ABSTRACT
Anthracyclines have been integral to cancer therapy for over 50 years, but their clinical use is constrained by dose-dependent cardiotoxicity. These agents target topoisomerase 2a, a critical enzyme for DNA replication, to exert their anticancer effects. Notably, while adult cardiomyocytes lack topoisomerase 2a, they do express topoisomerase 2b. Using genetic models, we demonstrated that deleting topoisomerase 2b in cardiomyocytes prevents anthracycline-induced DNA double-strand breaks, ROS production, mitochondrial dysfunction, and heart failure. These findings identify topoisomerase 2b as the key mediator of anthracycline-induced cardiotoxicity. Dexrazoxane, a Food and Drug Administration (FDA)-approved agent for preventing doxorubicin-induced cardiotoxicity, acts as a catalytic inhibitor of topoisomerases. However, this activity could potentially diminish doxorubicin’s anticancer efficacy. New evidence, discovered serendipitously, shows that dexrazoxane selectively induces degradation of topoisomerase 2b without affecting topoisomerase 2a. A promising alternative approach involves pretreating with dexrazoxane prior to doxorubicin infusion. This strategy is supported by preclinical studies and is being tested in the PHOENIX trial.
INTRODUCTION
The overall cancer death rate in the United States has steadily declined since the early 1990s. According to a recent SEER Cancer Statistics Review, cancer death rates for men decreased by 1.8% annually between 2006 and 2015. During the same period, the death rates for women declined by 4% annually (1). Moreover, among children aged 0–19, there was a 1.4% annual decline in cancer death rates between 2011 and 2015. In 2016, there were 15.5 million cancer survivors in the United States, a number projected to rise to 20.3 million by 2026 (2). Despite these remarkable advances in cancer therapy, cancer survivors often experience long-term complications resulting from chemotherapy or radiation treatments (3–6). Among these complications, chemotherapy-induced cardiotoxicity, particularly heart failure, is the most dreaded. Of the various chemotherapy drugs, anthracyclines such as doxorubicin are among the worst offenders in causing cardiotoxicity.
Doxorubicin, an inhibitor of DNA topoisomerase 2a (Top2a), is routinely used in the treatment of breast cancer, sarcoma, and pediatric leukemia (3,7). Of the two topoisomerase 2 isozymes, Top2a is highly expressed in cancer cells and plays a critical role in cell division (8). In contrast, adult cardiomyocytes express only topoisomerase 2b (Top2b), which is involved in DNA transcription rather than replication (3). Doxorubicin was traditionally thought to induce cardiotoxicity through the generation of reactive oxygen species (ROS) (9,10). This drug exhibits a high affinity for cardiolipin, a negatively charged phospholipid abundantly present in mitochondria (11). A one-electron reduction of the quinone group in the anthracene structure of doxorubicin forms a semiquinone radical, which reacts with molecular oxygen to produce superoxide radicals. The semiquinone radical then reverts to the quinone form, enabling the continuation of this redox cycling process. This cycling generates significant amounts of superoxide radicals, which, in turn, lead to the formation of additional reactive oxygen and nitrogen species. Furthermore, the semiquinone form of doxorubicin and the superoxide radicals can release iron from ferritin and aconitase, increasing cellular free iron levels. This iron excess promotes the production of hydroxyl radicals via Fenton chemistry, thereby exacerbating oxidative stress (12). However, the ROS hypothesis has limitations. Neither ROS scavengers nor iron chelators have successfully prevented doxorubicin-induced cardiotoxicity in animal models or human studies (13,14). These findings highlight the need for an alternative explanation for doxorubicin-induced cardiotoxicity.
