Abstract
Unprecedented population declines and extinctions because of human activities, combined with a growing recognition that such losses affect the stability of ecosystems, underscore the need to better understand how populations persist naturally. We provide field experimental evidence that high biodiversity—in particular, the combined effects of predators and competitors—acts in a way that regulates the size of local fish populations within their coral-reef community. These results indicate that complex interactions among multiple species are necessary for the stability of a highly diverse community, and so forewarn that overexploiting such species may have cascading negative consequences for the entire system.
Keywords: community stability, competition, population dynamics, ; predation, recruitment
Precipitous population declines and species extinctions attributable to human activities (1, 2) are known to affect the stability of ecosystem structure and function (3–6). Knowing how the diversity of species interactions in local communities influences the persistence of populations is fundamental to our understanding of community stability. Given that populations cannot grow without bounds, they are able to persist in the face of environmental variability because per capita demographic rates (e.g., births, deaths) respond to trends in abundance with sufficient density dependence that fluctuating population sizes are unlikely to reach zero (7–11). Density-perturbation experiments provide the strongest evidence for the presence and mechanisms of density dependence (8), but there have been few conclusive field experiments designed to assess the consequences of multi-species interactions in regulating populations (12).
Populations of marine fishes are often characterized by dramatic fluctuations in abundance. Debate concerning the dynamics of reef fish populations has centered on the relative contributions of density-independent factors acting during larval dispersal and density-dependent processes following the larval stage (i.e., competition and possibly predation) (11, 14). Although a growing number of field studies have documented density-dependent mortality (14), very few studies have identified the actual mechanisms and conditions responsible for these patterns (15, 16), especially the role of multispecies interactions in systems of high biodiversity, such as coral reefs (17). Such mechanisms are difficult to detect in nature because they can act on any life stage of a species, on the condition of individuals, within or between populations, and at various spatial or temporal scales (18). An ideal approach to determine the causes of density-dependent mortality is to compare per capita rates of mortality of populations subjected to cross-factored manipulations of both density and the putative sources of mortality (13, 14, 17). Alternate and combined sources of density-dependent mortality can then be determined by testing for a significant relationship between the per capita mortality rate and population density in the presence of different combinations of potential causes.
We tested for the existence and source of density-dependent mortality in a coral-reef fish via orthogonal manipulations of the presence of predators (top-down effects) and territorial competitors (bottom-up effects). The bicolor damselfish (Stegastes partitus) lives in loosely territorial social groups on shallow coral reef systems throughout the tropical western Atlantic and Caribbean Sea. Aggression toward conspecifics is limited to situations when they constitute a threat to space occupancy, including food supplies, and in the case of males guarding nests against potential egg predators. Like almost all reef fishes, their larvae disperse in the plankton. Late-stage larvae settle to reef habitats at lengths of about 15 mm, after which they become site attached and aggressive toward conspecifics and other reef fishes (19). Upon settlement, these small juveniles (hereafter, “recruits”) are exposed to a suite of reef-dwelling predators (e.g., small groupers) as well as more transient predators (especially schooling jacks) that swim between and forage among reefs (17, 20, 21). Taking advantage of the strong site fidelity of S. partitus, other damselfishes, and resident predators, we manipulated independent local fish assemblages on a matrix of transplanted coral patch reefs isolated from one another by a featureless sand bottom in the central Bahamas (17, 22). To determine whether the effects of predators and competitors observed on isolated patch reefs were applicable to more continuous reef habitats, we repeated a subset of these manipulations on similar-sized plots of continuous fore-reef.
Methodology
Experimental Patch Reefs.
We manipulated the density of recently settled S. partitus as well as the presence and absence of their predators and competitors on 20 experimental reefs, which were part of a matrix of 36 patch reefs of living coral, each 9 m2 in area. These reefs had been transplanted to a sand plain on the Great Bahama Bank in the lee of Norman's Pond Cay in 1991–1993 so that neighboring reefs were separated by 200 m (17, 22). Reef isolation allowed us to maintain independent manipulations of resident predators, competitors (older territorial damselfish), and the density of recruits of S. partitus (see below). Orthogonal manipulations of the presence or absence of predators and competitors were achieved in 1998 by adding or removing (and excluding, see below) predators and competitors at the isolated experimental patch reefs at natural mean densities. Predator and competitor treatments were allocated among the 20 experimental patch reefs in a randomized block design.
