Abstract
The helix–loop–helix proteins E47 and E12, which are encoded by the E2A gene, regulate several stages of T cell development. In addition, mice deficient for E2A are highly susceptible to thymic lymphoma. Here we report that the development of lymphoma in E2A-deficient mice did not require pre- and recombinase-activating gene expression. Rather, we found that, whereas illegitimate DNA rearrangement did not play a major role in the development of these lymphomas, defects that prevented pre-T cell antigen receptor expression tended to accelerate lymphomagenesis in E2A-deficient mice. These data and previous observations also provide insight into the role of Notch in lymphoma development. Specifically, we propose that Notch activation indirectly modulates E2A activity through induction of pre-Tα expression, ultimately leading to the development of lymphoma.
Commitment and progression of CD4− CD8− double-negative (DN) thymocytes to the T lineage is largely associated with the induction of gene rearrangements at the T cell antigen receptor (TCR)β, -γ, and -δ loci (1). The generation of an in-frame TCRβ gene allows for the expression of the pre-TCR complex, which contains the TCRβ chain, the pre-Tα polypeptide, and the CD3 complex of signal transduction proteins. Signaling events dependent on the formation of a pre-TCR complex result in a developmental transition referred to as β selection, characterized by the cessation of gene rearrangement, the initiation of cellular proliferation, and differentiation of DN into CD4+ CD8+ double-positive (DP) thymocytes.
Early thymocyte development is regulated in part by the E proteins, a subset of helix–loop–helix proteins. Among the members of the E proteins are E12 and E47, which are encoded by the E2A gene. A deficiency in E2A results in a substantial block at the DN stage of T cell development before the onset of TCR gene rearrangement (2). E proteins have been shown to regulate expression of the pre-Tα and recombinase-activating (RAG) genes, and are also required to promote VDJ rearrangements within the TCRβ loci (3–6). Furthermore, E2A proteins are essential for proper TCRγ and δ DNA rearrangement (7). E2A proteins also act in DN thymocytes to prevent developmental progression in the absence of TCRβ rearrangement (8). In addition to its role in early thymocyte development, a large fraction of E2A-deficient mice develop thymic lymphoma, generally within 2.5–8 months of age (2, 9). Thus E2A proteins also function as suppressors of T lymphoma in mice.
A considerable body of evidence suggests that E2A proteins may function to suppress T lymphoma in humans as well. Most T cell acute lymphoblastic leukemia and lymphoma isolates have been found to contain translocations that result in the aberrant expression of type II helix–loop–helix genes such as TAL1, TAL2, or LYL1 in the T cell lineage (10–13). Type II helix–loop–helix transcription factors bind DNA as heterodimers with type I helix–loop–helix proteins encoded by the E2A, HEB, and E2–2 genes (14). Overexpression of TAL1 and LYL1 have been shown to inhibit E2A activity, as the resulting heterodimers have altered DNA-binding specificities and transactivation properties relative to E2A homodimers (15–19). A rare subset of pediatric T cell acute lymphoblastic leukemia and lymphoma isolates is associated with a genetic lesion involving the Notch locus. Specifically, Notch1 is truncated by a chromosomal translocation to encode for a constitutively active intracellular domain (20). Previous data have indicated that Notch signaling directly influences E2A activity by modulation of its transactivation potential (21, 22). These data suggest that Notch may promote lymphomagenesis by directly modulating the activity of the E2A proteins. Furthermore, recent evidence has indicated that Ras-mediated signaling inhibits E2A activity through activation of Id expression (23). Because activated Ras has been shown to be involved in a wide variety of human cancers, these data imply that inappropriate modulation of E protein activity may contribute not only to the development of T cell acute lymphoblastic leukemia and lymphoma, but other tumors as well.
Here we show that lymphoma development in E2A-deficient mice is not perturbed in the absence of pre-TCR expression. Rather, we demonstrate that homozygosity for the severe combined immunodeficiency (scid) or TCRβ mutations significantly accelerates the onset of morbidity from lymphoma in an E2A-deficient background. In addition, disruption of RAG function increases the susceptibility of E2A-deficient mice to thymic lymphoma. Mice that are heterozygous for the E47 mutation show a greatly increased susceptibility to lymphoma in a RAG-1-null background. Thus, although aberrant rearrangement does not play a major role in the development of these lymphomas, defects that prevent pre-TCR expression enhance the rate of lymphomagenesis in E2A-deficient mice. Finally, we propose a model in which oncogenic forms of Notch indirectly modulate E2A activity through the excessive induction of pre-Tα expression, ultimately leading to the development of lymphoma.
Materials and Methods
Mice, Genotyping, and Statistical Analyses.