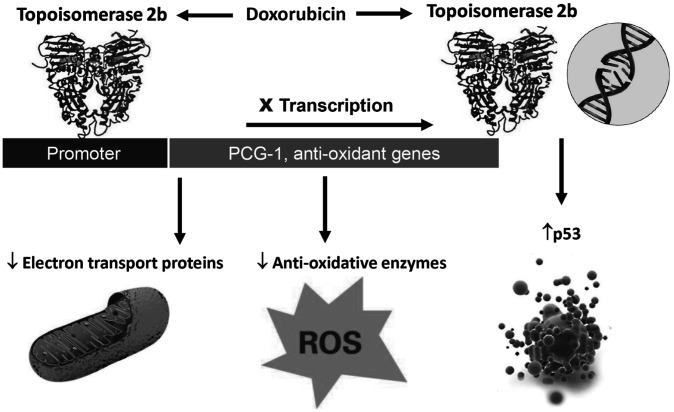
Recently, we demonstrated that Top2b is the molecular basis of doxorubicin-induced cardiotoxicity (15). Doxorubicin binds to Top2b and intercalates into DNA, causing double-strand breaks (DSBs) that activate p53 and initiate a cell death program in cardiomyocytes. Additionally, doxorubicin-bound Top2b binds to the promoters of PGC1α and PGC1β, suppressing the transcription of antioxidant enzymes and mitochondrial electron transport chain component genes. This suppression leads to a reduction in antioxidant enzymes, resulting in elevated ROS levels, while the impairment of mitochondrial electron transport chain components contributes to defective mitochondrial biogenesis and dysfunction. These effects are hallmark features of doxorubicin-induced cardiotoxicity, as observed in human heart samples. The Top2b hypothesis not only provides a molecular explanation for doxorubicin-induced cardiotoxicity but also accounts for the classic observations of increased ROS and mitochondrial dysfunction (Figure 1). Most importantly, cardiomyocyte-specific deletion of Top2b protects mice from developing doxorubicin-induced heart failure (15). In a new paradigm, Top2a mediates doxorubicin’s tumoricidal activity, whereas Top2b mediates doxorubicin’s cardiotoxic effect (Figure 2).
Fig. 1.
A unified theory of doxorubicin-induced cardiotoxicity based on topoisomerase 2b (15). Anthracycline-induced cardiotoxicity can be amplified by additional mechanisms, such as cellular metabolism of anthracycline and/or inherent mitochondrial pathology (16,17).
Fig. 2.
Doxorubicin kills cancer cells by poisoning Top2a and induces cardiotoxicity through Top2b.
Further support for the Top2b hypothesis comes from studies involving dexrazoxane. Dexrazoxane, approved by the FDA for the treatment of doxorubicin-induced cardiotoxicity and skin toxicity, was initially thought to exert its cardioprotective effects by chelating iron and acting as an antioxidant. However, other iron chelators and antioxidants, such as N-acetylcysteine, have failed to prevent doxorubicin-induced cardiotoxicity (13). Remarkably, dexrazoxane is a catalytic inhibitor of Top2b (18). Its cardioprotective effect is most likely due to its ability to inhibit the catalytic cycle of Top2b. Dexrazoxane binds to the ATPase domain of Top2b, locking the enzyme in a closed clamp conformation and preventing the passage of new double-stranded DNA. In contrast, doxorubicin interferes with the reannealing of cut double-stranded DNA, leading to the accumulation of double-strand breaks (DSBs) and subsequent cell death.
However, in a phase III trial, concerns were raised that dexrazoxane might reduce the efficacy of anthracyclines in treating breast cancer (19). This trial reported a significant difference in objective response rates, with the placebo group showing an unusually high response rate. Notably, other endpoints, such as survival and time to progression, were not affected by dexrazoxane in this study. The reported difference in objective response rates led to the FDA’s limited approval of dexrazoxane, resulting in its poor clinical utilization. In this paper, I would like to show that dexrazoxane can still be used in preventing doxorubicin-induced cardiotoxicity by early administration before doxorubicin infusion.
MATERIALS AND METHODS
Western Blot
Cells were lysed in cell lysis buffer [0.5M Tris-HCl, pH 7.4, 1.5M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10mM EDTA (Sigma, #20-188), and protease inhibitors (Thermo Scientific, #78438)]. Whole cell extracts were separated by gel electrophoresis on 4–20% Mini-PROTEAN® TGX™ Precast Protein Gels (Bio-rad, #4561093) and transferred to Trans-Blot Turbo Midi 0.2 µm Nitrocellulose Transfer Packs (Bio-rad, #1704159). Mouse anti-Actin (Sigma, #A1978), Rabbit anti-Top2b (abcam, #ab72334), and Rabbit anti-Top2a (Cell Signaling, #12286) were used for western blotting, and signals were detected with a chemiluminescent detection kit (Thermo Fisher Scientific, #PI34076).
DNA Double-Strand Analysis, TUNEL Staining, and Cardiac Magnetic Resonance Imaging (MRI)
Methods were described in a previous publication (15).