Experimental Plots on Continuous Reef.
To determine whether results from the experiments conducted on isolated patch reefs could be generalized to continuous coral-reef habitat, we manipulated the presence and absence of competitors, both in the presence of predators. Competitor manipulations on continuous reef were achieved in 1999 by removing all S. partitus and Stegastes leucostictus from half of 20 replicate 9-m2 plots of fore-reef habitat located along Great Exuma Sound on the windward side of Norman's Pond Cay. Treatments were allocated randomly and maintained by daily removals over the duration of the experiment, and very few immigrants were encountered.
Competitor and Predator Manipulations.
The manipulated competitors were the two most abundant territorial damselfish species on Bahamian reefs, both of which act aggressively toward recently settled S. partitus: S. leucostictus and older S. partitus (19). We manipulated the density of both species to reflect past (1995–1998) average densities observed on our experimental patch reefs and continuous reef habitat (two and three adults per reef or plot, respectively).
On experimental patch reefs randomly designated for predator removal, resident piscivores—squirrelfishes (Holocentrus ascensionis and Holocentrus rufus), groupers (Epinephelus striatus, Epinephelus guttatus, Epinephelus fulvus, Epinephelus cruentatus, and Serranus tigrinus), moray eels (Gymnothorax moringa and Gymnothorax vicinus), and soapfish (Rypticus bistrispinus)—were removed with hand nets. Transient predators—jacks (Caranx ruber and Caranx bartholomaei), snappers (Lutjanus synagris, Lutjanus apodus, Lutjanus mahogoni, and Ocyurus chrysurus), and lizardfish (Synodus intermedius)—were excluded by enclosing reefs with large circular cages of 15-mm nylon mesh that were 7 m in diameter and extended from the sand bottom to the sea surface (4-m depth; ref. 17). We did not include cage controls to ascertain the potential confounding effects of cages on settlement and postsettlement survival because, first, rates of natural settlement of S. partitus across all experimental reefs and plots were low, and second, previous assessments revealed no secondary effects of cages on postsettlement survival independent of predator exclusion (17).
Recruit Manipulations and per Capita Mortality.
Recently settled S. partitus (range 15–19 mm total length) were collected on the fore-reef with quinaldine fish anesthetic and hand nets, placed in plastic bags, transported to the experimental reefs and plots, and released immediately. Density levels of recruits (6, 9, 12, 15, and 18 fish per 9-m2 reef) spanned the range of recruitment observed previously on natural patch reefs.
Per capita mortality rates of experimental cohorts of new recruits were calculated from censuses over a 54-day period. After a 24-h acclimation period to ensure that mortality was not artificially induced by the translocation process, cohort survivorship on each reef was monitored at 2-day intervals the first week, 3-day intervals during the second week, 5-day intervals through the fifth week, and 7-day intervals through the eighth week (54 days total). Per capita rate of mortality was calculated as the proportional decline of the cohort on each reef or plot over 54 days.
One potential difficulty with this approach is distinguishing the experimental cohort from nonexperimental individuals that settle naturally over the course of the study. However, the experimental patch reefs were located in an area where natural rates of settlement of S. partitus were low, and the few recruits that settled during the experiment were readily distinguished by their smaller size. In addition, to distinguish experimental cohorts on patch reefs from any fish that settled naturally during the experiment, we measured natural settlement rates during the course of the experiment. Recruits of S. partitus were censused and removed at 2-day intervals on 16 additional patch reefs interspersed among the 20 manipulated reefs. All resident predators were excluded from these reefs with exclusion cages that did not interfere with natural settlement so that settlement was not underestimated by postsettlement mortality. Over the 44 days that these reefs were censused, settlement per reef was very low (mean of 1.8 new recruits ± 2.0 SE). Although natural recruitment was low, we removed when possible and excluded from our estimates of mortality those fish that clearly settled naturally during the experiment. Estimates of mean daily growth rates of recently settled S. partitus from the literature (0.36 mm × day−1; ref. 23) and from natural reefs near our study site (unpublished data) would result in a maximum size of ca. 35 mm for S. partitus that settled during the experiment [15 mm + (0.36 mm × day−1 × 54 days) = 34.5 mm total length]. Because of variable growth rates, we excluded from analysis only those individuals less than 25 mm total length at the end of the experiment, thereby ensuring inclusion of individuals from the experimental cohort. Even so, including natural settlers did not affect the conclusions of our statistical analyses.
