Abstract
Atopic dermatitis (AD) is a pruritic inflammatory skin disease. Because IL-18 directly stimulates T cells and mast cells to release AD-associated molecules, Th2 cytokines, and histamine, we investigated the capacity of IL-18 to induce AD-like inflammatory skin disease by analyzing KIL-18Tg and KCASP1Tg, which skin-specifically overexpress IL-18 and caspase-1, respectively. They spontaneously developed relapsing dermatitis with mastocytosis and Th2 cytokine accumulation accompanied by systemic elevation of IgE and histamine. Stat6-deficient KCASP1Tg displayed undetectable levels of IgE but manifested the same degree of cutaneous changes, whereas IL-18-deficient KCASP1Tg evaded the dermatitis, suggesting that IL-18 causes the skin changes in the absence of IgE/stat6. KIL-18Tg and IL-1-deficient KCASP1Tg took longer to display the lesion than KCASP1Tg. Thus, AD-like inflammation is initiated by overrelease of IL-18 and accelerated by IL-1. Our present study might provide insight into understanding the pathogenesis of and establishing therapeutics for chronic inflammatory skin diseases including AD.
Atopic dermatitis (AD) is a common inflammatory skin disease (1, 2), characterized by pruritus, chronic relapsing course, genetic background, and occasional association with high serum levels of IgE. Although the mechanism underlying AD is still elusive, activated T cells, basophils, and mast cells seem to play a crucial role in induction of AD. Allergen-dependent cross-linkage of FcɛR on basophils and mast cells activates them to produce Th2-related cytokines, such as IL-4, IL-13, and IL-5 and chemical mediators (3–8), suggesting the importance of antigen (Ag)/Ag-specific IgE in activation of basophils and mast cells (acquired type allergic response). However, we recently demonstrated the alternative, IgE-independent activation pathway (9, 10). IL-18 in the presence of IL-3 directly stimulates basophils and mast cells to produce these mediators in an IgE-independent manner in vitro (innate type allergic response) (9). IL-18 is a unique cytokine capable of strongly stimulating both IFN-γ and IL-4 production even in the absence of T cell antigen receptor engagement when it acts on freshly isolated T cells with IL-12 and IL-2, respectively (10–15). This innate style T cell activation is one of the outstanding properties of IL-18. Moreover, administration of IL-18 to normal BALB/c or C57BL/6 mice induces polyclonal IgE production in a CD4+ T cell-, stat6-, and IL-4-dependent manner (10). IL-18, like IL-1β, is stored as biologically inactive precursor form (pro) in various cell types, including macrophages and keratinocytes, and becomes active after cleavage with caspase-1 or caspase-1-like enzyme (16–22). Previously, we showed that KCASP1Tg that overexpress caspase-1 in their keratinocytes spontaneously release biologically active IL-18, produce IgE partly, but obviously depending on the action of IL-18, and manifest chronic dermatitis under specific pathogen-free (SPF) conditions (14, 23). Thus, IL-18 might induce allergic disorders, particularly intrinsic atopic diseases characterized by the absence of elevation of proper Ag-specific IgE (2, 24).
Because caspase-1 also activates other cytokines such as IL-1β, it is important to address whether IL-18-transgenic mice that keratinocyte-specifically overexpress mature IL-18 alone, designated as KIL-18Tg, disclose phenotypes similar to those in KCASP1Tg (23). In addition, IL-18-transgenic mice may allow us to clarify whether IL-18, but not IgE, is responsible for inducing atopic phenotypes in the absence of specific allergen. Here, we showed that both KCASP1Tg and KIL-18Tg developed AD-like skin disease, which is characterized by the presence of high plasma levels of histamine, frequent skin-scratching, and mast cell accumulation in the lesion. However, KIL-18Tg required much longer latency to manifest the cutaneous lesions as compared with KCASP1Tg. IL-1-deficient KCASP1Tg required almost the same incubation time to develop the skin lesion as did KIL-18Tg, suggesting the potent disease-accelerating action of IL-1. In contrast, depletion of IL-18 almost completely abrogated pruritic dermatitis by diminution of mast cell accumulation in the skin as well as plasma histamine levels. In addition, stat6-deficient KCASP1Tg, although containing undetectable levels of IgE in their sera, developed pruritic dermatitis without any delay. Therefore, IL-18 can induce such inflammatory skin lesions without inducing allergen-specific IgE production. These results clearly demonstrate the importance of IL-18-dependent but IgE/stat6-independent atopic skin inflammation and may provide us with a classification of allergic responses into IgE/stat6-dependent acquired type and IL-18-dependent innate type.
Experimental Procedures
DNA Construct and Transgenic Mice.
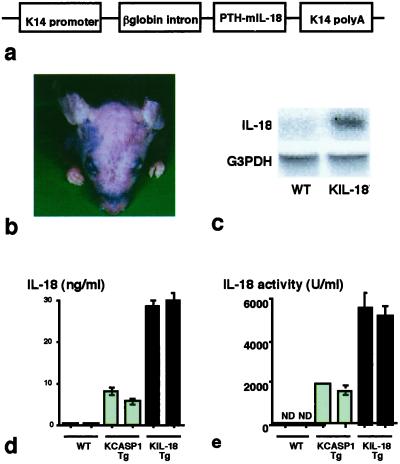
The cDNA-encoding region of the mouse mature IL-18 (25) was ligated into human keratin 14 (K14) promoter and rabbit β-globin intron, a kind gift from T. Tanaka of Kyoto University, by blunt-end ligation (Fig. 1a). The linear K14/IL-18 DNA fragment was injected into fertilized eggs of C57BL/6 mice as reported (23). A keratinocyte-specific mature IL-18-transgenic mice line (KIL-18Tg) was established. Of a total of 50 mice, two lines were transgenic for IL-18. Male KIL-18Tg were mated with C57BL/6 wild-type (WT) females, and generated KIL-18Tg and WT offspring in a 1:1 ratio. All experiments were performed on mice heterozygous for the transgene compared with nontransgenic WT littermates.
