these challenges, preclinical evidence continues to support curcumin’s therapeutic potential [247], underscoring the need for well-designed clinical trials to validate optimized formulations and establish clear dose-response relationships.
Advanced therapeutic development approaches
Despite its well-documented therapeutic potential for diabetes and its complications, curcumin’s clinical effectiveness is hindered by significant pharmacokinetic limitations. Clinical trials have shown that extremely high oral doses (up to 12,000 mg/day) result in minimal serum concentrations, which severely restricts its therapeutic utility [144]. This bioavailability challenge has prompted extensive research into advanced drug delivery systems designed to overcome curcumin’s poor absorption, rapid metabolism, and systemic elimination. Nanotechnology-based approaches have emerged as promising solutions; for example, dendritic nanoparticles have been shown to significantly enhance curcumin’s solubility and in vivo stability [248]. In vitro studies using solid lipid nanoparticles demonstrate prolonged curcumin release profiles extending up to 12 h while maintaining stability [249], as demonstrated by in vivo asthma models in which lipid nanocarriers substantially increased plasma and tissue curcumin concentrations [250]. Complementary strategies such as co-administering piperine have proven effective. Studies show that significantly higher serum curcumin levels are achieved in both rodents and humans when piperine is administered within one hour of curcumin dosing without reported adverse effects [237]. Structural analogues such as EF24 represent another innovative approach. They exhibit superior therapeutic activity against specific conditions, such as leukemia, while maintaining favorable safety profiles [251].
The development of various curcumin formulations has primarily focused on protecting the compound from chemical degradation and improving its absorption characteristics [252] (Fig. 7). Current advanced delivery systems use multiple technological approaches, including encapsulating curcumin in nanoparticles or microparticles to incorporate it into food products or supplements [253]. Curcumin can also be incorporated into diverse colloidal carriers, such as micellar aggregates, biopolymer particles, emulsion droplets, liposomes, and solid lipid particles [254]. These formulations address curcumin’s fundamental pharmacokinetic challenges, which stem stemming from poor intestinal absorption and rapid conjugation into glucuronide and sulfate metabolites in the intestine and liver, as well as extensive biliary excretion [255, 256]. The clinical impact of these limitations is substantial; human studies demonstrate persistently low bioavailability, even at doses as high as 12 g/day [256, 257]. Comparative evaluations of novel formulations reveal significant improvements, BCM-95CG (Biocurcumax) shows 6.93-fold greater bioavailability than standard curcumin, and 6.3-fold greater bioavailability than curcumin-lecithin-piperine combinations [258]. Theracurmin, which uses colloidal submicron particle dispersion technology, has demonstrated superior absorption characteristics compared to other delivery systems including BCM-95 and Meriva [259]. Combinations that incorporate hydrophilic carriers, cellulosic derivatives, and natural antioxidants have shown markedly improved blood absorption profiles compared to unformulated curcumin [260].
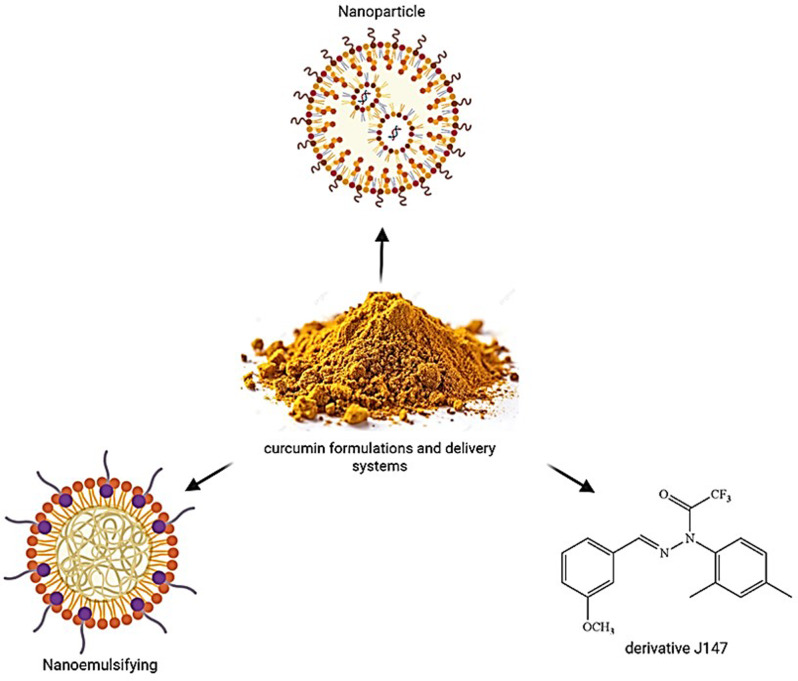
Fig. 7.
Curcumin Delivery Systems. Nanoparticle-based curcumin formulations, focusing on enhanced delivery systems
Despite limited clinical investigation to date, current research highlights the significant therapeutic potential of advanced curcumin formulations for managing neuropathic pain, particularly in diabetic neuropathies [252]. One such formulation, the synthetic curcumin derivative J147 which was originally developed for neurodegenerative diseases [261], has demonstrated remarkable neurogenic and neuroprotective effects in models of DPN. J147’s efficacy stems from its ability to modulate multiplepathogenic pathways [262], including the activation of AMPK signaling, which is oftenimpaired in DN [263]. J147 enhances AMPK protein and mRNA expression, resulting in measurable improvements in diabetes-induced mechanical allodynia, thermal hyperalgesia, and motor nerve conduction velocity [264]. Curcumin, the primary bioactive compound in turmeric, is widely recognized for its anti-inflammatory, antioxidant, and neuroprotective properties [265]. However, its clinical application faces substantial pharmacokinetic challenges, including poor oral bioavailability (less than1% in rat studies), rapid systemic metabolism, and negligible BBB penetration without advanced delivery systems, such as nanoparticles [266]. In contrast, J147 overcomes these limitations with superior pharmacokinetics, demonstrating 28% oral bioavailability in murine models and an extended plasma half-life of 2.5 h [267]. These advantages stem from its optimized molecular characteristics, including a reduced molecular weight of 351 Da and ideal lipophilicity (cLogP = 4.5). These characteristics collectively enhance BBB permeability and metabolic stability [268]. The two compounds have significantly different mechanisms of action. Curcumin exerts neuroprotective effects through its broad-spectrum, albeit nonspecific, anti-inflammatory and antioxidant activities. However, high concentrations (> 200nM) are often required for therapeutic effects [269]. For example, its modulation of targets, such as BDNF and β-secretase (BACE1) in Alzheimer’s disease models exhibitsindirect and imprecise activity [269]. In contrast, J147, operates with nanomolar potency (EC50 = 25nM) by directly inhibiting mitochondrial ATP synthase (ATP5A) and activating the AMPK/mTOR pathway [268, 270]. This targeted action more effectively mitigates oxidative stress, neuroinflammation, and amyloid-β accumulation, and robustly upregulates synaptic proteins and neurotrophic factors, such as BDNF [270]. The clinical potential of these compounds further underscores their differences. Curcumin has been studied for its potential to treat Alzheimer’s disease, depression, and cancer; however,, its poor pharmacokinetics have hindered its success in clinical applications [271, 272]. J147, shows promise in diverse conditions, including Alzheimer’s disease, diabetic neuropathy, ischemic stroke, and depression models, due to its broader therapeutic window and lower effective doses. In preclinical studies, J147 has been shown to reverse cognitive deficits, alleviate neuropathic pain, and enhance memory function in aging models [273, 274]. The safetyprofiles of these agents are also notable. Curcumin is well-tolerated but though it often requires nanoformulations to achieve clinical efficacy. In contrast, J147 has exhibited no significant toxicity in animal studies and has completed a Phase I clinical trial for Alzheimer’s disease (NCT03838185), though results are still pending [270]. Overall, J147 emerges as a superior pharmacological candidate due to its enhanced bioavailability, target specificity, and therapeutic potency [268, 270]. Nevertheless, further clinical validation is essential to establish its therapeutic utility in human populations.
Modern drug delivery platforms have transformed curcumin’s therapeutic potential for diabetes related complications. Among these, SNEDDS [143] and nano encapsulated formulations [275] show particular promise in addressing sensory neuropathy. The mechanisms involve dual modulation of P2Y12 receptors in dorsal root ganglia and suppression of inflammatory markers (IL-1β, connexin 43), effectively reducing neuronal hyperexcitability [275, 276]. Clinical evidence confirms these benefits, with nano-curcumin demonstrating significant improvements in both neuropathy scores (Toronto Clinical Scale) and metabolic parameters (fasting glucose, HbA1c) in T2DM patients [215]. This represents a major advancement over conventional curcumin, overcoming its notorious bioavailability challenges.
