ABSTRACT
Novel avian influenza viruses continue to circulate in animal species around the world and show a propensity to reassort and acquire virulence factors, which raises the concern that these viruses may adapt to humans. Pandemic preparedness has relied heavily on vaccine stockpiles. However, avian influenza strains genetically drift over time, and stockpiled vaccines often fail to elicit protective immunity for these genetic variants. Various strategies can help overcome immune imprinting and immunological hyporeactivity as well as broaden the immune response to variant viruses. Adjuvants remain a key strategy for improving the immunological response to avian influenza antigens. Today, three vaccines are approved in the United States for H5N1 influenza viruses though continued focus on surveillance and pandemic preparedness is essential to prepare for the possibility of human-to-human spread of this highly pathogenic influenza virus.
BACKGROUND
Influenza viruses contain eight separate ribonucleic acid (RNA) segments that can genetically reassort between virus strains, often creating major antigenic shifts. Though many animal influenza viruses have relatively little predilection for infecting humans, reassortment of RNA segments between animal and human strains can generate viruses more capable of infecting humans. Additionally, genetic drift occurs as circulating influenza strains acquire point mutations that change key surface antigen structures again allowing animal viruses to adapt and potentially acquire greater infectious potential for humans. The human population often is immunologically naïve to these predominantly animal-associated influenza virus surface antigens creating the potential for pandemic spread. Thus, worldwide efforts are in place to survey animal populations for emerging influenza viruses.
In the late 1990s, influenza A subtype H5N1 began circulating in aquatic wild bird populations in China and other parts of Asia. The virus then infected domestic poultry in Hong Kong, and a small number of humans became infected. The human infections demonstrated a 30% mortality, which caused significant worldwide concern. Through massive culling of poultry flocks and closure of live bird markets, the H5N1 influenza virus was eradicated from domestic flocks, but it continued to smolder in wild bird populations. The virus reemerged in mainland China in 2005 and through bird migration spread to many different countries. As the virus spread, the H5 hemagglutinin antigen, the main antigenically dominant surface protein for influenza viruses, adapted and mutated leading to two major antigenic clades (Clades 1 and 2) and many different subclades (Figure 1) (1). Hundreds of millions of wild birds and poultry have died, and, today, H5N1 is endemic in wild bird populations throughout the world including the United States.
Fig. 1.
Phylogenetic evolution of the H5N1 influenza virus hemagglutinin. This figure identifies the phylogenetic evolution of the hemagglutinin proteins from H5N1 influenza virus isolates collected across the world between 1995 and 2024. Clade 2.3.4.4b arose in 2021 in Europe and the Americas (1).
As H5N1 influenza spread widely through bird populations, it also spread to mammalian species worldwide with noted escalation in various animal populations over the past three years. Today, nearly 50 animal species have been infected ranging from marine to terrestrial species and carnivores to herbivores (2) signaling that H5N1 influenza is now a panzootic pathogen. H5N1 has shown significant escalation in Europe and the Americas since 2021. In the United States, 25 different mammalian and domestic poultry species have been infected with most infections attributed to influenza A H5N1 subclade 2.3.4.4b. In 2024, dairy farmers began to identify sick cows that were infected with H5N1 subclade 2.3.4.4b, and 15 states have identified infected dairy herds.
What garnered significant attention, though, has been the identification of human infections in individuals who have close contact with dairy cattle. Since 2003, 904 humans have tested positive for H5N1 virus with 464 cases succumbing to the infection, demonstrating a 51% case fatality rate. Nearly all cases have resulted from exposure to infected birds or bird-contaminated environments. The U.S. cases associated with dairy cattle are unusual. As of November 2024, the United States reported 46 confirmed human cases with 25 having exposure to cattle, 20 with exposure to poultry, and one case in Missouri with no known exposure to infected birds, animals, or environmental contamination (3).
H5N1 Viral Pathogenesis
As avian species continue to succumb to the H5N1 virus and many infected mammalian species and humans are identified, there is significant interest in whether the H5N1 viruses have developed mutations that may signal increased risk to humans and/or explain this unusual predilection for infecting cattle. The influenza virus surface hemagglutinin protein binds to host cell sialic acid receptors with different species showing a predilection for binding different forms of the sialic acid receptor. Avian species (including H5N1 Clade 2.3.4.4b) prefer sialic acids attached to galactose by an alpha 2,3 linkage, whereas human adapted strains prefer binding to alpha 2,6 linked receptors (4). Efficient human-to-human transmission may require avian species to recognize alpha 2,6 linkages. The sialic acid receptor linkage composition in cattle was never studied until 2024.
