Abstract
Background:
Closed-loop rhythmic auditory stimulation (RAS) systems show promise for improving gait quality in people with Parkinson disease (PD).
Objective:
To examine auditory-motor entrainment and spatiotemporal gait responses to system-controlled rhythm tempo increase before and after a community-based RAS walking intervention.
Methods:
Thirteen persons with PD used an autonomous closed-loop RAS system during 30 walking sessions. Baseline (BL) and post-intervention (POST) gait responses to tempo increase were assessed as participants walked with the system in a clinic hallway. Rhythm tempo, entrainment, cadence, stride length, gait speed, and stride time variability (STV) were measured during the first minute (tempo at preferred walking cadence, prior to increase) and fifth minute (tempo above preferred cadence, following increase) of each assessment. Within- and between-assessment responses of entrainment and spatiotemporal variables to tempo increase were evaluated.
Results:
During each assessment, participants entrained to rhythmic cues while significantly increasing their cadence and stride length in response to tempo increase. Gait speed increased significantly only during the POST assessment. Nearly 70% of participants had significantly lower mean STV at the POST assessment compared to BL, indicating increased gait rhythmicity. The between-assessment decrease in STV was associated with increased stride length.
Conclusions:
Study findings supported the potential of an autonomous closed-loop RAS system to induce entrainment and meaningful gait responses to system-controlled tempo increase in persons with PD. The system appeared to promote implicit motor learning processes during use. Associated post-intervention improvements in rhythmicity and stride length in a subset of participants were suggestive of fall prevention effects.
Trial registration:
Prospectively registered at ClinicalTrials.gov (NCT05421624)
Keywords: Gait, Motor learning, Parkinson’s disease, Rhythmic auditor stimulation, Technology, Walking
INTRODUCTION
Idiopathic Parkinson disease (PD) diminishes gait quality and walking function over many years.1,2 Progressive depletion of dopaminergic neurons in the basal ganglia disrupts movement rhythm and drive, resulting in a more variable gait pattern with reduced speed and stride length.3,4 Although gait speed and stride length respond modestly to pharmacological treatment, benefits are limited and decrease over time.5 Gait variability, a recognized fall risk indicator in older adults and people with PD,6,7 appears to be relatively dopamine-resistant especially as postural instability emerges.7,8 Limitations to pharmacologic management underscore the need for effective interventions that mitigate walking-related disability.
Rhythmic auditory stimulation (RAS) is a well-known clinical intervention used to facilitate a rhythmical and normalized gait pattern in people PD.9-13 RAS capitalizes on the human capacity to extract rhythm and synchronize movements to a steady beat, a process referred to as auditory-motor entrainment.14 In the traditional paradigm, rhythmic cues controlled by an “open-loop” system (e.g., metronome or music player) are presented at a fixed tempo regardless of how well the user entrains to the beat.15 Changes to fixed-tempo cues are made manually, typically by a trained evaluator (e.g., physical therapist), based on visual observation of gait quality. Although extensive evidence supports the effectiveness of open-loop, fixed tempo RAS interventions for improving cadence, stride length, and gait speed in the short-term,9-13 their impact on gait variability has been mixed.16 Moreover, fixed-tempo RAS systems are limited in how readily they can be adjusted in response to frequent fluctuations in PD medication states (i.e., on-off phenomenon) and the variable cadence demands of real-world walking.9,17 Taken together, these limitations suggest that walking interventions involving open-loop RAS systems may not be a practical long-term solution to managing progressive walking disability in PD.
Advances in wearable digital technology have led to the development of autonomous closed-loop RAS systems that adjust the timing of rhythmic cues based on real-time sensing of gait performance. Unlike open-loop systems, the interactive and adaptive nature of autonomous closed-loop systems allows them to efficiently deliver rhythmic cues across a range of real-world walking tempos, accommodate variable entrainment and walking abilities, and foster independent use by people with PD.18-21 We recently reported on the feasibility and proof-of-concept of using a music-based autonomous closed-loop system (MR-005, MedRhythms Inc, Portland, ME, USA) to improve gait quality, walking volume, and walking intensity in people with mild to moderate PD.22 Gait quality improvements in that study22 were observed during system use, as participants engaged in 20 unsupervised community-based walking sessions. Importantly, the system made tempo adjustments based on user entrainment and gait quality and inserted them seamlessly into the audio stream without explicit notification. Thus, although not assessed, the system may have promoted implicit motor learning (i.e., error-based recalibration) processes that have implications for the long-term retention of gait quality following intervention.23-25
Motor learning deficits in PD are an important consideration in rehabilitation.26,27 Implicit error-based recalibration processes may be less cognitively demanding than more explicit feedback methods,24 and therefore, potentially an important component of closed-loop RAS interventions used to mitigate walking-related disability. To better understand the potential of the MR-005 system to promote implicit motor learning processes in people with PD during use, the aims of this exploratory study were (1) to examine user entrainment and spatiotemporal gait responses to a system-controlled rhythm tempo increase, and (2) to compare entrainment and gait responses to tempo increase before and after a community-based RAS walking intervention.
METHODS
Study Design
The study was a quasi-experimental, exploratory analysis of treatment arm data from 13 participants enrolled in a prospective, single-blinded (assessor) randomized controlled trial, Amplifying Physical Activity Through Music in Parkinson Disease (Amped-PD).28 The Amped-PD trial, conducted at Boston University from August 2021 to November 2023, was designed to examine the effects of the MR-005 system on daily walking in persons with PD. Participants in the experimental “Amped-PD” group used the system during 30 unsupervised sessions of a community-based walking intervention over six weeks. Data for the exploratory analysis were collected from Amped-PD participants walking with the MR-005 system during baseline (BL) and post-intervention (POST) gait assessments (Figure 1).
Figure 1. Gait Assessment.