The pharmacokinetic limitations of native curcumin are effectively addressed through nanoformulation strategies. In diabetic neuropathy models, engineered nano-curcumin (4 mg/kg) exhibits targeted action on satellite glial cells within DRG tissues, specifically modulating overexpressed P2Y12 receptors. This targeted intervention cascades into reduced production of pain-associated proteins (Cx43) and inflammatory cytokines (IL-1β), while simultaneously downregulating p-Akt signaling pathways involved in pain perception [275]. Beyond receptor modulation, these formulations demonstrate remarkable neuroprotective effects, improving both mechanical allodynia and thermal hyperalgesia thresholds. The combined antioxidant and anti-inflammatory properties provide additional protection against oxidative nerve damage [147], though translation to human trials requires further validation.
Advanced nanoformulations (PLGA/lipid-based) combat diabetic neuropathy through multiple synergistic pathways. Their improved biodistribution enables effective modulation of DRG pain receptors (P2Y12, TRPV1, P2 × 3), reducing both inflammatory mediators (IL-1β, TNF-α) and associated thermal hyperalgesia [275, 277]. The engineered surface properties (nanoscale size, negative charge) facilitate superior nerve tissue penetration compared to free curcumin, allowing dramatic dose reduction. These systems also actively scavenge ROS while preventing intraepidermal nerve fiber degeneration, as quantified in clinical-relevant models [277] (Table 3).
Table 3.
Effects of different Curcumin formulations on neuropathic pain
| Animals(Sex, Strain) | Dose (mg/kg), Route of Administration, Duration of Treatment | Behavioral Evaluation/ Reference Other | Histopathological/ Biochemical/Molecular Parameters | Reference |
|---|---|---|---|---|
| Female Swiss Webstermiceor diabeticrats(strainandsexwere notspecified) | One group received only STZ (90 mg/kg), while the other received both STZ and the J147 compound, at doses of either 10 or 50 mg/kg, by injection or orally, for 20 weeks. | The treatment lowered blood sugar and HbA1c levels, reduced sensitivity to heat and painful touch. | The treatment blocked harmful, diabetes-related pathways and activated beneficial, metabolic pathways. It also reduced key inflammatory markers in the brain and body. | [264] |
| Male SPFrats | Animals received STZ alone or in combination with one of two J147 doses (10 or 100 mg/kg) for five days. | The treatment increased pain sensitivity. | The treatment protected nerve cells from damage, boosting survival and reducing death. It also activated AMPK, a key metabolic regulator, at the genetic and protein levels. | [262] |
| Male Sprague-Dawley | One group received an intraperitoneal injection of STZ (30 mg/kg). Another group received STZ plus curcumin nanoparticles (16 mg/kg), injection via sublingual vein during weeks 7 and 8). | The treatment reduced sensitivity to both types of pain. There was a smaller reaction to pressure, and a smaller reaction to heat. | The treatment targeted the P2Y12 receptor specifically, reducing its activity in nerve cells. This resulted in reduced inflammation and diminished communication between pain-signaling cells. | [278] |
| Male Sprague–Dawleyrats | The study had three groups. STZ group received only (55 mg/kg injection), other two groups received STZ plus either regular curcumin (30, 100, or 300 mg/kg orally). All treatments were given daily for two weeks. | The treatment stabilized body weight and blood sugar levels while significantly reducing pain sensitivity to pain from heat and cold and to pressure. | The SNEDDS-formulated treatment significantly reduced oxidative stress (MDA) and suppressed key inflammatory factors by lowering NF-κB, COX-2, and iNOS activity. It also decreased pro-inflammatory cytokines (IL-6 and TNF-α) in the sciatic nerves. | [279] |
(1) STZ: Streptozotocin (2) HbA1c: Hemoglobin A1c (3) AMPK: AMP-activated protein kinase (4) RSC96: Rat Schwann Cell line 96 (5) P2Y12: Purinergic receptor P2Y12 (6) AKT: Protein Kinase B (PKB) (7) NF-κB: Nuclear Factor-kappa B (8) COX-2: Cyclooxygenase-2 (9) iNOS: Inducible Nitric Oxide Synthase (10) TNF-α: Tumor Necrosis Factor-alpha 11. IKK-β: Inhibitor of Nuclear Factor Kappa-B Kinase subunit beta 12. SNEDDS: Self-Nanoemulsifying Drug Delivery System 13. MDA: Malondialdehyde
Limitations and future perspectives
As it is evident from a growing number of studies, exploring the prospective benefits of herbal remedies in managing the complexities of DN holds a great promise for developing novel therapuetic options, such as combination therapies and personalized herbal medicine. Therefore, more investigations are needed to identify specific herbal compounds with powerful antioxidant and anti-inflammatory properties.Moreover, it is necessary to conduct precise clinical trials to establish the safety and efficacy of currently recommended herbal treatments, providing a foundation for evidence-based practices. Finally, the gradual integration of confirmed herbal treatments into healthcare protocols for DN patients seems to be promising for improving outcomes. Nonetheless, several limitations and research gaps require attention.One substantial limitation is the relatively small sample size in many current studies, restricting the generalizability of results. Additionally, some research has lacked appropriate control groups and rigorous adherence to standardized methodologies and accurate analytical procedures.
Conclusion
DN is a debilitating diabetes complication, characterized by chronic pain, oxidative stress, and inflammation. Currently, there are limited effective treatments available. This review highlights the therapeutic potential of curcumin, a natural polyphenol derived from turmeric, in addressing the pathogenesis of DN. Curcumin exerts its beneficial effects by modulating key molecular pathways, such as suppressing NF-κB, reducing of pro-inflammatory cytokines, and enhancing of antioxidant defenses. Curcumin’s ability to regulate microRNAs, such as miR-21, miR-146a, and miR-155, offers a novel mechanism to mitigate neuroinflammation and promote neuronal repair. Despite its low bioavailability, advancements in nano formulations and delivery systems have significantly improved its pharmacokinetic profile, enhancing its clinical applicability. Preclinical and clinical studies demonstrate curcumin’s efficacy in alleviating neuropathic pain, improving nerve function, and restoring metabolic balance independently of glycemic control. Its excellent safety profile further supports its use as a complementary therapy. Future research should focus on optimizing curcumin formulations and conducting large-scale clinical trials to validate its long-term benefits. Due to its multi-target approach, curcumin shows promise as a cornerstone in DN management, offering hope for an improved quality of life for affected patients.
Acknowledgements
The authors sincerely appreciate the saveh University of Medical Science that support us in this review.
Author contributions
E.R.A.and Sh.B. designed the main idea and title.Manuscript preparation, Manuscript editing, Manuscript review: E.R.A. and Sh.B. and A.Y. and M.Sh. and F.A and F.T and M.R.R. and S.K.Preparation of figures and tables: A.Y.
Funding
In the current study, there was no funding source to be mentioned.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
The study was approved by the ethical committee of the saveh University of Medical Sciences, saveh, Iran.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. [DOI] [PubMed] [Google Scholar]
- 2.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16. [DOI] [PubMed] [Google Scholar]
- 3.Chernikov AA, Severina AS, Shamhalova MS, Shestakova MV. The role of «metabolic memory» mechanisms in the development and progression of vascular complications of diabetes mellitus. Diabetes Mellitus. 2017;20(2):126–34. [Google Scholar]
- 4.Ceriello A, Testa R, Genovese S. Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr Metabolism Cardiovasc Dis. 2016;26(4):285–92. [DOI] [PubMed] [Google Scholar]
- 5.Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Cao Q, Xiong X, Zhao P, Shen D, Zhang Y, et al. Fluoxetine regulates glucose and lipid metabolism via the PI3K–AKT signaling pathway in diabetic rats. Mol Med Rep. 2020;22(4):3073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Tang N, Hadden TJ, Rishi AK, Akt. FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978–86. [DOI] [PubMed] [Google Scholar]
- 8.Yung JHM, Giacca A. Role of c-Jun N-terminal kinase (JNK) in obesity and type 2 diabetes. Cells. 2020;9(3). [DOI] [PMC free article] [PubMed]
- 9.Pivari F, Mingione A, Brasacchio C, Soldati L. Curcumin and type 2 diabetes mellitus: prevention and treatment. Nutrients. 2019;11(8):1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barclay LR, Vinqvist MR, Mukai K, Goto H, Hashimoto Y, Tokunaga A, et al. On the antioxidant mechanism of curcumin: classical methods are needed to determine antioxidant mechanism and activity. Org Lett. 2000;2(18):2841–3. [DOI] [PubMed] [Google Scholar]
- 11.Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, et al. Curcumin blocks cytokine-mediated NF-kappa B activation and Proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol. 1999;163(6):3474–83. [PubMed] [Google Scholar]
- 12.Camacho-Barquero L, Villegas I, Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S, Motilva V, et al. Curcumin, a curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int Immunopharmacol. 2007;7(3):333–42. [DOI] [PubMed] [Google Scholar]
- 13.El-Bahr SM. Curcumin regulates gene expression of insulin like growth factor, B-cell cll/lymphoma 2 and antioxidant enzymes in streptozotocin induced diabetic rats. BMC Complement Altern Med. 2013;13:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aldebasi YH, Aly SM, Rahmani AH. Therapeutic implications of Curcumin in the prevention of diabetic retinopathy via modulation of anti-oxidant activity and genetic pathways. Int J Physiol Pathophysiol Pharmacol. 2013;5(4):194–202. [PMC free article] [PubMed] [Google Scholar]
- 15.Nakmareong S, Kukongviriyapan U, Pakdeechote P, Donpunha W, Kukongviriyapan V, Kongyingyoes B, et al. Antioxidant and vascular protective effects of Curcumin and Tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2011;383(5):519–29. [DOI] [PubMed] [Google Scholar]
- 16.Rasmi Y, di Bari I, Faisal S, Haque M, Aramwit P, da Silva A, et al. Herbal-based therapeutics for diabetic patients with SARS-Cov-2 infection. Mol Biol Rep. 2024;51(1):316. [DOI] [PubMed] [Google Scholar]
- 17.Zandi K, Ramedani E, Mohammadi K, Tajbakhsh S, Deilami I, Rastian Z, et al. Evaluation of antiviral activities of Curcumin derivatives against HSV-1 in Vero cell line. Nat Prod Commun. 2010;5(12):1934578X1000501220. [PubMed] [Google Scholar]
- 18.Karolin Kamel A-A. Comparative evaluation of the anti-ulcer activity of curcumin and omeprazole during the acute phase of gastric ulcer—efficacy of curcumin in gastric ulcer prevention against omeprazole. Food and Nutrition Sciences. 2011;2011.
