Abstract
The Wiskott–Aldrich syndrome (WAS) is a primary immunodeficiency disorder caused by a mutation in WAS protein (WASp) that results in defective actin polymerization. Although the function of many hematopoietic cells requires WASp, the specific expression and function of this molecule in natural killer (NK) cells is unknown. Here, we report that WAS patients have increased percentages of peripheral blood NK cells and that fresh enriched NK cells from two patients with a WASp mutation have defective cytolytic function. In normal NK cells, WASp was expressed and localized to the activating immunologic synapse (IS) with filamentous actin (F-actin). Perforin also localized to the NK cell-activating IS but at a lesser frequency than F-actin and WASp. The accumulation of F-actin and WASp at the activating IS was decreased significantly in NK cells that had been treated with the inhibitor of actin polymerization, cytochalasin D. NK cells from WAS patients lacked expression of WASp and accumulated F-actin at the activating IS infrequently. Thus, WASp has an important function in NK cells. In patients with WASp mutations, the resulting NK cell defects are likely to contribute to their disease.
The Wiskott–Aldrich syndrome (WAS) is an X-linked combined immunodeficiency affecting 1 in 300,000 live male births caused by mutations in the gene coding for WASp (1). WASp is a member of the WASp/Scar/WAVE family of proteins expressed specifically in hematopoietic cells. It binds actin monomers as well as the actin-related protein (ARP) 2/3 complex to catalyze branching of filamentous actin (F-actin) (2). Mutations in WASp impairing its function result in reduced actin polymerization, filopodia formation, phagocytosis, and chemotaxis (3). Children with WAS suffer from susceptibility to a variety of infections including those caused by herpesviruses as well as greatly increased incidence of malignancies (4).
Natural killer (NK) cells are lymphocytes lacking expression of T cell receptors and Ig that are capable of producing cytokines and mediating cytolysis after being activated through germ-line-encoded receptors. NK cells use these activities to play important roles in antiviral defense and tumor surveillance (5). NK cells also express inhibitory receptors, e.g., the killer cell Ig-like receptor (KIR) family, that are capable of curtailing NK cell functions after ligation. Naturally occurring human deficiencies of NK cells or NK cell activity have been reported but are rare (6). Advances in the understanding of human primary immunodeficiency and identification of single gene defects as etiologies will provide opportunities to elucidate specific pathways in NK cell function. In this light, quantification of NK cell activity in peripheral blood mononuclear cell (PBMC) populations from WAS patients has yielded mixed results, with occasional reports of deficits in cytotoxic activity (7–10). However, the activities of isolated NK cells from patients with WAS have not been evaluated.
NK cell activation appears to require function of the actin cytoskeleton, as does T cell activation (11). Early work has shown that actin is polymerized in NK cells encountering susceptible, but not resistant, target cells and that inhibitors of actin polymerization interfere with cytolytic function (12, 13). More recent studies with immortalized NK cell tumor lines or NK cells propagated in vitro with IL-2 have shown that a variety of signaling molecules as well as lipid rafts and the cytoskeletal attachment protein, talin, localize to the activating NK cell immunologic synapse (IS) (14–16). Although there are similarities between the NK cell-activating and inhibitory IS (17, 18), molecules accumulate with different kinetics and lipid rafts are excluded from the inhibitory IS (14, 15, 18). Furthermore, KIRs and KIR signaling are essential to the inhibitory IS, and KIR accumulation does not require ATP or actin polymerization (15, 17). In contrast, raft polarization in NK cell-activating interactions is necessary for cytotoxicity (14) and requires actin polymerization as evidenced by its sensitivity to cytochalasin D (Cyt-D) (15). However, the roles of specific regulators of cytoskeletal formation including WASp have not been investigated in NK cell functional interactions.
Methods
Patients and Control Donors.
Diagnosis of WAS was based on the presence of established clinical findings (4) and mutation in the WASP gene. WASP sequence analysis was performed as described (19–21). Pre-bone marrow transplant immunophenotypes of all WAS patients diagnosed and treated at Children's Hospital between 1992 and 2002 were reviewed retrospectively. PBMC were evaluated by flow cytometry in the Children's Hospital Clinical Immunology Laboratory by using FITC-anti-CD3, PE-anti-CD56, and PE-anti-CD16 (Becton Dickinson). When these reagents are used, NK cells are PE+ and FITC−.
Further studies were performed with whole blood from patients 1 and 2. Patient 1 had thrombocytopenia, recurrent otitis media, cellulitis, and severe eczema. Patient 2, the younger brother of patient 1, was diagnosed postnatally and had not experienced infectious complications. Both patients were treated every 3 weeks with 500 mg/kg i.v. Ig. All studies were performed with informed consent and the approval of the Children's Hospital Committee on Clinical Investigation.
Control donors were healthy adolescents or adults and had a similar range of NK cell cytotoxicity as would individuals specifically age-matched to WAS patients 1 and 2 (22).
Reagents.