Recent studies showed that the bovine mammary gland expresses both alpha 2,3 and a2,6 linked sialic acid receptors, whereas alpha 2,3 linked receptors dominate in the bovine respiratory tract cells (5) (Figure 2) (6). The expression of avian preferred sialic acid receptors in the mammary gland may help explain the high levels of H5N1 Clade 2.3.4.4b virus found in milk from infected cows and suggests that local viral replication is occurring (7). The shared expression of both linkages may provide susceptibility for human and avian origin viruses, which could make bovine species a “mixing vessel” for genetic reassortment between viruses creating a more pathogenic human H5N1 virus.
Fig. 2.
Distribution of cell surface receptors where a sialic acid molecule is attached to a sugar chain via an a2,3 or a2,6 glycosidic linkage. Avian and human influenza species have different preferences for sialic acid linkages with avian influenza species typically binding strongly to a2,3 linked sialic acids and human influenza viruses usually preferring a2,6 linkages (dark gray). Recent human infections in dairy workers frequently have been associated with conjunctivitis with the human conjunctivae demonstrating cell receptors with a2,3 sialic acid linkage.
The other way to examine the spread from dairy cattle to humans is to understand whether the dairy H5N1 Clade 2.3.4.4b viruses bind more readily to alpha 2,6 linked sialic acid receptors. In a recent publication from Eisfeld, et al, an avian H5N1 avian strain from 2004 showed a preference for alpha 2,3 linked receptors. However, the more recent bovine-H5N1 virus bound to both alpha 2,3 and alpha 2,6 linked sialic acid receptors (6). The human distribution of variously linked sialic acid receptors is also interesting. The human conjunctiva predominantly expresses alpha 2,3 linked receptors, which may explain the predilection for conjunctivitis as a common presenting symptom in dairy workers infected with H5N1 (8).
Unadjuvanted H5N1 Vaccines
Vaccines remain a key component for pandemic planning, and, unlike coronavirus vaccines, influenza vaccines are well integrated into the public health infrastructure. Early avian influenza vaccine studies evaluated traditional inactivated, H5 hemagglutinin-based, subunit vaccines manufactured using technology approved for seasonal influenza vaccines. The data showed that H5 inactivated, subunit vaccines were poorly immunogenic at the traditional 15 mg dose even after two doses (9). A multicenter trial later found that a two-dose regimen of 90 mg of H5 subvirion vaccine generated hemagglutination inhibition antibody titers of 1:40 or greater to the vaccination strain of influenza in over 58% of vaccinated individuals (10). A titer of 1:40 or higher is generally considered to be protective for influenza and adheres to the Food and Drug Administration (FDA) guidance for clinical data needed to support licensure of seasonal inactivated influenza vaccine (11). Unfortunately, the cross-reactivity against drifted strains (new clades and subclades) of H5 viruses was poor and spurred the evaluation of new strategies to boost overall titers and cross-clade protection.
Vaccine Adjuvants and H5N1 Vaccines
Vaccine adjuvants are added to vaccines to enhance the immunogenicity of the vaccine antigens. They can improve the magnitude of the antibody response, expand the duration of circulating antibodies, improve cross-protection toward genetically drifted pathogens, and improve cell-mediated immune responses (12). The mechanisms of action of adjuvants are partially understood. In many cases, they prolong exposure time of the antigen to the immune system, often acting as a depot for the antigen. In addition, they can enhance delivery of antigen to antigen-presenting cells and provide immunostimulatory signals that potentiate immune responses (13). Alum, one of the first explored adjuvants, was discovered decades ago, yet we only recently have learned some of the mechanisms behind how it stimulates the immune response. Most adjuvants work through innate immune response pathways that function through pattern recognition receptors (PRRs). These receptors are involved in the response to pathogen-associated molecular patterns (PAMPs) often found in microbial pathogens and foreign substances. PRRs activate innate immune cells and can also stimulate adaptive immune responses. These PRRs appear to play an important role in the improved antibody and cell-mediated immune responses induced by vaccine adjuvants.