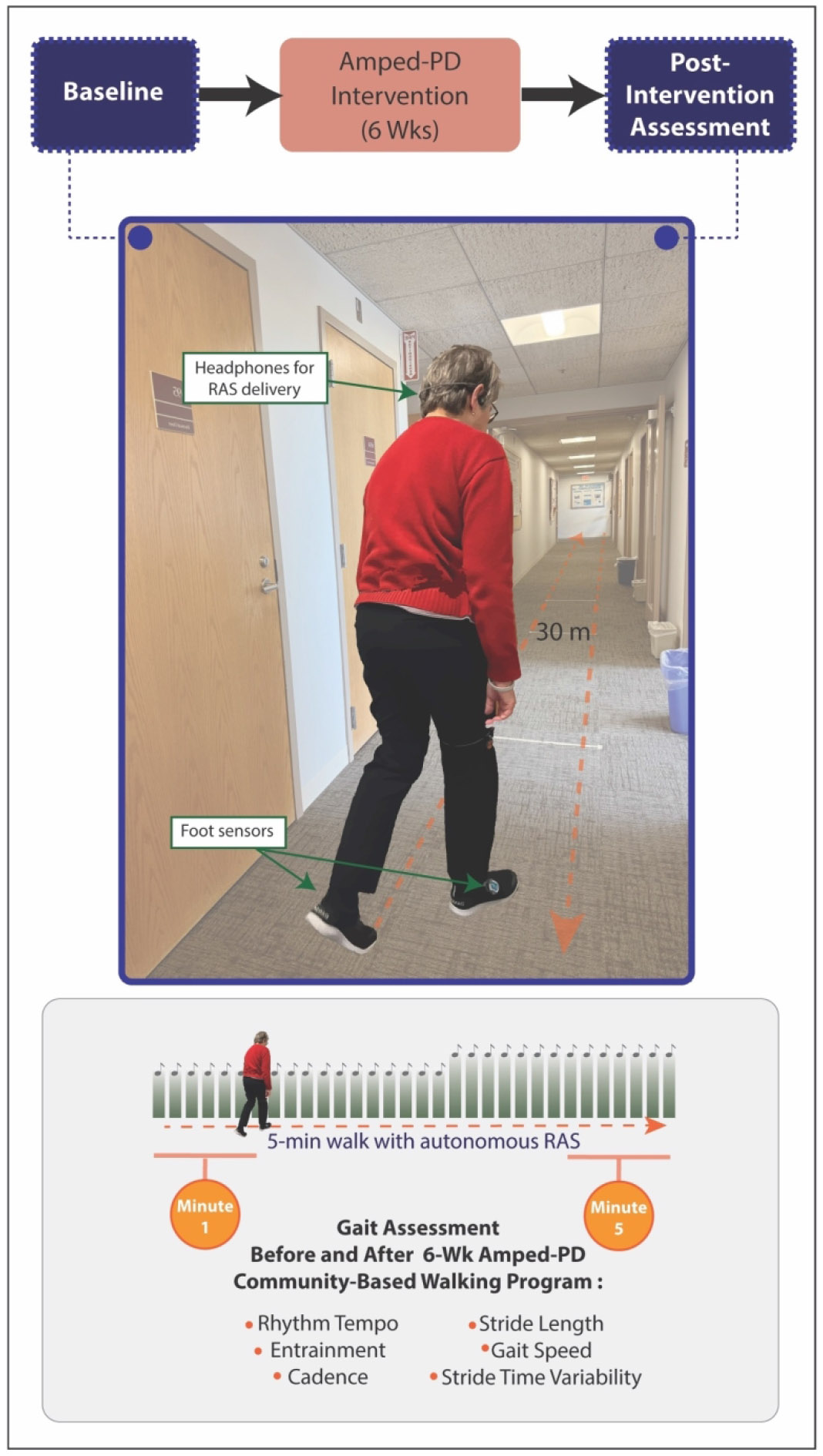
At baseline and post-intervention, gait responses to system-controlled tempo increase were assessed by having participants walk with the MR-005 system for five minutes in an indoor clinic hallway. Rhythm tempo, entrainment, cadence, stride length, gait speed, and stride time variability data collected by the system were evaluated during the first minute (tempo at preferred walking cadence, prior to increase) and fifth minute (tempo above preferred cadence, following increase) of each assessment.
Participants
Participants included in the Amped-PD trial were community-dwelling adults with idiopathic PD (H&Y stages 1-3), aged 40-85 years, who were able to walk for at least 10 minutes independently without physical assistance and who had stable PD medications for at least 2 weeks prior to enrollment. The trial excluded individuals with atypical Parkinsonism, moderately or significantly disturbing freezing of gait episodes per item #7 on the New Freezing of Gait Questionnaire,29 cognitive impairment (i.e., Mini-Mental State Exam (MMSE) score <24), history of ≥1 fall over the previous three months, the inability to walk independently at a comfortable speed ≥ 0.4 m/s, self-reported significant hearing impairment, self-reported regular walking exercise >3x/week for 30 minutes per session, or the presence of cardiac or orthopedic conditions that limited the ability to safely walk in the community. The trial complied with the standards of the Declaration of Helsinki and was approved by the Boston University Institutional Review Board. For the current analysis, we included Amped-PD participants who had finished the trial and had a complete set of BL and POST gait assessment data.
Amped-PD Intervention
The MR-005 system was comprised of two shoe-worn sensors, a bone-conducting headset, and a touchscreen device pre-loaded with a proprietary software application. Described previously,22 the software algorithm autonomously monitored real-time sensor data and adjusted rhythmic cues toward normative cadence values based on its evaluation of auditory-motor entrainment and step-to-step temporal variability and symmetry. Participants in the Amped-PD group were instructed to use the system during 30 dedicated community-based walking intervention sessions (one 30-minute session per day, five times per week for six weeks). To promote safety, participants were instructed to walk with the system on uncluttered, generally level surfaces suitable for straight-line forward walking. The system played popular and familiar music based on each user’s preferred music genre (e.g. classic rock, country, oldies, etc.), with songs proprietarily screened for therapeutic utility by the MR-005 manufacturer. The system prompted participants to “walk to the beat of the music.”
Measures
BL and POST gait assessments were conducted during the “on” phase of each participant’s medication cycle by instructing them to walk with the MR-005 system for five minutes in an uncluttered clinic hallway (Figure 1). All assessments were supervised by research personnel. The five-minute duration was chosen to allow for at least one system-controlled tempo increase. At the start of each assessment, the system briefly sampled participants’ natural gait at their preferred walking cadence and provided a music-based rhythmic cue at a matching tempo. For the remainder of the assessment, the system autonomously monitored gait performance and increased the rhythm tempo at two-minute intervals based on user entrainment and gait quality.
