Abstract
The botulinum neurotoxins (BoNTs) cause the paralytic human disease botulism and are one of the highest-risk threat agents for bioterrorism. To generate a pharmaceutical to prevent or treat botulism, monoclonal antibodies (mAbs) were generated by phage display and evaluated for neutralization of BoNT serotype A (BoNT/A) in vivo. Although no single mAb significantly neutralized toxin, a combination of three mAbs (oligoclonal Ab) neutralized 450,000 50% lethal doses of BoNT/A, a potency 90 times greater than human hyperimmune globulin. The potency of oligoclonal Ab was primarily due to a large increase in functional Ab binding affinity. The results indicate that the potency of the polyclonal humoral immune response can be deconvoluted to a few mAbs binding nonoverlapping epitopes, providing a route to drugs for preventing and treating botulism and diseases caused by other pathogens and biologic threat agents.
Keywords: monoclonal antibody, immunotherapy, antibody engineering, ; vaccine, phage display
The spore-forming bacteria Clostridium botulinum secrete botulinum neurotoxin (BoNT), the most poisonous substance known (1). The protein toxin consists of a heavy and light chain that contain three functional domains (2–4). The C-terminal portion of the heavy chain (HC) comprises the binding domain, which binds to a sialoganglioside receptor and a putative protein receptor on presynaptic neurons, resulting in toxin endocytosis (5, 6). The N-terminal portion of the heavy chain (HN) comprises the translocation domain, which allows the toxin to escape the endosome. The light chain is a zinc endopeptidase that cleaves different members of the SNARE complex, depending on serotype, resulting in blockade of neuromuscular transmission (7, 8).
There are seven BoNT serotypes (A–G; ref. 9), four of which (A, B, E, and F) cause the human disease botulism (10). Botulism is characterized by flaccid paralysis, which if not immediately fatal requires prolonged hospitalization in an intensive care unit and mechanical ventilation. The potent paralytic ability of the toxin has resulted in its use in low doses as a medicine to treat a range of overactive muscle conditions including cervical dystonias, cerebral palsy, posttraumatic brain injury, and poststroke spasticity (11). BoNTs are also classified by the Centers for Disease Control (CDC) as one of the six highest-risk threat agents for bioterrorism (the “Class A agents”), because of their extreme potency and lethality, ease of production and transport, and need for prolonged intensive care (10). Both Iraq and the former Soviet Union produced BoNT for use as weapons (12, 13), and the Japanese cult Aum Shinrikyo attempted to use BoNT for bioterrorism (10). As a result of these threats, specific pharmaceutical agents are needed for prevention and treatment of intoxication.
No specific small-molecule drugs exist for prevention or treatment of botulism, but an investigational pentavalent toxoid is available from the CDC (14) and a recombinant vaccine is under development (15). Regardless, mass civilian or military vaccination is unlikely because of the rarity of disease or exposure and the fact that vaccination would prevent subsequent medicinal use of BoNT. Postexposure vaccination is useless because of the rapid onset of disease. Toxin neutralizing antibody (Ab) can be used for pre- or postexposure prophylaxis or for treatment (16). Small quantities of both equine antitoxin and human botulinum immune globulin exist and are currently used to treat adult (17, 18) and infant botulism (19), respectively. Recombinant monoclonal antibody (mAb) could provide an unlimited supply of antitoxin free of infectious disease risk and not requiring human donors for plasmapheresis. Such mAbs must be of high potency to provide an adequate number of doses at reasonable cost. In some instances, the potency of polyclonal Ab can be recapitulated in a single mAb (20). In the case of BoNT, potent neutralizing mAbs have yet to be produced: single mAb neutralizing at most 10 to 100 times the 50% lethal dose (LD50) of toxin in mice (21, 22). In this work, we report that BoNT serotype A (BoNT/A) can be very potently neutralized in vitro and in vivo by combining two or three mAbs, providing a route to drugs for preventing and treating botulism and diseases caused by other pathogens and biologic threat agents.
Methods
IgG Construction.