- 19.Wu J, Li Q, Wang X, Yu S, Li L, Wu X, et al. Neuroprotection by Curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS ONE. 2013;8(3):e59843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao S, Xia J, Chen Z, Zhang S, Ahmad A, Miele L, et al. Inhibitory effect of Curcumin on oral carcinoma CAL-27 cells via suppression of Notch‐1 and NF‐κB signaling pathways. J Cell Biochem. 2011;112(4):1055–65. [DOI] [PubMed] [Google Scholar]
- 21.Farombi EO, Shrotriya S, Na H-K, Kim S-H, Surh Y-J. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of Heme oxygenase-1. Food Chem Toxicol. 2008;46(4):1279–87. [DOI] [PubMed] [Google Scholar]
- 22.Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23(8):1599–607. [DOI] [PubMed] [Google Scholar]
- 23.Jain SK, Rains J, Croad J, Larson B, Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal. 2009;11(2):241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiménez-Flores LM, López-Briones S, Macías-Cervantes MH, Ramírez-Emiliano J, Pérez-Vázquez VA, PPARγ. NF-κB and AMPK-dependent mechanism May be involved in the beneficial effects of Curcumin in the diabetic db/db mice liver. Molecules. 2014;19(6):8289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzales AM, Orlando RA. Curcumin and Resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr Metab (Lond). 2008;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atabaki M, Shariati-Sarabi Z, Tavakkol-Afshari J, Taghipour A, Jafari MR, Nikpoor AR, et al. Curcumin as an effective suppressor of MiRNA expression in patients with knee osteoarthritis. Avicenna J Phytomedicine. 2022;12(4):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mudduluru G, George-William JN, Muppala S, Asangani IA, Kumarswamy R, Nelson LD, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. 2011;31(3):185–97. [DOI] [PubMed] [Google Scholar]
- 28.Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol. 2016;31(1):5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26(6):1790–5. [DOI] [PubMed] [Google Scholar]
- 30.Sloan G, Shillo P, Selvarajah D, Wu J, Wilkinson ID, Tracey I, et al. A new look at painful diabetic neuropathy. Diabetes Res Clin Pract. 2018;144:177–91. [DOI] [PubMed] [Google Scholar]
- 31.Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJM. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care. 2018;41(4):645–52. [DOI] [PubMed] [Google Scholar]
- 32.Boulton AJM, Armstrong DG, Kirsner RS, Attinger CE, Lavery LA, Lipsky BA, et al. ADA clinical compendia series. Diagnosis and management of diabetic foot complications. Arlington (VA). American diabetes association © 2018 by American diabetes association. All rights reserved. None of the contents may be reproduced without the written permission of the American Diabetes Association.; 2018.
- 33.Miller JW, Proton P, Inhibitors. H2-Receptor antagonists, metformin, and vitamin B-12 deficiency: clinical implications. Adv Nutr. 2018;9(4):s511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell DSH. Metformin-induced vitamin B12 deficiency can cause or worsen distal symmetrical, autonomic and cardiac neuropathy in the patient with diabetes. Diabetes Obes Metab. 2022;24(8):1423–8. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, et al. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab. 2018;28(3):337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enders J, Elliott D, Wright DE. Emerging nonpharmacologic interventions to treat diabetic peripheral neuropathy. Antioxid Redox Signal. 2023;38(13–15):989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. 2021;17(7):400–20. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in Mainland China using 2018 diagnostic criteria from the American diabetes association: National cross sectional study. BMJ. 2020;369:m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gwathmey KG, Pearson KT. Diagnosis and management of sensory polyneuropathy. BMJ. 2019;365:l1108. [DOI] [PubMed] [Google Scholar]
- 40.Weisman A, Bril V, Ngo M, Lovblom LE, Halpern EM, Orszag A, et al. Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parameters. PLoS ONE. 2013;8(3):e58783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan Q, Li Q, Deng W, Zhao D, Qi L, Huang W, et al. Prevalence of and risk factors for peripheral neuropathy in Chinese patients with diabetes: A multicenter Cross-Sectional study. Front Endocrinol (Lausanne). 2018;9:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan Q, Li Q, Deng W, Zhao D, Qi L, Huang W, et al. Prevalence and diagnosis of diabetic cardiovascular autonomic neuropathy in beijing, china: A retrospective multicenter clinical study. Front Neurosci. 2019;13:1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Guan R, Pan L. Mechanism of Schwann cells in diabetic peripheral neuropathy: A review. Med (Baltim). 2023;102(1):e32653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elafros MA, Andersen H, Bennett DL, Savelieff MG, Viswanathan V, Callaghan BC, et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 2022;21(10):922–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin T, Gargya A, Singh H, Sivanesan E, Gulati A. Mechanism of peripheral nerve stimulation in chronic pain. Pain Med. 2020;21(Suppl 1):S6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosch-Queralt M, Fledrich R, Stassart RM. Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis. 2023;176:105952. [DOI] [PubMed] [Google Scholar]
- 47.Previtali SC. Peripheral nerve development and the pathogenesis of peripheral neuropathy: the sorting point. Neurotherapeutics. 2021;18(4):2156–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoke A, Cerri F, Fisgin A, Riva N, Quattrini A. Normal structure and pathological features in peripheral neuropathies. J Peripher Nerv Syst. 2021;26(Suppl 2):S11–20. [DOI] [PubMed] [Google Scholar]
- 49.Du W, Wang N, Li F, Jia K, An J, Liu Y, et al. STAT3 phosphorylation mediates high glucose-impaired cell autophagy in an HDAC1-dependent and -independent manner in Schwann cells of diabetic peripheral neuropathy. Faseb J. 2019;33(7):8008–21. [DOI] [PubMed] [Google Scholar]
- 50.Liu YP, Shao SJ, Guo HD. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020;248:117459. [DOI] [PubMed] [Google Scholar]
- 51.Cernea S, Raz I. Management of diabetic neuropathy. Metabolism. 2021;123:154867. [DOI] [PubMed] [Google Scholar]
- 52.Shen J. Plasticity of the central nervous system involving peripheral nerve transfer. Neural Plast. 2022;2022:5345269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93(6):1296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiAntonio A. Axon degeneration: mechanistic insights lead to therapeutic opportunities for the prevention and treatment of peripheral neuropathy. Pain. 2019;160(Suppl 1):S17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Modrak M, Talukder MAH, Gurgenashvili K, Noble M, Elfar JC. Peripheral nerve injury and myelination: potential therapeutic strategies. J Neurosci Res. 2020;98(5):780–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mrowicka M, Mrowicki J, Majsterek I. Relationship between biochemical pathways and non-coding RNAs involved in the progression of diabetic retinopathy. J Clin Med. 2024;13(1). [DOI] [PMC free article] [PubMed]
- 57.Anandhanarayanan A, Teh K, Goonoo M, Tesfaye S, Selvarajah D. Diabetic neuropathies. Endotext [Internet]; 2022.