Fig 1.
Spontaneous development of chronic dermatitis in KIL-18Tg. (a) Schematic structure of the transgene for IL-18. The mouse mature IL-18 cDNA was ligated to the human keratin 14 promoter to drive its basal keratinocyte-specific expression. The transgene construct also contained the rabbit β-globin intron sequence and the K14 polyadenylation signal to aid in processing the transcript. (b) Cutaneous changes in KIL-18Tg. Skin alterations were observed at 24 weeks at the earliest. A result representative of three mice is shown. Similar results were obtained in three independent experiments. (c) Northern blotting analysis. Total RNA was extracted from the skin of KIL-18Tg (48 weeks old) or WT. Messenger RNA expressions for human preproPTH (IL-18) that was ligated to cDNA that encodes mature IL-18 and G3PDH were determined by Northern blotting analysis. (d and e) High serum levels of IL-18 in KIL-18Tg. Sera were sampled from KCASP1Tg, KIL-18Tg, or WT littermates at 36 weeks after birth. IL-18 concentration in each serum was determined by ELISA (d) or biological assay (e). Data are represented as mean ± SD of triplicates. Similar results were obtained in three independent experiments.
Mice.
Transgenic mice that keratinocyte-specifically overexpress human precursor caspase-1 gene containing the same human K14 promoter (female, 4–60 weeks old), designated as KCASP1Tg, were used for this study (23). IL-1α/β-deficient KCASP1Tg were generated by the cross of KCASP1Tg with IL-1α/β double-knockout mice with C57BL/6 background (26). Stat6-deficient KCASP1Tg or IL-18-deficient KCASP1Tg were generated by the cross of KCASP1Tg with stat6-deficient mice (27), and IL-18-deficient mice with C57BL/6 background (28), respectively.
Reagents.
FITC-conjugated anti-CD3, phycoerythrin (PE)-conjugated anti-B220, FITC-conjugated anti-CD4, Cy-conjugated anti-CD8, FITC-conjugated anti-Mac-1, PE-conjugated anti-Gr-1, biotinylated anti-CD154 [CD40 ligand (L)], and PE-streptavidin were purchased from PharMingen. Culture medium generally used in this study was RPMI 1640 supplemented with 10% FCS, 100 units/ml of penicillin, 100 μg/ml of streptomycin, 50 μM 2-mercaptoethanol, and 2 mM L-glutamine.
Assay for IL-18 Activity.
IL-18 activity in the serum of various mice was determined by using IL-18-responsive NK cell clone named LNK cells according to the method shown elsewhere (22, 29).
Northern Blotting Analysis.
Total RNA was extracted from skin of KIL-18 and WT mice by using Isogen reagent (Nippon Gene, Toyama, Japan). Northern blotting analysis was performed by using 32P-labeled cDNAs encoding murine IL-18 or glyceraldehyde-3-phosphate dehydrogense (G3PDH) according to the method described (23).
Flow Cytometry.
Thymocytes or spleen cells from various mutant and WT mice were stained with various combinations of mAbs. Stained cells were analyzed by using a dual-laser FACScalibur (Becton Dickinson). Ten thousand cells were analyzed and data were processed with CELLQUEST (Becton Dickinson) (14, 22).
Histological Study.
Skin specimens were sampled from various types of transgenic mice, fixed, and stained with hematoxylin and eosin. In some experiments skin specimens were stained with toluidine blue to identify mast cells by their positive metachromasia.
Cell Preparation.
Splenic CD4+ T cells were isolated by magnetic cell sorting after incubation with anti-CD4-binding magnetic beads (Miltenyi Biotec, Auburn, CA). Splenic CD4+ T cells (1 × 106 per ml) were incubated in anti-CD3-bound 96-well plates for 48 h. Cytokine concentration in each supernatant was determined by ELISA.
ELISA for Cytokines and Ig.
IL-4 and IFN-γ levels were measured by corresponding ELISA kits (Genzyme TECHNE). IL-18 concentration was determined by an ELISA kit from MBL (Nagoya, Japan). Plasma histamine levels were measured by RIA (SRL, Osaka). Serum levels of IgE, IgG1, and IgM were also measured by ELISA according to the methods described (14).
Frequency of Skin-Scratching.
Mice were kept calm at least for 1 h, and then monitored by a video camera for 1 h. Frequency of scratching was counted at three distinct time points of 10-min duration as randomly selected.
Score for Skin Alterations.
Skin alterations were observed with a week interval. Score was estimated by relative evaluation of skin changes to the maximum skin alterations of each line with individual genetic backgrounds at each time point.
Percent Levels of AD-Associated Parameter.
Percent levels of serum IL-18 amounts, serum IgE and IgG1 concentration, plasma concentration of histamine, mast cell numbers in the skin specimens, and skin-scratching frequency observed in various transgenic mice compared with those in KCASP1Tg were calculated.
Results and Discussion
Transgenic Mice Overexpressing Mature IL-18 in Their Skin.