Experimental cohorts established in plots on continuous reef were tagged externally to distinguish them from natural settlers, which were few (mean ± SEM per reef over entire duration of experiment was 2.0 ± 0.47). All natural settlers were removed during censuses throughout the course of the experiment. Experimental cohorts of S. partitus recruits on the continuous reef were marked with single externally visible s.c. injections of colored elastomer (Northwest Marine Technology, Shaw Island, WA). A tagging control experiment conducted simultaneously with the main experiment indicated no effect of tagging on the mortality rate of individuals (unpublished data). Surveys of reef habitat adjacent to the experimental plots indicated no emigration of experimental fish after initial densities were established. Therefore, we attributed any losses between censuses to mortality.
Results
Experimental Patch Reefs.
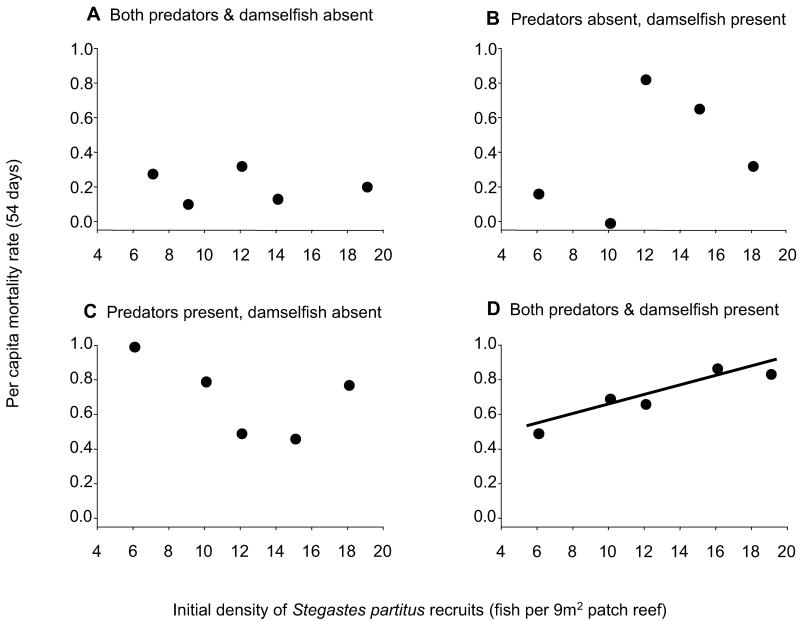
Experimental cohorts of S. partitus recruits on patch reefs without predators or competitors experienced low mortality rates that were independent of initial recruit density (Fig. 1A). Across density levels, per capita mortality averaged about 20% over the 54-day duration of the experiment. In the presence of competitors and absence of predators, mortality rates were higher, highly variable among density levels, and also independent of density (Fig. 1B). Similarly, cohorts on patch reefs with predators but no competitors experienced higher mortality rates, which were again not significantly related to density (Fig. 1C). The relationship in this particular case seemed to be negative (i.e., inversely density-dependent) but was far from statistically significant. Moreover, the high mortality (100%) suffered by the lowest density level (six fish) in this treatment is problematic because at low densities each fish represented a large proportion (0.17) of the total mortality (24, 25).
Fig 1.
Relationship between cohort per capita mortality and initial recruit density on patch reefs in the absence of both predators and competitors (older territorial damselfishes) (A), absence of predators and presence of competitors (B), presence of predators and absence of competitors (C), and presence of both predators and competitors (D). These relationships indicate low and density-independent mortality of recruits in the absence of both predators and competitors (A, r2 < 0.001, P = 0.78), higher and density-independent mortality in the presence of competitors only (B, r2 < 0.001, P = 0.42), still greater density-independent mortality in the presence of predators only (C, r2 = 0.22, P = 0.24), and strong density-dependent mortality only in the combined presence of both predators and competitors, which is the unmanipulated natural state of the system (D, r2 = 0.75, P = 0.036). Linear regression analyses are based on arcsin(square root) transformed per capita mortality rates.