- 58.Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic foot ulcers: A review. JAMA. 2023;330(1):62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Zhao B, Wang Y, Lan H, Liu X, Hu Y, et al. Diabetic neuropathy: cutting-edge research and future directions. Signal Transduct Target Ther. 2025;10(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.González P, Lozano P, Ros G, Solano F. Hyperglycemia and oxidative stress: an integral, updated and critical overview of their metabolic interconnections. Int J Mol Sci. 2023;24(11). [DOI] [PMC free article] [PubMed]
- 61.Wilcox G. Insulin and insulin resistance. Clin Biochemist Reviews. 2005;26(2):19. [PMC free article] [PubMed] [Google Scholar]
- 62.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med. 2005;11(2):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ibrahim MA, Isah MS, Ibrahim S. Inhibition of JAK-STAT and NF-κB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci. 2019;239:117045. [DOI] [PubMed] [Google Scholar]
- 64.Zhu J, Hu Z, Luo Y, Liu Y, Luo W, Du X, et al. Diabetic peripheral neuropathy: pathogenetic mechanisms and treatment. Front Endocrinol (Lausanne). 2023;14:1265372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agrawal R, Reno CM, Sharma S, Christensen C, Huang Y, Fisher SJ. Insulin action in the brain regulates both central and peripheral functions. Am J Physiol Endocrinol Metab. 2021;321(1):E156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23(3):133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant States. Cold Spring Harb Perspect Biol. 2014;6(1). [DOI] [PMC free article] [PubMed]
- 68.Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, et al. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24(30):6724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aghanoori MR, Smith DR, Roy Chowdhury S, Sabbir MG, Calcutt NA, Fernyhough P. Insulin prevents aberrant mitochondrial phenotype in sensory neurons of type 1 diabetic rats. Exp Neurol. 2017;297:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grote CW, Groover AL, Ryals JM, Geiger PC, Feldman EL, Wright DE. Peripheral nervous system insulin resistance in ob/ob mice. Acta Neuropathol Commun. 2013;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garber AJ. The metabolic syndrome. Med Clin North Am. 2004;88(4):837–46. ix. [DOI] [PubMed] [Google Scholar]
- 72.Dey D, Basu D, Roy SS, Bandyopadhyay A, Bhattacharya S. Involvement of novel PKC isoforms in FFA induced defects in insulin signaling. Mol Cell Endocrinol. 2006;246(1–2):60–4. [DOI] [PubMed] [Google Scholar]
- 73.Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51(10):2929–35. [DOI] [PubMed] [Google Scholar]
- 74.A ISS, C AB. A JS. Changes in plasma free fatty acids associated with Type-2 diabetes. Nutrients. 2019;11(9). [DOI] [PMC free article] [PubMed]
- 75.Platnich JM, Muruve DA. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. 2019;670:4–14. [DOI] [PubMed] [Google Scholar]
- 76.Roustit M, Loader J, Deusenbery C, Baltzis D, Veves A. Endothelial dysfunction as a link between cardiovascular risk factors and peripheral neuropathy in diabetes. J Clin Endocrinol Metab. 2016;101(9):3401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. [DOI] [PubMed] [Google Scholar]
- 78.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322(5907):1539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tahmasebi F, Barati S, Kashani IR. Effect of CSF1R inhibitor on glial cells population and remyelination in the Cuprizone model. Neuropeptides. 2021;89:102179. [DOI] [PubMed] [Google Scholar]
- 80.Al-Amily IM, Dunér P, Groop L, Salehi A. The functional impact of G protein-coupled receptor 142 (Gpr142) on pancreatic β-cell in rodent. Pflugers Arch. 2019;471(4):633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490(7418):107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, Papavassiliou AG. Crosstalk between advanced glycation and Endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metabolism. 2012;97(7):2231–42. [DOI] [PubMed] [Google Scholar]
- 83.Rabbani N, Thornalley PJ. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018;93(4):803–13. [DOI] [PubMed] [Google Scholar]
- 84.Hong J-N, Li W-W, Wang L-L, Guo H, Jiang Y, Gao Y-J, et al. Jiangtang Decoction ameliorate diabetic nephropathy through the regulation of PI3K/Akt-mediated NF-κB pathways in KK-Ay mice. Chin Med. 2017;12:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanajou D, Haghjo AG, Argani H, Aslani S. AGE-RAGE axis Blockade in diabetic nephropathy: current status and future directions. Eur J Pharmacol. 2018;833:158–64. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y-H, Chen Z-W, Li H-M, Yan X-F, Feng B. AGE/RAGE-induced EMP release via the NOX‐derived ROS pathway. J Diabetes Res. 2018;2018(1):6823058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rouhiainen A, Kuja-Panula J, Tumova S, Rauvala H. RAGE-mediated cell signaling. Calcium-binding proteins and RAGE: From structural basics to clinical applications. 2013:239– 63. [DOI] [PubMed]
- 88.Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee SR, An EJ, Kim J, Bae YS. Function of NADPH oxidases in diabetic nephropathy and development of nox inhibitors. Biomolecules Ther. 2020;28(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jha JC, Gray SP, Barit D, Okabe J, El-Osta A, Namikoshi T, et al. Genetic targeting or Pharmacologic Inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J Am Soc Nephrol. 2014;25(6):1237–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parwani K, Mandal P. Role of advanced glycation end products and insulin resistance in diabetic nephropathy. Arch Physiol Biochem. 2023;129(1):95–107. [DOI] [PubMed] [Google Scholar]
- 92.Asiri MMH, Engelsman S, Eijkelkamp N, Höppener JWM. Amyloid proteins and peripheral neuropathy. Cells. 2020;9(6). [DOI] [PMC free article] [PubMed]
- 93.Kawano T, Inokuchi J, Eto M, Murata M, Kang JH. Activators and inhibitors of protein kinase C (PKC): their applications in clinical trials. Pharmaceutics. 2021;13(11). [DOI] [PMC free article] [PubMed]
- 94.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15(5):585–94. [DOI] [PubMed] [Google Scholar]
- 95.Pinto-Junior DC, Silva KS, Michalani ML, Yonamine CY, Esteves JV, Fabre NT, et al. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci Rep. 2018;8(1):8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf. 2018;16(1):45–57. [DOI] [PubMed] [Google Scholar]
- 97.Wang X, Li Q, Han X, Gong M, Yu Z, Xu B. Electroacupuncture alleviates diabetic peripheral neuropathy by regulating Glycolipid-Related glo/ages/rage axis. Front Endocrinol (Lausanne). 2021;12:655591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fang J, Zhou S-H, Fan J, Yan S-X. Roles of glucose transporter-1 and the phosphatidylinositol 3–kinase/protein kinase B pathway in cancer radioresistance. Mol Med Rep. 2015;11(3):1573–81. [DOI] [PubMed] [Google Scholar]
- 99.Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feng S-Y, Wu S-J, Chang Y-C, Ng L-T, Chang S-J. Stimulation of GLUT4 glucose uptake by anthocyanin-rich extract from black rice (Oryza sativa L.) via PI3K/Akt and AMPK/p38 MAPK signaling in C2C12 cells. Metabolites. 2022;12(9):856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15(3):155–62. [DOI] [PubMed] [Google Scholar]
- 102.Ardestani A, Lupse B, Kido Y, Leibowitz G, Maedler K. mTORC1 signaling: A Double-Edged sword in diabetic β cells. Cell Metab. 2018;27(2):314–31. [DOI] [PubMed] [Google Scholar]
- 103.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114(7):963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, Liu F. Tissue-specific insulin signaling in the regulation of metabolism and aging. IUBMB Life. 2014;66(7):485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bartell SM, Kim HN, Ambrogini E, Han L, Iyer S, Serra Ucer S, et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun. 2014;5:3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farhan M, Wang H, Gaur U, Little PJ, Xu J, Zheng W. FOXO signaling pathways as therapeutic targets in cancer. Int J Biol Sci. 2017;13(7):815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sajan MP, Ivey RA III, Farese RV. BMI-related progression of atypical PKC-dependent aberrations in insulin signaling through IRS-1, akt, FoxO1 and PGC-1α in livers of obese and type 2 diabetic humans. Metabolism. 2015;64(11):1454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281(15):10105–17. [DOI] [PubMed] [Google Scholar]
- 109.Langlet F, Haeusler RA, Lindén D, Ericson E, Norris T, Johansson A, et al. Selective Inhibition of FOXO1 activator/repressor balance modulates hepatic glucose handling. Cell. 2017;171(4):824–35. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miyazaki S, Minamida R, Furuyama T, Tashiro F, Yamato E, Inagaki S, et al. Analysis of Foxo1-regulated genes using Foxo1‐deficient pancreatic β cells. Genes Cells. 2012;17(9):758–67. [DOI] [PubMed] [Google Scholar]
- 111.Xu B-Q, Yang P, Zhang Y-Q. Hypoglycemic activities of lyophilized powder of Gynura divaricata by improving antioxidant potential and insulin signaling in type 2 diabetic mice. Food Nutr Res. 2015;59(1):29652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback Inhibition of the insulin signaling cascade. J Biol Chem. 2003;278(5):2896–902. [DOI] [PubMed] [Google Scholar]