Where specified cells were maintained in RPMI complete medium (RPMI-CM), consisting of 1× RPMI (GIBCO/BRL), 10% heat-inactivated FBS (Atlanta Biologicals, Norcross, GA), 0.1 mM nonessential amino acids, 10 mM Hepes, 1 mM Na Pyruvate, 50 units/ml Penicillin G, 50 μg/ml streptomycin sulfate, and 2 mM l-glutamine (GIBCO/BRL). Cyt-D (Sigma) was dissolved in DMSO (EM Science) for use as an inhibitor.
Cell Preparation and Analysis.
Lymphocytes were separated from blood by using Ficoll/Hypaque (Amersham Pharmacia). Negative selection of NK from PBMC was performed by using Stem-Sep NK (StemCell Technologies, Vancouver) or from blood by using Rosette-Sep NK (StemCell Technologies) according to the manufacturer's instructions. Enrichment was assessed with a FACScalibur (Becton Dickinson) by using directly conjugated FITC-anti-CD3, PE-anti-CD56, and CY5-anti-CD16 and in all cases resulted in >85% CD56+/CD3− NK cells with <0.5% contaminating CD3+ T cells. Enriched NK cells were used directly in experiments or incubated at 37°C in RPMI-CM with 0.1% DMSO or 10 μM Cyt-D/0.1% DMSO for 30 min before use. For gene array analysis, highly purified NK cells were prepared by sorting CD56+/CD16+/CD3− cells with a Moflo (Cytomation, Fort Collins, CO). Total RNA was prepared from sorted cells with Trizol reagent (GIBCO) and double-stranded cDNA was synthesized and transcribed as biotin-labeled cRNA, which was hybridized to Human Genome U95Av2 GeneChip Arrays (Affymetrix, Santa Clara, CA) and prepared further according to the manufacturer's recommendations. Analysis of hybridization was performed by using NetAffyx and microarray 5.0 software (Affymetrix). For Western blotting, PBMC or NK cell preparations were lysed in 1% Nonidet P-40 lysis buffer and 20 μg protein separated on a 10% polyacrylamide gel. Proteins were transferred to nitrocellulose membranes, which were incubated with murine anti-WASp mAb 5A5 (23). Bound antibody was detected with peroxidase-conjugated goat-anti-mouse (Jackson ImmunoResearch) and enhanced chemiluminescence detection system (Amersham Pharmacia).
Cytotoxicity Assays.
NK cell cytotoxicity was evaluated by 51Cr-release assay by using K562 erythroleukemia target cells. K562 cells in RPMI-CM were labeled by incubation with 100 μCi (1 Ci = 37 GBq) 51Cr (Na2CrO4) (Perkin–Elmer) per 1 × 106 cells at 37°C for 1 h followed by four washes in RPMI medium. Labeled target cells (1 × 104) in RPMI-CM were placed in each well of a 96-well, round-bottomed polystyrene plate (Corning), mixed with NK cells at specified effector-to-target cell ratios and incubated for 4 h at 37°C. 51Cr released into supernatants (experimental cpm) was measured with a Lumiplate and TopCount XL gamma detector (Packard). Percentage lysis was calculated as follows: (exp. cpm − spontaneously released cpm)/(total cpm − spontaneously released cpm). Total cpm were assessed by complete lysis of target cells by using 1% Nonidet P-40 in water. Lytic units were defined as the number of effector cells required to mediate 20% lysis of target cells expressed as the inverse normalized to 1 × 104 cells and were calculated by using the slopes of curves generated by 51Cr-release assay over the range of effector-to-target cell ratios. For experiments using Cyt-D- and DMSO-treated cells, cytotoxicity assay was performed in the presence of 5 μM Cyt-D/0.05% DMSO or 0.05% DMSO, respectively.
Confocal Microscopy.
NK cell/target cell conjugates were formed by suspending NK effector cells at 5 × 106/ml and K562 erythroleukemia target cells at 2.5 × 106/ml in RPMI-CM. Equal volumes of effector and target cell suspensions were mixed and incubated for 15 min at 37°C. When cells were first pretreated with Cyt-D or DMSO, conjugates were allowed to form in the presence of 5 μM Cyt-D/0.05% DMSO or 0.05% DMSO, respectively. NK cells or NK cell/K562 cell conjugates were resuspended gently and 60 μl was incubated on Polyprep microscope slides (Sigma) for 15 min at 37°C. Slides were rinsed in 2× PBS, and adherent cells were fixed and permeabilized with 4% formaldehyde, 0.1% saponin, and 0.1% Triton X-100 in PBS for 15 min. Slides were washed in 2× PBS with 0.1% saponin (PBS-s) and incubated for 1 h with anti-WASp mAb, with control mouse IgG2a clone MOPC31 (Becton Dickinson) used as a control. Slides were washed with 2× PBS-s, incubated for 1 h with ALEXA-568-conjugated, highly cross-adsorbed goat-anti-mouse (Molecular Probes), washed with 2× PBS-s, and incubated with 10% heat-inactivated mouse serum (Sigma) for 30 min to block subsequent-nonspecific binding. After two PBS-s washes, murine FITC-anti-perforin IgG2b or FITC-IgG2b control (Becton Dickinson) was added with or without ALEXA-647-conjugated phalloidin (Molecular Probes) or ALEXA-647-conjugated streptavidin (as a negative control) for 1 h. Slides were washed with 2× PBS-s, covered with 0.15-mm glass coverslips (VWR Scientific), and mounted with cytoseal (Richard-Allan Scientific, Kalamazoo, MI). All antibodies were used in the range of 0.2–20 μg/ml.