VH genes of C25, S25, and 3D12 single-chain fragment variable (scFv) were amplified using PCR from the respective phagemid DNA with the primer pairs GTCTCCTGAGCTAGCTGAGGAGACGGTGACCGTGGT and either GTACCAACGCGTGTCTTGTCCCAGGTCCAGCTGCAGGAGTCT (C25), GTACCAACGCGTGTCTTGTCCCAGGTGAAGCTGCAGCAGTCA (S25), or GTACCAACGCGTGTCTTGTCCCAGGTGCAGCTGGTGCAGTCT (3D12). DNA was digested with Mlu1 and NheI, ligated into N5KG1Val-Lark (gift of Mitch Reff, IDEC Pharmaceuticals, San Diego) and clones containing the correct VH identified by DNA sequencing. Vκ genes of C25, S25, and 3D12 scFv were amplified from the respective phagemid DNA with the primer pairs TCAGTCGTTGCATGTACTCCAGGTGCACGATGTGACATCGAGCTCACTCAGTCT and CTGGAAATCAAACGTACGTTTTATTTCCAGCTTGGT (C25), TCAGTCGTTGCATGTACTCCAGGTGCACGATGTGACATCGAGCTCACTCAGTCT and CTGGAAATCAAACGTACGTTTGATTTCCAGCTTGGT (S25), or TCAGTCGTTGCATGTACTCCAGGTGCACGATGTGACATCGTGATGACCCAGTCT and CTGGAAATCAAACGTACGTTTTATCTCCAGCTTGGT (3D12), cloned into pCR-TOPO (Invitrogen) and clones containing the correct Vκ identified by DNA sequencing. Vκ genes were excised from pCR-TOPO with DraIII and BsiWI and ligated into DraIII- and BsiWI-digested N5KG1Val-Lark DNA containing the appropriate VH gene. Clones containing the correct VH and Vκ gene were identified by DNA sequencing, and vector DNA was used to transfect CHO DG44 cells by electroporation. Stable cell lines were established by selection in G418 and expanded into 1L spinner flasks. Supernatant containing IgG was collected, concentrated by ultrafiltration, and purified on Protein G (Pharmacia).
Measurement of IgG Affinity and Binding Kinetics.
IgG binding kinetics were measured using surface plasmon resonance in a BIAcore (Pharmacia Biosensor) and used to calculate the Kd. Approximately 200–400 response units of purified IgG (10–20 μg/ml in 10 mM acetate, pH 3.5–4.5) was coupled to a CM5 sensor chip by using N-hydroxysuccinimide–N-ethyl-N′-(dimethylaminopropyl)-carbodiimide chemistry. The association rate constant for purified BoNT/A HC was measured under continuous flow of 15 μl/min, using a concentration range of 50–800 nM. The association rate constant (kon) was determined from a plot of (ln (dR/dt))/t vs. concentration. The dissociation rate constant (koff) was determined from the dissociation part of the sensorgram at the highest concentration of scFv analyzed using a flow rate of 30 μl/min to prevent rebinding. Kd was calculated as koff/kon.
Measurement of in Vitro Toxin Neutralization.
Phrenic nerve-hemidiaphragm preparations were excised from male CD-1 mice (25–33g) and suspended in 135 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 15 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 11 mM glucose. The incubation bath was bubbled with 95% O2/5% CO2 and maintained at 36°C. Phrenic nerves were stimulated at 0.05 Hz with square waves of 0.2 ms duration. Isometric twitch tension was measured using a force-displacement transducer (Model FT03, Grass Instruments, Quincy, MA). Purified IgG were incubated with BoNT/A for 30 min at room temperature and then added to the tissue bath resulting in a final IgG concentration of 6.0 × 10−8 M (S25 and 3D12 alone) or 2.0 × 10−8 M (C25 alone) and a final BoNT/A concentration of 2.0 × 10−11 M. For pairs of IgG, the final concentration of each IgG was decreased 50%, and for studies of a mixture of all 3 IgG, the concentration of each IgG was decreased by 67%.
Measurement of in Vivo Toxin Neutralization.
Fifty micrograms of the appropriate IgG were added to the indicated number of mouse LD50 of BoNT/A neurotoxin (Hall strain) in a total volume of 0.5 ml of gelatin phosphate buffer and incubated at RT for 30 min. For pairs of Ab, 25 μg of each Ab was added, and for the combination of 3 Ab, 16.7 μg of each Ab was added. The mixture was then injected i.p. into female CD-1 mice (16–22 g). Mice were studied in groups of ten and were observed at least daily. The final death tally was determined 5 days after injection.
