Abstract
Research into population declines of North American bird species has mainly focused on the fragmentation of habitat on the breeding or wintering grounds [Robinson, S. K., Thompson, F. R., Donovan, T. M., Whitehead, D. R. & Faaborg, J. (1995) Science 267, 1987–1990]. In contrast, research into declines of European species has mainly focused on intensification of agriculture [Donald, P. F., Green, R. E. & Heath, M. F. (2001) Proc. R. Soc. London Ser. B 268, 25–29] and the role played by the atmospheric deposition of pollutants, in particular, acid rain [Graveland, J. (1998) Environ. Rev. 6, 41–54]. However, despite widespread unexplained declines of bird populations in regions of heavy wet acid ion deposition [Sauer, J. R., Hines, J. E. & Fallon, J. (2001) The North American Breeding Bird Survey Results and Analysis 1966–2000 (Patuxent Wildlife Research Center, Laurel, MD)], no North American studies have presented evidence linking such widespread terrestrial bird declines to acid rain. To address the question of the role played by acid rain in population declines of eastern North American songbird species, we combine data from several sources. We use a multiple logistic regression model to test for adverse effects of acid rain on the Wood Thrush, while controlling for regional abundance, landscape-level habitat fragmentation, elevation, soil pH, and vegetation. We show a strong, highly significant, negative effect of acid rain on the predicted probability of breeding by this species, and interactions with elevation, low pH soils, and habitat fragmentation that worsen these negative effects. Our results suggest an important role for acid rain in recent declines of some birds breeding in the eastern United States, particularly in high elevation zones with low pH soils, and show the need to consider other large-scale influences, in addition to habitat fragmentation, when addressing bird population declines.
In North America, research into bird population declines has mainly focused on habitat fragmentation as the process leading to the observed patterns (1). However, in Europe, where fragmentation of native habitat may have occurred centuries earlier, research has focused on intensification of agricultural practices (2) and on the effects of pollution, such as acid rain (3). We integrate these approaches to test for the contribution of acid rain, over and above any effects of habitat fragmentation, to declines of a Neotropical migrant bird species in North American regions experiencing heavy wet deposition of acid ions (acid rain). We use data on the distribution of the Wood Thrush (Hylocichla mustelina), which breeds across a range of acid ion wet deposition values, and which displays variably negative regional population trends, to address the question of the impacts of acid rain on forest birds. To do so rigorously, we combine data on the presence or absence of breeding Wood Thrushes across their range with data on wet atmospheric deposition of a number of ions, soil pH, regional abundance of thrushes, landscape-level forest fragmentation, and site characteristics. This combination of data sources allows us to present strong evidence implicating acid rain in the decline of the Wood Thrush and other northeastern bird species.
Effects of Acidic Deposition.
Atmospheric deposition of acid ions has been shown to negatively affect the biota of European forests (3, 5, 6) in a number of ways. These negative effects range from the loss of needles or leaves resulting in a more open canopy (5, 7), to the complete die-off of forest tree species (5, 6). Thinning of the canopy may result in lowered abundance of preferred prey for insectivorous birds (7, 8) or in an increase in scanning for predators that decreases time spent actively feeding (7). Further, the tree die-off may render an area unsuitable for forest birds owing to the loss of favored nesting, roosting, and foraging sites (6). Airborne pollutants such as acid rain may also alter the soil fauna, leading to the depauperation of soils (9) and to attendant changes in the availability of food for birds, like the Wood Thrush, that forage on invertebrates in the soil litter layer.
Calcium Depletion.
Many of the changes seen in acidified regions have also been linked to depletion of calcium in the soil (3). This is potentially a serious problem, because a female bird laying a clutch of eggs experiences calcium demand 10–15 times greater than that of a similar-sized mammal with developing embryos (10). Songbirds cannot meet this need from skeletal sources (11) and hence must obtain calcium in their food during the egg-laying period. Failure to sequester sufficient calcium during laying can result in eggs with thin, brittle eggshells that break before hatching, porous eggshells that cause the desiccation of the embryo, and complete reproductive failure (3, 12). Taken together, all of these effects of acid precipitation have resulted in tremendous loss of avian biodiversity in affected areas of Europe (6).
Despite clean-air legislation, many eastern regions of North America continue to experience heavy wet acidic deposition (13), and many bird species breeding in these areas show unexplained population declines (4, 14). Further, long-term acid deposition has depleted the available calcium in acid-sensitive soils, and current emission standards may be insufficient to ensure the recovery of these soils (13). As depleted soil pools of calcium are implicated in European bird declines, it is crucial for effective conservation planning to determine the role of acid rain in the decline of North American bird species (12). However, as yet there have been no North American studies that present evidence linking terrestrial bird population declines to acid rain. To explore the potential linkage between acid rain and declines, we chose the Wood Thrush as our focal species.