For generation of colonies carrying E47 and either scid or TCRβ mutations, mice carrying the E47-null mutation in an FVB/N background were bred with scid and TCRβ mutant mice in C57BL/6J backgrounds that were purchased from The Jackson Laboratory (24–26). The E47 and RAG-1 mutant colony was expanded from E47+/− RAG-1+/− mice in an FVB/N × 129 background. Mice were genotyped for the E47, RAG-1, scid, and TCRβ mutations as described (24, 27–29). Mice that seemed outwardly ill, exhibiting cachexia and/or breathing difficulty, were killed and dissected to confirm the presence of thymic lymphoma. Mice that died and were not confirmed by necropsy to have thymic lymphoma were excluded from the data presented. Statistical analyses of the rates of morbidity due to lymphoma were performed by means of a Log-Rank test, whereas comparison of the fractions of thymic lymphomas that spread to the periphery was done by use of the Fisher's Exact test.
Immunoblot and Flow Cytometry.
Lymphoma suspensions, nuclear and whole-cell extracts were prepared as described (8). For the immunoblot, 50 μg of nuclear extract or 100 μg of whole-cell extract was resolved on a SDS-7.5% polyacrylamide gel and transferred to Immobilon (Millipore) by electrophoresis. The blot was analyzed with anti-E47 (PharMingen) as described (8). Staining for surface antigens was performed as described (8). For analysis of DNA content, 106 cells were fixed in 70% ethanol/PBS, washed one time with PBS, and then resuspended in PBS plus 10 μg/ml propidium iodide (Sigma) and 100 μg/ml RNase A (Qiagen, Valencia, CA). Samples were incubated for 3 h at room temperature before analyzing on a FACscalibur (Becton Dickinson Immunocytometry Systems).
Results
The scid Mutation Increases the Rate of Lymphoma Morbidity in E47-Deficient Mice.
We interbred mice with the E47-null allele with mice carrying the scid mutation to generate a colony with E47−/− scid/scid and control genotypes. The scid defect is caused by a mutation that results in a severe reduction in DNA-dependent protein kinase (DNA-PK) activity that is necessary for normal nonhomologous end joining (30). Mice that were homozygous for both the E47 and scid mutations were found to have a greatly increased rate of morbidity from lymphoma than their E47−/− scid/+ cohorts (Fig. 1A). Of 30 E47−/− scid/+ mice 18 became ill because of thymic lymphoma within one year of age, with the median age of morbidity being approximately 230 days (Fig. 1A). Similar rates of lymphoma onset were also observed in scid/scid mice that were either E47+/− or +/+ (Fig. 1B). In contrast, all 11 mice with an E47−/− scid/scid genotype became morbid because of lymphoma, and the median age of illness was 102 days (Fig. 1A). No mice that were wild type or heterozygous for both E47 and DNA-PK developed lymphoma (data not shown). These data demonstrate that the scid mutation accelerates lymphoma morbidity in E47-deficient mice (P < 10−4).
Fig 1.
Morbidity from thymic lymphoma in E47-null mice is accelerated by homozygosity for the scid mutation. (A) Plot comparing the rate of lymphoma morbidity in E47−/− mice with scid/+ and scid/scid genotypes. (B) Plot of lymphoma morbidity in scid/scid mice with E47+/− and E47+/+ genotypes. The age at which mice were killed or died of lymphoma is plotted against the fraction of mice that remained outwardly healthy. The number of mice of each genotype is indicated in parentheses in the symbol legend. The data represent those mice that either were shown to have become ill or died because of lymphoma or survived for at least 1 year without becoming ill. Two E47−/− scid/scid, two E47−/− scid/+, four E47+/− scid/scid, and four E47+/+ scid/scid mice were found dead and were unable to be necropsied, whereas three E47+/− scid/scid and one E47 wild-type scid/scid mice died or were euthanized because of illnesses other than lymphoma; these mice were excluded from the data presented.
Lymphoma Morbidity in E2A-Deficient Mice Is Accelerated in a Homozygous TCRβ-Null Background.
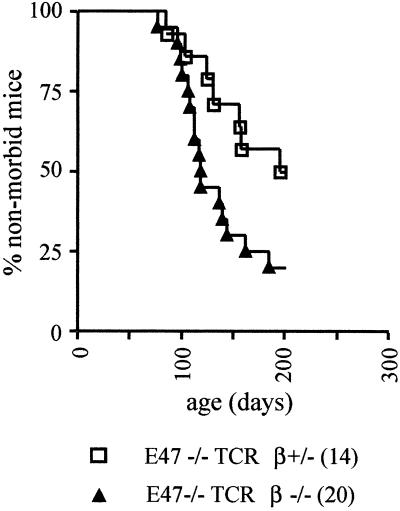
The increase in lymphomagenesis in E47-null mice induced by the scid mutation could be due to the resulting deficiency in nonhomologous end joining, or a consequence of the developmental arrest found in scid thymocytes. To help distinguish between these possibilities, we crossed E47-deficient mice into a background carrying a null mutation in the TCRβ gene, and examined the rate of lymphomagenesis in the resulting colony. The TCRβ mutation prevents the development of αβ T cells, but does not affect either nonhomologous end joining or RAG enzymatic activity (26). Because mice deficient for TCRβ are susceptible to a B cell-dependent autoimmune disorder after about six months of age, we chose to terminate this study after 200 days (31). Seven of 14 mice with an E47−/− TCRβ+/− genotype became ill within the time frame of this study, with the median point reached at day 195. In contrast, 16 of 20 mice that were homozygous for the E47 and TCRβ null mutations developed lymphoma, with the median age of illness being 118 days (Fig. 2). Thus homozygosity for the TCRβ mutation also acted to accelerate lymphoma morbidity in E2A-deficient mice, although the effect seemed somewhat less dramatic than that observed for the scid mutation (P = 0.04).