- 113.Carvalho BM, Oliveira AG, Ueno M, Araújo TG, Guadagnini D, Carvalho-Filho MA, et al. Modulation of double-stranded RNA-activated protein kinase in insulin sensitive tissues of obese humans. Obes (Silver Spring). 2013;21(12):2452–7. [DOI] [PubMed] [Google Scholar]
- 114.Shen J, Yang T, Xu Y, Luo Y, Zhong X, Shi L et al. δ-Tocotrienol, isolated from rice bran, exerts an Anti-Inflammatory effect via MAPKs and PPARs signaling pathways in Lipopolysaccharide-Stimulated macrophages. Int J Mol Sci. 2018;19(10). [DOI] [PMC free article] [PubMed]
- 115.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–8. [DOI] [PubMed] [Google Scholar]
- 116.Ormazabal P, Scazzocchio B, Varì R, Santangelo C, D’Archivio M, Silecchia G, et al. Effect of Protocatechuic acid on insulin responsiveness and inflammation in visceral adipose tissue from obese individuals: possible role for PTP1B. Int J Obes (Lond). 2018;42(12):2012–21. [DOI] [PubMed] [Google Scholar]
- 117.Kiechl S, Wittmann J, Giaccari A, Knoflach M, Willeit P, Bozec A, et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19(3):358–63. [DOI] [PubMed] [Google Scholar]
- 118.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. [DOI] [PubMed] [Google Scholar]
- 119.Hosooka T, Noguchi T, Kotani K, Nakamura T, Sakaue H, Inoue H, et al. Dok1 mediates high-fat diet-induced adipocyte hypertrophy and obesity through modulation of PPAR-gamma phosphorylation. Nat Med. 2008;14(2):188–93. [DOI] [PubMed] [Google Scholar]
- 120.Boughanem H, Cabrera-Mulero A, Millán-Gómez M, Garrido-Sánchez L, Cardona F, Tinahones FJ et al. Transcriptional analysis of FOXO1, C/EBP-α and PPAR-γ2 genes and their association with Obesity-Related insulin resistance. Genes (Basel). 2019;10(9). [DOI] [PMC free article] [PubMed]
- 121.Li PY, Hsu CC, Yin MC, Kuo YH, Tang FY, Chao CY. Protective effects of red guava on inflammation and oxidative stress in Streptozotocin-Induced diabetic mice. Molecules. 2015;20(12):22341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G. Curcumin–from molecule to biological function. Angew Chem Int Ed Engl. 2012;51(22):5308–32. [DOI] [PubMed] [Google Scholar]
- 123.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32(6):1053–64. [DOI] [PubMed] [Google Scholar]
- 124.Wright JS. Predicting the antioxidant activity of Curcumin and Curcuminoids. J Mol Struct (Thoechem). 2002;591(1–3):207–17. [Google Scholar]
- 125.McQuaid SE, Hodson L, Neville MJ, Dennis AL, Cheeseman J, Humphreys SM, et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heger M, van Golen RF, Broekgaarden M, Michel MC. The molecular basis for the pharmacokinetics and pharmacodynamics of Curcumin and its metabolites in relation to cancer. Pharmacol Rev. 2014;66(1):222–307. [DOI] [PubMed] [Google Scholar]
- 127.Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Woźniak K, Aprotosoaie AC, et al. Bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr. 2020;60(4):626–59. [DOI] [PubMed] [Google Scholar]
- 128.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim C-K, Luo J-L, et al. Characterization of metabolites of the chemopreventive agent Curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–64. [PubMed] [Google Scholar]
- 129.Pan M-H, Huang T-M, Lin J-K. Biotransformation of Curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27(4):486–94. [PubMed] [Google Scholar]
- 130.Maradana MR, Thomas R, O’Sullivan BJ. Targeted delivery of Curcumin for treating type 2 diabetes. Mol Nutr Food Res. 2013;57(9):1550–6. [DOI] [PubMed] [Google Scholar]
- 131.Sadeghi A, Rostamirad A, Seyyedebrahimi S, Meshkani R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology. 2018;26(5):1265–72. [DOI] [PubMed] [Google Scholar]
- 132.Kong F, Ye B, Cao J, Cai X, Lin L, Huang S, et al. Curcumin Represses NLRP3 Inflammasome Activation via TLR4/MyD88/NF-κB and P2X7R Signaling in PMA-Induced Macrophages. Front Pharmacol. 2016;7:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pescosolido N, Giannotti R, Plateroti AM, Pascarella A, Nebbioso M. Curcumin: therapeutical potential in ophthalmology. Planta Med. 2014;80(04):249–54. [DOI] [PubMed] [Google Scholar]
- 134.Panahi Y, Khalili N, Sahebi E, Namazi S, Karimian MS, Majeed M, et al. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology. 2017;25(1):25–31. [DOI] [PubMed] [Google Scholar]
- 135.Matei A-M, Caruntu C, Tampa M, Georgescu SR, Matei C, Constantin MM, et al. Applications of nanosized-lipid-based drug delivery systems in wound care. Appl Sci. 2021;11(11):4915. [Google Scholar]
- 136.Hajavi J, Momtazi AA, Johnston TP, Banach M, Majeed M, Sahebkar A. Curcumin: a naturally occurring modulator of adipokines in diabetes. J Cell Biochem. 2017;118(12):4170–82. [DOI] [PubMed] [Google Scholar]
- 137.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of Curcumin. Adv Exp Med Biol. 2007;595:105–25. [DOI] [PubMed] [Google Scholar]
- 138.Mahesh T, Balasubashini MMS, Menon VP. Photo-irradiated Curcumin supplementation in streptozotocin-induced diabetic rats: effect on lipid peroxidation. Therapies. 2004;59(6):639–44. [DOI] [PubMed] [Google Scholar]
- 139.Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol Immunol. 2019;116:29–37. [DOI] [PubMed] [Google Scholar]
- 140.Marton LT, Pescinini ESLM, Camargo MEC, Barbalho SM, Haber J, Sinatora RV, et al. The effects of Curcumin on diabetes mellitus: A systematic review. Front Endocrinol (Lausanne). 2021;12:669448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gilbert ER, Liu D. Epigenetics: the missing link to Understanding β-cell dysfunction in the pathogenesis of type 2 diabetes. Epigenetics. 2012;7(8):841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Prior M, Dargusch R, Ehren JL, Chiruta C, Schubert D. The neurotrophic compound J147 reverses cognitive impairment in aged alzheimer’s disease mice. Alzheimers Res Ther. 2013;5:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Joshi RP, Negi G, Kumar A, Pawar YB, Munjal B, Bansal AK et al. SNEDDS curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: an insight into its mechanism for neuroprotection. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9(6):776–85. [DOI] [PubMed]
- 144.Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greeshma N, Prasanth K, Balaji B. Tetrahydrocurcumin exerts protective effect on vincristine induced neuropathy: behavioral, biochemical, neurophysiological and histological evidence. Chemico-Biol Interact. 2015;238:118–28. [DOI] [PubMed] [Google Scholar]
- 146.Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Therapeutic role of Curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci Lett. 2012;511(1):18–22. [DOI] [PubMed] [Google Scholar]
- 147.Zhao W-C, Zhang B, Liao M-J, Zhang W-X, He W-Y, Wang H-B, et al. Curcumin ameliorated diabetic neuropathy partially by Inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett. 2014;560:81–5. [DOI] [PubMed] [Google Scholar]
- 148.Silveira RS, Baldoni AO, Couto RO, Mendonca TS, Domingueti CP. In vivo efficacy of turmeric (Curcuma longa L.) in the treatment of peripheral neuropathy: A systematic review of animal models. An Acad Bras Cienc. 2023;95(3):e20200447. [DOI] [PubMed] [Google Scholar]
- 149.Akaishi T, Abe K. CNB-001, a synthetic pyrazole derivative of curcumin, suppresses lipopolysaccharide-induced nitric oxide production through the Inhibition of NF-κB and p38 MAPK pathways in microglia. Eur J Pharmacol. 2018;819:190–7. [DOI] [PubMed] [Google Scholar]
- 150.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74. [DOI] [PubMed] [Google Scholar]
- 151.Niederberger E, Kynast K, Lötsch J, Geisslinger G. MicroRNAs as new players in the pain game. Pain. 2011;152(7):1455–8. [DOI] [PubMed] [Google Scholar]
- 152.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 153.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of MicroRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. [DOI] [PubMed] [Google Scholar]
- 154.La Marca V, Fierabracci A. Insights into the diagnostic potential of extracellular vesicles and their MiRNA signature from liquid biopsy as early biomarkers of diabetic micro/macrovascular complications. Int J Mol Sci. 2017;18(9):1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McClelland AD, Kantharidis P. MicroRNA in the development of diabetic complications. Clin Sci. 2014;126(2):95–110. [DOI] [PubMed] [Google Scholar]
- 156.ElSharawy A, Keller A, Flachsbart F, Wendschlag A, Jacobs G, Kefer N, et al. Genome-wide MiRNA signatures of human longevity. Aging Cell. 2012;11(4):607–16. [DOI] [PubMed] [Google Scholar]
- 157.Wang J, Tan L, Tan L, Tian Y, Ma J, Tan C-C, et al. Circulating MicroRNAs are promising novel biomarkers for drug-resistant epilepsy. Sci Rep. 2015;5(1):10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Deiuliis JA. MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes (Lond). 2016;40(1):88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McClelland AD, Kantharidis P. MicroRNA in the development of diabetic complications. Clin Sci (Lond). 2014;126(2):95–110. [DOI] [PubMed] [Google Scholar]
- 160.Herder C, Bongaerts BW, Rathmann W, Heier M, Kowall B, Koenig W, et al. Association of subclinical inflammation with polyneuropathy in the older population: KORA F4 study. Diabetes Care. 2013;36(11):3663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kovacs B, Lumayag S, Cowan C, Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Investig Ophthalmol Vis Sci. 2011;52(7):4402–9. [DOI] [PubMed] [Google Scholar]
- 162.Wilson N, Wright D. Inflammatory mediators in diabetic neuropathy. J Diabetes Metab. 2011;5(004).