Study Species.
The Wood Thrush is a widespread, forest-dwelling, Neotropical migrant that breeds most abundantly along the slopes of the Appalachian Mountains from northern New England to the Smoky Mountains in the South, and winters from southeastern Mexico to Panama (15). It nests in forest patches ≤1 ha and in fragmented landscapes (15), and our earlier work on this species demonstrated no significant negative effect of habitat fragmentation (16). Predation on the eggs or young may be a major cause of nest failure in fragmented landscapes (15). However, both nest predation and brood parasitism by the Brown-headed Cowbird (Molothrus ater) may have little effect on the demography of these thrushes (15) in the East, where parasitism rates are often low (17), because this species often nests successfully twice per breeding season (15). Nevertheless, Wood Thrush populations show a significant, long-term, negative trend range-wide (−1.7% ± 0.5% yr−1 from 1966 to 1998), with large variation in long-term trends between regions (4).
Methods
Data Sources and Preparation.
We combine data from a number of sources to test whether the probability of breeding by the Wood Thrush decreases with increases in the deposition of acid ions and whether this effect varies with elevation, soil pH, or habitat fragmentation. The Cornell Laboratory of Ornithology's Birds in Forested Landscapes (BFL) project provided the response variable for our regression model, attempted breeding, at 650 study sites across the Wood Thrush's range. Data from the National Atmospheric Deposition Project (NADP) (18) allow us to use estimates of long-term acidic wet deposition as the predictor variable in our model. Other BFL data provided fine-scale descriptions of the vegetation and topography at each study site, as well as landscape measures of habitat fragmentation, which allow us to statistically control for the effects of these covariates (19). In addition, we use estimates of the soil pH across the area of interest from the Natural Resources Conservation Service (20) to control for the effect of soil chemistry, as well as data from the Breeding Bird Survey (4) to correct for variation in thrush abundance based on location within its geographic range alone. Further details for each dataset are provided below, but in general, data were transformed as necessary to meet the assumptions of individual analysis methods, and Principal Components Analysis (PCA) was used to reduce the dimensionality of multivariate datasets (21). The resulting Principal Components (Factors) were standardized to a mean of zero and a standard deviation of one before being used as predictors or covariates in the regression analysis.
BFL Project.
BFL is a continent-wide study of the effects of habitat fragmentation on forest-dwelling birds in North America. At each randomly chosen 7-ha study site, volunteers follow a simple, but rigorous, protocol that uses playback of Wood Thrush territorial calls to elicit a response from any resident thrush attempting to breed. Volunteers visit each site twice during the breeding season and gather data on the type and structure of the vegetation, the hydrology, and the topology at the site. They also use topographic maps to calculate a number of measures of habitat fragmentation and return all data to the Laboratory of Ornithology for collation and analysis (see ref. 22 for details of data collection and analysis). PCA was used to derive easily interpretable factors (22) that describe characteristics of the site and the surrounding landscape, which were used as covariates in the regression analysis.
Breeding Bird Survey Data.
Independent measures of Wood Thrush abundance were drawn from the Breeding Bird Survey, an annual survey of bird populations that takes place at the height of the breeding season and is conducted by volunteers under the aegis of the United States Geological Survey and the Canadian Wildlife Service (4). The survey is made up of over 3,700 routes of 39.4 km, each of which contains 50 3-min stops. The total number of birds seen and heard on each route is recorded for each species. For the purposes of this analysis, we chose all routes on which a mean of ≥1 Wood Thrush route−1 year−1 was detected; all routes also met Breeding Bird Survey data-quality criteria (4). We calculated a mean number of thrushes detected for each route over the years 1995–1999 and used these data to fit a polynomial trend surface (23) to provide an estimate of the probability of detecting a thrush at each study site, based on geographic location alone. This estimated probability was used as a covariate in the regression model.
Acid Deposition and Soil pH.