Measurement of Solution Affinity of mAbs.
Equilibrium binding studies were conducted using a KinExA flow fluorimeter to quantify the antibodies with unoccupied binding sites in reaction mixtures of the antibody with the antigen. Studies with reaction mixtures comprised of one, two, or three different antibodies were conducted in Hepes-buffered saline, pH 7.4, with total antibody concentrations of 342, 17.2, and 17.2 pM, respectively. In all cases, the concentration of soluble toxin was varied from less than 0.1 to greater than 10-fold the value of the apparent Kd (twelve concentrations, minimum). Reaction mixtures comprised of one, two, or three different antibodies were incubated at 25°C for 0.5, 3, and 17 h, respectively, to ensure that equilibrium was achieved.
Results
To generate mAbs capable of neutralizing BoNT/A, we previously generated scFv phage antibody libraries from mice immunized with recombinant BoNT/A binding domain (HC) and from humans immunized with pentavalent botulinum toxoid (23, 24). After screening more than 100 unique mAbs from these libraries, three groups of scFv were identified that bound nonoverlapping epitopes on BoNT/A HC and that neutralized toxin in vitro (prolonged the time to neuroparalysis in a murine hemidiaphragm model; refs. 23 and 24). In vitro toxin neutralization increased significantly when two scFv binding nonoverlapping epitopes were combined. In vivo toxin neutralization could not be determined because of the rapid clearance of the 25-kDa scFv from serum (25).
To evaluate in vivo BoNT neurotoxin neutralization, IgG were constructed from the VH and Vκ genes of three BoNT/A scFv that neutralized toxin in vitro. VH and Vκ genes were sequentially cloned into a mammalian expression vector, resulting in the fusion of the human Cκ gene to the Vκ and the human γ1 gene to the VH. Stable expressing cell lines were established and IgG purified from supernatant yielding chimeric IgG with murine V-domains and human C-domains, for the murine scFv C25 and S25, and a fully human IgG for the human scFv 3D12. IgG equilibrium binding constants (Kd) were measured and found to be at least comparable to the binding constants of the scFv from which they were derived (Table 1). The antigen binding affinity of two of the IgG (S25 and 3D12) was significantly higher (lower Kd) than for the corresponding scFv, largely because of an increase in the association rate constant (kon). We presume this reflects an increase in the stability of the molecule and hence an increase in the functional antibody concentration.
Table 1.
Association (kon) and dissociation (koff) rate constants and equilibrium dissociation constants (Kd) for BoNT/A IgG and scFv from which the IgG were derived
| Antibody
|
IgG | scFv | ||||
|---|---|---|---|---|---|---|
| Kd (M−1) | kon (M−1⋅s−1) | koff (s−1) | Kd (M−1) | kon (M−1⋅s−1) | koff (s−1) | |
| C25 | 1.69 × 10−9 | 1.32 × 106 | 2.24 × 10−3 | 1.10 × 10−9 | 3.00 × 105 | 3.30 × 10−4 |
| S25 | 3.90 × 10−9 | 1.46 × 106 | 5.70 × 10−3 | 7.30 × 10−8 | 1.10 × 104 | 8.10 × 10−4 |
| 3D12 | 5.62 × 10−11 | 2.26 × 106 | 1.27 × 10−4 | 3.69 × 10−8 | 1.30 × 104 | 5.0 × 10−4 |
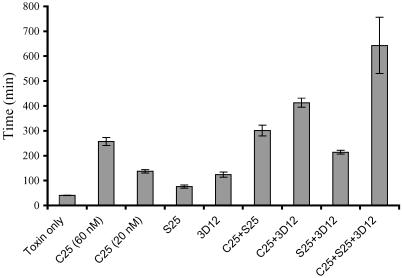
In vitro toxin neutralization by IgG was determined in the mouse hemidiaphragm assay (26). Compared with toxin alone, each of the three IgG significantly increased the time to neuroparalysis, with C25 being the most potent (Fig. 1). Significant synergy in toxin neutralization was observed when pairs of IgG were studied. For these studies, it was necessary to decrease the concentration of C25 IgG studied 3-fold to 20 nM because of its high potency and the fact that the hemidiaphragm preparations have an 8-h lifespan. Each pair of IgG significantly increased the time to neuroparalysis compared with the time for either single IgG (Fig. 1). A mixture of all three IgG further increased the time to neuroparalysis, although this difference did not reach statistical significance compared with antibody pairs because of the small number of diaphragms studied.