Images were acquired with a Zeiss LSM 510 confocal microscope equipped with argon (488 nm) and dual-helium neon (543 nm, 633 nm) lasers, and analysis was performed with associated LSM 510 software (Zeiss). To compensate the microscope, single stains with the aforementioned negative controls were performed. Microscope settings were adjusted to eliminate nonspecific fluorescence and cross-channel bleed-through.
Evaluation of NK Cell/Target Cell Conjugates.
Slides were scanned by using Nomarski optics, and conjugates were identified as NK cells (small cells) and K562 cells (large cells) that were in obvious contact. Once conjugates were identified, fluorescence was examined in all three channels and the presence of an NK cell was confirmed by detection of perforin expression. F-actin, WASp, and perforin were evaluated as nonsynaptic or having synaptic accumulation in 50–100 conjugates randomly selected on each slide. The percentage of conjugates with synaptic accumulation of each marker was calculated as well as the percentage colocalization of WASp or perforin with F-actin. Mean data were compared by using Student's t test. In experiments with normal NK cells, at least five conjugates demonstrating synaptic F-actin and WASp localization were evaluated for three-dimensional alignment by the acquisition of 16–20 images at 0.5-μm intervals in the z axis, which then were projected and rotated for z, x or z, y viewing by using the LSM 510 software package.
Results
WASp Is Expressed in Normal NK Cells but Is Absent in NK Cells from Patients with WAS.
Although expression of WASp is limited to hematopoietic cells, information regarding the NK cell-specific expression of WASp is limited. This question initially was addressed by WASp probe hybridization in a genome microarray that had been incubated with mRNA from highly purified, normal fresh peripheral CD56+CD3− NK cells. In NK cells from three separate control donors, WASp mRNA hybridization to two distinct probe sets was present, demonstrating WASp mRNA in normal NK cells. Hybridization of mRNAs for other genes expected to be expressed in normal NK cells also was found including CD16, CD45, and perforin (L.K. and J.L.S., unpublished results).
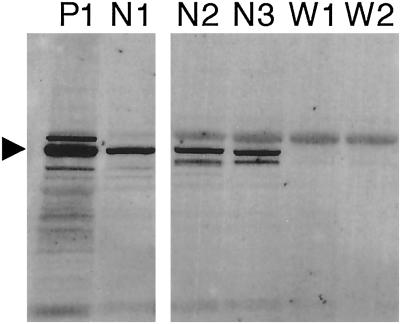
To determine whether the presence of WASp mRNA correlates with detectable levels of WASp, Western blotting was performed with cell lysates from enriched peripheral blood NK cells. High levels of WASp protein were detected easily in PBMC as well as in NK cells prepared from the same healthy individual (Fig. 1, P1 and N1). Similar levels of WASp also were found in NK cells prepared from other normal donors (Fig. 1, N2 and N3). Although WASp expression can be variable in PBMC from WAS patients (20), WASp was not detectable in NK cells from WAS patients 1 and 2 (Fig. 1, W1 and W2). Culture of patient NK cells in 500 units/ml IL-2 for 3 weeks failed to reverse this finding (data not shown). Thus, WASp was expressed and produced in normal NK cells but was absent in two WAS patient NK cells.
Fig 1.
WASp is expressed in normal but not in WAS NK cells. Total cell lysates were prepared, proteins were separated, and Western blotting was performed by using mAb 5A5. PBMC lysate from control donor 1 (P1) is shown for comparison with enriched NK cell lysates from control donor 1 (N1), control donor 2 (N2), control donor 3 (N3), and WAS patients 1 (W1) and 2 (W1). The arrowhead points to the band correlating with the expected size of WASp.
Increased NK Cell Populations with Deficient Cytotoxic Activity in WAS.
Previous reports of NK cell cytolytic activity in total PBMC from WAS patients have not yielded consistent deficiencies (7–9). To determine whether the observed heterogeneity might be a result of expanded NK cell populations in WAS patients, the immunophenotype of all patients treated at Children's Hospital in the past decade was reviewed. The frequency of NK cells was evaluated before bone marrow transplantation in nine patients diagnosed with WAS who had demonstrable mutation in the WASP gene (Table 1). The mean percentage of NK cells in PBMC from WAS patients was elevated significantly as compared with age-specific means for healthy children (24). In addition, mean NK cell percentages of WAS patients were ≈2 SD above the age mean. The increased percentages of peripheral blood NK cells in WAS patients may account for heterogeneity in cytotoxic activity previously observed in WAS patient total PBMC.