Fig 2.
Morbidity from thymic lymphoma in E47-null mice is accelerated by homozygosity for the TCRβ mutation. The age at which mice were killed or died of lymphoma is plotted against the fraction of mice that remained outwardly healthy. The number of mice of each genotype is indicated in parentheses in the symbol legend. The data represent those mice that either were shown to have become ill or died because of lymphoma or survived for at least 1 year without becoming ill. Three E47−/− TCRβ+/− and five E47−/− TCRβ−/− that were found dead and not subjected to necropsy were excluded from the data presented.
Deficiencies in RAG-1 Increase the Rate of Lymphoma Morbidity in E47−/− Mice.
To assess the role of aberrant rearrangement in inducing lymphoma in E2A-deficient mice, we generated a colony of mice carrying mutations in E47 and RAG-1.
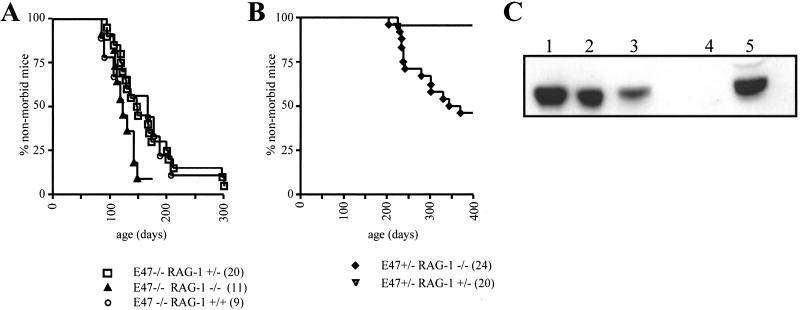
As expected, most E47-deficient mice that were wild-type or heterozygous for the RAG-1 gene developed lymphoma (Fig. 3A). Mice homozygous for both the E47 and RAG-1 null mutations also developed lymphoma (Fig. 3A). In addition, the overall rate of onset seemed to be faster than the rate observed in E47-null mice that were heterozygous or wild-type for RAG-1 (Fig. 3A). The ages of earliest and median onset of lymphoma morbidity in the E47−/− RAG-1−/− and E47−/− RAG-1+/− colonies were fairly similar. However, a complete deficiency in RAG-1 seemed to decrease the numbers of relatively long-term survivors with an E47−/− genotype. Whereas 9 of 20 mice with an E47−/− RAG-1+/− genotype and 5 of 9 E47−/− RAG-1+/+ mice did not display morbidity from lymphoma until more than 160 days of age, only 1 of 11 E47−/− RAG-1−/− did not become morbid by 150 days of age. The one relatively long-term E47−/− RAG-1−/− survivor was killed for an experiment at 173 days of age. When this mouse was analyzed it was found to have a small thymoma, although it did not seem moribund when killed. These observations indicate that aberrant DNA rearrangement events are not a critical factor in promoting lymphoma in E47-null mutant mice. In fact, a RAG-1 deficiency actually seems to promote the rate of lymphomagenesis in E47-null mice.
Fig 3.
Effect of RAG-1 deficiency on morbidity from thymic lymphoma in E47-null mice. (A) Plot comparing the rate of lymphoma morbidity in E47−/− mice with RAG-1+/+, +/−, and −/− genotypes. (B) Plot comparing the rate of lymphoma morbidity in E47+/− mice with RAG-1−/− and RAG-1+/− genotypes. The age at which mice were killed or died of lymphoma is plotted against the fraction of mice that remained outwardly healthy. The number of mice of each genotype is indicated in parentheses in the symbol legend. The data represent those mice that either were shown to have become ill or died because of lymphoma or survived for at least 1 year without becoming ill. Five E47−/− RAG-1+/−, two E47−/− RAG-1−/−, and ten E47+/− RAG-1−/− were found dead and were not subjected to necropsy, whereas one E47−/− RAG-1+/− and three E47+/− RAG-1−/− mice were found not to have thymic lymphoma upon necropsy; these mice were excluded from the data presented. (C) Thymic lymphomas from E47+/− RAG-1−/− mice express E47. Immunoblot with nuclear extracts from two E47+/− RAG-1−/− lymphomas (lanes 1–2), whole cell extract from a third E47+/− RAG-1−/− lymphoma, nuclear extract from a E47−/− scid/scid lymphoma (lane 4), and whole-cell extract from the p53−/− T lymphoma line 16610D9 (49) (lane 5) was probed with the anti-E47 antibody G127–32 (PharMingen).