Statistical Analysis
All experiments were independently repeated at least three times. Data were represented as mean ± SD. Statistical significance was calculated for Student’s t-test, using GraphPad Prism 6.0 (GraphPad Software, Inc.). A p value less than 0.05 was considered statistically significant.
RESULTS
Dexrazoxane Induces Degradation of Top2b, but Not Top2a, in a Proteasome-Dependent Manner
Dexrazoxane (100 μM) induced degradation of Top2b, but not Top2a in HCT116 cells (Figure 3). This degradation is mediated by the ubiquitin-proteasome system because degradation is inhibited by MG132, a proteasome inhibitor. Dexrazoxane-induced degradations of Top2b, but not Top2a, were observed in human breast cancer cell lines (MCF-7, MDA-MB-231), lymphoma cancer cell lines (MAVER-1, BC-3), sarcoma cancer cell lines (RD, HT-1080), leukemia cell lines (HL-60, MOLT-4), prostate cancer cell lines (PC-3, LNCaP), a kidney cell line (293FT), and a cervical carcinoma cell line (HeLa) (data not shown). Thus, dexrazoxane selectively degraded Top2b, but not Top2a, in all cancer cell lines tested.
Fig. 4.
Mice were treated with dexrazoxane (100 mg/kg, IP) for different time periods before the heart was removed and Top2b expression determined by western blot analysis with GAPDH as loading control.
Fig. 3.
Top2a and Top2b expression in HCT116 cells after dexrazoxane treatment (100 µM) with or without Mg132 (10 µM).
Dexrazoxane-Induced Degradation of Top2b in Cardiomyocyte Cell Lines and Murine Heart
Dexrazoxane also induced degradation of Top2b, but not Top2a, in a mouse cardiomyocyte cell line (HL-1) and a human cardiomyocyte cell line (AC-16) (data not shown). When dexrazoxane was administered intraperitoneal (IP) to mice at a dose of 100 mg/kg, Top2b in the murine heart was also decreased in a time-dependent manner (Figure 4). As shown, Top2b was not detectable six hours after dexrazoxane administration, but gradually returned to baseline by 48 hours.
Fig. 5.
Dexrazoxane (Dex) pretreatment eight hours before doxorubicin ameliorates doxorubicin (Dox)-induced DSBs in the heart.
*p < 0.05
**p < 0.001, compared with the doxorubicin-treated group; N = 6
Dexrazoxane Pretreatment Reduced DNA Double-Strand Break and Apoptosis in Doxorubicin-Treated Mouse Hearts
DNA double-strand breaks and apoptosis are two hallmarks of doxorubicin-induced acute cardiotoxicity. Since genetic depletion of Top2b ameliorated doxorubicin’s acute cardiotoxicity, pharmacologic depletion of Top2b by dexrazoxane is expected to reduce doxorubicin’s acute cardiotoxicity. For this purpose, mice were injected with dexrazoxane (100 mg/kg) eight hours prior to doxorubicin treatment. Hearts were removed 16 hours after doxorubicin treatment, embedded in OCT, and snap frozen in liquid nitrogen. Heart sections were stained with anti-ɣH2AX antibody to detect DNA DSBs and a TUNEL assay kit to detect apoptosis. Figures 5 and 6 show that pretreatment with dexrazoxane eight hours before doxorubicin protected the heart from developing DSBs and apoptosis.
Fig. 6.
Dexrazoxane (Dex) pretreatment eight hours before doxorubicin ameliorates doxorubicin (Dox)-induced apoptosis in the heart.
*p < 0.05
**p < 0.001, compared with the doxorubicin-treated group; N = 6
Pretreatment with Dexrazoxane Reduces Doxorubicin-Induced Cardiotoxicity in a Chronic Heart Failure Model
A chronic doxorubicin-administration model was used to show that the pretreatment strategy is effective in preventing doxorubicin-induced heart failure. Mice underwent pretreatment cardiac MRI to assess their ejection fraction (EF). They were divided into four groups. Group 1: Doxorubicin (5 mg/kg IP), Group 2: Dexrazoxane (100 mg/kg) IP, Group 3: Dexrazoxane (100 mg/kg) IP, given eight hours before doxorubicin (5 mg/kg) IP, Group 4: Saline control. N=7 for each group. This protocol was repeated weekly for five weeks. At the end of the study, mice underwent cardiac MRI again for EF determination. As shown, five weekly treatments of doxorubicin reduced the EF from 55 to 45% (Figure 7). The dexrazoxane pretreated group had complete protection from doxorubicin-induced EF drop.