Fig 1.
In vitro toxin neutralization by mAb, pairs of mAbs, and oligoclonal Ab. Time to 50% twitch reduction was measured in isolated mouse hemidiaphragms and reported for toxin only control, single mAb (C25, S25, or 3D12), pairs of mAbs (C25+S25, C25+3D12, or 3D12+S25), and oligoclonal Ab (C25+3D12,+S25). Single mAb significantly prolonged time to neuroparalysis compared with toxin only. Pairs of mAbs significantly prolonged time to neuroparalysis compared with single mAbs.
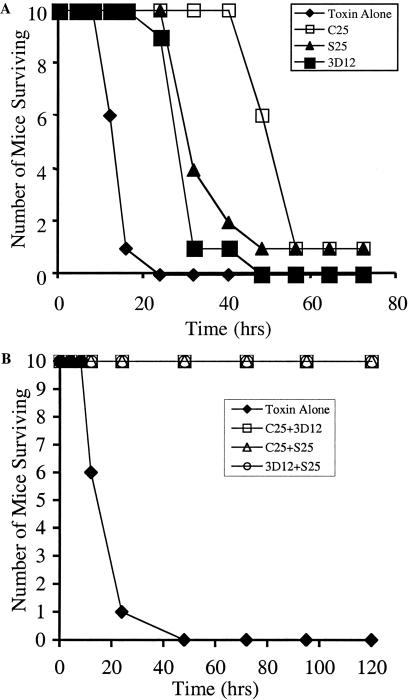
In vivo toxin neutralization was studied using a mouse assay in which toxin and Ab are premixed and injected i.p., and time to death and number of surviving mice determined (27). Fifty micrograms of each single mAb prolonged the time to death but failed to protect mice challenged with 20 LD50s (Fig. 2A). In contrast, any pair of mAbs completely protected mice challenged with 100 LD50s of toxin (Fig. 2B). At 500 LD50s, the majority of mice receiving two of the pairs of mAbs (S25 + 3D12 or C25 + S25) died, whereas 80% of mice receiving the pair of C25 + 3D12 survived (Fig. 3). All mice receiving a mixture of all three mAbs (oligoclonal Ab) survived challenge with 500 LD50s of toxin (Fig. 3). In these studies, the total amount of Ab administered was kept constant at 50 μg per mouse. To determine potency, mAb pairs and oligoclonal Ab were studied at increasing doses of toxin (Fig. 3). The most potent mAb pair (C25 + 3D12) protected 90% of mice challenged with 1,000 LD50s, with no mice surviving challenge with 2,500 LD50s. In contrast, oligoclonal Ab completely protected all mice challenged with 5,000 LD50s of toxin, with five of ten mice surviving challenge with 20,000 LD50s of toxin. The potency of the oligoclonal Ab was titrated using a modification of the standard mouse neutralization bioassay (28) and was determined to be 45 international units (IU)/mg of Ab, 90 times more potent than the human botulinum immune globulin used to treat infant botulism (19). By definition, one IU neutralizes 10,000 LD50s of BoNT/A toxin (29).
Fig 2.
In vivo toxin neutralization by mAbs and pairs of mAbs. Fifty micrograms total Ab was mixed with 20 or 100 mouse LD50s of toxin and injected i.p. Time to death and number of surviving mice was determined. No single mAb showed significant protection against 20 LD50s. All mice survived challenge with 100 LD50s when given any pair of mAbs.
Fig 3.
In vivo toxin neutralization by mAbs, pairs of mAbs, and oligoclonal Ab. In vivo toxin neutralization was determined for mAbs, pairs of mAbs, and oligoclonal Ab at increasing toxin challenge doses. No single mAb showed significant protection. In contrast all mAb pairs neutralized at least 100 LD50s, with approximately 50% of mice surviving challenge with 1,500 LD50s of toxin for the most potent pair (C25+3D12). Oligoclonal Ab was even more potent with approximately 50% of mice surviving challenge with 20,000 LD50s of toxin.