Although KIL-18Tg were born normally and were healthy, they eventually developed skin diseases at about 6 months after birth under SPF conditions (Fig. 1b). In contrast, KCASP1Tg manifested skin disorders within 8 weeks (23). KIL-18Tg, like KCASP1Tg, frequently scratched their skin, particularly the skin lesion, which will be detailed later (see Fig. 5). Northern blotting analysis revealed the presence of mature IL-18 in the skin of KIL-18Tg (Fig. 1c) and 105 bp bigger endogenous IL-18 mRNA encoding proIL-18 in the skin of both WT and KIL-18Tg (data not shown). Like KCASP1Tg (23), KIL-18Tg contained the transgene selectively in the skin, but not in the liver, kidney, colon, lung, brain, or spleen (data not shown).
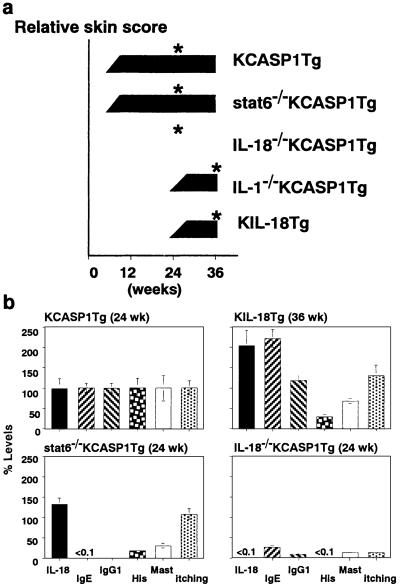
Fig 5.
IL-18-dependent, but stat6-independent pruritic dermatitis. (a) IL-18 is an initiating and IL-1, promoting factor for AD-like dermatitis. KCASP1Tg (n = 5), stat6-deficient KCASP1Tg (n = 2), IL-18-deficient KCASP1Tg (n = 2), or IL-1α/β-deficient KCASP1Tg (n = 2) and KIL-18Tg (n = 5) were kept under SPF conditions. Their skin alterations were scored at a week interval. A representative result of two to five mice in each experimental group is shown. Asterisks indicate the time points at which the indicated experiments (b; Table 1) were performed. The similar results were obtained in two independent experiments. ND, not detected. (b) Elimination of AD-associated parameters in IL-18-deficient KCASP1Tg. Percent levels of individual parameters in each experimental group compared with those in the KCASP1Tg group as shown in Table 1 were calculated. Data are represented as percent mean ± percent SD of each experimental group.
KIL-18Tg, having had high serum levels of IL-18 even at birth, persistently displayed much higher levels of IL-18 than did KCASP1Tg (Fig. 1d). Because their sera induced IFN-γ in LNK cells, a IL-18-responsive cell line (29), IL-18 in the serum of KIL-18Tg or KCASP1Tg is biologically active (Fig. 1e) (23). Both types of transgenic mice showed high levels of IL-18 in their sera (23). Downstream cytokines of IL-18, such as IL-4, IL-13, and IFN-γ, were not detected in either KCASP1Tg or KIL-18Tg serum by commercially available ELISA kits.
Neutrophil Accumulation and Th2 Deviation in the Spleen of KIL-18Tg and KCASP1Tg.
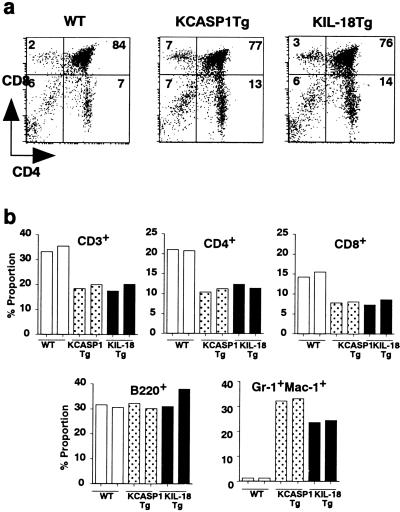
Although both KIL-18Tg and KCASP1Tg showed almost intact T cell development in their thymus (Fig. 2a), their splenic lymphocytes showed relatively lower proportion of T cells as compared with WT (Fig. 2b). However, CD4+ T cells/CD8+ T cells ratio and proportion of B cells in their spleens seemed to be equal to those in WT (Fig. 2b). Proportion of neutrophils determined by Gr-1+ Mac-1+ cells was tremendously elevated in the spleen of KIL-18Tg or KCASP1Tg (Fig. 2b), which might be partly because of granulocyte/macrophage colony-stimulating factor, because T cells can produce granulocyte/macrophage colony-stimulating factor in response to IL-18 in vitro (30). Before the onset, neutrophil accumulation in the spleen of both types of transgenic mice was only slight, but was strikingly enhanced after the onset (data not shown), suggesting a possible contribution of neutrophils to the development of such cutaneous changes.
Fig 2.
Increase of splenic neutrophil proportion in both types of transgenic mice. Thymocytes were prepared from KCASP1Tg, KIL-18Tg, and WT littermates at 36 weeks after birth, and their expression of CD4 or CD8 was determined by flow cytometry (a). Spleen cells from various genotype mice were stained with PE-anti-B220, FITC-anti-CD3, FITC-anti-CD4 or PE-anti-CD8, or PE-anti-Gr-1 plus FITC-anti-Mac-1, and the percent proportion of each cell type was calculated (b). A representative result is shown. Similar results were obtained in three independent experiments.