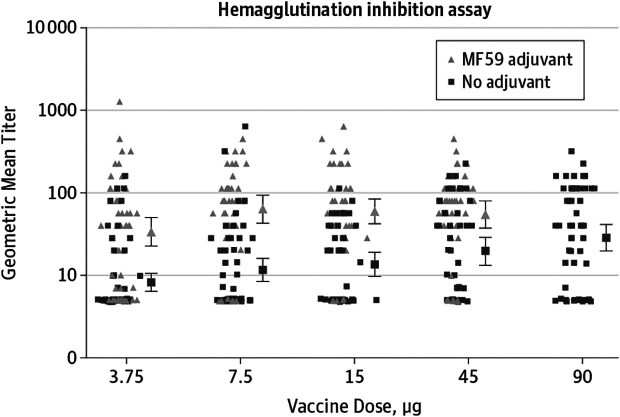
One of the early adjuvants evaluated with the H5 vaccines was MF59, which is an oil-in-water adjuvant. The addition of MF59 generated hemagglutination inhibition titers of 1:40 or greater in 63% of participants vaccinated with 15 mg of vaccine adjuvanted with MF59, whereas 45 mg of unadjuvanted H5 vaccine only generated similar titers in 29% of participants (14). Subsequent studies have determined that doses as low as 3.75 mg when mixed with MF59 can be as immunogenic as 90 mg of unadjuvanted H5 hemagglutinin antigen though 7.5 mg appears to induce a slightly higher antibody titer (15). Similar studies have been undertaken with another oil-in-water adjuvant, AS03, with similar significant improvements in antibody response to smaller doses of H5 antigen (16). Few head-to-head comparisons have been performed between MF59 and AS03 adjuvanted H5 vaccines, though one study with H5 antigen isolated from an H5N8 strain of virus (H5 Clade 2.3.4.4c) demonstrated 89% seroprotection (hemagglutination inhibition titers ≥1:40) in the 7.5 mg dose of AS03 adjuvanted H5 vaccine versus 56% seroprotection in those dosed with 7.5 mg of MF59 adjuvanted vaccine (17) (Figure 3) (15).
Fig. 3.
The addition of the adjuvant MF59 (triangles) demonstrates improved hemagglutinin inhibition antibody titers compared to unadjuvanted influenza virus hemagglutinin protein (squares). 3.75 mg of hemagglutinin protein mixed with MF59 demonstrated comparable immunogenicity to 90 mg of unadjuvanted hemagglutinin protein (15).
Also of critical importance is the ability to induce cross-clade protection given the significant genetic drifting seen with the H5N1 influenza strains. Adjuvants have been shown to induce greater breadth of antibody responses to heterologous vaccine strains. MF59 expanded cross-reactive antibodies against genetically drifted Clade 2 variants of H5N1 influenza viruses (18) (Figure 4). Some have advocated for proactive priming of one clade of H5 vaccine with a late booster with a newer clade of vaccine to induce a more rapid and cross-protective immune response. Data do suggest that early priming can induce a pool of cross-reactive memory B cells that can be boosted rapidly years later by a mismatched H5 antigen (19). Priming can also increase the duration of T cell responses induced by a heterologous H5 booster vaccination (20).
Fig. 4.
H5N1 vaccine mixed with MF59 produced cross-reactive antibody responses against four heterologous H5N1 viruses (18).
These and other studies have led to FDA approval of three H5N1 vaccines: 90 mg of unadjuvanted H5 hemagglutinin given at two doses 21 days apart and H5 hemagglutinin proteins mixed with either MF59 or AS03 (dose unspecified and would be determined in a pandemic situation), also given as two-dose series 21 days apart. Today, the United States has national stockpiles of H5 Clade 1 hemagglutinin antigen, Clade 2 antigen, MF59, and AS03. The separation of the antigen from the adjuvant is intentional allowing for point of use mixing using the antigen with the greatest cross reactivity with a circulating pandemic strain of H5N1 virus.
Currently, H5N1 Clade 2.3.4.4b virus circulates in birds and dairy cattle and has incidentally infected humans. The stockpiled Clades 1 and 2 H5 hemagglutinin antigens are significantly drifted from today’s H5N1 viruses. This raises the question of whether vaccination with Clade 1 or earlier Clade 2 subclade antigens provides any protection for Clade 2.3.4.4b. Serum samples from many of the H5N1 clinical trials have been stored for future use. Samples from three different studies that analyzed high-dose (90 mg) unadjuvanted Clade 1 vaccines, Clade 1 antigens with or without MF59, and Clade 2.1 antigens with or without AS03 have been utilized to explore cross-protection for this new subclade of H5N1 virus (Figure 5) (21). Sixty-four percent of individuals vaccinated with two doses of AS03 adjuvanted Clade 2.1 vaccine demonstrated hemagglutination inhibition titer of greater than or equal to 1:40 (or a four-fold rise in titer if the pre-vaccination titer was greater than 1:10) to the Clade 2.3.4.4b antigen. MF59 adjuvanted Clade 1 vaccine required three doses to generate cross-neutralizing hemagglutination inhibiting antibodies.