Estimates of the predictor, acid deposition, and the covariate soil pH were obtained from two different sources. We estimated atmospheric deposition using data from NADP's National Trend Network (18), which collects weekly samples of wet deposition at over 200 sites across the United States. These samples are returned to a central laboratory and analyzed for the presence of inorganic ions. Because calcium availability may depend at least partially on past, as well as present, acid ion deposition (13, 24), we chose only data from sites that were in continuous operation between 1984 and 1999 and calculated the mean deposition of H+, Ca2+, NO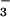 , SO
, SO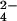 , NH
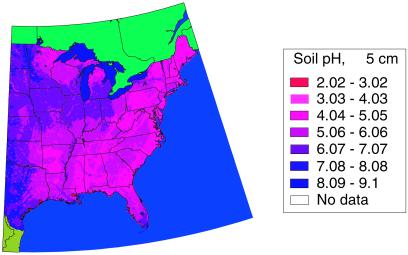
, NH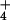 , and precipitation for each site over those years. We then used PCA to derive two uncorrelated factors from these means that were used as predictors in the regression analysis (see Table 1). To provide estimates of these composite variables at unsampled locations, we interpolated these data using inverse distance weighting (ref. 23; Figs. 1 and 2). We also obtained data on the pH of the top 5 cm of soil across the range of the Wood Thrush, from STATSGO, a nationwide database of soil properties in the United States that is compiled by the Natural Resources Conservation Service (20). We used these data to provide estimates of the acid-neutralizing capacity at each BFL study site (see Fig. 3).
, and precipitation for each site over those years. We then used PCA to derive two uncorrelated factors from these means that were used as predictors in the regression analysis (see Table 1). To provide estimates of these composite variables at unsampled locations, we interpolated these data using inverse distance weighting (ref. 23; Figs. 1 and 2). We also obtained data on the pH of the top 5 cm of soil across the range of the Wood Thrush, from STATSGO, a nationwide database of soil properties in the United States that is compiled by the Natural Resources Conservation Service (20). We used these data to provide estimates of the acid-neutralizing capacity at each BFL study site (see Fig. 3).
Table 1.
Orthogonal factors derived from principal components analysis (with varimax rotation) of NADP deposition data, showing the coefficients for each ion
| Variable
|
Standardized principal components | |
|---|---|---|
| PC1 | PC2 | |
| Ca2+ (kg/ha) | −0.251 | 0.647 |
| H+ lab (kg/ha) | 0.359 | |
NH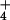 (kg/ha) (kg/ha) |
0.434 | |
NO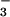 (kg/ha) (kg/ha) |
0.221 | |
SO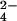 (kg/ha) (kg/ha) |
0.277 | |
| Precipitation | 0.310 | −0.209 |
| Eigenvalue | 3.952 | 1.18 |
| Cumulative variance explained | 0.659 | 0.855 |
Only coefficients ≥0.20 shown to simplify interpretation of the table.
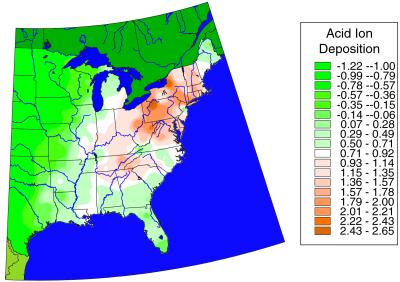
Fig 1.
Estimated wet atmospheric deposition of acid ions measured as the (standardized) composite variable PC1 derived from PCA (see Table 1) on mean deposition of focal ions for the years 1984–1999. Map derived from interpolated point values of PCA scores for each NADP National Trend Network site.
Fig 2.
Estimated wet atmospheric deposition of base ions measured as the (standardized) composite variable PC2 derived from PCA (see Table 1) on mean deposition of focal ions for the years 1984–1999. Map derived from interpolated point values of PCA scores for each NADP National Trend Network site.
Fig 3.
Estimated pH of the topmost 5 cm of soil, based on the Natural Resources Conservation Service statsgo database. Note regions of highest deposition and low pH soils along the spine of the Appalachians, which coincides with the regions of highest abundance for the Wood Thrush.
Analyses.
Data from each source were imported into an ARCVIEW (25) geographic information system and reprojected as necessary. All point data were interpolated (23) as described above to provide estimates of each variable at unsampled locations (interpolation was based on all data east of the −100th meridian of longitude). All data were then spatially joined (23) based on their geographical coordinates. Subsequent analysis was performed using SAS PROC LOGISTIC (26) to fit a multiple logistic regression model to these data.