- 163.Kosik KS. The neuronal MicroRNA system. Nat Rev Neurosci. 2006;7(12):911–20. [DOI] [PubMed] [Google Scholar]
- 164.Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28(17):4322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hébert SS, De Strooper B. Alterations of the MicroRNA network cause neurodegenerative disease. Trends Neurosci. 2009;32(4):199–206. [DOI] [PubMed] [Google Scholar]
- 166.Smalheiser NR, Lugli G. MicroRNA regulation of synaptic plasticity. Neuromol Med. 2009;11:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chakraborty C, Priya D, Bandyopadhyay S. MiRNAs in insulin resistance and diabetes-associated pancreatic cancer: the ‘minute and miracle’molecule moving as a monitor in the ‘genomic galaxy’. Curr Drug Targets. 2013;14(10):1110–7. [DOI] [PubMed] [Google Scholar]
- 168.Sakai A, Suzuki H. Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochem Biophys Res Commun. 2013;435(2):176–81. [DOI] [PubMed] [Google Scholar]
- 169.Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28(17):5369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Song B, Wang C, Liu J, Wang X, Lv L, Wei L, et al. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res. 2010;29(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou X, Zhang J, Jia Q, Ren Y, Wang Y, Shi L, et al. Reduction of miR-21 induces glioma cell apoptosis via activating caspase 9 and 3. Oncol Rep. 2010;24(1):195–201. [DOI] [PubMed] [Google Scholar]
- 172.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14(3):331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, et al. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol Biol Cell. 2008;19(8):3272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hatley ME, Patrick DM, Garcia MR, Richardson JA, Bassel-Duby R, van Rooij E, et al. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell. 2010;18(3):282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu M, Wu H, Liu T, Li Y, Wang F, Wan H, et al. Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Res. 2009;19(7):828–37. [DOI] [PubMed] [Google Scholar]
- 176.Mei Y, Bian C, Li J, Du Z, Zhou H, Yang Z, et al. miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J Cell Biochem. 2013;114(6):1374–84. [DOI] [PubMed] [Google Scholar]
- 177.Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, et al. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24(45):10211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ruan Q, Wang T, Kameswaran V, Wei Q, Johnson DS, Matschinsky F et al. The microRNA-21– PDCD4 axis prevents type 1 diabetes by blocking pancreatic β cell death. Proceedings of the National Academy of Sciences. 2011;108(29):12030-5. [DOI] [PMC free article] [PubMed]
- 179.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133(2):647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhou S, Shen D, Wang Y, Gong L, Tang X, Yu B, et al. microRNA-222 targeting PTEN promotes neurite outgrowth from adult dorsal root ganglion neurons following sciatic nerve transection. PLoS ONE. 2012;7(9):e44768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xu JT, Tu HY, Xin WJ, Liu XG, Zhang GH, Zhai CH. Activation of phosphatidylinositol 3-kinase and protein kinase b/akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol. 2007;206(2):269–79. [DOI] [PubMed] [Google Scholar]
- 182.Liu R, Liu C, He X, Sun P, Zhang B, Yang H, et al. MicroRNA-21 promotes pancreatic β cell function through modulating glucose uptake. Nat Commun. 2022;13(1):3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jazdzewski K, Murray EL, Franssila K, Jarzab B, Schoenberg DR, de la Chapelle A. Common SNP in pre-miR-146a decreases mature MiR expression and predisposes to papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2008;105(20):7269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Alipoor B, Ghaedi H, Meshkani R, Torkamandi S, Saffari S, Iranpour M, et al. Association of MiR-146a expression and type 2 diabetes mellitus: A Meta-Analysis. Int J Mol Cell Med. 2017;6(3):156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2007;21(11):2785–94. [DOI] [PubMed] [Google Scholar]
- 186.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of MicroRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang H, Zhang Y, Wu X, Wang Y, Cui H, Li X, et al. Regulation of human natural killer cell IFN-γ production by MicroRNA-146a via targeting the NF-κB signaling pathway. Front Immunol. 2018;9:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Feng Y, Chen L, Luo Q, Wu M, Chen Y, Shi X. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Design, Development and Therapy. 2018:171-7. [DOI] [PMC free article] [PubMed]
- 189.Liu XS, Fan B, Szalad A, Jia L, Wang L, Wang X, et al. MicroRNA-146a mimics reduce the peripheral neuropathy in type 2 diabetic mice. Diabetes. 2017;66(12):3111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Feng Y, Chen L, Luo Q, Wu M, Chen Y, Shi X. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Des Devel Ther. 2018;12:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fan B, Chopp M, Zhang ZG, Liu XS. Treatment of diabetic peripheral neuropathy with engineered mesenchymal stromal cell-derived exosomes enriched with microRNA-146a provide amplified therapeutic efficacy. Exp Neurol. 2021;341:113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kubota K, Nakano M, Kobayashi E, Mizue Y, Chikenji T, Otani M, et al. An enriched environment prevents diabetes-induced cognitive impairment in rats by enhancing Exosomal miR-146a secretion from endogenous bone marrow-derived mesenchymal stem cells. PLoS ONE. 2018;13(9):e0204252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sha M, Ye J, Zhang L-x, Luan Z-y, Chen Y-b. Celastrol induces apoptosis of gastric cancer cells by miR-146a Inhibition of NF-κB activity. Cancer Cell Int. 2013;13:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ciccacci C, Latini A, Colantuono A, Politi C, D’Amato C, Greco C, et al. Expression study of candidate MiRNAs and evaluation of their potential use as biomarkers of diabetic neuropathy. Epigenomics. 2020;12(7):575–85. [DOI] [PubMed] [Google Scholar]
- 195.Kim NH, Ahn J, Choi YM, Son HJ, Choi WH, Cho HJ, et al. Differential Circulating and visceral fat MicroRNA expression of non-obese and obese subjects. Clin Nutr. 2020;39(3):910–6. [DOI] [PubMed] [Google Scholar]
- 196.Mostahfezian M, Azhir Z, Dehghanian F, Hojati Z. Expression pattern of micrornas, miR-21, miR-155 and miR-338 in patients with type 1 diabetes. Arch Med Res. 2019;50(3):79–85. [DOI] [PubMed] [Google Scholar]
- 197.Jankauskas SS, Gambardella J, Sardu C, Lombardi A, Santulli G. Functional role of miR-155 in the pathogenesis of diabetes mellitus and its complications. Noncoding RNA. 2021;7(3). [DOI] [PMC free article] [PubMed]
- 198.Lu J, Hamze Z, Bonnavion R, Herath N, Pouponnot C, Assade F, et al. Reexpression of oncoprotein MafB in proliferative β-cells and Men1 insulinomas in mouse. Oncogene. 2012;31(31):3647–54. [DOI] [PubMed] [Google Scholar]
- 199.Guay C, Kruit JK, Rome S, Menoud V, Mulder NL, Jurdzinski A, et al. Lymphocyte-derived Exosomal MicroRNAs promote pancreatic β cell death and May contribute to type 1 diabetes development. Cell Metabol. 2019;29(2):348–61. e6. [DOI] [PubMed] [Google Scholar]
- 200.Shao S, He F, Yang Y, Yuan G, Zhang M, Yu X. Th17 cells in type 1 diabetes. Cell Immunol. 2012;280(1):16–21. [DOI] [PubMed] [Google Scholar]
- 201.Chen A, Wen J, Lu C, Lin B, Xian S, Huang F, et al. Inhibition of miR–155–5p attenuates the valvular damage induced by rheumatic heart disease. Int J Mol Med. 2020;45(2):429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Asangani IA, Rasheed SA, Nikolova D, Leupold J, Colburn N, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–36. [DOI] [PubMed] [Google Scholar]
- 203.Wu H, Liu Q, Cai T, Chen YD, Wang ZF. Induction of microRNA-146a is involved in curcumin-mediated enhancement of Temozolomide cytotoxicity against human glioblastoma. Mol Med Rep. 2015;12(4):5461–6. [DOI] [PubMed] [Google Scholar]