Table 1.
Increased NK cell percentages in WAS patients
| Patient | Age in months | WASP mutation | Ref. | % NK | % NK age mean |
|---|---|---|---|---|---|
| 1 | 44 | G252A | 34 | 10 (26) | |
| 2 | 7 | G252A | 28 | 5 (17) | |
| 3 | 2 | ΔA763 | 28 | 6 (13) | |
| 4 | 3 | Insert T (+2) intron 3 | 20 | 26 | 6 (13) |
| 5 | 11 | G→A (+5) intron 6 | 19 | 19 | 7 (16) |
| 6 | 11 | C665T | 19 | 10 | 7 (16) |
| 7 | 11 | G431A | 22 | 7 (16) | |
| 8 | 15 | 1055 insert T | 7 | 7 (16) | |
| 9 | 134 | Large deletion | 21 | 23 | 15 (31) |
| Average | 26 ± 15 | 22 ± 3 | 8 ± 1 |
M. I. Lutskiy, F.S.R., and E.R.-O., unpublished data.
Average % NK cells for age, with 95th percentile, ≈2 SD above mean, in parentheses.
Mean ± SE.
Increased relative to age mean %; P = 0.0003.
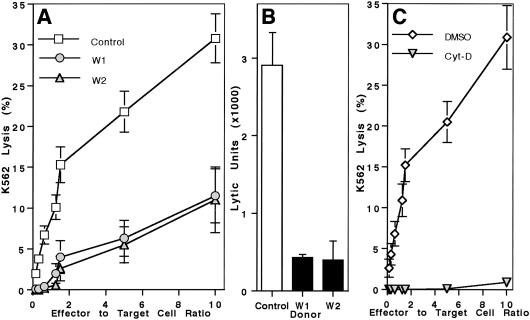
To determine whether NK cells in WAS patients have an intrinsic defect in cytolytic activity, two patients who had not yet received a bone marrow transplant were studied in greater detail. PBMC from patients 1 and 2 were assessed for NK cell cytotoxicity against K562 target cells and were found to have activity present (data not shown). To more accurately investigate NK cell activity on a per cell basis, NK cells were enriched by negative selection and evaluated for lysis of K562 cells. When similarly pure populations of NK cells were used from control donors and WAS patients, NK cell cytotoxicity was decreased significantly in both boys with WAS (Fig. 2A). A severe deficit in activity of enriched NK cells also was reflected in lytic units: WAS patients 1 and 2 only had 17% and 16% of controls, respectively (Fig. 2B). Thus, NK cell cytotoxicity is defective in WAS. The low activity may be compensated by increases in the PBMC NK cell populations.
Fig 2.
Deficiency of NK cell cytotoxicity in WAS and Cyt-D-treated NK cells. (A) Cytotoxic activity against K562 target cells by enriched NK cells from controls (□) and WAS patients 1 (○) and 2 (▵) is shown. Lysis mediated by WAS patient samples was different from controls (P < 0.005). Mean data for 10 controls and 3 individual donations for each WAS patient are shown ± SE. (B) K562 lytic units were calculated from individual curves shown in A. Mean data + SE are shown. Values for WAS patients 1 and 2 were less than controls (P < 0.01). (C) Normal enriched NK cells were incubated with either 10 μM Cyt-D (▿) or DMSO vehicle (⋄). Lytic activity against K562 target cells ± SE in four separate experiments is shown. Lysis by Cyt-D-treated cells was essentially eliminated as compared with DMSO control-treated cells (P < 0.001).
WASp and F-Actin Colocalize to the Activating NK Cell IS.
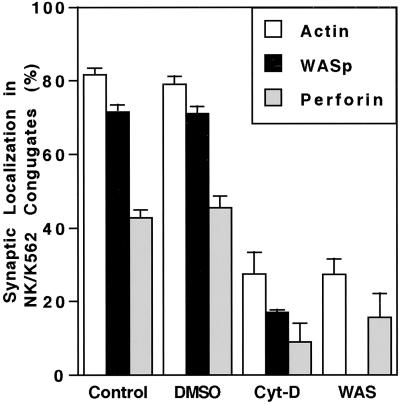
F-actin is known to accumulate at contact points between NK cells and susceptible target cells (12). Because NK cell cytotoxicity was deficient in WAS patients and WASp catalyzes actin polymerization, localization of WASp in healthy donor NK cells forming an activating IS was examined. In freshly isolated normal NK cells, both F-actin and WASp were visualized at the NK cell side of the interface with K562 target cells by laser-scanning confocal microscopy (Figs. 3 and 4A). F-actin and WASp accumulated at the activating IS in 82% and 72% of NK cell/target cell conjugates, respectively (Fig. 3). When F-actin was located at the IS, WASp was found to colocalize 86% of the time. Perforin also could be visualized in conjugated NK cells and was found either at the NK cell IS along with F-actin and WASp (Fig. 4A) or at some other location in the cell (Fig. 4B). Perforin was found only at the IS in 43% of conjugates (Fig. 3). Similarly, when F-actin was found at the IS, perforin also was there 50% of the time. Three-dimensional reconstruction of the NK cell IS with accumulated F-actin and WASp by using Z sections routinely demonstrated colocalization of the two molecules (Fig. 4C). When present at the IS, perforin only colocalized with part of the F-actin/WASp complex. Thus, F-actin and WASp were located in the vast majority of NK cell-activating IS, and perforin was present at the IS in a subset.