The response variable for the regression was the presence or absence of attempted breeding by the Wood Thrush, and the predictor variable was the estimated deposition of acid ions or base cations at each study site. Covariates included landscape-level measures of forest fragmentation or site-level vegetation type and structure, as well as estimates of soil pH and of the regional abundance of the Wood Thrush at each site. We fit the full model containing all of the predictors, covariates, and two-way interactions and then used manual backward elimination of uninformative predictors, based on Wilks χ2 and examination of the difference in the −2 log likelihoods of the two nested models, one containing the variable in question and one without it (19). Predictors were retained in the model if the P value for the Wilks χ2 was <0.10. We also examined the Akaike Information Criterion and the Hosmer and Lemeshow goodness-of-fit at each step (19, 27). The final best-fit model yielded a predicted probability of detecting a breeding attempt at each study site, based on the estimated acid deposition, while controlling for the covariates (19, 27). To test for the overall effect of acid deposition, the −2 log likelihood of final model was compared with that of the nested model with all of the terms containing the acid deposition variable removed (19).
Results
PCAs.
PCA on the site vegetation and landscape-level fragmentation variables derived from the BFL dataset yielded six principal components with eigenvalues of approximately one or greater, which together explained 71% of the variability in the dataset (see Table 2). The first principal component, FC1, may be interpreted as a measure of landscape-level habitat fragmentation, which increases with increasing amounts of forest/non-forest edge and with increasing isolation of the patch containing the study point, and decreases with increasing proportion of forested land in the surrounding 1,000 ha. FC2 is a measure of coniferous dominance of the canopy at the study site and increases with increasing proportion of the canopy composed of coniferous trees. FC3 may be considered a measure of the proportion of the study site within core habitat and increases with increasing patch size and distance from the edge of the patch. FC4 increases with increases in the proportion of low vegetation that is composed of deciduous shrubs and decreases with increasing amounts of low vegetation cover. FC5 increases with increases in the proportion of saplings and with increases in the proportion of low vegetation at the site. Finally, FC6 may be interpreted as a measure of elevation-related changes to the study site that decreases with increasing elevation and increases with increasing canopy height and increasing proportion of coniferous shrubs.
Table 2.
Orthogonal factors derived from principal components analysis (with varimax rotation) of BFL data, showing the coefficients for each habitat variable
| Variable
|
Standardized rotated principal components | |||||
|---|---|---|---|---|---|---|
| FC1 | FC2 | FC3 | FC4 | FC5 | FC6 | |
| Ln (patch size) | 0.57 | |||||
| Asqrt (% forest) | −0.42 | |||||
| Ln (edge density) | 0.40 | |||||
| Ln (isolation) | 0.34 | |||||
| Ln (distance from edge) | 0.67 | |||||
| Ln (elevation) | −0.49 | |||||
| Ln (canopy height) | 0.69 | |||||
| Asqrt (% con. canopy) | 0.41 | |||||
| Asqrt (% dec. canopy) | −0.44 | |||||
| Asqrt (% con. shrubs) | 0.38 | |||||
| Asqrt (% dec. shrubs) | 0.76 | |||||
| Asqrt (% saplings) | −0.25 | 0.65 | ||||
| Asqrt (% total low veg) | 0.32 | 0.54 | ||||
| Eigenvalue | 2.99 | 1.65 | 1.30 | 1.16 | 1.06 | 0.97 |
| Cumulative variance explained | 0.23 | 0.35 | 0.46 | 0.55 | 0.62 | 0.71 |
Only coefficients ≥0.25 shown to simplify interpretation of the table. Asqrt, arcsin − square root; % Con. canopy, proportion of coniferous trees in the canopy; % Dec. canopy, proportion of deciduous trees in the canopy; % Con. shrubs, proportion of coniferous shrubs in understory; % Dec. shrubs, proportion of deciduous shrubs in understory; % total low veg, total proportion of ground covered by low vegetation.
PCA on the 16-year mean ion deposition data from the NADP sites yielded two principal components with eigen values equal to one or greater (see Table 1), which together explained 85% of the variability in the deposition dataset. The first principal component, PC1, increases with increasing precipitation and increasing deposition of H+, NO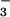 , and SO
, and SO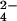 and decreases with deposition of Ca2+ and NH
and decreases with deposition of Ca2+ and NH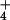 and hence provides a measure that reflects the deposition of acidifying ions. PC2, on the other hand, increases with increases in deposition of Ca2+ and NH
and hence provides a measure that reflects the deposition of acidifying ions. PC2, on the other hand, increases with increases in deposition of Ca2+ and NH and decreases with increasing precipitation.
and decreases with increasing precipitation.
Interpolation of Deposition Data.