Only in the presence of both competitors and predators—the natural state of the system—were mortality rates significantly related to the density of recruit cohorts (Fig. 1D). Therefore, over a natural range of densities of S. partitus recruits, the combined effects of their natural competitors and predators—not one or the other alone—induced density-dependent mortality. The impact of this mortality was a dramatic (about one order of magnitude) dampening of variation in numbers of recruits at the end of the experiment. Coefficients of variation for the four treatments (predator absent/competitor absent, predator absent/competitor present, predator present/competitor absent, and predator present/competitor present) were 1.6, 2.5, 2.5, and 0.2, respectively.
Continuous Reef Plots.
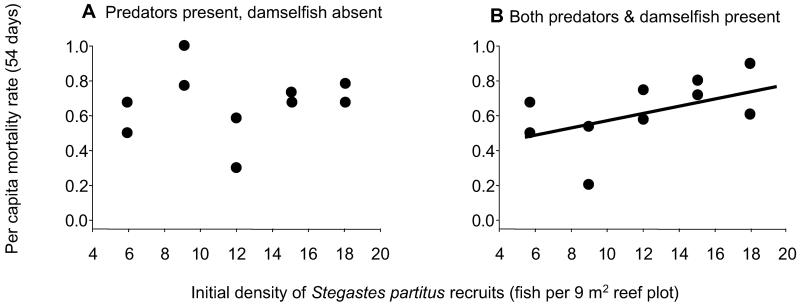
Experimental results on continuous reef were qualitatively similar to those on patch reefs (Fig. 2). In the presence of predators only, the per capita mortality rate was not related to initial recruit density and was more variable than in the combined presence of predators and competitors (Fig. 2A). In the presence of both predators and competitors, the relationship between mortality rate and initial recruit density was nearly identical to that observed under similar conditions on patch reefs, although not quite statistically significant (Figs. 2B and 1D, respectively). We attribute the higher variance to the more variable movement of predators on continuous vs. patch reefs.
Fig 2.
Relationship between cohort per capita mortality and initial recruit density on plots of continuous reef in the presence of predators and absence of competitors (older territorial damselfishes) (A) and presence of both predators and competitors (B). These relationships indicate qualitatively similar patterns as observed on patch reefs: density-independent mortality of recruits in the presence of predators and absence of competitors (A, r2 < 0.001, P = 0.95) and density-dependent mortality in the combined presence of both predators and competitors (B, r2 = 0.25, P = 0.08). Note the similarity between Figs. 1D and 2B. Linear regression analyses are based on arcsin(square root) transformed per capita mortality rates.
Discussion
These results contribute to a growing number of studies demonstrating experimentally that recently settled marine fish experience mortality at rates dependent on their density (14). Although density-dependent mortality occurred within 54 days after settlement, it was manifested more gradually than has been reported in other studies (14). For instance, strong density-dependent mortality occurred in three species of more social damselfish in French Polynesia within the first 1–2 weeks after settlement (25), as well as a schooling damselfish species at our site within a week of settlement (17). The relative delay in appearance of density dependence in the present study suggests that its timing and duration varies among species. That these differences are related to differences between species is underscored by the fact that marked differences in timing of density-dependent mortality between the schooling damselfish studied previously (within only a few days of settlement; ref. 17) and the more bottom-oriented damselfish in this study (over nearly 2 months) were observed on the very same system of experimental reefs. Evidence that settlement of S. partitus is independent of conspecific density (26) suggests that postsettlement processes play a particularly important role in regulating local populations. That the synergistic effect of predators and competitors was strikingly similar on both patch reefs and continuous reefs indicates that combined interactions contribute to population regulation irrespective of patchiness or isolation of reef habitat.
Why did density-dependent mortality occur only in the combined presence of both predators and competitors? First, because recruits and adults of several species of damselfishes compete for common shelter from predation (27), the presence of adults of other species increases per capita displacement of S. partitus recruits from shelter (28). Second, agonistic interactions between recruits and older damselfishes could increase susceptibility to predators through distraction and reduced vigilance. Third, increases in activity owing to increased aggressive interactions between older damselfish and recruits could draw greater attention of predators. All three mechanisms may act separately or in combination to increase mortality with higher densities of recruits. It is also possible that density dependence owing to within-species competition would be found in the absence of either predators or adult competitors if recruitment occurred at densities higher than the natural maximum used in these experiments.