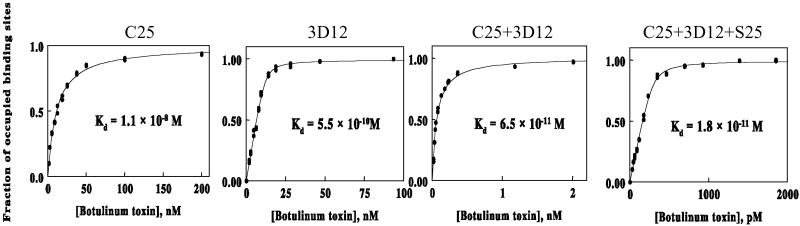
Two potential mechanisms could account for the increase in potency observed when mAbs were combined: an increase in the functional binding affinity of the Ab mixture for toxin and/or an increase in the blockade of the toxin surface that binds to cellular receptor(s). To determine the effect of combining antibodies on the functional binding affinities, apparent Kd were determined for each single mAb, pairs of mAbs, and the mixture of all three mAbs by using a flow fluorimeter to quantify the free antibody that remained in solution reaction mixtures. For single mAbs, the antigen binding affinities measured in homogeneous solution (both antigen and antibody in solution; Fig. 4) were lower (higher Kd) than those measured by surface plasmon resonance in a BIAcore (Table 1), where the antibody is immobilized and only the antigen is in solution. When antibody C25, which showed the greatest in vitro potency, was mixed in equimolar amounts with antibody 3D12, the resulting Ab combination bound to the toxin with an apparent Kd of 65 pM, an affinity 200- and 10-fold higher (lower Kd) than those observed with the individual antibodies alone. Addition of equimolar amounts of a third mAb (S25) to the mixture increased the apparent affinity further to 18 pM. An equimolar mixture of C25 with S25 yielded only a minor 2-fold increase in affinity, which may explain why this pair is less potent in vivo than the combination of C25 and 3D12. The increase in functional affinity observed with multiple mAbs may be due to either a conformational change in toxin that occurs on binding of the first mAb, resulting in higher affinity binding of the second and third mAbs, or from mAb binding changing the toxin from a monovalent to a multivalent antigen (30). This results in an “avidity effect” and an increase in affinity. Avidity effects have been well recognized and characterized for IgG binding to multivalent antigens (31), such as cell surfaces, but are not well appreciated as occurring in solution.
Fig 4.
Solution equilibrium dissociation constants (Kd) of antibodies. The solution Kd of single mAb C25 and 3D12 were determined in a flow fluorimeter by measuring the amount of free Ab present as a function of increasing BoNT HC toxin. Combining C25 and 3D12 mAb in equimolar amounts decreased the C25 Kd more than 100-fold. Adding a third Ab (S25) decreased the Kd another 4-fold to 18 pM.
The increments in measured Kd are consistent with the increase in in vivo potency observed for mAb pairs and oligoclonal Ab. Rearranging the equilibrium binding equation:
 |
Assuming a 2-ml mouse blood volume, the serum antibody concentration is 160 nM when mice receive 50 μg of Ab. Because the administered amount of toxin is a large multiple of the LD50, bound toxin ∼ administered toxin. Thus, the above equation simplifies to
 |
To determine the amount of administered toxin that results in death of 50% of mice, one substitutes 1 LD50 for the amount of free toxin and solves for administered toxin, yielding the equation:
 |
Using the solution Kdfor C25, the predicted toxin dose at which 50% of the mice survive is 16 LD50s (administered toxin = 1 LD50 × 160 nM/10 nM). When this calculation is applied to the C25 and 3D12 Ab pair, and to oligoclonal Ab, the magnitude of the increase in potency on combining antibodies parallels the increase in functional affinity (Table 2).
Table 2.