Estimates of atmospheric deposition expressed as the principal component scores for PC1 and PC2 were interpolated from approximately 200 points and plotted as maps (see Figs. 1 and 2). Fig. 1 shows the geographic distribution of estimated PC1 values. It reveals little deposition in the West, with increases eastward through the Midwest, and highest acid ion deposition along the spine of the Appalachians, from northern New England southward to the Smoky Mountains. Fig. 2 shows the geographic distribution of the estimated PC2 values. In contrast to PC1, deposition of calcium and ammonium ions, as measured by PC2 values, is predominantly a Midwestern phenomenon, with the heaviest deposition occurring to the west of the Mississippi River. The zone of heavy deposition stretches southeastward from the southern shore of Lake Michigan, through Iowa, and then southward through Nebraska, Kansas, Oklahoma, and Texas. The highest PC2 deposition levels occur in Iowa, and little deposition occurs in the eastern states where acid deposition is highest. Thus, atmospheric calcium deposition, which could partially offset acid ion deposition, is generally lowest where soil calcium depletion is potentially the highest.
Logistic Regression Analysis.
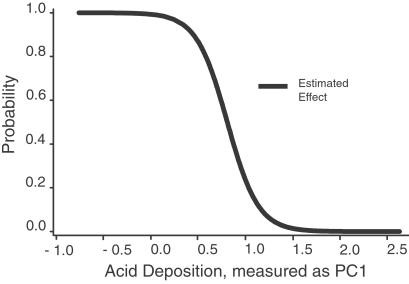
The final regression model (see Table 3) was based on data from 653 sites and contained PC1, the composite measure of acid ion deposition, as the predictor variable, nine covariates, and nine interaction terms, three of which included PC1. The regression was highly significant overall (−2 log L χ2 = 118.43, df = 19, P = 0.0001) and an adequate fit to the data (Hosmer and Lemeshow goodness-of-fit = 5.61, df = 8, P = 0.69; concordance = 74.1%). The model indicates an extremely strong, highly significant, negative effect of acidic deposition on the predicted probability of breeding by the Wood Thrush (estimated odds ratio 0.02, 95% confidence interval for odds ratio: <0.001, 0.46; −2 log L χ2 = 41.44, df = 5, P < 0.00001; see Fig. 4). The model also predicts a reduced probability of breeding at higher elevation and under lower canopies, as well as increases in probability with increases in forest patch size and distance from forest/non-forest edge. Three interactions with acid deposition PC1 × FC1 (deposition × fragmentation), PC1 × FC5 (deposition × sapling density), and PC1 × FC6 (deposition × elevation) further reduce the probability of breeding. Thus, after controlling for covariates such as geography, soil pH, and vegetation, the model predicts that the probability of Wood Thrushes attempting to breed at a study site decreases with increasing deposition, particularly at higher elevations. Further, the negative effects of acid deposition are strengthened in the presence of fragmentation, thus providing a mechanism by which fragmentation may negatively impact a species not otherwise known to be highly fragmentation sensitive.
Table 3.
Results of logistic regression of Wood Thrush occurrence on environmental variables
| Variable | Est. param. | Wald χ2 | Pr. >χ2 | Std. est. | Odds ratio |
|---|---|---|---|---|---|
| Intercept | 2.82 | 0.63 | 0.429 | ||
| FC1 | 0.38 | 1.80 | 0.180 | 0.21 | 1.46 |
| FC2 | −0.47 | 10.03 | 0.002 | −0.17 | 0.63 |
| FC3 | 0.26 | 6.54 | 0.011 | 0.14 | 1.30 |
| FC4 | 2.47 | 6.53 | 0.011 | 1.26 | 56.18 |
| FC5 | 0.94 | 15.37 | 0.000 | 0.52 | 2.55 |
| FC6 | 4.03 | 6.73 | 0.010 | 1.90 | 2.56 |
| PC1 | −6.12 | 5.01 | 0.025 | −2.31 | 0.002 |
| PC2 | −0.11 | 0.15 | 0.696 | −0.05 | 0.90 |
| pH | −0.82 | 1.59 | 0.206 | −0.28 | 0.44 |
| Trend | 0.02 | 0.10 | 0.749 | 0.05 | 1.02 |
| FC4 × pH | −0.42 | 5.76 | 0.016 | −1.19 | 0.66 |
| FC6 × pH | −0.67 | 6.46 | 0.011 | −1.73 | 0.514 |
| PC1 × pH | 1.27 | 6.61 | 0.010 | 2.65 | 3.560 |
| FC1 × PC1 | −0.73 | 4.93 | 0.026 | −0.47 | 0.483 |
| FC5 × PC1 | −0.76 | 15.54 | 0.000 | −0.49 | 0.468 |
| FC6 × PC1 | 0.72 | 5.13 | 0.024 | 0.37 | 2.064 |
| Trend × FC1 | −0.15 | 6.84 | 0.009 | −0.39 | 0.863 |
| Trend × FC6 | 0.22 | 9.83 | 0.002 | 0.54 | 1.242 |
| Trend × PC2 | 0.12 | 4.90 | 0.027 | 0.24 | 1.128 |
Est. param., estimated parameter; Std. est., standardized estimate; pH, pH of the top 5 cm of soil; Trend, natural log of estimated abundance of Wood Thrush at any site; AIC intercept only, 841.792; AIC intercept and covariates, 761.130. FC1 reflects landscape-level forest fragmentation; FC2, coniferous dominance; FC3, patch size and distance-from-edge; FC4, deciduous shrubs; FC5, sapling layer; FC6, decreases with increasing altitude and decreasing canopy height (22). PC1 represents acid ion deposition; PC2, calcium and ammonium deposition (see Tables 1 and 2).