Deficiency in RAG-1 Does Not Delay the Development of Lymphomas in E47+/− Mice.
Mice that are heterozygous for E2A or E47-null mutations are not generally susceptible to lymphoma (refs. 2 and 9; data not shown). Furthermore, none of the E47+/+ RAG-1−/− in our colony developed lymphoma (data not shown). Unexpectedly, 13 of the 24 mice that were generated carrying an E47+/− RAG-1−/− genotype became ill because of thymic lymphoma (Fig. 3B). These lymphomas arose much later than those observed in an E47-null background, because no morbidity in the E47+/− RAG-1−/− mice was observed before 200 days of age, whereas the E47−/− mice in this study began to become ill from lymphoma after less than 100 days (Fig. 3B). We did observe one thymic lymphoma in an E47+/− RAG-1+/− mouse in our colony; however, it was the only one of 21 mice affected with this genotype (Fig. 3B). Taken together, these data indicate that deficiencies in the E47 and RAG genes act in concert to promote the development of lymphoma.
We also observed a lymphoma in one E47+/− TCRβ−/− mouse out of a colony of 23. As described above, the E47 TCRβ mutant colony study was terminated after about 200 days, which is before the first lymphomas were observed in E47+/− RAG-1−/− mice. Thus it is possible that E47+/− TCRβ−/− mice are also susceptible to this relatively late onset lymphoma.
Heterozygosity for mutations in tumor suppressor genes has been shown often to result in an increased susceptibility to several types of cancer (32). Tumors that arise in these genetic contexts have generally been found to contain spontaneous mutations in the wild-type tumor suppressor allele. This loss of heterozygosity is thought to be a required early event in the eventual transformation of these cells. To test whether loss of heterozygosity is a common mechanism in the generation of thymic lymphoma in E47+/− RAG-1−/− mice, we examined extracts prepared from three E47+/− RAG-1−/− lymphomas for the presence of E47 by immunoblot analysis. We found that all three lymphomas expressed E47 (Fig. 3C). Thus loss of heterozygosity does not seem to be necessary for the development of lymphoma in an E47+/− RAG-1−/− background.
Phenotypic Analysis of Lymphomas.
To compare the surface phenotype with that previously observed for E2A-deficient lymphomas, thymic lymphoma isolates were analyzed for the presence of T-cell surface antigens by flow cytometry. Lymphomas derived from E47-deficient mice and carrying either the RAG-1-null or scid mutations typically expressed high but frequently heterogeneous surface levels of CD4, CD8, CD25, and CD44, as well as high levels of CD24 and Thy-1 and moderate levels of CD5 (Fig. 4 and data not shown). Lymphomas from E47+/− RAG-1−/− mice also appeared to have a similar surface phenotype to that of the E47-null lymphomas (Fig. 4). These expression patterns were consistent with that previously observed for lymphomas derived from E2A-deficient mice (data not shown; refs. 2 and 9).
Fig 4.
Phenotypic analysis of lymphomas from E47-deficient mice with defects in TCRβ expression. Lymphomas from mice with E47−/− scid/scid, E47+/− RAG-1−/−, E47−/− RAG-1−/−, E47−/− TCRβ−/−, and E47−/− TCRβ+/− genotypes were analyzed by flow cytometry for surface expression of CD25, CD44, CD8, and CD4 as well as for DNA content. Each column of plots represents the analysis of one lymphoma with the genotype indicated at the top. (Top) Dot plots depicting surface CD25 and CD44 expression; (Middle) Dot plots indicating surface CD8 and CD4 levels. (Bottom) Histograms showing the DNA content measured by using propidium iodide, with the percentage of cells with greater than 2N listed in the top right corner of each histogram. The DNA content histogram from wild-type thymocytes is also provided for comparison.
All of the E2A-deficient lymphomas we have analyzed consisted of relatively large cells, suggesting that they were mitotically active (data not shown). To comfirm that these cells were indeed actively cycling the DNA content of several lymphoma isolates was analyzed. At least 20% of the cells in all of the isolates that were analyzed showed a DNA content indicative of the S or G2/M phases (Fig. 4 and data not shown). A few of the isolates were grossly aneuploid in character, a characteristic also observed occasionally in E2A-deficient lymphomas without defects in TCRβ rearrangement and expression (data not shown). Thus lymphomas from E2A-deficient mice, with or without mutations in RAG-1 or DNA-PK, tend to be highly mitotic.
Effect of TCRβ and RAG-1 Mutations on Spread of Lymphomas to Periphery.