Fig 3.
Quantification of F-actin, WASp, and perforin in the NK cell-activating IS. NK cell/K562 target cell conjugates were evaluated as detailed in Methods. Synaptic localization of F-actin (open bars), WASp (solid bars), and perforin (shaded bars) is shown. Means of five individual experiments for control donors, four each for DMSO and Cyt D, and three for WAS patients are shown. Error bars show SE. Differences between Cyt-D and DMSO treatment as well as differences between WAS patients and control donors were statistically significant (P < 0.001).
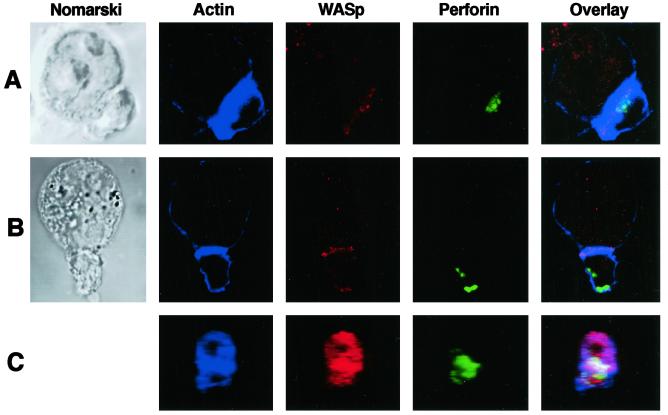
Fig 4.
Cellular localization of F-actin, WASp, and perforin in conjugates of normal NK cells and K562 target cells. NK cell (small cell) and K562 target cell (large cell) conjugates were visualized by three-color laser-scanning confocal microscopy. Images were captured by using Nomarski optics (Left) and fluorescent detectors localizing F-actin (blue), WASp (red), perforin (green), or an overlay of all fluorescent channels (Right in which white areas represent colocalization of all three fluorescent signals and purple areas represent colocalization of WASp and F-actin). Examples are shown for control donor NK cell/target cell conjugates with synaptic localization of perforin (A) and nonsynaptic localization of perforin (B). Three-dimensional supramolecular organization in Z sections of an NK cell/K562 cell interface with synaptic localization of all three signals is shown projected and rotated for two-dimensional viewing (C).
Cyt-D Inhibits NK Cell Cytotoxicity as Well as Actin and WASp Accumulation at the Activating NK Cell IS.
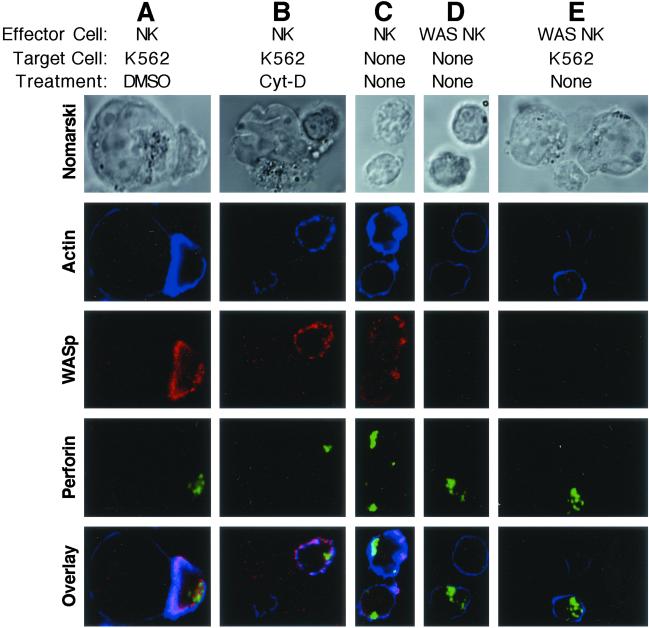
The importance of actin polymerization on and its role in WASp localization to the NK cell-activating IS was investigated by using Cyt-D as a polymerization inhibitor. Cyt-D previously has been demonstrated to prevent NK cell cytotoxic activity (13). Pretreatment of fresh control donor NK cells with Cyt-D for 30 min completely inhibited lytic activity (Fig. 2C), as reported previously. Treatment of cells with DMSO (the solvent for Cyt-D) did not affect NK cytotoxicity. Microscopically, DMSO-treated cells had a normal appearance of the activating NK cell IS (Fig. 5A), but Cyt-D-treated cells had discontinuous F-actin and WASp and synaptic accumulation was not seen (Fig. 5B). In DMSO-treated cells, F-actin and WASp were accumulated at the IS in 79% and 71% of conjugates, whereas in Cyt-D-treated cells they were accumulated in only 27% and 17% of conjugates (Fig. 3). Perforin accumulation also was decreased in conjugates formed with Cyt-D- compared with DMSO-treated NK cells, suggesting that this process depends on effective actin polymerization as well. Thus, actin polymerization is required for NK cell cytotoxic activity and accumulation of WASp and perforin at the activating IS.