That mortality rates were density-dependent only in the presence of both predators and competitors has important implications for understanding the role of community-wide biodiversity in maintaining ecologically viable populations and, by extension, community stability. In the absence of either suite of strong interactors, populations of S. partitus are likely to fluctuate more widely, reflecting more the spatial and temporal vagaries of larval supply from the plankton (23). This result suggests that relatively high diversity of a local community can contribute to ecosystem stability by maintaining the relative abundance of a species in that community. Although our manipulations of interference competitors included both interspecific (older S. leucostictus) and intraspecific (older S. partitus) interactions, prior studies indicate that the highly aggressive nature of S. leucostictus was probably most responsible for mortality of new recruits of S. partitus (19). Recent research suggests that increased species diversity within a trophic level or ecologically functional group may contribute to the persistence of community structure by compensating for the differential response of species to varying environmental conditions (4, 29, 30). In such cases, ecological redundancy does not act to dampen population fluctuations, but differences between species in their susceptibility to specific environmental perturbations act to compensate for one another's fluctuations. In contrast, our study suggests that diversity within and among trophic levels can act to dampen variability of populations, thereby contributing to community stability. Thus, multiple feedback mechanisms between community-wide diversity and stability may exist both within and among communities (31).
Density-dependent mortality identified in this study has different implications for population regulation and persistence at the local (within-reef) vs. regional (among-reef) scale. At the local scale, spatial density-dependent mortality identified here may act to dampen (i.e., regulate) temporal fluctuations of reef-fish populations. However, persistence of local populations is nonetheless reliant on larvae supplied from the more regional collection of subpopulations distributed among reefs, i.e., the metapopulation (11, 13). Collectively, local density-dependent mortality acts both to limit mean larval production across subpopulations as well as to dampen variability in the regional larval pool by regulating numbers of adults. Thus, whereas mortality reduces regional larval production and population size, thereby potentially reducing the probability of persistence, the density-dependent nature of that mortality is nonetheless likely to enhance the persistence of the metapopulation (11, 32, 33).
Understanding species interactions that contribute to density dependence and population regulation identifies factors that contribute to persistence and must be protected for effective conservation (11). In coral-reef fish communities, overexploitation of important predators or competitors may act to destabilize other populations and the community overall. Indeed, the predators (groupers, jacks, etc.) and competitors (territorial damselfishes) in our experiments are heavily targeted by fisheries and occasionally by the aquarium trade, respectively. The compensatory mortality identified in this and related studies (16, 17) should not be interpreted to suggest that marine fish populations are inherently capable of rebounding from reductions caused by overexploitation. To the contrary, our results indicate that reductions in the abundance and intensity of multispecies interactions in a community of high biodiversity allow greater population fluctuations, perhaps destabilizing local community structure and increasing the likelihood of both local and regional extinctions.
Acknowledgments
We thank A. Altieri, D. Frerich, K. Kroeker, B. McLeod, and the Caribbean Marine Research Center, especially B. Kakuk. G. Almany shared data on recruitment rates. D. Doak, J. Estes, P. Raimondi, and C. Syms provided constructive reviews. This work was supported in part by U.S. National Science Foundation Grants OCE-96-17483 and OCE-00-93976 (to M.A.H.) and OCE-96-18012 (to M.H.C.), U.S. National Oceanic and Atmospheric Administration–National Undersea Research Program Grant CMRC-97-3109 (to M.A.H.), and the Caribbean Marine Research Center.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vitousek P. M., Mooney, H. A., Lubchenco, J. & Melillo, J. M. (1997) Science 277, 494-499. [Google Scholar]
- 2.Chapin F. S., Zavaleta, E. S., Eviner, V. T., Naylor, R. L., Vitousek, P. M., Reynolds, H. L., Hooper, D. U., Lavorel, S., Sala, O. E., Hobbie, S. E., et al. (2000) Nature (London) 405, 234-242. [DOI] [PubMed] [Google Scholar]
- 3.Schultze E. D. & Mooney, H. A., (1993) Biodiversity and Ecosystem Function (Springer, Berlin).