All of the lymphomas we observed in these studies, like lymphomas characterized in E2A-deficient mice, seemed to originate in the thymus, which was invariably grossly enlarged (2, 9). However, a high percentage of E2A-deficient mice also exhibited varying degrees of lymphoma colonization of the spleen, kidneys, lymph nodes, and liver (2, 9). We have examined the mice affected with lymphoma in this study to see if deficiencies in rearrangement and TCRβ expression can affect the frequency with which E2A-deficient lymphomas spread to the periphery by macroscopic analysis of peripheral organs upon necropsy. Lymphomas deficient for either RAG-1 or TCRβ had a reduced tendency to spread to peripheral organs (Table 1). One of the TCRβ-null lymphomas that was found to colonize peripheral organs expressed surface TCRγδ, thus tightening the correlation between TCR expression and the capability to invade the periphery (P = 0.006). Thus, TCR or pre-TCR expression seemed to be an important contributing factor to the efficiency of peripheral invasion.
Table 1.
Deficiencies in RAG-1 and TCRβ correlate with decreased spread of E47-null thymic lymphomas to peripheral organs
| Genotype | RAG-1+/+ | RAG-1+/− | RAG-1−/− | TCRβ +/− | TCRβ −/− |
|---|---|---|---|---|---|
| Peripheral spread | 3 | 12 | 1 | 5 | 4 (1 γδ+) |
| No peripheral spread | 5 | 8 | 9 | 2 | 10 (1 γδ+) |
Mice moribund because of lymphoma were dissected and examined for evidence of colonization of peripheral organs. This table lists the numbers of E47−/− thymic lymphomas from mice with the indicated genotypes that either exhibited (top row) or lacked (bottom row) clear indications of invasion of peripheral organs by lymphoma. Two lymphomas from E47−/−, TCRβ −/− mice expressed the γδ TCR, of which one did not exhibit spread to the periphery, as indicated in parentheses in the appropriate boxes.
Discussion
Previous studies have indicated that the E2A gene products act to regulate the rearrangement of TCR gene segments (6, 7). These observations raised the possibility that a deficiency in E2A could result in an increase in aberrant rearrangement events and perhaps explain the susceptibility of E47 and E2A-null mice to thymic lymphoma. Another possibility, however, is that lymphomagenesis in E2A-deficient mice is related to the roles that E2A proteins play in regulating thymocyte developmental progression. In particular, recent evidence has demonstrated that E2A proteins are required to prevent the aberrant maturation of thymocytes that have defects in TCRβ expression (8). The data presented here demonstrate that deficiencies in TCRβ, RAG-1, and DNA-PK, regardless of their effect on the rearrangement process, all act to accelerate lymphoma morbidity in the context of a deficiency for E47, suggesting that interference with pre-TCR expression acts to promote lymphomagenesis in E2A-deficient mice.
Acceleration of Lymphoma in E2A-Deficient Mice by scid, TCRβ, and RAG-1 Mutations.
Mice homozygous for the scid mutation are susceptible to T lymphoma, but most scid mice do not exhibit lymphoma morbidity until an age of at least 7 months (33). However, the scid mutation has been shown to cooperate with a deficiency of p53 in promoting the onset of lymphoma, although one study failed to observe such an effect (27, 34, 35). Synergy between p53 and scid mutations in the promotion of lymphoma is consistent with what is known about the function of DNA-PK in nonhomologous end joining and p53 in promoting the death or growth arrest of cells that have incurred double-strand DNA breaks (30). Our data suggest that the cooperation between the scid mutation and E47 deficiency with respect to the acceleration of lymphoma is similar in magnitude to that between scid and p53 mutations (Fig. 1). However, we note that the rate of lymphoma morbidity in E47−/− mice is also accelerated significantly by homozygosity for a null mutation in the TCRβ or the RAG locus (Figs. 2 and 3), which suggests that the increased rate of lymphoma in E47−/− scid mutant mice may be due more to the effect of the scid mutation on TCRβ and pre-TCR expression, rather than be a direct consequence of the deficiency in double-strand DNA breaks repair.
Our data suggest that other important cofactors for lymphomagenesis must exist besides RAG-mediated DNA cleavage, at least in mouse mutant model systems. One possible candidate for such a cofactor could be the rapid rate of mitosis that accompanies the transition from DN to DP thymocyte. Deficiencies in E2A, p53, and Ikaros, as well as overexpression of Lck or Pim-1, have been shown to increase the susceptibility to thymic lymphoma and to promote the DN-to-DP transition in the absence of pre-TCR expression (2, 8, 9, 36–43). On the basis of these observations and the data described here we propose that defects in the regulation of thymocyte maturation, rather than genetic damage arising from errors in DNA rearrangement, function as the critical initiators of thymic lymphoma in E2A-deficient mice.
Lymphoma in E47+/− RAG-1−/− Mice.