Fig. 5.
Hemagglutination inhibition antibody titers to Clade 2.3.4.4b hemagglutinin antigen from individuals vaccinated with H5 hemagglutinin antigens from Clade 1 virus (H5N1 A/Vietnam) with or without MF59 (panel e) or H5 hemagglutinin antigens from Clade 2.1 virus (H5N1 A/Indonesia) with or without AS03 (panel f) (21).
SUMMARY
H5N1 influenza viruses are endemic in animal populations and have acquired some of the characteristics that could enhance infection in humans. However, to date, there has been no human-to-human transmission. Pandemic planning is ongoing and with the escalation of animal and human infections in the United States, the federal government has authorized production of new vaccine antigens that target the new circulating strain of H5N1 Clade 2.3.4.4b. Compared to the recent coronavirus pandemic, the existing knowledge of influenza vaccine immune responses and manufacturing processes is a significant advantage. In addition, many studies have been undertaken to understand the potential efficacy of H5N1 vaccines.
Adjuvants enhance the immune response to the novel H5 hemagglutinin through various mechanisms including creation of a depot reservoir for the vaccine antigen and the innate immune response pathways that involve pathogen-associated molecular patterns recognized by pattern recognition receptors and tissue damage pathways. We are just beginning to understand the intricate differences between various adjuvants but have excellent data to support the use of MF59 and AS03 oil-in-water adjuvants with H5 antigens. These adjuvants allow small quantities of vaccine antigen to effectively stimulate protective immune responses (dose-sparing).
DISCUSSION
Lowell, San Francisco: As an immunologist, I’m going to comment on RNA-based vaccines. The reason RNA vaccines work so well is that RNA is an adjuvant itself.
Winokur, Iowa City: That is absolutely true, though I will tell you that the preliminary data with the flu mRNAs aren’t fabulous. I would say the data are okay, but we’re still not seeing the breadth and depth we would like to see. The federal government has contracted with several pharmaceutical companies to create new vaccines that target Clade 2.3.4.4b, and at least one company is pursuing an mRNA vaccine. We will learn more as they start testing a prototype vaccine.
Bray, Salt Lake City: Can you imagine what it would take to get human-to-human transmission?
Winokur, Iowa City: I think you’re going to see the evolution of either of two things: Clade 2 is going to evolve to getting mutations that allow the virus to bind better to the alpha 2,6 sialic acid cell receptors or they’re going to swap genes. Remember flu has the ability to swap entire segments of the RNA in the virus and pick up one of the human RNA pieces that allow it to bind to human epithelial cells and spread.
Bray, Salt Lake City: Maybe I’m not understanding immunology, but if we were kissing with our eyeballs …
Winokur, Iowa City: I think you probably don’t need to do that. The upper airway, for the most part, has alpha 2,6 glycosylated cell receptors, whereas the conjunctivae have alpha 2,3 glycosylated cell receptors, which favor binding avian types of influenza virus. When farmers are milking their cows, they come in contact with the cows, rub their eyes, and get eye infections. For human-to-human spread, the virus must develop the ability to bind into those upper respiratory tract cells. You’re going to have aerosols, and you’re going to spread from person to person.
Freeman, Boston: What infection signal would you need to see in the population to initiate a vaccine campaign? Also, once you saw that signal, how quickly do you think it would become a major health problem?
Winokur, Iowa City: Well, as soon as it starts spreading person to person, it is going to happen fast. I think those are really interesting questions right now. With our current knowledge, it is time to think about whether we should start to vaccinate some high-risk people like dairy farmers and poultry farmers. I think there are a lot of such questions going on in the health system. Finland had larger mink farm outbreaks so it is now vaccinating mink farmers and trying to prevent this from spreading and to tamp it down in humans so that they can’t start to get that evolution and maybe swap whole segments of RNA. If someone becomes coinfected with H1N1 or H3N2 that starts swapping, that may be a problem. Some experts believe it is crucial to vaccinate farm workers for typical circulating seasonal human strains of influenza to reduce the possibility of coinfection between avian and human strains of influenza virus.
Morris, Gainesville: When we’re talking about new epidemics, we need to be aware of some of the political and social issues. One of the big problems we’re having in trying to track H5N1 is that we are being blocked from access to dairy farms. Dairy farms frequently have illegal aliens working there, the workers are not allowed to be tested, and they have no access to medical care—there’s a whole series of social issues. We may have the vaccine, but I think we need to be cognizant of what we’ve learned with SARS-CoV-2—the social components and the political components (in states like Florida) may serve as a major barrier to our ability to scientifically address the problems that may well arise.