Fig 4.
Estimated effect of atmospheric acid ion deposition on the probability of breeding for the Wood Thrush, controlling for covariates. Graph derived by solving the regression equation (derived from the best-fit model; see Table 3) for each data point, setting all variables except PC1 at their mean values. Model predicts a sharp decline in the probability of breeding over a relatively short range of acid deposition values.
Discussion
Our results suggest an important role for the atmospheric wet deposition of acid ions in recent declines of some birds breeding in the eastern United States, particularly in high elevation zones with low pH soils. Calcium depletion in such areas could affect birds in a number of ways. For example, decreases in the soil's pool of available calcium may profoundly alter both the quality and the quantity of available prey (3, 5, 28, 29). Acid inputs combined with this low calcium availability may also lead to toxicity because of increased uptake of aluminum, cadmium, and lead from the food supply (28, 30). In addition, the normal diet of insectivorous and granivorous birds contains insufficient calcium for reproduction (31), and these birds are dependent on calcium-rich supplementary food items such as snail shells (10, 31, 32), isopods and millipedes (31), or earthworms (31, 33), which may be scarce in areas with heavy acidic deposition (9, 31, 32, 34, 35). Further, growing nestlings also have an extremely high calcium demand and must be provided with calcium-rich supplements (10, 35). Procuring these food items may represent a considerable burden on the parents in acidified regions (12, 35).
The reduction in availability of prey with high calcium content represents not only a loss of a calcium source needed for reproduction but also a substantial decrease in the biomass of available prey organisms in the litter (9). For example, the exact contribution of snails and slugs (both of which decrease in abundance in the face of acidification) to the pool of prey available to the Wood Thrush is not known, but they may represent up to 6% of the animal biomass of boreal forests (36). Likewise, in healthy sugar maple (Acer saccharum) stands earthworms are up to eight times as abundant and represent up to 34 times the biomass of earthworms in declining maple stands with calcium-poor, acidic soils (34). Nevertheless, the decreases in the abundance of calcium-rich prey represents only one aspect of profound alteration of the fauna of the soil and leaf litter layer seen with the acidification owing to atmospheric deposition (9). Decreased rates of decomposition in the litter layer and a concomitant decrease in soil fauna diversity and abundance are both seen in polluted soils (9). Thus, substantial reduction of the prey base owing to acidic deposition may provide another mechanism to make affected habitat much less suitable for breeding thrushes.
Although the proximate cause of decreases in the probability of breeding is as yet unclear, it seems unlikely to be eggshell abnormalities, as the studies that address this issue in North American terrestrial bird species have not shown eggshell defects severe enough to cause their failure (3, 37–41). Decreased probability of breeding may also be the result of demographic changes less drastic than complete reproductive failure, although most studies (37, 40–42) have not demonstrated such changes (but see also ref. 38). However, even more subtle effects caused by decreased winter survival of fledglings (42, 43) or lower return rates of adults in acidified regions (42, 43) would be sufficient to generate the patterns seen in the Wood Thrush (17, 42, 43). Such effects could be caused either by nestlings that are not provided sufficient calcium-rich food items to thrive or by adults that provide sufficient food and calcium to their young but do so only with extraordinary effort and at the expense of their own physical condition (43). In fact, St. Louis et al. (38) showed that Tree Swallows (Tachycineta bicolor) nesting near experimentally acidified lakes had young that grew more slowly and fledged fewer and smaller young per clutch. Further, they also showed that the breeding adults had to travel up to 650 m from the nest site to obtain calcium-rich supplemental food items for their nestlings (35). However, decreased probability of breeding may also simply be the cumulative result of decisions by individual thrushes not to attempt breeding in areas that, although otherwise suitable, show decreased availability of prey, particularly of calcium-rich prey items such as slugs, snails, and earthworms.