Fig. 7.
Dexrazoxane pretreatment eight hours before doxorubicin is effective in preventing chronic doxorubicin therapy-induced heart failure. EF was determined by cardiac MRI before (white bar) and after doxorubicin treatment (gray bar). N = 7. EF values were means ± standard deviations.
*p < 0.05
DISCUSSION
Dexrazoxane is the only FDA-approved drug for preventing doxorubicin-induced cardiotoxicity. Currently, it is administered concurrently with doxorubicin. However, because dexrazoxane also binds to Top2α, it has the potential to interfere with doxorubicin’s tumoricidal effects. As a result, the FDA restricts its use to breast cancer patients who have received a cumulative doxorubicin dose of 300 mg/m² and require continued treatment to maintain tumor control. Despite these guidelines, subclinical cardiotoxicity from doxorubicin is known to occur much earlier, potentially from the initiation of therapy, as demonstrated by more sensitive detection methods such as cardiac biopsies or echo strain imaging (20,21). The lack of cardio-protection at this critical early stage is a missed opportunity for preventing long-term cardiac damage. Based on preclinical studies, we propose a novel strategy to optimize dexrazoxane’s use by altering its dosing schedule. Administering dexrazoxane as a pretreatment eight hours before doxorubicin is expected to provide effective cardio-protection by inducing Top2b degradation while preserving doxorubicin’s anticancer efficacy through the retention of Top2a and clearance of dexrazoxane.
We are conducting the National Institutes of Health (NIH)-sponsored PHOENIX trial (Prevention of Heart Failure Induced by Doxorubicin with Early Administration of Dexrazoxane) to evaluate this novel strategy in breast cancer patients (Figure 8). Since Top2β degradation has not yet been demonstrated in humans, we will begin with a dose and time-course study. This preliminary phase aims to determine the timeline of dexrazoxane-induced Top2β degradation and to identify the dosage required for significant Top2β reduction in human tissues. Given the impracticality of using cardiac tissue in this study, phase I will utilize human peripheral blood leukocytes as a surrogate. We will also assess whether Top2α is resistant to dexrazoxane-induced degradation, ensuring specificity in its effects.
Fig. 8.
Schematic diagram for the PHOENIX study. As shown, dexrazoxane induces degradation of Top2b, but not Top2a. Furthermore, dexrazoxane is markedly reduced eight hours after infusion.
We aim to reestablish the clinical use of dexrazoxane for preventing doxorubicin-induced cardiotoxicity by administering it early, prior to doxorubicin infusion. This approach is grounded in a robust mechanistic foundation and supported by preclinical studies. As dexrazoxane is off-patent, its cost for breast cancer patients undergoing a four-cycle anthracycline-containing regimen is estimated to be under $3,000 (ranging from $305.31 to $463.74 per 500 mg vial). This represents a significantly more affordable alternative to repeated echocardiograms, which average $1,500 per scan in the United States, and the substantial long-term costs associated with managing doxorubicin-induced congestive heart failure.
ACKNOWLEDGMENTS AND FINANCIAL SUPPORT
Supported by NIH grants HL126916 and HL151993. Cancer Prevention Research Institute of Texas, RP110486. Edward T. H. Yeh is a scholar of the Arkansas Research Alliance.
DISCUSSION
Deweese, Baltimore: Thank you for the fantastic talk, particularly the translational element. For other reasons, we think a lot about Top2 alpha and beta and how it relates to hormone regulation, particularly in the context of testosterone and men with prostate cancer. As it turns out, there’s a very important relationship that we have just published on these SWI/SNF subunits BRG1 and 2. If those were involved in this process and there are ways to modulate these SWI/SNF complexes, we could get away from the risks of that particular drug and its inhibition of Top2 by the chemotherapeutic.
Yeh, Little Rock: I do not know about those. I focused on dexrazoxane because it is FDA approved and very inexpensive to administer the drug. We basically just give it any time before dexrazoxane: one hour before actually worked pretty well. If there is still dexrazoxane around, it may affect the efficacy, and that’s why we did this phase I study.
Deweese, Baltimore: Well, it’s been helpful to me since we’re going to study that. Thank you very much.
Yeh, Little Rock: Okay.
Blumenthal, Baltimore: Could you give us any insight on the use of statin therapy, ACE inhibitors, and angiotensin receptor blockers to also augment that effect? Are there any data showing that they can help?