Approximately half of the E47 heterozygotes in our colony that were also RAG-1−/− became ill unexpectedly because of lymphoma (Fig. 3B). These observations suggest that the developmental block caused by the RAG deficiency raises the threshold of E2A activity required to prevent lymphoma. We have found no evidence of a loss-of-heterozygosity mechanism operating in the development of RAG-1−/− E47+/− lymphomas, because the isolates we examined were found to express E47 protein (Fig. 3C). It is possible, however, that these lymphomas may have incurred other secondary events that act to inhibit E2A activity. Lymphoma morbidity in RAG-1-deficient E47 heterozygotes occurs with a significantly longer latency than in an E47-null background, which might suggest that a larger number of secondary events are necessary for lymphoma progression in E47+/− RAG-1−/− mice. It will be interesting to determine how E2A activity is regulated in E47+/− RAG-1−/− lymphomas.
Neither heterozygosity for E2A mutations nor a complete deficiency of RAG-1 alone has been previously found to promote lymphoma susceptibility in the absence of other mutations. RAG-1-null mice with a transgene directing thymocyte-specific expression of a dominant negative form of the FADD gene (FADD-DN), however, have also been reported to develop lymphoma (44). Both FADD-DN RAG-1-null and E47+/− RAG-1−/− mice have been found to contain DP thymocytes, which is indicative of defects in the β selection checkpoint that regulates the DN-to-DP transition (8, 44). These observations provide additional correlative data suggesting that defects in the regulation of thymocyte maturation, rather than genetic damage arising from errors in DNA rearrangement, function as the critical initiators of thymic lymphoma in mutant models such as E2A-deficient mice.
Acceleration of Lymphoma in E2A-Deficient Mice by Defects in TCRβ Expression.
One of the issues raised by our data is how mutations that block TCRβ expression act to accelerate lymphoma morbidity in E2A-deficient mice through mechanisms other than defects in the rearrangement process. Thymocytes that are homozygous for the RAG-1, scid, or TCRβ mutations normally exhibit a complete developmental block in the DN stage because of the inability to express the pre-TCR complex (1). Such a disruption of development could in theory contribute to an increase in oncogenic potential. However, we have shown recently that E47−/− thymocytes with defects in TCRβ expression are overwhelmingly DP, indicating that the normal developmental checkpoint has been completely abrogated (8). Thus, it does not seem that the increased rate of lymphomagenesis in E47-null TCRβ-deficient backgrounds can be a direct consequence of an arrest in development. However, our data suggest that some differences must exist between the E2A-deficient thymocytes that mature in a TCRβ-deficient background and those that develop in the context of normal pre-TCR expression. We are currently investigating whether any unique aspects of thymocytes deficient for both E47 and TCRβ could potentially render them more susceptible to oncogenic transformation.
Roles of E2A and Notch in Lymphomagenesis.
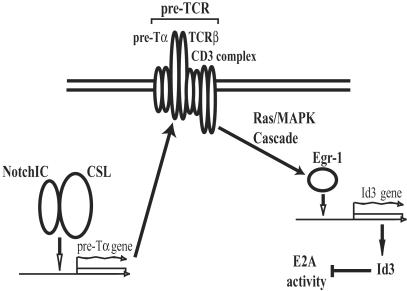
In contrast to the malignancies observed in E2A-deficient mice, lymphoma development in mice expressing constitutively active forms of Notch is blocked by null mutations in genes required for pre-TCR expression (45, 46). These data and our observations suggest that activated Notch does not promote lymphomagenesis through direct effects on E2A, even though ectopic Notch activity has been reported to inhibit directly the function of E2A in vitro (21). However, we would like to consider the possibility that Notch and E2A function are indirectly connected (Fig. 5). Notch signaling has been shown to activate pre-Tα gene expression both in T lineage cells and in bone marrow cells (47, 48). It is thus possible that activated forms of Notch could induce aberrantly high levels of the pre-TCR complex. Taken together with data showing that pre-TCR signaling negatively regulates E47 DNA binding, these observations suggest that activated Notch could promote lymphoma through a pre-TCR-dependent effect on E2A activity (8). It thus may be interesting to determine how lymphomagenesis induced by activated Notch is affected by other mutations that disrupt the connection between pre-TCR signaling and E2A activity.
Fig 5.
Model depicting an indirect pathway linking expression of activated Notch (NotchIC) with the inhibition of E2A. NotchIC has been shown to activate the pre-Tα promoter through association with the CSL transcription factor, and thus may induce aberrantly high levels of the pre-TCR complex (48). Signaling through the antigen receptor complex in thymocytes acts to inhibit E2A activity, at least in part through the induction of Id3 transcription by a Ras/mitogen-activated protein kinase (Ras/MAPK)-dependent pathway that activates the early growth response gene 1 (Egr-1) (23).