Fig 5.
Effect of Cyt-D or WASP mutation on cellular localization of F-actin, WASp, and perforin in NK cells or NK cell/K562 target cell conjugates. NK cells (small cells) were imaged by three-color laser-scanning confocal microscopy alone or after conjugation with K562 target cells (large cells). Images using Nomarski optics (top row) and fluorescent detectors for F-actin (blue), WASp (red), or perforin (green) were acquired. An overlay of all fluorescent channels is shown (bottom row). The effect of DMSO vehicle (A) or Cyt-D (B) incubation on the accumulation of molecules in conjugates between control donor NK cells and K562 target cells is shown. Individual NK cells from controls (C) and WAS patient 2 (D) are compared. An NK cell from WAS patient 2 is shown in conjugation with a target cell (E). Faint or barely visible blue signal in WAS patient cells is consistent with low F-actin content in cells containing WASP mutation.
Actin Accumulation at the NK Cell-Activating Synapse Is Decreased in WAS Patients.
Although defective actin polymerization is a hallmark of WAS, this process has not been evaluated in NK cells of patients with WASp mutations. In addition, a requirement for WASp in perforin mobilization is unknown. To address these questions, WAS patient NK cells were evaluated by laser-scanning confocal microscopy. Patient NK cells were strikingly different from control because they lacked detectable WASp and appeared to have only faint rings of F-Actin (Fig. 5 C and D). Perforin staining, however, was robust and similar to controls. WASp was not induced in patient NK cells conjugating with K562 cells, and F-actin failed to accumulate at the NK cell IS (Fig. 5E). F-actin and perforin were found at the IS in only 27% and 16% of conjugates (Fig. 3). Thus, similar to Cyt-D treatment, patients with WAS have strikingly decreased or absent F-actin accumulation and proportionally decreased perforin accumulation at the NK cell IS. Furthermore, polarization of perforin granules depended on effective WASp-driven actin polymerization.
Discussion
In the few studies of NK cells in WAS reported, some patients had normal and others had deficient K562 killing (4, 7, 9, 10). The observed variability appears to be a result of increased NK cell percentages in PBMC (Table 1), because isolated NK cells from two WAS patients were defective on a per cell basis (Fig. 2). In murine models and humans with NK cell deficiencies, NK cells are important in the defense against herpesviruses (25). The preponderance of herpesgroup virus infections in WAS patients, including a disproportionate number of invasive, recurrent, and drug-resistant occurrences (4, 26, 27), can be understood in this light. Furthermore, children with WAS have an extraordinarily high rate of malignancies (4) that may be related to a role of NK cells in tumor cell surveillance (5). Thus, the functional defects found in WAS patient NK cells appear to be clinically significant.
WASp was expressed in normal NK cells (Fig. 1) and was located diffusely near the surface of normal unactivated NK cells (Fig. 5). After interaction with a susceptible target cell, WASp accumulated at the activating IS with F-actin (Figs. 3 and 4). This localization was required for functional activating IS formation and cytotoxic activity, as shown both by Cyt-D treatment of normal NK cells and by studies of WAS patients deficient in WASp. The signals required for WASp targeting to membrane activation domains also are being investigated in T cells (28, 29). Because of the differences in signaling cascades on activation of T cells and NK cells, pathways leading to WASp localization and function in these two cell types could be distinct. Pursuit of WASp regulation in NK cells, therefore, may provide hitherto unappreciated mechanisms of WASp localization.
The nature of both the inhibitory and activating human NK cell IS continues to be elucidated structurally, spatially, and temporally (14–18). In addition to WASp and F-actin, talin accumulates at the NK cell-activating IS and forms a variety of three-dimensional arrangements (16). Moreover, rearrangement at the NK cell IS occurs with great speed (18). These studies, however, used IL-2-driven NK cell cultures. To avoid the effect of activating factors required for in vitro propagation that have well known and potent effects on NK cells, the present studies used freshly isolated NK cells. In vivo treatment of a WAS patient and stimulation of WAS patient lymphocytes in vitro with IL-2 suggest that IL-2 still can induce lymphocyte activation (30, 31). Moreover, preliminary attempts to culture WAS patient NK cells in IL-2 resulted in some normalization of cytolytic activity (unpublished data), and, thus, in vitro IL-2 stimulation was avoided in trying to assess the role of WASp in NK cells. Because IL-2 can induce activation of cells with WASp mutations, IL-2 may promote cytoskeletal rearrangement independently of WASp. Thus, temporal and even spatial events in cultured cells might differ markedly from those in an unstimulated NK cell encountering a susceptible target. The effects of IL-2 on Cyt-D-treated NK cells have not been evaluated, but, presumably, the direct effect of this inhibitor on actin would prevent IL-2 from overcoming Cyt-D-mediated inhibition.