- 4.Tilman D. (1999) Ecology 80, 1455-1474. [Google Scholar]
- 5.McCann K. S. (2000) Nature (London) 405, 228-233. [DOI] [PubMed] [Google Scholar]
- 6.Jackson J. B. C., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., Bradbury, R. H., Cooke, R., Erlandson, J., Estes, J. A., et al. (2001) Science 293, 629-638. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson A. J. (1933) J. Anim. Ecol. 2, 132-178. [Google Scholar]
- 8.Nicholson A. J. (1957) Cold Spring Harbor Symp. Quant. Biol. 22, 326-335. [Google Scholar]
- 9.Murdoch W. W. (1994) Ecology 75, 271-287. [Google Scholar]
- 10.Turchin P. (1995) in Population Dynamics: New Approaches and Synthesis, eds. Cappucino, N. & Price, P. W. (Academic, San Diego), pp. 19–40.
- 11.Hixon M. A., Pacala, S. W. & Sandin, S. A. (2002) Ecology 83, 1490-1508. [Google Scholar]
- 12.Cappuccino N. & Harrison, S. (1996) in Frontiers of Population Ecology, eds. Floyd, R. B., Sheppard, A. W. & De Barro, P. J. (Commonwealth Scientific and Industrial Research Organisation, Collingwood, Australia), pp. 53–64.
- 13.Caley M. J., Carr, M. H., Hixon, M. A., Hughes, T. P., Jones, G. P. & Menge, B. A. (1996) Annu. Rev. Ecol. System. 27, 477-500. [Google Scholar]
- 14.Hixon M. A. & Webster, M. S. (2002) in Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem, ed. Sale, P. F. (Academic, San Diego), pp. 303–325.
- 15.Forrester G. F. & Steele, M. A. (2000) Ecology 81, 2416-2427. [Google Scholar]
- 16.Anderson T. W. (2001) Ecology 82, 245-257. [Google Scholar]
- 17.Hixon M. A. & Carr, M. H. (1997) Science 277, 946-949. [Google Scholar]
- 18.Belovsky G. E. & Joern, A. (1995) in Population Dynamics: New Approaches and Synthesis, eds. Cappucino, N. & Price, P. W. (Academic, San Diego), pp. 359–386.
- 19.Robertson D. R. (1996) Ecology 77, 885-899. [Google Scholar]
- 20.Randall J. E. (1967) Stud. Trop. Oceanogr. 5, 665-847. [Google Scholar]
- 21.Nemeth R. S. (1998) Environ. Biol. Fishes 53, 129-141. [Google Scholar]
- 22.Carr M. H. & Hixon, M. A. (1995) Marine Ecol. Progr. Ser. 124, 31-42. [Google Scholar]
- 23.Sponaugle S. & Cowen, R. J. (1996) Marine Freshwater Res. 47, 433-447. [Google Scholar]
- 24.Forrester G. F. (1995) Oecologia 103, 275-282. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt R. J. & Holbrook, S. J. (1999) Ecology 80, 35-50. [Google Scholar]
- 26.Booth D. J. & Beretta, G. A. (1994) Coral Reefs 13, 81-89. [Google Scholar]
- 27.Harrington M. E. (1993) Copeia 1993, 67-74. [Google Scholar]
- 28.Hixon M. A. (1991) in The Ecology of Fishes on Coral Reefs, ed. Sale, P. F. (Academic, New York), pp. 475–508.
- 29.Tilman D. (1996) Ecology 77, 350-363. [Google Scholar]
- 30.Brown J. H., Whitham, T. G., Ernest, S. K. M. & Gehring, C. A. (2001) Science 293, 643-650. [DOI] [PubMed] [Google Scholar]
- 31.Naeem S. (2002) Ecology 83, 1537-1552. [Google Scholar]
- 32.Hanski I., Foley, P. & Hassell, M. P. (1996) J. Anim. Ecol. 65, 274-282. [Google Scholar]
- 33.Chesson P. (1996) in Frontiers of Population Ecology, eds. Floyd, R. B., Sheppard, A. W. & De Barro, P. J. (Commonwealth Scientific and Industrial Research Organisation, Collingwood, Australia), pp. 353–368.