Observed and predicted toxin neutralization by recombinant antibody
| Antibody | Predicted toxin neutralization | Observed toxin neutralization |
|---|---|---|
| C25 | 16 LD50s | <20 LD50s |
| C25+3D12 | 2,500 LD50s | 1,500 LD50s |
| C25+3D12+S25 | 8,900 LD50s | 20,000 LD50s |
The second potential mechanism for potent toxin neutralization by oligoclonal Ab is the need to block multiple epitopes on the toxin binding domain surface that bind to cellular receptors. It has been hypothesized that the toxin binds to cellular receptors via at least two sites on the toxin binding domain (5, 6). These include a ganglioside binding site and a putative protein receptor binding site. In fact, two spatially separated ganglioside binding sites have been observed in the co-crystal structure of the homologous tetanus toxin (32), and mAbs binding nonoverlapping tetanus toxin epitopes can block binding of toxin to GT1b ganglioside (33). Our prior epitope mapping studies are consistent with multiple mAbs blocking a large portion of the BoNT binding domain (HC) (34). Two of the mAbs (S25 and 3D12) bind the C-terminal subdomain of BoNT HC. The C25 mAb binds a conformational epitope that consists of sequence from the N- and C-terminal subdomains of BoNT HC. One model consistent with the epitope mapping places the three mAb epitopes on the same HC face and overlapping the known docking sites for the putative cellular ganglioside receptor GT1b (34). Validation of this model awaits finer epitope mapping studies.
Discussion
In conclusion, we have shown that one of the six class A biowarfare agents, BoNT/A, can be potently neutralized by an oligoclonal Ab consisting of only three mAbs. Oligoclonal Ab is 90 times more potent than hyperimmune human globulin and approaches the potency of hyperimmune mono-serotype horse type A antitoxin (27). Thus, the potency of polyclonal serum can be deconvoluted, or reduced, to mAbs binding only three nonoverlapping epitopes. This synergistic effect results in a more than 20,000-fold increase in potency for the three mAbs compared with the potency of any of the single mAbs. Others have previously shown synergy between monoclonal antibodies in neutralizing tetanus toxin or HIV infection. In the case of tetanus toxin, combining three to four monoclonal antibodies increased the potency of in vivo toxin neutralization up to 200-fold (35). In the case of HIV, combining three or four mAbs increased the potency of viral neutralization 10-fold compared with individual mAbs (36). Thus, our observation is likely to prove general in many systems. We show, however, that the increased potency in the case of toxin neutralization likely results from a large increase in the functional affinity of the mixture antibodies. Whether such a mechanism holds true for viral neutralization is unclear.
One can hypothesize that the polyclonal humoral immune response to toxin is functionally dominated by Ab binding only a few nonoverlapping epitopes. The increase in potency appears to result primarily from a large decrease in the Kd of oligoclonal Ab compared with the individual mAb, and also to greater blockade of the toxin surface that interacts with cellular receptors. Such mechanisms may be generally applicable to many antigens in solution, suggesting that oligoclonal Ab may offer a general route to more potent antigen neutralization than mAb. Although it might be possible to achieve a similar potency by engineering the Kd of the C25 mAb to near pM, oligoclonal Ab offers a simpler, more rapid route to a potent antitoxin.
Oligoclonal Ab also offers a safe and unlimited supply of drug for prevention and treatment of BoNT/A intoxication. Because the Ab consists of either chimeric or human IgG, production could be immediately scaled to produce a stockpile of safe antitoxin. Alternatively, we have already replaced the chimeric S25 IgG with a fully human IgG and increased potency of the oligoclonal Ab more than 2-fold. Work is ongoing to replace chimeric C25 with a fully human homologue. Chimeric, humanized, and human mAb represent an increasingly important class of therapeutic agents whose means of production are known. Ten mAbs have been approved by the FDA for human therapy and more then 70 other mAb therapeutics are in clinical trials (37). With an elimination half-life of up to 4 weeks, Ab could provide months of protection against toxin or be used for treatment. Oligoclonal Ab would be applicable to the other BoNT toxin serotypes, as well as to other class A agents. Anthrax is a toxin-mediated disease, and Ab has been shown to be protective for this agent (38, 39). Vaccinia immune globulin can be used to prevent or treat smallpox or complications arising from vaccination of immunocompromised hosts (40). Ab may also be useful for plague and disease caused by the hemorrhagic fever viruses (41, 42). Our data support the rapid development and evaluation of oligoclonal Ab for countering BoNT and other agents of biowarfare and bioterrorism.