A difference between synaptic localization of WASp/F-actin and perforin in normal cells was demonstrated. Even though 82% of NK cell/target cell conjugates had F-actin and 72% had WASp at the IS, perforin was found to localize to the synapse in only 43% (Fig. 4). Perforin accumulation was also concomitantly decreased in NK cells with impaired actin polymerization resulting from Cyt-D treatment. Actin cytoskeletal rearrangements, therefore, probably precede perforin mobilization. If the purpose of cytoskeletal rearrangement is to allow approximation of lipid raft domains and signaling partners (11), perhaps only signals generated after actin reorganization result in perforin mobilization. Conversely, organization of microtubule structures presumably important in granule movements (32) might occur as an independent and slower process. The former model, however, is suggested by the demonstrated requirement for actin polymerization and WASp function both in NK cell cytotoxic activity and perforin mobilization to the NK cell-activating IS.
Thus, WASp is expressed in human NK cells and localizes to the activating IS with F-actin, and intact WASp function is required for NK cell cytotoxicity. Patients with WASP mutations and treatment with cytochalasin D, an actin-depolymerizing agent, demonstrate the importance of cytoskeletal activation and reorganization in the activation of NK cells. Moreover, the present data reinforce the conclusion that formation of the activating and inhibitory NK cell IS occurs by fundamentally distinct mechanisms, the actin cytoskeleton being required for the former, as shown here, but not for the latter synapse (15, 17).
Acknowledgments
We thank the patients and families affected by WAS for their devotion to research as well as N. Martinez-Quiles and M. Fassett for advice. This work was supported by National Institutes of Health Grant AI-50207 (to J.L.S.), a March of Dimes Foundation grant (to N.R.), and the Jeffery Modell Foundation Center of Excellence and National Institutes of Health Grant HL-59561 (to R.S.G. and F.S.R.). J.S.O. was supported by National Institutes of Health Training Grant AI-07512.
Abbreviations
IS, immunologic synapse
WAS, Wiskott–Aldrich syndrome
WASp, WAS protein
WASP, the gene mutated in WAS
NK, natural killer
KIR, killer cell immunoglobulin-like receptor
F-actin, filamentous actin
Cyt-D, Cytochalasin-D
PBMC, peripheral blood mononuclear cell
RPMI-CM, RPMI complete medium
PBS-s, PBS with 0.1% saponin
References
- 1.Derry J. M., Ochs, H. D. & Francke, U. (1994) Cell 78, 635-644. [DOI] [PubMed] [Google Scholar]
- 2.Higgs H. N. & Pollard, T. D. (2001) Annu. Rev. Biochem. 70, 649-676. [DOI] [PubMed] [Google Scholar]
- 3.Thrasher A. J., Burns, S., Lorenzi, R. & Jones, G. E. (2000) Immunol. Rev. 178, 118-128. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan K. E., Mullen, C. A., Blaese, R. M. & Winkelstein, J. A. (1994) J. Pediatr. 125, 876-885. [DOI] [PubMed] [Google Scholar]
- 5.Miller J. S. (2001) Exp. Hematol. 29, 1157-1168. [DOI] [PubMed] [Google Scholar]
- 6.Biron C. A., Byron, K. S. & Sullivan, J. L. (1989) N. Engl. J. Med. 320, 1731-1735. [DOI] [PubMed] [Google Scholar]
- 7.Lipinski M., Virelizier, J. L., Tursz, T. & Griscelli, C. (1980) Eur. J. Immunol. 10, 246-249. [DOI] [PubMed] [Google Scholar]
- 8.Lopez C., Kirkpatrick, D., Read, S. E., Fitzgerald, P. A., Pitt, J., Pahwa, S., Ching, C. Y. & Smithwick, E. M. (1983) J. Infect. Dis. 147, 1030-1035. [DOI] [PubMed] [Google Scholar]
- 9.Messina C., Kirkpatrick, D., Fitzgerald, P. A., O'Reilly, R. J., Siegal, F. P., Cunningham-Rundles, C., Blaese, M., Oleske, J., Pahwa, S. & Lopez, C. (1986) Clin. Immunol. Immunopathol. 39, 394-404. [DOI] [PubMed] [Google Scholar]