Rhythm tempo, entrainment, and spatiotemporal data were extracted from gait cycle data collected by the MR-005 system during each gait assessment (Figure 1). Data collected during turns at the end of the hallway were excluded. Rhythm tempo values were expressed in beats per minute. Entrainment values were expressed as the unit-less ratio of rhythm tempo to walking cadence, with 1.0 indicating perfect temporal alignment. Cadence (steps/min), stride length (m), and gait speed (m/s) values were derived from foot sensor data. Gait variability was measured in terms of stride time variability (STV), expressed as the coefficient of variation (CV) of stride-to-stride gait cycle duration (%). Lower STV values indicated a more rhythmical gait pattern.
To characterize the sample, demographic and clinical data were obtained by research personnel during the BL assessment. The Movement Disorders Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III (motor) score was used as an indicator of disease severity.30 The Ten Meter Walk Test (10MWT) was used to characterize comfortable and fast-paced gait speed.31 Distance walked during the Six-Minute Walk Test (6MWT) was used to characterize walking endurance.31
Statistical Analysis
Statistical analyses were conducted using SPSS statistical software program version 29.0 (IBM Corp, Armonk, New York). Descriptive statistics were used to characterize the sample. Preliminary analysis of BL and POST assessment data revealed that the timing of tempo adjustments varied among participants. Therefore, to capture all gait responses to tempo increase, we first calculated mean values for rhythm tempo, entrainment, cadence, stride length, gait speed, and STV during the first minute (tempo at preferred walking cadence, prior to increase) and fifth minute (tempo above preferred walking cadence, following increase) of each assessment (Figure 1). We then used a series of two-factor (assessment x minute) repeated measures analysis of variance (ANOVA) to evaluate (1) the magnitude of the tempo increase during each assessment, (2) the response of entrainment and spatiotemporal variables to the tempo increase during each assessment, and (3) differences between BL and POST gait responses (α=.05). Partial eta squared (ηp2) was used to assess effect size in the ANOVA models (<.01 = negligible effect; .01 = small effect; .06 = medium effect; .14 = large effect).32 Based our previous work,22 we used post-hoc pairwise comparisons and correlation analyses to further evaluate between-assessment differences and potential interdependencies among changes in spatiotemporal parameters. Bonferroni corrections were used to adjust for multiple comparisons.
RESULTS
The sample was comprised of 13 adults (68.2 ±9.5 years, 7 males, 6 females) with mild to moderate PD severity [Hoehn & Yahr Stage 2 (N=10), 2.5 (N=2), 3 (N=1); MDS UPDRS III score = 21.3±10.7]. Mean time since PD onset was 4.2±2.5 years. Participants’ comfortable gait speed (1.16±.19 m/s), fast-paced gait speed (1.74±.34 m/s), and walking endurance (536 ± 88.4 m) reflected their relatively high walking capacity. Participants collectively completed 31.4±4.3 Amped-PD walking sessions over six weeks. No falls related to the intervention were reported.
Rhythm Tempo
All participants experienced system-controlled rhythm tempo increase between the first and fifth minutes of the BL and POST assessments (Table 1). Each tempo increase (BL: Δ=16.5±1.2 beats/minute; POST: Δ=17.0±1.7 beats/minute) was significant, as revealed by a main effect of minute (p<.01). The magnitude of tempo increase at the POST assessment was not significantly different from baseline (p>.05).
Table 1.
Gait Responses to Tempo Increase with Repeated Measures ANOVA Results (Full Sample)
| Baseline Assessment |
Post- Assessment |
Main Effect: Assessment |
Main Effect: Minute |
Interaction: Assessment X Minute |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | F (1,12) |
Sign. (p) |
Effect Size (np2) |
Obs. Power |
F (1,12) |
Sign. (p) |
Effect Size (np2) |
Obs. Power |
F (1,12) |
Sign. (p) |
Effect Size (np2) |
Obs. Power |
|
| Rhythm Tempo (beats/min) | ||||||||||||||
| Minute 1 | 101.1 (11.9) | 103.2 (10.1) | 1.07 | .32 | .089 | .159 | 175.51 | <.01 | .936 | 1.000 | 0.12 | .73 | .010 | .062 |
| Minute 5 | 117.5 (10.7) | 120.2 (8.3) | ||||||||||||
| Entrainment Value | ||||||||||||||
| Minute 1 | 0.99 (.02) | .99 (.04) | 2.64 | .13 | .180 | .322 | 24.54 | <.01 | .672 | .995 | 1.99 | .18 | .142 | .255 |
| Minute 5 | 1.06 (.05) | 1.03 (.04) | ||||||||||||
| Cadence (steps/min) | ||||||||||||||
| Minute 1 | 103.9(11.4) | 105.2 (8.9) | 1.84 | .20 | .133 | .240 | 23.21 | <.01 | .659 | .993 | 3.29 | .09 | .215 | .386 |
| Minute 5 | 110.7(10.7) | 115.3 (8.4) | ||||||||||||
| Stride Length (m) | ||||||||||||||
| Minute 1 | 1.24 (.19) | 1.25 (.14) | 0.59 | .46 | .047 | .109 | 7.36 | .02 | .380 | .702 | 0.26 | .62 | .021 | .075 |
| Minute 5 | 1.28 (.19) | 1.31 (.18) | ||||||||||||
| Gait Speed (m/s) | ||||||||||||||
| Minute 1 | 1.14 (.31) | 1.11 (.14) | 0.49 | .50 | .039 | .099 | 10.91 | .01 | .476 | .858 | 3.47 | .09 | .224 | .403 |
| Minute 5 | 1.19 (.24) | 1.31 (.23) | ||||||||||||
| STV (%) | ||||||||||||||
| Minute 1 | 4.13 (1.7) | 3.43 (.85) | 3.08 | .11 | .204 | .365 | 2.12 | .17 | .150 | .268 | 0.18 | .68 | .015 | .068 |
| Minute 5 | 3.96 (1.9) | 2.94 (.64) | ||||||||||||
Spatiotemporal gait characteristics were assessed during a 5-minute walking bout with MR-005, before and after 30 sessions of the Amped-PD intervention (grey cells). Minute 1 values during each assessment coincided with tempo at preferred walking cadence, prior to increase. Minute 5 values during each assessment coincided with tempo at elevated walking cadence, following increase. Entrainment value was expressed as the unitless ratio of rhythm tempo to walking cadence. Stride time variability (STV) was expressed as the coefficient of variation of gait cycle duration. Repeated Measures ANOVA Within-Subjects Effects (white cells) revealed a significant main effect of minute for rhythm tempo, entrainment value, cadence, stride length, and gait speed (bolded results). Effect size interpretation for partial eta squared (ηp2): <.01 = negligible effect; .01 = small effect; .06 = medium effect; .14 = large effect.32 Sign. = significance; Obs.=observed.