In any case, these results speak for a reevaluation of avian population analyses and for more intensive studies to elucidate the processes leading to observed patterns of decline. Until now, most research on North American bird population declines, including the Cornell Laboratory of Ornithology's BFL project (16), was focused on studying the effects of habitat fragmentation on the breeding grounds and on habitat loss in the wintering grounds (44). These remain fruitful areas for study, but other large-scale influences must also be considered when seeking the mechanisms that lead to population declines in birds. We note that the analyses in this study are only made possible by the combination of four large datasets, each of which was collected for other purposes. It is clear that large-scale, volunteer-based projects such as the Breeding Bird Survey and the BFL project, along with data from long-term monitoring projects such as the NADP, are critical for answering questions about the effects of widespread human-caused environmental change.
Acknowledgments
Special thanks to Diane E. Black for asking the questions, providing the impetus for this research, and starting the ball rolling. We gratefully acknowledge the fieldwork of hundreds of dedicated volunteer participants in the Cornell Laboratory of Ornithology's BFL project and in the Breeding Bird Survey. We also thank Therese M. Donovan, Patrick J. Sullivan, and Wesley M. Hochachka for helpful comments on early drafts of this manuscript. Funding for this research was provided by the National Fish and Wildlife Foundation, the U.S. Department of Agriculture Forest Service, the Archie and Grace Berry Charitable Foundation, the Florence and John Schumann Foundation, the Packard Foundation, and an Institute for Ecosystem Studies-Cornell University Human Accelerated Environmental Change grant.
Abbreviations
BFL, Birds in Forested Landscapes
NADP, National Atmospheric Deposition Project
PCA, principal components analysis
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Robinson S. K., Thompson, F. R., Donovan, T. M., Whitehead, D. R. & Faaborg, J. (1995) Science 267, 1987-1990. [DOI] [PubMed] [Google Scholar]
- 2.Donald P. F., Green, R. E. & Heath, M. F. (2001) Proc. R. Soc. London Ser. B 268, 25-29. [Google Scholar]
- 3.Graveland J. (1998) Environ. Rev. 6, 41-54. [Google Scholar]
- 4.Sauer J. R., Hines, J. E. & Fallon, J., (2001) The North American Breeding Bird Survey Results and Analysis 1966–2000 (Patuxent Wildlife Research Center, Laurel, MD).
- 5.Graveland J. (1990) Experientia 46, 962-970. [Google Scholar]
- 6.Flousek J., Hudek, K. & Glotz von Blotzheim, U. N. (1996) in Handbuch der Vogels Mitteleuropas, ed. von Blotzheim, U. N. G. (AULA, Wiesbaden, Germany), pp. 11–30.
- 7.Brotons L., Magrans, M., Ferrus, L. & Nadal, J. (1998) Can. J. Zool. 76, 556-565. [Google Scholar]
- 8.Zang H. (1998) J. Ornithol. 139, 263-268. [Google Scholar]
- 9.Rusek J. & Marshall, V. G. (2000) Annu. Rev. Ecol. Syst. 31, 395-423. [Google Scholar]
- 10.Graveland J. (1996) Can. J. Zool. 74, 1035-1044. [Google Scholar]
- 11.Pahl R., Winkler, D. W., Graveland, J. & Batterman, B. W. (1997) Proc. R. Soc. London Ser. B 264, 239-244. [Google Scholar]
- 12.Graveland J. & Drent, R. H. (1997) J. Anim. Ecol. 66, 279-288. [Google Scholar]
- 13.Driscoll C. T., Lawrence, G. B., Bulger, A. J., Butler, T. J., Cronan, C. S., Eagar, C., Lambert, K. F., Likens, G. E., Stoddard, J. L. & Weathers, K. C. (2001) Bioscience 51, 180-198. [Google Scholar]
- 14.James F. C., McCullogh, C. E. & Wiedenfeld, D. A. (1996) Ecology 77, 13-27. [Google Scholar]
- 15.Roth R. R., Johnson, M. S. & Underwood, T. J. (1996) in The Birds of North America, No. 246, eds. Poole, A. & Gill, F. (Academy of Natural Sciences, Philadelphia).