- 10.Plebani A., Airo, P., Brugnoni, D., Lebowitz, M., Cattaneo, R., Monafo, V., Meini, A., Notarangelo, L. D., Duse, M. & Ugazio, A. G. (1995) Haematologica 80, 521-525. [PubMed] [Google Scholar]
- 11.Dustin M. L. & Cooper, J. A. (2000) Nat. Immunol. 1, 23-29. [DOI] [PubMed] [Google Scholar]
- 12.Carpen O., Virtanen, I., Lehto, V. P. & Saksela, E. (1983) J. Immunol. 131, 2695-2698. [PubMed] [Google Scholar]
- 13.Katz P., Zaytoun, A. M. & Lee, J. H., Jr. (1982) J. Immunol. 129, 2816-2825. [PubMed] [Google Scholar]
- 14.Lou Z., Jevremovic, D., Billadeau, D. D. & Leibson, P. J. (2000) J. Exp. Med. 191, 347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fassett M. S., Davis, D. M., Valter, M. M., Cohen, G. B. & Strominger, J. L. (2001) Proc. Natl. Acad. Sci. USA 98, 14547-14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vyas Y. M., Mehta, K. M., Morgan, M., Maniar, H., Butros, L., Jung, S., Burkhardt, J. K. & Dupont, B. (2001) J. Immunol. 167, 4358-4367. [DOI] [PubMed] [Google Scholar]
- 17.Davis D. M., Chiu, I., Fassett, M., Cohen, G. B., Mandelboim, O. & Strominger, J. L. (1999) Proc. Natl. Acad. Sci. USA 96, 15062-15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vyas Y. M., Maniar, H. & Dupont, B. (2002) J. Immunol. 168, 3150-3154. [DOI] [PubMed] [Google Scholar]
- 19.Kwan S. P., Hagemann, T. L., Blaese, R. M., Knutsen, A. & Rosen, F. S. (1995) Hum. Mol. Genet. 4, 1995-1998. [DOI] [PubMed] [Google Scholar]
- 20.Shcherbina A., Rosen, F. S. & Remold-O'Donnell, E. (1999) J. Immunol. 163, 6314-6320. [PubMed] [Google Scholar]
- 21.Lutskiy M. I., Jones, L. N., Rosen, F. S. & Remold-O'Donnell, E. (2002) Hum. Genet. 110, 515-519. [DOI] [PubMed] [Google Scholar]
- 22.Yabuhara A., Kawai, H. & Komiyama, A. (1990) Pediatr. Res. 28, 316-322. [DOI] [PubMed] [Google Scholar]
- 23.Kawai S., Minegishi, M., Ohashi, Y., Sasahara, Y., Kumaki, S., Konno, T., Miki, H., Derry, J., Nonoyama, S., Miyawaki, T., et al. (2002) J. Immunol. Methods 260, 195-205. [DOI] [PubMed] [Google Scholar]
- 24.Comans-Bitter W. M., de Groot, R., van den Beemd, R., Neijens, H. J., Hop, W. C., Groeneveld, K., Hooijkaas, H. & van Dongen, J. J. (1997) J. Pediatr. 130, 388-393. [DOI] [PubMed] [Google Scholar]
- 25.Biron C. A., Nguyen, K. B., Pien, G. C., Cousens, L. P. & Salazar-Mather, T. P. (1999) Annu. Rev. Immunol. 17, 189-220. [DOI] [PubMed] [Google Scholar]
- 26.Wade N. A., Lepow, M. L., Veazey, J. & Meuwissen, H. J. (1985) Pediatrics 75, 672-675. [PubMed] [Google Scholar]
- 27.Saijo M., Suzutani, T., Murono, K., Hirano, Y. & Itoh, K. (1998) Br. J. Dermatol. 139, 311-314. [DOI] [PubMed] [Google Scholar]
- 28.Cannon J. L., Labno, C. M., Bosco, G., Seth, A., McGavin, M. H., Siminovitch, K. A., Rosen, M. K. & Burkhardt, J. K. (2001) Immunity 15, 249-259. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Quiles N., Rohatgi, R., Anton, I. M., Medina, M., Saville, S. P., Miki, H., Yamaguchi, H., Takenawa, T., Hartwig, J. H., Geha, R. S., et al. (2001) Nat. Cell Biol. 3, 484-491. [DOI] [PubMed] [Google Scholar]
- 30.Molina I. J., Sancho, J., Terhorst, C., Rosen, F. S. & Remold-O'Donnell, E. (1993) J. Immunol. 151, 4383-4390. [PubMed] [Google Scholar]
- 31.Azuma H., Oshima, M., Ito, K., Okuno, A., Kawabata, I., Banba, K., Murahashi, H., Sekine, T., Kato, Y., Ikebuchi, K., et al. (2000) Eur. J. Pediatr. 159, 633-634. [DOI] [PubMed] [Google Scholar]
- 32.Burkhardt J. K., McIlvain, J. M., Jr., Sheetz, M. P. & Argon, Y. (1993) J. Cell Sci. 104, 151-162. [DOI] [PubMed] [Google Scholar]