- 204.Wang L, Xu Y, Yu Q, Sun Q, Xu Y, Gu Q, et al. H-RN, a novel antiangiogenic peptide derived from hepatocyte growth factor inhibits inflammation in vitro and in vivo through PI3K/AKT/IKK/NF-κB signal pathway. Biochem Pharmacol. 2014;89(2):255–65. [DOI] [PubMed] [Google Scholar]
- 205.Rajaram MV, Ni B, Morris JD, Brooks MN, Carlson TK, Bakthavachalu B, et al. Mycobacterium tuberculosis Lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and MicroRNA miR-125b. Proc Natl Acad Sci. 2011;108(42):17408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Caillaud M, Aung Myo YP, McKiver BD, Osinska Warncke U, Thompson D, Mann J, et al. Key developments in the potential of Curcumin for the treatment of peripheral neuropathies. Antioxidants. 2020;9(10):950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14:583–600. [DOI] [PubMed] [Google Scholar]
- 208.Nasri H, Shirzad H, Baradaran A, Rafieian-Kopaei M. Antioxidant plants and diabetes mellitus. J Res Med Sci. 2015;20(5):491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Khajehdehi P, Zanjaninejad B, Aflaki E, Nazarinia M, Azad F, Malekmakan L, et al. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Ren Nutr. 2012;22(1):50–7. [DOI] [PubMed] [Google Scholar]
- 211.Salehi B, Stojanović-Radić Z, Matejić J, Sharifi-Rad M, Kumar NVA, Martins N, et al. The therapeutic potential of curcumin: A review of clinical trials. Eur J Med Chem. 2019;163:527–45. [DOI] [PubMed] [Google Scholar]
- 212.Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of Curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol. 2014;723:202–6. [DOI] [PubMed] [Google Scholar]
- 213.Zhou H, Beevers S, Huang C. The targets of Curcumin. Curr Drug Targets. 2011;12(3):332–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang L, Liu Y, Wang X, Li C, Shen M, Di L, et al. Curcumin alleviates TGF-β1-induced fibrosis in NRK-49F cells via suppression of miR-21 expression, and regulation of the TGF-β1/smad3 signaling pathway. J Traditional Chin Med Sci. 2020;7(1):68–74. [Google Scholar]
- 215.Asadi S, Gholami MS, Siassi F, Qorbani M, Khamoshian K, Sotoudeh G. Nano Curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo-controlled clinical trial. Complement Ther Med. 2019;43:253–60. [DOI] [PubMed] [Google Scholar]
- 216.Na LX, Li Y, Pan HZ, Zhou XL, Sun DJ, Meng M, et al. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double‐blind, placebo‐controlled trial. Mol Nutr Food Res. 2013;57(9):1569–77. [DOI] [PubMed] [Google Scholar]
- 217.Panahi Y, Khalili N, Hosseini MS, Abbasinazari M, Sahebkar A. Lipid-modifying effects of adjunctive therapy with curcuminoids–piperine combination in patients with metabolic syndrome: results of a randomized controlled trial. Complement Ther Med. 2014;22(5):851–7. [DOI] [PubMed] [Google Scholar]
- 218.Basu P, Maier C, Basu A. Effects of Curcumin and its different formulations in preclinical and clinical studies of peripheral neuropathic and postoperative pain: A comprehensive review. Int J Mol Sci. 2021;22(9):4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Neerati P, Devde R, Gangi AK. Evaluation of the effect of Curcumin capsules on glyburide therapy in patients with type-2 diabetes mellitus. Phytother Res. 2014;28(12):1796–800. [DOI] [PubMed] [Google Scholar]
- 220.Kulkarni R, Patki P, Jog V, Gandage S, Patwardhan B. Treatment of osteoarthritis with a herbomineral formulation: a double-blind, placebo-controlled, cross-over study. J Ethnopharmacol. 1991;33(1–2):91–5. [DOI] [PubMed] [Google Scholar]
- 221.Kuptniratsaikul V, Thanakhumtorn S, Chinswangwatanakul P, Wattanamongkonsil L, Thamlikitkul V. Efficacy and safety of curcuma domestica extracts in patients with knee osteoarthritis. J Altern Complement Med. 2009;15(8):891–7. [DOI] [PubMed] [Google Scholar]
- 222.Pinsornsak P, Niempoog S. The efficacy of curcuma longa L. extract as an adjuvant therapy in primary knee osteoarthritis: a randomized control trial. J Med Assoc Thai. 2012;95(Suppl 1):S51–8. [PubMed] [Google Scholar]
- 223.Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, Buntragulpoontawee M, Lukkanapichonchut P, Chootip C et al. Efficacy and safety of curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014:451–8. [DOI] [PMC free article] [PubMed]
- 224.Kertia N, Asdie AH, Rochmah W. Ability of curcuminoid compared to diclofenac sodium in reducing the secretion of cycloxygenase-2 enzyme by synovial fluid’s monocytes of patients with osteoarthritis. Acta Med Indones. 2012;44(2):105–13. [PubMed] [Google Scholar]
- 225.Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A, Sahebkar A. Curcuminoid treatment for knee osteoarthritis: A randomized double-blind placebo‐controlled trial. Phytother Res. 2014;28(11):1625–31. [DOI] [PubMed] [Google Scholar]
- 226.Satoskar R, Shah S, Shenoy S. Evaluation of anti-inflammatory property of Curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Therapy Toxicol. 1986;24(12):651–4. [PubMed] [Google Scholar]
- 227.Usharani P, Mateen A, Naidu M, Raju Y, Chandra N. Effect of NCB-02, Atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D. 2008;9:243–50. [DOI] [PubMed] [Google Scholar]
- 228.Sahebkar A, Mohammadi A, Atabati A, Rahiman S, Tavallaie S, Iranshahi M, et al. Curcuminoids modulate pro-oxidant–antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother Res. 2013;27(12):1883–8. [DOI] [PubMed] [Google Scholar]
- 229.Nieman DC, Cialdella-Kam L, Knab AM, Shanely RA. Influence of red pepper spice and turmeric on inflammation and oxidative stress biomarkers in overweight females: a metabolomics approach. Plant Foods Hum Nutr. 2012;67(4):415–21. [DOI] [PubMed] [Google Scholar]
- 230.Di Pierro F, Bressan A, Ranaldi D, Rapacioli G, Giacomelli L, Bertuccioli A. Potential role of bioavailable Curcumin in weight loss and omental adipose tissue decrease: preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur Rev Med Pharmacol Sci. 2015;19(21):4195–202. [PubMed] [Google Scholar]
- 231.Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A, et al. Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo‐controlled trial. Phytother Res. 2016;30(9):1540–8. [DOI] [PubMed] [Google Scholar]
- 232.Ganjali S, Sahebkar A, Mahdipour E, Jamialahmadi K, Torabi S, Akhlaghi S, et al. Investigation of the effects of Curcumin on serum cytokines in obese individuals: a randomized controlled trial. Sci World J. 2014;2014(1):898361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kocaadam B, Şanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57(13):2889–95. [DOI] [PubMed] [Google Scholar]
- 234.Lao CD, Ruffin MTt, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–54. [DOI] [PubMed] [Google Scholar]
- 236.Wahlström B, Blennow G. A study on the fate of Curcumin in the rat. Acta Pharmacol Et Toxicologica. 1978;43(2):86–92. [DOI] [PubMed] [Google Scholar]
- 237.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas P. Influence of Piperine on the pharmacokinetics of Curcumin in animals and human volunteers. Planta Med. 1998;64(04):353–6. [DOI] [PubMed] [Google Scholar]
- 238.Ravindranath V, Chandrasekhara N. Metabolism of curcumn-studies with [3H] Curcumin. Toxicology. 1981;22(4):337–44. [DOI] [PubMed] [Google Scholar]
- 239.Yang K-Y, Lin L-C, Tseng T-Y, Wang S-C, Tsai T-H. Oral bioavailability of Curcumin in rat and the herbal analysis from curcuma longa by LC–MS/MS. J Chromatogr B. 2007;853(1–2):183–9. [DOI] [PubMed] [Google Scholar]
- 240.Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of Curcumin in rats. Toxicology. 1980;16(3):259–65. [DOI] [PubMed] [Google Scholar]
- 241.Ravindranath V, Chandrasekhara N. In vitro studies on the intestinal absorption of Curcumin in rats. Toxicology. 1981;20(2–3):251–7. [DOI] [PubMed] [Google Scholar]
- 242.Jankun J, Wyganowska-Świątkowska M, Dettlaff K, Jelińska A, Surdacka A, Wątróbska-Świetlikowska D, et al. Determining whether Curcumin degradation/condensation is actually bioactivation (Review). Int J Mol Med. 2016;37(5):1151–8. [DOI] [PubMed] [Google Scholar]