Acknowledgments
We thank Randall Johnson (University of California at San Diego) and Barbara Kee (University of Chicago) for providing mice. Statistical analyses were performed by the University of California at San Diego Cancer Center Biostatistics Shared Resource. I.E. is a Lymphoma Research Foundation Fellow. This research was supported by grants from the National Institutes of Health to (C.M.).
Abbreviations
DN, CD4−, CD8− double-negative thymocyte
DP, double positive
RAG, recombinase-activating genes
scid, severe combined immunodeficiency
DNA-PK, DNA-dependent protein kinase
TCR, T cell antigen receptor
References
- 1.Fehling H. J. & von Boehmer, H. (1997) Curr. Opin. Immunol. 9, 263-275. [DOI] [PubMed] [Google Scholar]
- 2.Bain G., Engel, I., Maandag, E. C. R., Riele, H. P. J. t., Voland, J. R., Sharp, L. L., Chun, J., Huey, B., Pinkel, D. & Murre, C. (1997) Mol. Cell. Biol. 17, 4782-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blom B., Heemskerk, M. H. M., Verschuren, M. C. M., Dongen, J. J. M. v., Stegmann, A. P. A., Bakker, A. Q., Couwenberg, F., Res, P. C. M. & Spits, H. (1999) EMBO J. 18, 2793-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi A., Yamasaki, S., Takase, K., Nakatsu, F., Arase, H., Onodera, M. & Saito, T. (2001) J. Immunol. 167, 2157-2163. [DOI] [PubMed] [Google Scholar]
- 5.Reizis B. & Leder, P. (2001) J. Exp. Med. 194, 979-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barndt R. J., Dai, M. & Zhuang, Y. (2000) Mol. Cell. Biol. 20, 6677-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bain G., Romanow, W. J., Albers, K., Havran, W. L. & Murre, C. (1999) J. Exp. Med. 189, 289-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel I., Johns, C., Bain, G., Rivera, R. R. & Murre, C. (2001) J. Exp. Med. 194, 733-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan W., Young, A. Z., Soares, V. C., Kelley, R., Benezra, R. & Zhuang, Y. (1997) Mol. Cell. Biol. 17, 7317-7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bash R. O., Hall, S., Timmons, C. F., Crist, W. M., Amylon, M., Smith, R. G. & Baer, R. (1995) Blood 86, 666-676. [PubMed] [Google Scholar]
- 11.Xia Y., Brown, L., Tsan, J., Yang, C., Siciliano, M., Espinosa, R., Lebeau, M. & Baer, R. (1991) Proc. Natl. Acad. Sci. USA 88, 11416-11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellentin J. D., Smith, S. D. & Cleary, M. L. (1989) Cell 58, 77-83. [DOI] [PubMed] [Google Scholar]
- 13.Ferrando A. A., Neuberg, D. S., Staunton, J., Loh, M. L., Huard, C., Raimondi, S. C., Behm, F. G., Pui, C.-H., Downing, J. R., Gilliland, D. G., et al. (2002) Cancer Cells 1, 75-87. [DOI] [PubMed] [Google Scholar]
- 14.Massari M. E. & Murre, C. (2000) Mol. Cell. Biol. 20, 429-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu H. L., Huang, L., Tsan, J. T., Frank, W., Wright, W. E., Hu, J. S., Kingston, R. E. & Baer, R. (1994) Mol. Cell. Biol. 14, 1256-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu H.-L., Wadman, I., Tsan, J. T. & Baer, R. (1994) Proc. Natl. Acad. Sci. USA 91, 5947-5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voronova A. F. & Lee, F. (1994) Proc. Natl. Acad. Sci. USA 91, 5952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto A., Cui, X., Naumovski, L. & Cleary, M. (1996) Mol. Cell. Biol. 16, 2394-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S. T. & Sun, X.-H. (1998) J. Biol. Chem. 273, 7030-7037. [DOI] [PubMed] [Google Scholar]
- 20.Ellisen L., Bird, J., West, D., Soreng, A., Reynolds, T., Smith, S. & Sklar, J. (1991) Cell 66, 649-661. [DOI] [PubMed] [Google Scholar]
- 21.Ordentlich P., Lin, A., Shen, C. P., Blaumueller, C., Matsuno, K., Artavanis-Tsakanos, S. & Kadesch, T. (1998) Mol. Cell. Biol. 18, 2230-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izon D. J., Aster, J. C., He, Y., Weng, A., Karnell, F. G., Patriub, V., Xu, L., Bakkour, S., Rodriguez, C., Allman, D. & Pear, W. S. (2002) Immunity 16, 231-243. [DOI] [PubMed] [Google Scholar]
- 23.Bain G., Cravatt, C. B., Loomans, C., Alberola-Ila, J., Hedrick, S. M. & Murre, C. (2001) Nat. Immunol. 2, 165-171. [DOI] [PubMed] [Google Scholar]