Entrainment
Participants generally aligned their walking cadence to rhythm tempo during the BL and POST assessments, as indicated by entrainment values close to 1.0 [grand mean (SE) =1.02 (.006)] (Table 1). However, compared to nearly perfect temporal synchrony during the first minute of each assessment (when tempo was set to preferred walking cadence), participants tended to walk slightly behind the beat of the faster tempo during the fifth minute (i.e., entrainment value >1.0). Each entrainment value increase (BL: Δ=6.5±1.3%; POST: Δ=4.3±1.4%) was significant, as indicated by a main effect of minute (p<.01). The magnitude of entrainment value increase at the POST assessment was not significantly different from baseline (p>.05).
Cadence, Stride Length, and Gait Speed
Cadence, stride length, and gait speed increased in response to system-controlled tempo increase during the BL and POST assessments (Table 1). The increases were significant, as revealed by a main effect of minute (Cadence: BL Δ=6.8±1.4 steps/minute, POST Δ=10.1±2.4 steps/minute, p<.01; Stride Length: BL Δ=.043±.018 m, POST Δ=.053±.022 m, p=.02; Gait Speed: BL Δ=.05±..18 m/s, POST Δ=.20±.21 m/s, p=.01). The magnitude of cadence and stride length responses at POST assessment, respectively, were not significantly different from baseline (p>.05). For gait speed, however, although there was no interaction between assessment and minute (p=.09), post hoc comparisons used to better understand the main effect of minute revealed that only the gait speed increase during the POST assessment was significant (Bonferroni-adjusted p=.01, Cohen’s d = .94).
Gait Variability
The STV response to system-controlled rhythm tempo increases during the BL and POST assessments was relatively muted (Table 1). Although trending toward lower STV (i.e., increased rhythmicity) at a faster rhythm tempo, the decreases (BL Δ=−.18±.52%, POST Δ=−.48±.31%) were not significant (p>.05). The magnitude of STV response to tempo increase at the POST assessment was not significantly different from baseline (p>.05).
Given the pathognomonic relevance of gait rhythmicity in PD, we further explored the STV values of individual participants. In nearly 70% of the sample (9/13 participants), mean STV (i.e., the mean of first minute STV and fifth minute STV values) was lower at POST assessment compared to baseline. Considering only this subset of individuals (N=9), the decrease in mean STV from baseline (4.7±.4%) to POST assessment (3.2±.2%) was significant, as revealed by a main effect of assessment (F(1,8)=8.41, p=.02, partial eta squared (np2) =.512, observed power = .719). There was no coinciding main effect of minute (F(1,8)=0.58, p=.47, np2 = .07, observed power = .104) or interaction between assessment and minute (F(1,8)=0.04, p=.84, np2 = .005, observed power = .054) (Figure 2). Post-hoc comparisons used to better understand the main effect of assessment in the subset revealed that compared to the first minute of the BL assessment (tempo at preferred walking cadence), STV at both time points in the POST assessment were significantly lower (POST first minute Δ=−1.6± 1.8%, Bonferroni-adjusted p=.02, Cohen’s d = .91; POST fifth minute Δ=−1.8±1.6%, Bonferroni-adjusted p=.01, Cohen’s d = 1.14). In the remaining participants (n=4), mean STV increased from baseline (2.6±.6%) to POST assessment (3.2±.2%).
Figure 2:

Stride time Variability During Closed-Loop Rhythmic Auditory Stimulation, Before and After the Amped-PD Intervention.For a subset of participants (N=9), mean stride time variability (STV) during the post-intervention gait assessment (3.2±.2%) was significantly lower (−1.5% COV) than mean STV during the baseline assessment (4.7±.4%), as revealed by a main effect of assessment. Post-hoc comparisons used to better understand the main effect of assessment revealed that compared to the first minute of the BL assessment, STV at both time points in the POST assessment were significantly lower (POST first minute =−1.6±1.8%; POST fifth minute =−1.8±1.6%).
Post-hoc bivariate correlation analyses using the full sample (N=13) revealed that the difference in mean STV between BL and POST assessments was inversely related to difference in stride length (r=−.63, p=.02), but not differences in cadence (r=−.19, p=.54) or gait speed (r=−.48, p=.10) (Figure 3). In other words, a more rhythmical (i.e., less variable)) gait pattern following the Amped-PD intervention tended to be accompanied by longer strides, whereas a less rhythmical (i.e., more variable) gait pattern following intervention tended to be accompanied by shorter strides.