- 243.Baker M. Deceptive Curcumin offers cautionary Tale for chemists. Nature. 2017;541(7636):144–5. [DOI] [PubMed] [Google Scholar]
- 244.Bahadori F, Demiray M. A realistic view on the essential medicinal chemistry of Curcumin. ACS Med Chem Lett. 2017;8(9):893–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Khayyal MT, El-Hazek RM, El-Sabbagh WA, Frank J, Behnam D, Abdel-Tawab M. Micellar solubilisation enhances the antiinflammatory activities of Curcumin and Boswellic acids in rats with adjuvant-induced arthritis. Nutrition. 2018;54:189–96. [DOI] [PubMed] [Google Scholar]
- 246.Akbar MU, Zia KM, Nazir A, Iqbal J, Ejaz SA, Akash MSH. Pluronic-Based mixed polymeric micelles enhance the therapeutic potential of Curcumin. AAPS PharmSciTech. 2018;19(6):2719–39. [DOI] [PubMed] [Google Scholar]
- 247.Xu X-Y, Meng X, Li S, Gan R-Y, Li Y, Li H-B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients. 2018;10(10):1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Falconieri MC, Adamo M, Monasterolo C, Bergonzi MC, Coronnello M, Bilia AR. New dendrimer-based nanoparticles enhance Curcumin solubility. Planta Med. 2017;83(05):420–5. [DOI] [PubMed] [Google Scholar]
- 249.Tiyaboonchai W, Tungpradit W, Plianbangchang P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int J Pharm. 2007;337(1–2):299–306. [DOI] [PubMed] [Google Scholar]
- 250.Wang W, Zhu R, Xie Q, Li A, Xiao Y, Li K et al. Enhanced bioavailability and efficiency of Curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int J Nanomed. 2012:3667–77. [DOI] [PMC free article] [PubMed]
- 251.Skoupa N, Dolezel P, Ruzickova E, Mlejnek P. Apoptosis induced by the Curcumin analogue EF-24 is neither mediated by oxidative stress-related mechanisms nor affected by expression of main drug transporters ABCB1 and ABCG2 in human leukemia cells. Int J Mol Sci. 2017;18(11):2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pieretti S, Ranjan AP, Di Giannuario A, Mukerjee A, Marzoli F, Di Giovannandrea R, et al. Curcumin-loaded Poly (d, l-lactide-co-glycolide) nanovesicles induce antinociceptive effects and reduce pronociceptive cytokine and BDNF release in spinal cord after acute administration in mice. Colloids Surf B Biointerfaces. 2017;158:379–86. [DOI] [PubMed] [Google Scholar]
- 253.McClements DJ, Decker EA, Park Y, Weiss J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit Rev Food Sci Nutr. 2009;49(6):577–606. [DOI] [PubMed] [Google Scholar]
- 254.McClements DJ, Decker EA, Weiss J. Emulsion-Based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72(8):R109–24. [DOI] [PubMed] [Google Scholar]
- 255.Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M. Discovery of the Curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc Natl Acad Sci U S A. 2011;108(16):6615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Dei Cas M, Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential. Nutrients. 2019;11(9):2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4b):2895–900. [PubMed] [Google Scholar]
- 258.Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A pilot Cross-Over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), A novel bioenhanced Preparation of Curcumin. Indian J Pharm Sci. 2008;70(4):445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sunagawa Y, Hirano S, Katanasaka Y, Miyazaki Y, Funamoto M, Okamura N, et al. Colloidal submicron-particle Curcumin exhibits high absorption efficiency-a double-blind, 3-way crossover study. J Nutr Sci Vitaminol (Tokyo). 2015;61(1):37–44. [DOI] [PubMed] [Google Scholar]
- 260.Jäger R, Lowery RP, Calvanese AV, Joy JM, Purpura M, Wilson JM. Comparative absorption of Curcumin formulations. Nutr J. 2014;13(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Prior M, Goldberg J, Chiruta C, Farrokhi C, Kopynets M, Roberts AJ, et al. Selecting for neurogenic potential as an alternative for alzheimer’s disease drug discovery. Alzheimer’s Dement. 2016;12(6):678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lv J, Cao L, Zhang R, Bai F, Wei P. A Curcumin derivative J147 ameliorates diabetic peripheral neuropathy in streptozotocin (STZ)-induced DPN rat models through negative regulation AMPK on TRPA1. Acta Cir Bras. 2018;33(6):533–41. [DOI] [PubMed] [Google Scholar]
- 263.Roy Chowdhury SK, Smith DR, Saleh A, Schapansky J, Marquez A, Gomes S, et al. Impaired adenosine monophosphate-activated protein kinase signalling in dorsal root ganglia neurons is linked to mitochondrial dysfunction and peripheral neuropathy in diabetes. Brain. 2012;135(6):1751–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Daugherty DJ, Marquez A, Calcutt NA, Schubert D. A novel Curcumin derivative for the treatment of diabetic neuropathy. Neuropharmacology. 2018;129:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin: miniperspective. J Med Chem. 2017;60(5):1620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18. [DOI] [PubMed] [Google Scholar]
- 267.Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, et al. A novel neurotrophic drug for cognitive enhancement and alzheimer’s disease. PLoS ONE. 2011;6(12):e27865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, et al. The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell. 2018;17(2):e12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tang M, Taghibiglou C. The mechanisms of action of Curcumin in alzheimer’s disease. J Alzheimer’s Disease. 2017;58(4):1003–16. [DOI] [PubMed] [Google Scholar]
- 270.Qiu F, Wang Y, Du Y, Zeng C, Liu Y, Pan H, et al. Current evidence for J147 as a potential therapeutic agent in nervous system disease: a narrative review. BMC Neurol. 2023;23(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Banez MJ, Geluz MI, Chandra A, Hamdan T, Biswas OS, Bryan NS, et al. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr Res. 2020;78:11–26. [DOI] [PubMed] [Google Scholar]
- 272.Ramaholimihaso T, Bouazzaoui F, Kaladjian A. Curcumin in depression: potential mechanisms of action and current Evidence-A narrative review. Front Psychiatry. 2020;11:572533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Daugherty D, Goldberg J, Fischer W, Dargusch R, Maher P, Schubert D. A novel alzheimer’s disease drug candidate targeting inflammation and fatty acid metabolism. Alzheimers Res Ther. 2017;9(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Prior M, Dargusch R, Ehren JL, Chiruta C, Schubert D. The neurotrophic compound J147 reverses cognitive impairment in aged alzheimer’s disease mice. Alzheimers Res Ther. 2013;5(3):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jia T, Rao J, Zou L, Zhao S, Yi Z, Wu B, et al. Nanoparticle-encapsulated Curcumin inhibits diabetic neuropathic pain involving the P2Y12 receptor in the dorsal root ganglia. Front NeuroSci. 2018;11:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Katagiri A, Shinoda M, Honda K, Toyofuku A, Sessle BJ, Iwata K. Satellite glial cell P2Y12 receptor in the trigeminal ganglion is involved in lingual neuropathic pain mechanisms in rats. Molecular pain. 2012;8:1744-8069-8-23. [DOI] [PMC free article] [PubMed]
- 277.Bhandari R, Sharma A, Kuhad A. Novel Nanotechnological approaches for targeting dorsal root ganglion (DRG) in mitigating diabetic neuropathic pain (DNP). Front Endocrinol. 2022;12:790747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jia T, Rao J, Zou L, Zhao S, Yi Z, Wu B, et al. Nanoparticle-Encapsulated Curcumin inhibits diabetic neuropathic pain involving the P2Y12 receptor in the dorsal root ganglia. Front Neurosci. 2017;11:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Joshi RP, Negi G, Kumar A, Pawar YB, Munjal B, Bansal AK, et al. SNEDDS Curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: an insight into its mechanism for neuroprotection. Nanomedicine. 2013;9(6):776–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.