- 16.Hames R. S., Rosenberg, K. V., Lowe, J. D., Barker, S. E. & Dhondt, A. A. (2002) in The Effects of Habitat Fragmentation in Western North America, eds. George, L. & Dobkins, D. S. (Cooper Ornithological Society, Seattle), in press.
- 17.Weinberg H. J. & Roth, R. R. (1998) Auk 115, 879-889. [Google Scholar]
- 18.Lamb D. & Bowersox, V. (2000) Atmos. Environ. 34, 1661-1663. [Google Scholar]
- 19.Agresti A., (1996) An Introduction to Categorical Data Analysis (Wiley, New York).
- 20.USDA/NRCS, (1994) State Soil Geographic (STATSGO) Data Base: Data Use Information (USDA Natural Resources Conservation Service, National Soil Survey Center, Washington, DC).
- 21.Johnson R. A. & Wichern, D. W., (1982) Applied Multivariate Statistical Analysis (Prentice–Hall, Englewood Cliffs, NJ).
- 22.Hames R. S., (2001) Ph.D. dissertation (Cornell Univ., Ithaca, NY).
- 23.Burrough P. A. & McDonnell, R. A., (1998) Principles of Geographical Information Systems (Oxford Univ. Press, Oxford).
- 24.Likens G. E., Driscoll, C. T., Buso, D. C., Siccama, T. G., Johnson, C. E., Lovett, G. M., Fahey, T. J., Reiners, W. A., Ryan, D. F., Martin, C. W. & Bailey, S. W. (1998) Biogeochemistry 41, 89-173. [Google Scholar]
- 25.ESRI, (1996) arcview (Environmental Systems Research International, Redlands, CA), Version 3.2.
- 26.SAS Institute, (1996) SAS/STAT Software: Changes and Enhancements Through Release 6.11 (SAS Institute, Cary, NC).
- 27.Hosmer D. W. & Lemeshow, S., (1989) Applied Logistic Regression (Wiley, New York).
- 28.Swiergosz R., Sawicka-Kapusta, K., Nyholm, N. E. I., Zwolinska, A. & Orkisz, A. (1998) Environ. Poll. 102, 213-220. [Google Scholar]
- 29.Eeva T., Lehikoinen, E. & Ronka, M. (1998) Funct. Ecol. 12, 607-612. [Google Scholar]
- 30.Scheuhammer A. M. (1991) Environ. Poll. 71, 329-375. [DOI] [PubMed] [Google Scholar]
- 31.Graveland J. & Vangijzen, T. (1994) Ardea 82, 299-314. [Google Scholar]
- 32.Graveland J. & vanderWal, R. (1996) Oecologia 105, 351-360. [DOI] [PubMed] [Google Scholar]
- 33.Dekhuijzen H. M. & Schuijl, G. P. J. (1996) Limosa 69, 165-174. [Google Scholar]
- 34.Coderre D., Mauffette, Y., Gagnon, D., Tousignant, S. & Bessette, G. (1995) Pedobiologia 39, 86-96. [Google Scholar]
- 35.St. Louis V. L. & Breebaart, L. (1991) Condor 93, 286-294. [Google Scholar]
- 36.Hawkins J. W., Lankester, M. W., Lautenschlager, R. A. & Bell, F. W. (1997) Can. J. Zool. 75, 501-505. [Google Scholar]
- 37.Glooschenko V., Blancher, P., Herskowitz, J., Fulthorpe, R. & Rang, S. (1986) Water Air Soil Poll. 30, 553-567. [Google Scholar]
- 38.St. Louis V. L. & Barlow, J. C. (1993) Can. J. Zool. 71, 1090-1097. [Google Scholar]
- 39.Taliaferro E. H., Holmes, R. T. & Blum, J. D. (2001) Wildl. Soc. Bull. 113, 94-100. [Google Scholar]
- 40.Mahony N., Nol, E. & Hutchinson, T. (1997) Can. J. Zool. 75, 509-517. [Google Scholar]
- 41.Blancher P. J. & McNicol, D. K. (1988) Can. J. Zool. 66, 842-849. [Google Scholar]
- 42.Darveau M., Gauthier, G., Desgranges, J. L. & Mauffette, Y. (1993) Can. J. Zool. 71, 1592-1601. [Google Scholar]
- 43.Möckel R. (1992) Ökol. Vögel 14, 1-100. [Google Scholar]
- 44.Holmes R. T., Sherry, T. W. & Sturges, F. W. (1986) Ecol. Monogr. 56, 201-220. [Google Scholar]