Figure 3:
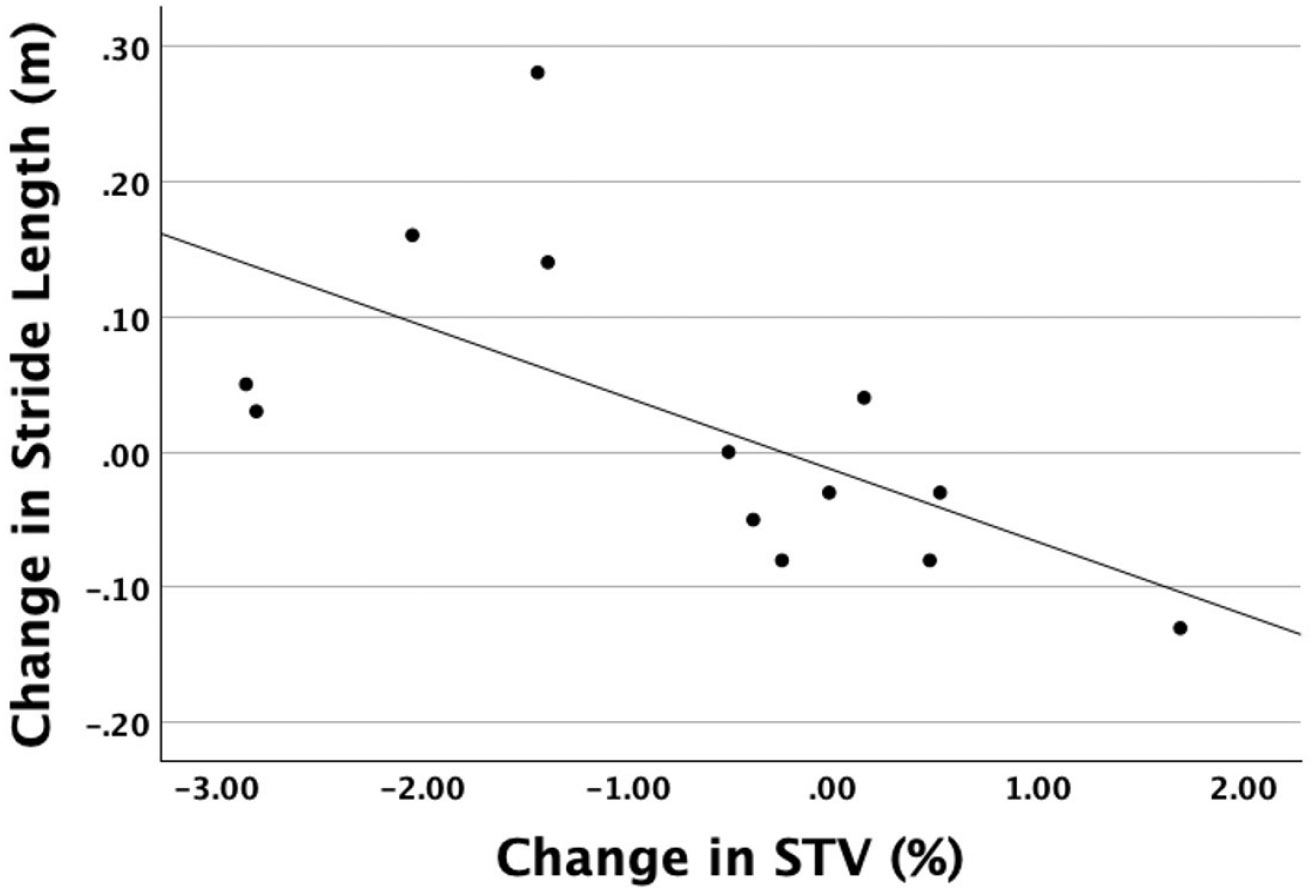
Association Between Change in Stride Length and Change in Stride Time Variability from Baseline to Post-Intervention.Change in mean STV was associated with change in mean stride length (r−.63, p=.02).
DISCUSSION
The goal of RAS is to help people with PD maintain a more efficient and stable walking rhythm, given their impaired internal rhythm generation and motor drive mechanisms.9-13 Previous research has suggested that closed-loop RAS systems, which offer a more interactive and adaptive approach to intervention, may produce improved walking outcomes.17-22,33,34 To date, two previous studies have investigated using a closed-loop music-based RAS system as a community-based PD walking intervention.20,22 In one of them, naturalistic gait quality (i.e., without RAS cues) was assessed before and after intervention.20 In the other, we evaluated gait quality, walking volume, and walking intensity during intervention.22 The current study expanded this line of inquiry by focusing on how people with PD interact with and adapt to an autonomous closed-loop RAS system. In the discussion that follows, we evaluate study findings and develop a series of propositions to guide future investigations of closed-loop RAS.
Entrainment
Entrainment of walking cadence to rhythm tempo in people with PD relies on a complex network of cortical and subcortical connections that either stimulate or bypass impaired internal rhythm and drive mechanisms involving the basal ganglia.14 Importantly, entrainment abilities in the PD population vary,19 thereby potentially limiting the success of RAS intervention for some individuals. In this context, the entrainment results in our study were promising. Participants generally aligned their cadence to the rhythm tempo during both sessions as they attempted to walk to the beat of the music. Unlike an open-loop RAS approach, in which users might receive verbal feedback about gait performance from a trained evaluator (e.g., physical therapist), participants using the MR-005 system received no explicit feedback about how well their cadence appeared to match the rhythm tempo. Instead, the high beat salience of MR-005 music selections and implicit system adjustments to beat salience (e.g., supplemental metronome overlay) may have contributed to entrainment success.
Entrainment values appeared to be influenced by rhythm tempo. Whereas participants quickly entrained at their preferred walking cadence (during the first minute of BL and POST assessments), they lagged modestly (4.3-6.5%) behind the tempo following a substantial (~17%) tempo increase (Table 1). Although the cadence lag was similar between the BL and POST assessments, there was a trend toward improved entrainment at the higher (fifth minute) tempo of the POST assessment. The extent to which prolonged use of closed-loop RAS improves entrainment abilities in people with PD (e.g., via longer intervention periods or longer individual intervention sessions) warrants further investigation.
Gait Responses to Tempo Increase
Participants immediately responded to system-controlled rhythm tempo increases during the BL and POST assessments by significantly increasing their cadence and stride length (Table 1). Following the six-week Amped-PD intervention, cadence and stride length responses to tempo increase were robust enough to result in a significant increase in gait speed. The responses were largely consistent with the body of evidence supporting the use of RAS to improve gait performance in people with PD9-13 Moreover, the POST assessment gait responses were clinically meaningful: the MR-005 system quickly moved the sample from preferred walking cadence and speed to a cadence (115 steps/min) and speed (1.3 m/s) associated with outdoor walking and crossing a street in the time allotted at signalized intersections.35,36 The cadences during the fifth minute of each assessment were well above an established threshold for moderate intensity walking,37 consistent with our previous work.22 These immediate motor drive and physical activity impacts support the potential benefit of routine walking with an autonomous closed-loop RAS system.28
Implications for Motor Learning
The cadence, stride length, and speed responses to system-controlled tempo increase during the BL and POST assessments may have represented an implicit short-term locomotor (i.e., sensorimotor) adaptation process.38 By definition,39 short-term adaptation is a modification of movement based on error feedback. The modification, which emerges gradually with continuous repetition, retains the basic identity of the original pattern but changes in terms of one or more parameters.39 Once adapted, individuals cannot retrieve the original pattern without first “de-adapting” from the modified pattern over a series of trials.39 In this context, we speculated that auditory-motor entrainment created the conditions necessary for an error-driven motor recalibration of the gait pattern to the higher system tempo.24,40 As a result, participants modified both temporal (i.e., cadence) and spatial (i.e., stride length) parameters over a series of gait cycles (i.e. trials),41 resulting in a concurrent increase in speed. Although beyond the scope of the study, we suspect that participants would have had difficulty de-adapting from the modified gait pattern until rhythmic cueing returned to the original tempo. The cadence and stride length responses to system-controlled tempo increase appeared to be largely consistent between BL and POST assessments, and thus, not significantly impacted by the six-week Amped-PD intervention.