Winokur, Iowa City: I couldn’t agree more. Even though we learned a lot during the Corona virus pandemic, we are not prepared.
REFERENCES
- 1.Webby RJ, Uyeki TM. An update on highly pathogenic avian influenza A(H5N1) virus, Clade 2.3.4.4b. J Infect Dis . 2024;230(3):533–42. doi: 10.1093/infdis/jiae379. [DOI] [PubMed] [Google Scholar]
- 2.Plaza PI, Gamarra-Toledo V, Eugui JR, Lambertucci SA. Recent changes in patterns of mammal infection with highly pathogenic avian influenza A(H5N1) virus worldwide. Emerg Infect Dis . 2024;30(3):444–52. doi: 10.3201/eid3003.231098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Control CfD. H5 Bird Flu: Current Situation. Available at: https://www.cdc.gov/bird-flu/situation-summary/index.html .
- 4.Zhao C, Pu J. Influence of host sialic acid receptors structure on the host specificity of influenza viruses. Viruses . 2022;14(10) doi: 10.3390/v14102141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristensen C, Jensen HE, Trebbien R, Webby RJ, Larsen LE. Avian and human influenza A virus receptors in bovine mammary gland. Emerg Infect Dis (In Eng.) . 2024;30(9) doi: 10.3201/eid3009.240696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisfeld AJ, Biswas A, Guan L, et al. Pathogenicity and transmissibility of bovine H5N1 influenza virus. Nature . 2024;633(8029):426–32. doi: 10.1038/s41586-024-07766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services FaDA. Maryland: Silver Springs; 2024. Updates of Highly Pathogenic Avian Influenza (HPAI) Available at: https://www.fda.gov/food/alerts-advisories-safety-information/investigation-avian-influenza-h5n1-virus-dairy-cattle . [Google Scholar]
- 8.Kumlin U, Olofsson S, Dimock K, Arnberg N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir Viruses . 2008;2(5):147–54. doi: 10.1111/j.1750-2659.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet . 2001;357(9272):1937–43. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 10.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. New Eng J Med . 2006;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 11.Services USDoHaH, Administration FaD. Guidance for Industry Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccine. Available at: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Guidance-for-Industry–Clinical-Data-Needed-to-Support-the-Licensure-of-Seasonal-Inactivated-Influenza-Vaccines.pdf .
- 12.Pulendran B, P SA, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov . 2021;20(6):454–75. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarkar I, Garg R, van Drunen Littel-van den Hurk S. Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev Vaccines . 2019;18(5):505–21. doi: 10.1080/14760584.2019.1604231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein DI, Edwards KM, Dekker CL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis . 2008;197(5):667–75. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 15.Belshe RB, Frey SE, Graham IL, et al. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. JAMA . 2014;312(14):1420–8. doi: 10.1001/jama.2014.12609. [DOI] [PubMed] [Google Scholar]
- 16.Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One . 2008;3(2):e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winokur PL, Hegmann TE, Keitel WA, et al. Safety and immunogenicity of a monovalent inactivated influenza A/H5N8 virus vaccine given with and without AS03 or MF59 adjuvants in healthy adults. Clin Infect Dis . 2023 doi: 10.1093/cid/ciac983. [DOI] [PubMed] [Google Scholar]
- 18.Mulligan MJ, Bernstein DI, Frey S, et al. Point-of-use mixing of influenza H5N1 vaccine and MF59 adjuvant for pandemic vaccination preparedness: antibody responses and safety. A phase 1 clinical trial. Open Forum Infect Dis . 2014;1(3):ofu102. doi: 10.1093/ofid/ofu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galli G, Hancock K, Hoschler K, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A . 2009;106(19):7962–7. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoft DF, Lottenbach K, Goll JB, et al. Priming vaccination with influenza virus H5 hemagglutinin antigen significantly increases the duration of T cell responses induced by a heterologous H5 booster vaccination. J Infect Dis (In Eng.) 2016;214(7):1020–9. doi: 10.1093/infdis/jiw310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khurana S, King LR, Manischewitz J, et al. Licensed H5N1 vaccines generate cross-neutralizing antibodies against highly pathogenic H5N1 Clade 2.3.4.4b influenza virus. Nat Med . 2024;30(10):2771–6. doi: 10.1038/s41591-024-03189-y. [DOI] [PubMed] [Google Scholar]