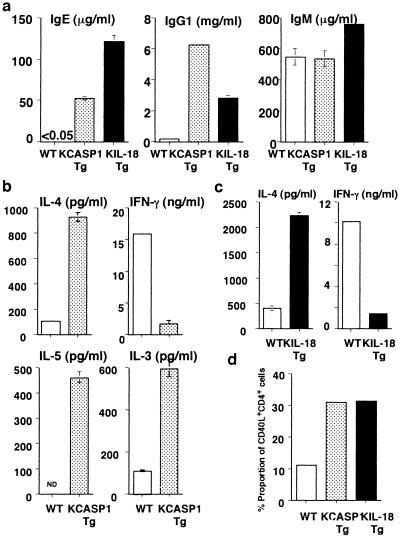
Next, we tested whether endogenously accumulated IL-18 in KIL-18Tg resulted in high-serum IgE/IgG1 and dominant Th2 response as did in KCASP1Tg (14). Consistently with previous reports (14, 15), both IgE and IgG1 levels were markedly elevated in KIL-18Tg and KCASP1Tg (Fig. 3a). We measured the capacity of freshly isolated splenic CD4+ T cells to produce cytokines in response to immobilized anti-CD3. CD4+ T cells from KCASP1Tg produced much more amounts of IL-3, IL-4, and IL-5 but smaller amounts of IFN-γ as compared with WT (Fig. 3b). CD4+ T cells from KIL-18Tg also produced greater amounts of IL-4 but less IFN-γ (Fig. 3c). We simultaneously examined whether their CD4+ T cells express CD40L, a critical molecule required for IgE production by B cells (31, 32). A 3-fold increase in the proportion of CD4+ T cells expressing CD40L was observed in both types of transgenic mice (Fig. 3d). These results indicate that IL-18 induces IgE/IgG1 response by causing CD4+ T cells to develop into Th2 cells and to express CD40L under SPF conditions.
Fig 3.
Spontaneous deviation of splenic CD4+ T cells into Th2 cells. (a) High serum levels of IgE and IgG1 in both types of transgenic mice. Sera were sampled from the various types of mice (36 weeks old) and their serum levels of various types of Ig were measured by ELISA. Data are represented as mean ± SD of triplicate cultures. Similar results were obtained in three independent experiments. (b and c) Spontaneous development of Th2 cells in both types of transgenic mice. Splenic CD4+ T cells from KCASP1Tg or WT littermates at 12 weeks of age (b) or from KIL-18Tg or WT littermates at 36 weeks of age (c) were isolated by membrane attack complexes and were incubated with immobilized anti-CD3 for 48 h. Concentration of IL-4 and IFN-γ in each supernatant was determined by ELISA. Data are represented as mean ± SD of triplicate cultures. Similar results were obtained in three independent experiments. (d) Increase of CD40L-expressing CD4+ T cells in both types of transgenic mice. Spleen cells were isolated from KCASP1Tg, KIL-18Tg, or WT littermate at 36 weeks of age, and their CD40L expression gated on CD4+ cells were identified by flow cytometry. A representative result is shown. Similar results were obtained in three independent experiments. ND, not detected.
Chronic Dermatitis in KIL-18Tg and KCASP1Tg.
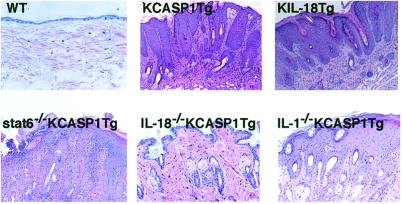
We next investigated the histology of AD-like skin lesions. KCASP1Tg showed remarkable skin alternations at 8 weeks, which started as severe erosive dermatitis, followed by reepithelization and lichenoid changes (Fig. 4; ref. 23). Finally, they developed prominent acanthosis and papillomatosis with intercellular edema and parakeratotic scale-crust in their epidermis (Fig. 4). In contrast, KIL-18Tg showed later onset of skin lesions, which started as focal skin alterations around their eyes but gradually extended to their face, head, and trunk (Figs. 1b and 5a). Skin alterations of KIL-18Tg slightly differed from those of KCASP1Tg and showed the presence of marked lichenification without scarring. Their epidermis is obviously acanthotic, although their dermis is severely infiltrated with lymphocytes and polymorphonuclear cells, such as neutrophils (Fig. 4; data not shown).
Fig 4.
AD-like inflammatory skin disease in KCASP1Tg and KIL-18Tg. Skins were sampled from WT (20 weeks old), KCASP1Tg (24 weeks old), KIL-18Tg (36 weeks old), stat6-deficient KCASP1Tg (24 weeks old), IL-18-deficient KCASP1Tg (24 weeks old), or IL-1 α/β-deficient KCASP1Tg (24 weeks old). The skin specimens were stained with hematoxylin and eosin. [Original magnification, ×100 (KCASP1Tg, stat6-deficient KCASP1Tg, IL-18-deficient KCASP1Tg, IL-1-deficient KCASP1Tg); ×400 (WT, KIL-18Tg).] Data are representative of three specimens sampled from two to five various mutant mice. The similar results were obtained in two independent experiments.