Yeh, Little Rock: Yes, a lot of agents have been shown either in animal models or in a small patient study to prevent this problem. I call them “shotgun therapy” where we use all that is in the cardiologist’s toolbox. I think we need to use a sort of mechanism-based therapy. Hopefully, I can convince you that Top2β is the culprit—any way you can inhibit or delete it is probably the best way to go around the problem. Obviously, you can give a statin or an ACE inhibitor, but I think mechanism-based therapy is probably the best approach.
Deswal, Houston: Thank you, Dr. Yeh, for a very nice work. Are there animal models studying the use of dexrazoxane and doxorubicin on the tumor because that’s the hesitancy?
Yeh, Little Rock: Yes, we actually have done those studies. We initially planned to use dexrazoxane as an anti-tumor agent so it actually has some anti-tumor activities. In our animal model, it doesn’t appear to have any adverse effect now. However, there’s a clinical study showing that it may decrease efficacy because of the theoretical consideration. People can’t avoid using them and that’s the problem.
Deswal, Houston: Thank you.
Reiser, Galveston: Very nice talk and congratulations. I am wondering about your perspective on biomarkers versus imaging, or longitudinal strain, to detect cardiotoxicity versus C-reactive protein (CRP) or other inflammatory markers. Where do you think the field is headed?
Yeh, Little Rock: People are trying to detect cardiotoxicity using biomarkers or imaging. I think these are all good, but they cost a lot of money. Longitudinal study is also very expensive. My proposal is that we prevent the problem, rather than causing it and then monitoring it. At the time of chemotherapy, if you delete Top2β, you do not cause a problem and then you don’t need to monitor it. That’s my hope and hopefully it will save the government some money.
Reiser, Galveston: It’s good to monitor though.
Yeh, Little Rock: I agree, but if we can do it without monitoring, that would be better.
Contributor Information
Edward T. H. Yeh, Little Rock, AR.
Hui-Ming Chang, Little Rock, AR.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. Betheda, MD: National Cancer Institute; 2014. SEER Cancer Statistics Review, 1975–2014. [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev . 2016;25:1029–36. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol . 2017;70:2536–51. doi: 10.1016/j.jacc.2017.09.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J Am Coll Cardiol . 2017;70:2552–65. doi: 10.1016/j.jacc.2017.09.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol . 2014;32:1218–27. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys . 2010;76:656–65. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science . 1984;226:466–8. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- 8.Azuma Y, Arnaoutov A, Dasso M. SUMO-2/3 regulates topoisomerase II in mitosis. J Cell Biol . 2003;163:477–87. doi: 10.1083/jcb.200304088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doroshow JH. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res . 1983;43:460–72. [PubMed] [Google Scholar]
- 10.Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest . 1980;65:128–35. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doroshow JH. Doxorubicin-induced cardiac toxicity. N Engl J Med . 1991;324:843–5. doi: 10.1056/NEJM199103213241210. [DOI] [PubMed] [Google Scholar]
- 12.Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, Sawyer DB. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol . 2008;26:3777–84. doi: 10.1200/JCO.2007.14.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers C, Bonow R, Palmeri S, et al. A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N-acetylcysteine. Semin Oncol . 1983;10:53–5. [PubMed] [Google Scholar]
- 14.Martin E, Thougaard AV, Grauslund M, et al. Evaluation of the topoisomerase II-inactive bisdioxopiperazine ICRF-161 as a protectant against doxorubicin-induced cardiomyopathy. Toxicol . 2009;255:72–9. doi: 10.1016/j.tox.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med . 2012;18:1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 16.Lipshultz SE, Lipsitz SR, Kutok JL, et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer . 2013;119:3555–62. doi: 10.1002/cncr.28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson LE, Bedja D, Alvey SJ, Cardounel AJ, Gabrielson KL, Reeves RH. Protection from doxorubicin-induced cardiac toxicity in mice with a null allele of carbonyl reductase 1. Cancer Res . 2003;63:6602–6. [PubMed] [Google Scholar]
- 18.Lyu YL, Kerrigan JE, Lin CP, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res . 2007;67:8839–46. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 19.Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol . 1997;15:1318–32. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 20.Ewer MS, Ali MK, Mackay B, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J Clin Oncol . 1984;2:112–7. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 21.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging . 2014;15:1063–93. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]