Acknowledgments
This work was partially funded by Department of Defense Contract DAMD-17-98-C-8030 and National Institute of Environmental Health Sciences Grant 5 S11 ES09996.
Abbreviations
BoNT, botulinum neurotoxin
BoNT/A, BoNT serotype A
HC, heavy chain C-terminal domain
scFv, single-chain Fv
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gill M. D. (1982) Microbiol. Rev. 46, 86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson L. L. (1980) J. Pharmacol. Exp. Ther. 212, 16-21. [PubMed] [Google Scholar]
- 3.Montecucco C. & Schiavo, G. (1995) Q. Rev. Biophys. 28, 423-472. [DOI] [PubMed] [Google Scholar]
- 4.Lacy D. B., Tepp, W., Cohen, A. C., DasGupta, B. R. & Stevens, R. C. (1998) Nat. Struct. Biol. 5, 898-902. [DOI] [PubMed] [Google Scholar]
- 5.Dolly J. O., Black, J., Williams, R. S. & Melling, J. (1984) Nature (London) 307, 457-460. [DOI] [PubMed] [Google Scholar]
- 6.Montecucco C. (1986) Trends Biochem. Sci. 11, 315-317. [Google Scholar]
- 7.Schiavo G., Benfenati, F., Poulain, B., Rossetto, O., Polverino de Lauretto, P., DasGupta, B. R. & Montecucco, C. (1992) Nature (London) 359, 832-835. [DOI] [PubMed] [Google Scholar]
- 8.Schiavo G., Rossetto, O., Catsicas, S., ., Polverino de Lauretto, P., DasGupta, B. R., Benfenati, F. & Montecucco, C. (1993) J. Biol. Chem. 268, 23784-23787. [PubMed] [Google Scholar]
- 9.Lacy D. B. & Stevens, R. C. (1999) J. Mol. Biol. 291, 1091-1104. [DOI] [PubMed] [Google Scholar]
- 10.Arnon S. S., Schechter, R., Inglesby, T. V., Henderson, D. A., Bartlett, J. G., Ascher, M. S., Eitzen, E., Fine, A. D., Hauer, J., Layton, M., et al. (2001) J. Am. Med. Assoc. 285, 1059-1070. [DOI] [PubMed] [Google Scholar]
- 11.Mahant N., Clouston, P. D. & Lorentz, I. T. (2000) J. Clin. Neurosci. 7, 389-394. [DOI] [PubMed] [Google Scholar]
- 12.United Nations Security Council, (1995) Tenth Report of the Executive Committee of the Special Commission Established by the Secretary-General Pursuant to Paragraph 9(b)(I) of Security Council Resolution 687 (1991), and Paragraph 3 of Resolution 699 (1991) on the Activities of the Special Commission (United Nations Security Council, New York).
- 13.Bozheyeva G., Kunakbayev, Y. & Yeleukenov, D., (1999) Former Soviet Biological Weapons Facilities in Kazakhstan: Past, Present, and Future (Center for Nonproliferation Studies, Monterey Institute of International Studies, Monterey, CA).
- 14.Siegel L. S. (1988) J. Clin. Microbiol. 26, 2351-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne M. P. & Smith, L. A. (2000) Biochimie 82, 955-966. [DOI] [PubMed] [Google Scholar]
- 16.Franz D. R., Pitt, L. M., Clayton, M. A., Hanes, M. A. & Rose, K. J. (1993) in Botulinum and Tetanus Neurotoxins: Neurotransmission and Biomedical Aspects, ed. DasGupta, B. R. (Plenum, New York), pp. 473–476.
- 17.Black R. E. & Gunn, R. A. (1980) Am. J. Med. 69, 567-570. [DOI] [PubMed] [Google Scholar]
- 18.Hibbs R. G., Weber, J. T., Corwin, A., Allos, B. M., Abd el Rehim, M. S., Sharkawy, S. E., Sarn, J. E. & McKee, K. T., Jr. (1996) Clin. Infect. Dis. 23, 337-340. [DOI] [PubMed] [Google Scholar]
- 19.Arnon S. S. (1993) in Botulinum and Tetanus Neurotoxins: Neurotransmission and Biomedical Aspects, ed. DasGupta, B. R. (Plenum, New York), pp. 477–482.