Mast cell number is remarkably and preferentially increased in the dermal infiltrates (Table 1, Fig. 5b) but not in the spleen, lungs, peripheral blood, or intestine (data not shown) of KCASP1Tg or KIL-18Tg, suggesting the contribution of local production of IL-18 to this mast cell accumulation. Because mast cells play a critical role in induction of skin-itching, we counted frequency of skin-scratching. After development of dermatitis, KIL-18Tg and KCASP1Tg much more frequently scratched their skin including the erosive lesion than did WT (Table 1, Fig. 5b). However, before onset of skin changes, both types of transgenic mice showed almost comparable frequencies of skin-scratching as did WT (data not shown). We showed previously that IL-18 stimulates CD4+ T cells to produce IL-3, which together with IL-18 activates basophils and mast cells to release histamine in vitro (9, 30). Therefore, we measured plasma histamine levels. Histamine levels were obviously elevated in both types of transgenic mice (Table 1, Fig. 5b), suggesting IL-18-dependent activation of mast cells in the dermal infiltrates of both types of transgenic mice. Histamine concentration in KCASP1Tg is much higher than that in KIL-18Tg (Table 1, Fig. 5b), possibly reflecting the fact that mast cell numbers in KCASP1Tg were larger than those in KIL-18Tg (Table 1, Fig. 5b). These results suggested that their skin lesions might be intimately associated with itching and that IL-18 may cause the inflammatory cutaneous disease by activation of cutaneous mast cells, and possibly of lymphocytes and neutrophils.
Table 1.
Characteristics of pruritic chronic dermatitis in KCASP1Tg
| IL-18, ng/ml | IgE, μg/ml | IgG1, μg/ml | Histamine, nM | Mast cell no., per 5 fields | Skin-scratching, per 10 min | |
|---|---|---|---|---|---|---|
| WT | 0.12 ± 0.05 | ND | 78 ± 51 | ND | 3.5 ± 1.3 | 11 ± 7 |
| KCASP1Tg | 9.5 ± 1.7 | 33.2 ± 4.1 | 6,178 ± 1,325 | 1,272 ± 257 | 56.3 ± 11.3 | 385 ± 48 |
| KIL-18Tg | 19.3 ± 3.6 | 73.5 ± 16.3 | 6,812 ± 2,133 | 360 ± 66 | 38.3 ± 4.3 | 500 ± 92 |
| Stat6−/−KCASP1Tg | 12.5 ± 1.88 | ND | 2 ± 0.3 | 150 ± 37 | 16.9 ± 3.3 | 416 ± 84 |
| IL-18−/−KCASP1Tg | ND | 8.6 ± 2.6 | 1,347 ± 266 | ND | 4.7 ± 2.3 | 23 ± 5 |
Serum, plasma, and skin specimens were sampled from five WT (20 weeks old), five KCASP1Tg (24 weeks old), five KIL-18Tg (36 weeks old), two stat6-deficient (stat6−/−) KCASP1Tg (24 weeks old), or two IL-18-deficient (IL-18−/−) KCASP1Tg (24 weeks old). IL-18, IgG1, and IgE serum levels were measured by ELISA. Plasma histamine levels were determined by RIA. Mast cell numbers were counted in the distinct five fields of skin specimens from various transgenic mice after being stained with toluidine blue at high magnification (×400). Various transgenic mice were kept under calm conditions and were recorded by a video camera for 60 min. Skin-itching was evaluated by skin-scratching frequency counted from three scenes (10 min) selected at random. Data are shown as mean ± SD (of triplicate culture) of two to five samples in each experimental group. ND, not detected.
IgE/stat6-Independent but IL-18-Dependent Pruritic Dermatitis.
It is well established that allergen-specific IgE plays a critical role in hay fever or other allergic disorders (33). However, involvement of allergen-specific IgE in AD is not well defined, and involvement of Th1 cytokines has been suggested (34). KCASP1Tg and KIL-18Tg spontaneously develop inflammatory skin lesion with high concentration of IgE even without obvious immunization or infection, prompting us to examine the possibility that polyclonal IgE in these transgenic mice is not involved in the development of these skin alterations. To address this possibility and to take advantage of prompt development of the inflammatory skin alterations, we constructed KCASP1Tg without IgE production by depletion of stat6 gene. As reported, stat6-deficient KCASP1Tg containing high serum levels of IL-18 showed no detectable levels of IgE and very little IgG1 (Table 1, Fig. 5b; ref. 14). Furthermore, these mice showed significant reduction in the number of accumulated mast cells and plasma level of histamine as compared with KCASP1Tg (Table 1, Fig. 5b). Defects in Th2 cell development, IL-4 production, and IL-4 signaling in stat6-deficient KCASP1Tg may result in such reduction in mast cell accumulation and plasma histamine levels, because IL-4 is an important mast cell growth factor (35, 36). Nevertheless, the cutaneous changes in stat6-deficient KCASP1Tg started almost at the same time as did those in KCASP1Tg (Fig. 5a). These results suggest that high levels of IL-18 are principally responsible for inducing skin alterations associated with itching. To prove this possibility, we crossed KCASP1Tg with IL-18-deficient mice. Resultant IL-18-deficient KCASP1Tg showed neither frequent skin-scratching nor manifestation of dermatitis even at 6 months, although had low, but significant levels of IgE and IgG1 in their sera (Fig. 5). These results allowed us to conclude that IL-18 is principally responsible for inducing skin lesions in KCASP1Tg mice independently of elevation of IgE/IgG1.