In contrast, gait variability responded minimally to system-controlled tempo increase during the BL and POST assessments. However, nearly 70% of the sample had a lower mean STV (i.e., increased gait rhythmicity) during the POST assessment in comparison to baseline (Figure 2). This subset of participants (N=9) tended to have higher baseline gait variability than the remainder of the sample (N=4), suggesting that they had greater opportunity for improvement.42 The STV decrease was most apparent when comparing the first minute of the BL assessment to both POST assessment time points (Figure 2). The mean STV decrease (−1.5% COV) for these participants was twice a published Minimal Clinically Important Difference value for gait variability in people with PD (MCID = 0.67% COV).43
Although reasons for the between-assessment decrease in STV were unclear, one explanation may have been that the six-week Amped-PD intervention resulted in long-term adaptive learning, an implicit motor learning process representing the stored ability to predict a locomotor perturbation and adapt gait parameters accordingly.38 We propose that in the context of Amped-PD walking sessions, the sudden switch from naturalistic walking to walking with the MR-005 system constituted a rhythmic perturbation to the motor control system. The adaptive closed-loop system autonomously controlled the rhythmic perturbation by leveraging auditory-motor entrainment to minimize gait variability across a variety of tempos. After 30 cycles of adapting to and de-adapting from the closed-loop RAS environment, participants may have learned to quickly switch between two rhythm calibrations (i.e., a higher variability calibration walking without cues and a lower variability calibration walking with cues).41
Our findings supported the idea that the MR-005 closed-loop RAS system, which silently evaluated user gait performance and adjusted rhythm tempo without providing explicit feedback, may be well suited for promoting implicit sensorimotor adaptation and adaptive learning processes during use.44 The potential influence of these processes on drive and rhythm impairments in PD might be relevant to consider when evaluating the utility of closed-loop RAS systems for mitigating walking disability.24,26,27 Drive-related impairments (i.e., decreased stride length and speed) arise from overactive inhibitory output from the basal ganglia to cortical motor areas.3 These parameters appeared to adapt quickly to closed-loop RAS tempo increase but may have been less likely to undergo substantial long-term adaptive learning through repetitive system use. Their effective management, therefore, ultimately may be enhanced by long-term use of closed-loop RAS as a walking aid, especially once responsiveness to pharmacologic treatment has waned. Simply starting to walk with the system could result in relatively immediate increases in cadence, stride length, and speed that would be dependent on the system-controlled rhythm tempo. In contrast, rhythm-related impairment (i.e., increased gait variability) is thought to result from deteriorating automatic rhythm control and a subsequent shift to voluntary rhythm control.3 Gait rhythmicity appeared to improve relatively slowly (but not in response to within-assessment tempo increase) and may have been dependent on long-term adaptive learning processes associated with repetitive system use. Thus, only with extended practice might users display improved gait rhythmicity when walking with the system.
Implications for Fall Prevention
The associated change in gait rhythmicity and stride length following the Amped-PD intervention was surprising, given the fundamentally different effects of the MR-005 system on both parameters (see above). Although both are key markers of gait impairment in PD, reflecting the deterioration of rhythm and drive mechanisms, respectively, 3,4 the effect of RAS on gait variability appears to be at least somewhat independent of its effects on stride length.45 Our findings, however, seemed to suggest a linkage between rhythmicity and stride length changes (but not cadence or speed changes) resulting from closed-loop RAS intervention. Participants whose gait was more rhythmical following the Amped-PD intervention tended to take longer strides, potentially lowering their fall risk when using the MR-005 system (Figure 3).7,46 Importantly, however, the association between increased rhythmicity and stride length was relatively moderate, leaving much of variance in each variable unexplained. Nonetheless, the extent to which closed-loop RAS may have a fall prevention advantage over open-loop RAS during community-based walking warrants further investigation.
Study Limitations
Unlike the Amped-PD parent trial, which examined six-week naturalistic (“un-cued”) walking outcomes in participants using MR-005 in comparison to an active control group, the quasi-experimental design of this exploratory analysis narrowed the focus to gait changes only during MR-005 use. It remains unclear if the findings extend to walking without RAS following intervention, and any inferences suggesting causality should be interpreted with caution. Because MR-005 use was likely to have elicited a substantial amount of moderate intensity walking,22 the potential effect of exercise on motor learning could not be ruled out.47 In addition, study findings were based on a small sample of relatively high-functioning individuals with mild-to-moderate PD. The unbalanced distribution of participants based on STV changes following intervention (N=9 vs. N=4) made it impracticable to identify those individuals who might respond better to RAS training. The Amped-PD parent trial excluded individuals with greater disease severity, more disabling gait patterns (e.g., freezing of gait), and cognitive impairments, which reportedly are a group that responds well to RAS.16 Given that the six-week MR-005 intervention was implemented in the home/community setting without supervision, we also excluded individuals with relatively higher fall risk. Gait assessments took place in a supervised, uncluttered clinic hallway with minimal distractions, which reduced measurement error but may have limited the generalizability of the results to real-world walking. The six-week duration of the Amped-PD intervention may not have been long enough to see more robust POST assessment improvements in entrainment at tempos above preferred cadence, decreased gait variability following tempo increase, or long-term changes in preferred walking cadence, stride length, and speed during MR-005 system use.
CONCLUSIONS
Study findings supported the potential of an autonomous closed-loop RAS system to rapidly induce entrainment and meaningful gait responses to system-controlled tempo increase in persons with mild to moderate PD. The two-way, ongoing, adaptive interaction between participants and the closed-loop MR-005 system appeared to show promise for promoting implicit motor learning processes during use. Associated post-intervention improvements in rhythmicity and stride length in a subset of participants were suggestive of fall prevention effects.