- 20.Lang A. B., Cryz, S. J., Schurch, U., Ganss, M. T. & Bruderer, U. (1993) J. Immunol. 151, 466-472. [PubMed] [Google Scholar]
- 21.Pless D. D., Torres, E. R., Reinke, E. K. & Bavari, S. (2001) Infect. Immun. 69, 570-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallis B., Fooks, S., Shone, C. & Hambleton, P. (1993) in Botulinum and Tetanus Neurotoxins: Neurotransmission and Biomedical Aspects, ed. DasGupta, B. R. (Plenum, New York), pp. 433–436.
- 23.Amersdorfer P., Wong, C., Smith, T., Chen, S., Deshpande, S., Sheridan, R. & Marks, J. D. (1997) Infect. Immun. 65, 3743-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amersdorfer P., Wong, C., Smith, T., Chen, S., Deshpande, S., Sheridan, R. & Marks, J. D. (2002) Vaccine 20, 1640-1648. [DOI] [PubMed] [Google Scholar]
- 25.Colcher D., Bird, R., Roselli, M., Hardman, K. D., Johnson, S., Pope, S., Dodd, S. W., Pantoliano, M. W., Milenic, D. E. & Schlom, J. (1990) J. Natl. Cancer Inst. 82, 1191-1197. [DOI] [PubMed] [Google Scholar]
- 26.Desphande S. S., Sheridan, R. E. & Adler, M. (1995) Toxicon 33, 551-557. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan R. E., Deshpande, S. S., Amersdorfer, P., Marks, J. D. & Smith, T. (2001) Toxicon 39, 651-657. [DOI] [PubMed] [Google Scholar]
- 28.Hatheway C. L. & Dang, C. (1994) in Therapy with Botulinum Toxin, ed. Jankovic, J. (Dekker, New York.), pp. 93–107.
- 29.Bowmer E. J. (1963) Bull. W. H. O. 29, 701-709. [PMC free article] [PubMed] [Google Scholar]
- 30.Moyle W. R., Anderson, D. M. & Ehrlich, P. H. (1983) J. Immunol. 131, 1900-1905. [PubMed] [Google Scholar]
- 31.Crothers D. M. & Metzger, H. (1972) Immunochemistry 9, 341-357. [DOI] [PubMed] [Google Scholar]
- 32.Fotinou C., Emsley, P., Black, I., Ando, H., Ishida, H., Kiso, M., Sinha, K. A., Fairweather, N. F. & Isaacs, N. W. (2001) J. Biol. Chem. 276, 32274-32281. [DOI] [PubMed] [Google Scholar]
- 33.Fitzsimmons S. P., Clark, K. C., Wilkerson, R. & Shapiro, M. A. (2000) Vaccine 19, 114-121. [DOI] [PubMed] [Google Scholar]
- 34.Mullaney B. P., Pallavicini, M. G. & Marks, J. D. (2001) Infect. Immun. 69, 6511-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volk W. A., Bizzini, B., Snyder, R. M., Bernhard, E. & Wagner, R. R. (1984) Infect. Immun. 45, 604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwick M. B., Wang, M., Poignard, P., Stiegler, G., Katinger, H., Burton, D. R. & Parren, P. W. (2001) J. Virol. 75, 12198-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichert J. M. (2001) Nat. Biotechnol. 19, 819-822. [DOI] [PubMed] [Google Scholar]
- 38.Little S. F., Ivins, B. E., Fellows, P. F. & Friedlander, A. M. (1997) Infect. Immun. 65, 5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beedham R. J., Turnbull, P. C. B. & Williamson, E. D. (2001) Vaccine 19, 4409-4416. [DOI] [PubMed] [Google Scholar]
- 40.Feery B. J. (1976) Vox Sang. 31, 68-76. [DOI] [PubMed] [Google Scholar]
- 41.Hill J., Leary, S. E., Griffin, K. F., Williamson, E. D. & Titball, R. W. (1997) Infect. immun. 65, 4476-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson J. A., Hevey, M., Bakken, R., Guest, S., Bray, M., Schmaljohn, A. L. & Hart, M. K. (2000) Science 287, 1664-1666. [DOI] [PubMed] [Google Scholar]