Because IL-18-deficient KCASP1Tg showed striking diminution in the number of cutaneous mast cells and the levels of serum histamine (Table 1, Fig. 5b), IL-18, but not IgE, might account for the development of the inflammatory dermatitis by activation of mast cells and T cells. IL-18 acts on T cells and mast cells to produce IL-3 and IL-4 (9, 14, 30), which in combination might play an important role in mast cell accumulation. A significant relationship exists between reduction in mast cell number and histamine level (P < 0.05), although no obvious correlation exists between histamine levels or mast cell numbers and manifestation of itching. These results may indicate the presence of the threshold of histamine level that is required for induction of skin-itching. We suspect that KIL-18Tg and stat6-deficient KCASP1Tg have higher amounts of histamine than this threshold. Indeed, they displayed still much higher levels of mast cell numbers and histamine levels as compared with WT and also developed pruritic skin alterations (Figs. 4 and 5b). However, this finding does not deny the possibility that other factor(s), such as leukotrienes (37), might be involved in the development of skin-itching. Alternatively, IL-18-driven IL-4/IL-13 and/or some other factors might activate mast cells and/or other inflammatory cells to induce itching independently of stat6. We need further study to clarify the role of stat6-independent action of IL-4/IL-13 in this skin disease.
IL-1, as an Accelerator for AD.
KIL-18Tg took much longer to develop their cutaneous alterations than did KCASP1Tg. Although KIL-18Tg displayed higher levels of serum IL-18 than did KCASP1Tg (Fig. 1d), KIL-18Tg started to manifest skin changes much later than KCASP1Tg (Fig. 5a), which led us to propose the possibility that IL-1β, another product of keratinocytes overexpressing caspase-1 (23), might be involved in the reduction of the incubation time. To investigate this possibility, we crossed KCASP1Tg with IL-1α/β double knockout mice (26). IL-1α/β-deficient KCASP1Tg, like KIL-18Tg, showed late onset of the skin alterations (Fig. 5a), suggesting that IL-1 might play a promoting role in the development of these atopic changes. The histopathological changes of IL-1α/β-deficient KCASP1Tg skin lesions are similar to those changes of KIL-18Tg but milder than those changes of KCASP1Tg (Fig. 4). Therefore, IL-1 over-release seems to play a role in accelerating the skin alterations initiated by abnormally accumulated IL-18.
In this study we demonstrate that high accumulation of IL-18 is primarily responsible for inducing allergen/allergen-specific IgE-independent AD-like inflammatory skin disease in these transgenic mice. They frequently scratch their skin including the lesion and display high plasma levels of histamine, suggesting pruritic cutaneous changes (Fig. 5b). Like AD patients, their skin changes start to appear in their face and then expand to their trunks and extremities. All of the KCASP1Tg and KIL-18Tg early or lately suffer from the skin disease. They have high serum levels of IgE (Figs. 3 and 5b). Thus, the skin disease in both types of transgenic mice seems to fulfill the criteria of AD, although few eosinophils are found in the skin lesion (Fig. 4). Both types of transgenic mice are at least mouse models for inflammatory skin disease. To date, it is believed that allergen-specific IgE is essential to induce atopic phenotypes (4, 5, 9). However, our transgenic mice spontaneously develop AD-like inflammatory dermatitis without encountering with specific allergen (Figs. 1b, 4, and 5), suggesting that their polyclonal IgE and IgG1 play a minor role in activation of mast cells to produce the inflammatory changes in their skin. In fact, stat6-deficient KCASP1Tg that had very low levels of IgE and IgG1 in their sera still developed pruritic dermatitis similar to that in KCASP1Tg (Figs. 4 and 5). These results strongly suggest that neither IgE nor IgG1 is involved in the development of the cutaneous pathological changes in KCASP1Tg, although Ag-specific IgG1 as well as IgE are profoundly involved in the occurrence of anaphylaxis (38, 39). In human cases, a subgroup of AD patients exists who have low levels of IgE (2, 24). The depletion of the IL-18 gene in KCASP1Tg resulted in elimination of pathological cutaneous alterations (Fig. 4). These results strongly indicated that overrelease of IL-18 in the skin gives rise to the inflammatory cutaneous changes independently of IgE or stat6-mediated signaling. Thus, measurement of serum IL-18 levels may be important in the cases of AD. However, it apparently is more informative to measure serum levels of both IL-18 and IL-12 in patients with AD to determine their therapeutics whether against IgE or IL-18, because IL-18 shows IFN-γ-inducing activity in the presence of IL-12 (10).
Transgene-encoding human caspase-1 or active form murine IL-18 was selectively expressed in keratinocytes of KCASP1Tg and KIL-18Tg, respectively, under control of keratin 14 (ref. 23 and data not shown). Although the transgene is locally expressed in the skin but not in other tissues including spleen (23; data not shown), splenic CD4+ T cells of these mutant mice spontaneously developed into Th2 cells under SPF conditions (Fig. 3 b and c). This development may be partly attributable to systemic elevation of IL-18 (Fig. 1 d and e), which has potential to induce Th2 cell development upon occasional exposure to intrinsic Ag, such as bacterial flora (10, 14). Alternatively, cutaneous dendritic cells might become Th2 cell-driving antigen-presenting cells in the circumstances of high IL-18 concentration and might migrate into peripheral immune organs including the spleen to participate in Th2 cell development. Further study is required.
After development of the cutaneous alterations, both KCASP1Tg and KIL-18Tg increased neutrophil numbers in their spleens (Fig. 2b). Comparable levels of neutrophil accumulation were observed in the skin lesions of stat6-deficient and normal KCASP1Tg (data not shown). Indeed, IL-18 has capacity to induce proliferation of neutrophils by induction of granulocyte/macrophage colony-stimulating factor (30), to activate neutrophils directly (40), and possibly to recruit neutrophils by induction of neutrophil chemotactic factor, such as IL-8 in human (41). Activated neutrophils might participate in the development of inflammatory skin changes in these mutant mice by releasing various potent effector molecules (42).