Acknowledgements:
The authors wish to thank research assistants Samantha Giles, Yuuki Hori, Stephen Natola, Ruby Perez, and Benjamin Pollock for their contributions to the study. In addition, authors would like to thank MedRhythms, Inc. for supplying the MR-005 devices and providing access to sensor data.
Funding:
The study was funded by NIH/NIA Boston Roybal Center for Active Lifestyle Interventions P30AG048785 (F.P) and supported by 1F31D110123-01 (J.A.Z) and FPTR PODS II Scholarship (J.A.Z).
Footnotes
Ethics Considerations: The Amped-PD trial complied with the standards of the Declaration of Helsinki and was approved by the Boston University Institutional Review Board.
Consent to Participate: All participants provided written informed consent.
Declaration of Conflicting Interest: L.N.A. was a paid advisor to MedRhythms, Inc. The remaining authors declare no competing interests.
Data Availability:
The datasets used and/or analyzed during the exploratory analysis are available from the corresponding author on reasonable request.
REFERENCES
- 1.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The evolution of disability in Parkinson disease. Mov Disord Off J Mov Disord Soc. 2008;23(6):790–796. doi: 10.1002/mds.21879 [DOI] [PubMed] [Google Scholar]
- 2.Lord S, Galna B, Rochester L. Moving forward on gait measurement: toward a more refined approach. Mov Disord Off J Mov Disord Soc. 2013;28(11):1534–1543. doi: 10.1002/mds.25545 [DOI] [PubMed] [Google Scholar]
- 3.Peterson DS, Horak FB. Neural Control of Walking in People with Parkinsonism. Physiol Bethesda Md. 2016;31(2):95–107. doi: 10.1152/physiol.00034.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirelman A, Bonato P, Camicioli R, et al. Gait impairments in Parkinson’s disease. The LancetNeurology. 2019;18(7):697–708. doi: 10.1016/S1474-4422(19)30044-4 [DOI] [PubMed] [Google Scholar]
- 5.Grabli D, Karachi C, Welter ML, et al. Normal and pathological gait: what we learn from Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83(10):979–985. doi: 10.1136/jnnp-2012-302263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893 [DOI] [PubMed] [Google Scholar]
- 7.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212(1-2):47–53. doi: 10.1016/s0022-510x(03)00104-7 [DOI] [PubMed] [Google Scholar]
- 8.Blin O, Ferrandez AM, Pailhous J, Serratrice G. Dopa-sensitive and dopa-resistant gait parameters in Parkinson’s disease. J Neurol Sci. 1991;103(1):51–54. doi: 10.1016/0022-510x(91)90283-d [DOI] [PubMed] [Google Scholar]
- 9.Ghai S, Ghai I, Schmitz G, Effenberg AO. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci Rep. 2018;8(1):506–5. doi: 10.1038/s41598-017-16232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forte R, Tocci N, Vito GD. The Impact of Exercise Intervention with Rhythmic Auditory Stimulation to Improve Gait and Mobility in Parkinson Disease: An Umbrella Review. Brain Sci. 2021;11(6):685. doi: 10.3390/brainsci11060685. doi:10.3390/brainsci11060685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scataglini S, Dyck ZV, Declercq V, Cleemput GV, Struyf N, Truijen S. Effect of Music Based Therapy Rhythmic Auditory Stimulation (RAS) Using Wearable Device in Rehabilitation of Neurological Patients: A Systematic Review. Sensors. 2023;23(13):5933. doi: 10.3390/s23135933. doi:10.3390/s23135933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Peng JL, Ou-Yang JB, et al. Effects of Rhythmic Auditory Stimulation on Gait and Motor Function in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Clinical Randomized Controlled Studies. Front Neurol. 2022;13:818559. doi: 10.3389/fneur.2022.818559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye X, Li L, He R, Jia Y, Poon W. Rhythmic auditory stimulation promotes gait recovery in Parkinson’s patients: A systematic review and meta-analysis. Front Neurol. 2022;13:940419. doi: 10.3389/fneur.2022.940419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thaut MH, McIntosh GC, Hoemberg V. Neurobiological foundations of neurologic music therapy: rhythmic entrainment and the motor system. Front Psychol. 2015;5:1185. doi: 10.3389/fpsyg.2014.01185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muthukrishnan N, Abbas JJ, Shill HA, Krishnamurthi N. Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors. 2019;19(24):5468. doi: 10.3390/s19245468. doi:10.3390/s19245468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison EC, Earhart GM. The effect of auditory cues on gait variability in people with Parkinson’s disease and older adults: a systematic review. Neurodegener Dis Manag. 2023;13(2):113–128. doi: 10.2217/nmt-2021-0050 [DOI] [PubMed] [Google Scholar]
- 17.Hove MJ, Suzuki K, Uchitomi H, Orimo S, Miyake Y. Interactive rhythmic auditory stimulation reinstates natural 1/f timing in gait of Parkinson’s patients. PloS One. 2012;7(3):e32600. doi: 10.1371/journal.pone.0032600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginis P, Heremans E, Ferrari A, Dockx K, Canning CG, Nieuwboer A. Prolonged Walking with a Wearable System Providing Intelligent Auditory Input in People with Parkinson’s Disease. Front Neurol. 2017;8:128. doi: 10.3389/fneur.2017.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bella SD, Dotov D, Bardy B, de Cock VC. Individualization of music-based rhythmic auditory cueing in Parkinson’s disease. Ann N Y Acad Sci. Published online June 4, 2018. doi: 10.1111/nyas.13859 [DOI] [PubMed] [Google Scholar]
- 20.Cock VCD, Dotov D, Damm L, et al. BeatWalk: Personalized Music-Based Gait Rehabilitation in Parkinson’s Disease. Front Psychol. 2021;12:655121. doi: 10.3389/fpsyg.2021.655121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baram Y, Aharon-Peretz J, Badarny S, Susel Z, Schlesinger I. Closed-loop auditory feedback for the improvement of gait in patients with Parkinson’s disease. J Neurol Sci. 2016;363:104–106. doi: 10.1016/j.jns.2016.02.021 [DOI] [PubMed] [Google Scholar]