- 24.Bain G., Maandag, E. C. R., Riele, H., Feeney, A. J., Sheehy, A., Schlissel, M., Shinton, S. A., Hardy, R. R. & Murre, C. (1997) Immunity 6, 145-154. [DOI] [PubMed] [Google Scholar]
- 25.Bosma G. C., Custer, R. P. & Bosma, M. J. (1983) Nature (London) 301, 527-530. [DOI] [PubMed] [Google Scholar]
- 26.Mombaerts P., Clarke, A. R., Rudnicki, M. A., Iacomini, J., Itohara, S., Lafaille, J. J., Wang, L., Ichikawa, Y., Jaenisch, R., Hooper, M. L. & Tonegawa, S. (1992) Nature (London) 360, 225-231. [DOI] [PubMed] [Google Scholar]
- 27.Liao M. J., Zhang, X. X., Hill, R., Gago, J. J., Qumsiyeh, M. B., Nichols, W. & VanDyke, T. (1998) Mol. Cell. Biol. 18, 3495-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blunt T., Gell, D., Fox, M., Taccioli, G. E., Lehmann, A. R., Jackson, S. P. & Jeggo, P. A. (1996) Proc. Natl. Acad. Sci. USA 93, 10285-10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng S. L., Robert, M. E., Hayday, A. C. & Craft, J. (1996) J. Exp. Med. 184, 1149-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danska J. S. & Guidos, C. J. (1997) Semin. Immunol. 9, 199-206. [DOI] [PubMed] [Google Scholar]
- 31.Mombaerts P., Mizoguchi, E., Grusby, M. J., Glimcher, L. H., Bhan, A. K. & Tonegawa, S. (1993) Cell 75, 274-282. [DOI] [PubMed] [Google Scholar]
- 32.Hakem R. & Mak, T. W. (2001) Annu. Rev. Genet. 35, 209-241. [DOI] [PubMed] [Google Scholar]
- 33.Bosma M. J. & Carroll, A. M. (1991) Annu. Rev. Immunol. 9, 323-350. [DOI] [PubMed] [Google Scholar]
- 34.Guidos C. J., Williams, C. J., Grandal, I., Knowles, G., Huang, M. T. F. & Danska, J. S. (1996) Genes Dev. 10, 2038-2054. [DOI] [PubMed] [Google Scholar]
- 35.Nacht M., Strasser, A., Chan, Y. R., Harris, A. W., Schlissel, M., Bronson, R. T. & Jacks, T. (1996) Genes Dev. 10, 2055-2066. [DOI] [PubMed] [Google Scholar]
- 36.Bogue M. A., Zhu, C., Aguilar-Cordova, E., Donehower, L. A. & Roth, D. B. (1996) Genes Dev. 10, 553-565. [DOI] [PubMed] [Google Scholar]
- 37.Jiang D., Lenardo, M. J. & Zuniga-Pflucker, J. C. (1996) J. Exp. Med. 183, 1923-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacks T., Remington, L., Williams, B. O., Schmitt, E. M., Halachmi, S., Bronson, R. T. & Weinberg, R. A. (1994) Curr. Biol. 4, 1-7. [DOI] [PubMed] [Google Scholar]
- 39.Winandy S., Wu, L., Wang, J.-H. & Georgopoulos, K. (1999) J. Exp. Med. 190, 1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abraham K. M., Levin, S. D., Marth, J. D., Forbush, K. A. & Perlmutter, R. M. (1991) Proc. Natl. Acad. Sci. USA 88, 3977-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mombaerts P., Anderson, S. J., Perlmutter, R. M., Mak, T. W. & Tonegawa, S. (1994) Immunity 1, 261-267. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt T., Karsunky, H., Rodel, B., Zevnik, B., Elsasser, H. & Moroy, T. (1998) EMBO J. 17, 5349-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Lohuizen M., Verbeek, S., Krimpenfort, P., Domen, J., Saris, C., Radaszkiewics, T. & Berns, A. (1989) Cell 56, 673-682. [DOI] [PubMed] [Google Scholar]
- 44.Newton K., Harris, A. W. & Strasser, A. (2000) EMBO J. 19, 931-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allman D., Karnell, F. G., Punt, J. A., Bakkour, S., Xu, L., Myung, P., Koretzky, G. A., Pui, J. C., Aster, J. C. & Pear, W. S. (2001) J. Exp. Med. 194, 99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellavia D., Campese, A. F., Checquolo, S., Balestri, A., Biondi, A., Cazzaniga, G., Lendahl, U., Fehling, H. J., Hayday, A. C., Frati, L., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 3788-3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deftos M. L., Huang, E., Ojala, E. W., Forbush, K. A. & Bevan, M. J. (2000) Immunity 13, 73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reizis B. & Leder, P. (2002) Genes Dev. 16, 295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bain G., Quong, M. W., Soloff, R. S., Hedrick, S. M. & Murre, C. (1999) J. Exp. Med. 190, 1605-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]