Induction and/or activation of AD are strongly influenced by bacterial infection (1, 2). Some microbe products induce IL-18 secretion by activation of Toll-like receptors, recently identified signaling receptors that specifically recognize microbe-derived molecules, leading to IL-18-dependent AD-like skin lesions (21). In fact, macrophages secrete IL-18 upon infection with various microbes including bacteria, virus, and protozoa (2, 43, 44). We are now investigating the microbe products that induce IL-18 release from keratinocytes.
Acknowledgments
We thank Dr. H. Tahara at the Institute of Medical Science (University of Tokyo) for providing us with cDNA of murine mature IL-18, Drs. S. Ootake and H. Tsumura (Mie University) for helpful technical assistance, and Dr. E. V. Fuchs (University of Chicago) for providing us with K14 promoter DNA. This work was supported in part by grants, grants-in-aid, and Hitec Research Center grant from the Ministry of Education, Science and Culture, Japan, and a grant-in-aid from the Mie Medical Research Foundation.
Abbreviations
AD, atopic dermatitis
Ag, antigen
SPF, specific pathogen-free
K14, keratin 14
WT, wild type
CD40L, CD40 ligand
PE, phycoerythrin
References
- 1.Leung D. Y. M. (1995) J. Allergy Clin. Immunol. 96, 302-318. [DOI] [PubMed] [Google Scholar]
- 2.Rudikoff D. & Lebwohl, M. (1998) Lancet 351, 1715-1721. [DOI] [PubMed] [Google Scholar]
- 3.Serafin W. E. & Austen, K. F. (1987) N. Engl. J. Med. 317, 30-34. [DOI] [PubMed] [Google Scholar]
- 4.Plaut M., Pierce, J. H., Watson, C. J., Hanley-Hyde, J., Nordan, R. P. & Paul, W. E. (1989) Nature (London) 339, 64-67. [DOI] [PubMed] [Google Scholar]
- 5.Paul W. E., Seder, R. A. & Plaut, M. (1993) Adv. Immunol. 53, 1-29. [PubMed] [Google Scholar]
- 6.Okayama Y., Petit-Frere, C., Kassel, O., Semper, A., Quint, D., Tunon-de-Lara, M. J., Bradding, P., Hogate, S. T. & Church, M. K. (1995) J. Immunol. 155, 1796-1808. [PubMed] [Google Scholar]
- 7.Bischoff S. C., Sellge, G., Lorentz, A., Sebald, W., Raab, R. & Manns, M. P. (1999) Proc. Natl. Acad. Sci. USA 96, 8080-8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorentz A., Schwengberg, S., Sellge, G., Manns, M. P. & Bischoff, S. C. (2000) J. Immunol. 164, 43-48. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimoto T., Tsutsui, H., Tominaga, K., Hoshino, K., Okamura, H., Akira, S., Paul, W. E. & Nakanishi, K. (1999) Proc. Natl. Acad. Sci. USA 96, 13962-13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakanishi K., Yoshimoto, T., Tsutsui, H. & Okamura, H. (2001) Annu. Rev. Immunol. 19, 423-474. [DOI] [PubMed] [Google Scholar]
- 11.Okamura H., Tsutsui, H., Komatsu, T., Yutsudo, M., Hakura, A., Tanimoto, T., Torigoe, K., Okura, T., Nukada, Y., Hattori, K., et al. (1995) Nature (London) 378, 88-91. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto T., Takeda, K., Tanaka, T., Ohkusu, K., Kashiwamura, S., Okamura, H., Akira, S. & Nakanishi, K. (1998) J. Immunol. 161, 3400-3407. [PubMed] [Google Scholar]
- 13.Yang J., Murphy, T. L., Ouyang, W. & Murphy, K. M. (1999) Eur. J. Immunol. 29, 548-555. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimoto T., Mizutani, H., Tsutsui, H., Noben-Trauth, N., Yamanaka, K., Tanaka, M., Izumi, S., Okamura, H., Paul, W. E. & Nakanishi, K. (2000) Nat. Immunol. 1, 132-137. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino T., Kawase, Y., Okamoto, M., Yokota, K., Yoshino, K., Yamamura, K., Miyazaki, J., Young, H. A. & Oizumi, K. (2001) J. Immunol. 166, 7014-7018. [DOI] [PubMed] [Google Scholar]
- 16.Mizutani H., Black, R. & Kupper, T. S. (1991) J. Clin. Invest. 87, 1066-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoll S., Muller, G., Kurimoto, M., Saloga, J., Tanimoto, T., Yamauchi, H., Okamura, H., Knop, J. & Enk, A. H. (1997) J. Immunol. 159, 298-302. [PubMed] [Google Scholar]
- 18.Dinarello C. A. (1996) Blood 87, 2095-2147. [PubMed] [Google Scholar]