- 22.Zajac JA, Porciuncula F, Cavanaugh JT, et al. Feasibility and Proof-of-Concept of Delivering an Autonomous Music-Based Digital Walking Intervention to Persons with Parkinson’s Disease in a Naturalistic Setting. J Park Dis. 2023;13(7):1253–1265. doi: 10.3233/JPD-230169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forner-Cordero A, Pinho JP, Umemura G, et al. Effects of supraspinal feedback on human gait: rhythmic auditory distortion. J Neuroengineering Rehabil. 2019;16(1):159. doi: 10.1186/s12984-019-0632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duppen CP, Sachdeva N, Wrona H, Dayan E, Browner N, Lewek MD. Blending motor learning approaches for short-term adjustments to gait in people with Parkinson disease. Exp Brain Res. Published online October 3, 2024. doi: 10.1007/s00221-024-06933-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duppen CP, Wrona H, Dayan E, Lewek MD. Evidence of Implicit and Explicit Motor Learning during Gait Training with Distorted Rhythmic Auditory Cues. J Mot Behav. 2024;56(1):42–51. doi: 10.1080/00222895.2023.2231874 [DOI] [PubMed] [Google Scholar]
- 26.Olson M, Lockhart TE, Lieberman A. Motor Learning Deficits in Parkinson’s Disease (PD) and Their Effect on Training Response in Gait and Balance: A Narrative Review. Front Neurol. 2019;10:62. doi: 10.3389/fneur.2019.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul SS, Dibble LE, Peterson DS. Motor learning in people with Parkinson’s disease: Implications for fall prevention across the disease spectrum. Gait Posture. 2018;61:311–319. doi: 10.1016/j.gaitpost.2018.01.026 [DOI] [PubMed] [Google Scholar]
- 28.Oosterhof TH, Schootemeijer S, de Vries NM. Clinical Trial Highlights - Interventions Promoting Physical Activity in Parkinson’s Disease. J Park Dis. 2023;13(3):311–322. doi: 10.3233/JPD-239001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30(4):459–463. doi: 10.1016/j.gaitpost.2009.07.108 [DOI] [PubMed] [Google Scholar]
- 30.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord Off J Mov Disord Soc. 2008;23(15):2129–2170. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 31.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88(6):733–746. doi: 10.2522/ptj.20070214 [DOI] [PubMed] [Google Scholar]
- 32.Richardson JTE. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. 2011;6(2):135–147. doi: 10.1016/j.edurev.2010.12.001 [DOI] [Google Scholar]
- 33.Ashoori A, Eagleman DM, Jankovic J. Effects of Auditory Rhythm and Music on Gait Disturbances in Parkinson’s Disease. Front Neurol. 2015;6:234. doi: 10.3389/fneur.2015.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dotov DG, Bayard S, de Cock VC, et al. Biologically-variable rhythmic auditory cues are superior to isochronous cues in fostering natural gait variability in Parkinson’s disease. Gait Posture. 2017;51:64–69. doi: 10.1016/j.gaitpost.2016.09.020 [DOI] [PubMed] [Google Scholar]
- 35.Murtagh EM, Mair JL, Aguiar E, Tudor-Locke C, Murphy MH. Outdoor Walking Speeds of Apparently Healthy Adults: A Systematic Review and Meta-analysis. Sports Med Auckl NZ. 2021;51(1):125–141. doi: 10.1007/s40279-020-01351-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langlois JA, Keyl PM, Guralnik JM, Foley DJ, Marottoli RA, Wallace RB. Characteristics of older pedestrians who have difficulty crossing the street. Am J Public Health. 1997;87(3):393–397. doi: 10.2105/ajph.87.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tudor-Locke C, Han H, Aguiar EJ, et al. How fast is fast enough? Walking cadence (steps/min) as a practical estimate of intensity in adults: a narrative review. Br J Sports Med. 2018;52(12):776–788. doi: 10.1136/bjsports-2017-097628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roemmich RT, Nocera JR, Stegemöller EL, Hassan A, Okun MS, Hass CJ. Locomotor adaptation and locomotor adaptive learning in Parkinson’s disease and normal aging. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2014;125(2):313–319. doi: 10.1016/j.clinph.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain J Neurol. 1996;119 ( Pt 4)(Pt 4):1199–1211. doi: 10.1093/brain/119.4.1199 [DOI] [PubMed] [Google Scholar]
- 40.Gamble KR, Cummings TJ, Lo SE, Ghosh PT, Howard JH, Howard DV. Implicit sequence learning in people with Parkinson’s disease. Front Hum Neurosci. 2014;8:563. doi: 10.3389/fnhum.2014.00563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol. 2008;21(6):628–633. doi: 10.1097/WCO.0b013e328315a293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tosserams A, Keijsers N, Kapelle W, et al. Evaluation of Compensation Strategies for Gait Impairment in Patients With Parkinson Disease. Neurology. 2022;99(20):e2253–e2263. doi: 10.1212/WNL.0000000000201159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baudendistel ST, Haussler AM, Rawson KS, Earhart GM. Minimal clinically important differences of spatiotemporal gait variables in Parkinson disease. Gait Posture. 2024;108:257–263. doi: 10.1016/j.gaitpost.2023.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leech KA, Roemmich RT, Gordon J, Reisman DS, Cherry-Allen KM. Updates in Motor Learning: Implications for Physical Therapist Practice and Education. Phys Ther. 2022;102(1):pzab250. doi: 10.1093/ptj/pzab250. doi:10.1093/ptj/pzab250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease. Eur J Neurosci. 2007;26(8):2369–2375. doi: 10.1111/j.1460-9568.2007.05810.x [DOI] [PubMed] [Google Scholar]
- 46.Thaut MH, Rice RR, Janzen TB, Hurt-Thaut CP, McIntosh GC. Rhythmic auditory stimulation for reduction of falls in Parkinson’s disease: a randomized controlled study. Clin Rehabil. 2019;33(1):34–43. doi: 10.1177/0269215518788615 [DOI] [PubMed] [Google Scholar]
- 47.Duchesne C, Lungu O, Nadeau A, et al. Enhancing both motor and cognitive functioning in Parkinson’s disease: Aerobic exercise as a rehabilitative intervention. Brain Cogn. 2015;99:68–77. doi: 10.1016/j.bandc.2015.07.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the exploratory analysis are available from the corresponding author on reasonable request.