- 19.Gu Y., Kuida, K., Tsutusi, H., Ku, G., Hsiao, K., Fleming, M. A., Hayashi, N., Okamura, H., Nakanishi, K., Kurimoto, M., et al. (1997) Science 275, 206-209. [DOI] [PubMed] [Google Scholar]
- 20.Fantuzzi G. & Dinarello, C. A. (1999) J. Clin. Immunol. 19, 1-11. [DOI] [PubMed] [Google Scholar]
- 21.Seki E., Tsutsui, H., Nakano, H., Tsuji, N. M., Hoshino, K., Adachi, O., Adachi, K., Futatsugi, S., Kuida, K., Takeuchi, O., et al. (2001) J. Immunol. 166, 2651-2657. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsui H., Kayagaki, N., Kuida, K., Nakano, H., Hayashi, N., Takeda, K., Matsui, K., Kashiwamura, S.-I., Hada, T., Akira, S., et al. (1999) Immunity 11, 359-367. [DOI] [PubMed] [Google Scholar]
- 23.Yamanaka K., Tanaka, M., Tsutsui, H., Kupper, T. S., Asahi, K., Okamura, H., Nakanishi, K., Suzuki, M., Kayagaki, N., Black, R. A., et al. (2000) J. Immunol. 165, 997-1003. [DOI] [PubMed] [Google Scholar]
- 24.Werfel T. & Kapp, A. (1999) Curr. Probl. Dermatol. 28, 29-36. [DOI] [PubMed] [Google Scholar]
- 25.Osaki T., Hashimoto, W., Gambotto, A., Okamura, H., Robbins, P. D., Kurimoto, M., Lotze, M. T. & Tahara, H. (1999) Gene Ther. 6, 808-815. [DOI] [PubMed] [Google Scholar]
- 26.Horai R., Asano, M., Sudo, K., Kanuka, H., Suzuki, M., Nishihara, M., Takahashi, M. & Iwakura, Y. (1998) J. Exp. Med. 187, 1463-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K., Tanaka, T., Shi, W., Matsumoto, M., Minami, M., Kashiwamura, S., Nakanishi, K., Yoshida, N., Kishimoto, T. & Akira, S. (1996) Nature (London) 380, 627-630. [DOI] [PubMed] [Google Scholar]
- 28.Takeda K., Tsutsui, H., Yoshimoto, T., Adachi, O., Yoshida, N., Kishimoto, T., Okamura, H., Nakanishi, K. & Akira, S. (1998) Immunity 8, 383-390. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsui H., Nakanishi, K., Matsui, K., Higashino, K., Okamura, H., Miyazawa, Y. & Kaneda, K. (1996) J. Immunol. 157, 3967-3973. [PubMed] [Google Scholar]
- 30.Ogura T., Ueda, H., Hosohara, K., Tsuji, R., Nagata, Y., Kashiwamura, S. & Okamura, H. (2001) Blood 98, 2101-2107. [DOI] [PubMed] [Google Scholar]
- 31.Armitage R. J., Fanslow, W. C., Strockbine, L., Sato, T. A., Clifford, K. N., Macduff, B. M., Anderson, D. M., Gimpel, S. D., Davis-Smith, T. & Maliszewski, C. R. (1992) Nature (London) 357, 80-82. [DOI] [PubMed] [Google Scholar]
- 32.Flavell R. A. & Grewal, I. S. (1996) Immunol. Today 17, 410-414. [DOI] [PubMed] [Google Scholar]
- 33.Ishizaka K. & Ishizaka, T. (1978) Immunol. Rev. 41, 109-148. [DOI] [PubMed] [Google Scholar]
- 34.Werfel T., Morita, A., Grewe, M., Renz, H., Wahn, U., Krutmann, J. & Kapp, A. (1996) J. Invest. Dermatol. 107, 871-876. [DOI] [PubMed] [Google Scholar]
- 35.Mosmann T. R., Bond, M. W., Coffman, R. L., Ohara, J. & Paul, W. E. (1986) Proc. Natl. Acad. Sci. USA 83, 5654-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura Y. (1989) Annu. Rev. Immunol. 7, 59-76. [DOI] [PubMed] [Google Scholar]
- 37.Andoh T. & Kuraishi, Y. (1998) Eur. J. Pharmacol. 17, 93-96. [DOI] [PubMed] [Google Scholar]
- 38.Dombrowicz D., Flamand, V., Brigman, K. K., Koller, B. H. & Kinet, J.-P. (1993) Cell 75, 969-976. [DOI] [PubMed] [Google Scholar]
- 39.Miyajima I., Dombrowicz, D., Martin, T. R., Ravetch, J. V., Kinet, J.-P. & Galli, S. J. (1997) J. Clin. Invest. 99, 901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung B. P., Culshaw, S., Gracie, J. A., Hunter, D., Canetti, C. A., Campbell, C., Cunha, F., liew, F. Y. & McInnes, I. B. (2001) J. Immunol. 167, 2879-2886. [DOI] [PubMed] [Google Scholar]
- 41.Wang W., Tanaka, T., Okamura, H., Sugita, M., Higa, S., Kishimoto, T. & Suemura, M. (2001) Eur. J. Immunol. 31, 1010-1016. [DOI] [PubMed] [Google Scholar]
- 42.Weissmann G., Smalen, J. E. & Korchak, H. M. (1980) N. Engl. J. Med. 303, 27-34. [DOI] [PubMed] [Google Scholar]
- 43.Akira S., Takeda, K. & Kaisho, T. (2001) Nat. Immunol. 2, 675-681. [DOI] [PubMed] [Google Scholar]
- 44.Adachi K., Tsutsui, H., Kashiwamura, S., Seki, E., Nakano, H., Takeuchi, O., Takeda, K., Okumura, K., Van Kaer, L., Okamura, H., et al. (2001) J. Immunol. 167, 5928-5934. [DOI] [PubMed] [Google Scholar]