Abstract
Recent investigation has focused on identifying signaling pathways that inhibit cardiac hypertrophy, a major risk factor for cardiovascular morbidity and mortality. In this context, nitric oxide (NO), signaling via cGMP and cGMP-dependent protein kinase type I (PKG I), has been recognized as a negative regulator of cardiac myocyte (CM) hypertrophy. However, the underlying mechanisms are poorly understood. Here, we show that PKG I inhibits CM hypertrophy by targeting the calcineurin-NFAT signaling pathway. Calcineurin, a Ca2+-dependent phosphatase, promotes hypertrophy in part by activating NFAT transcription factors which induce expression of hypertrophic genes, including brain natriuretic peptide (BNP). Activation of PKG I by NO/cGMP in CM suppressed NFAT transcriptional activity, BNP induction, and cell enlargement in response to α1-adrenoreceptor stimulation but not in response to adenoviral expression of a Ca2+-independent, constitutively active calcineurin mutant, thus demonstrating NO-cGMP-PKG I inhibition of calcineurin-NFAT signaling upstream of calcineurin. PKG I suppressed single L-type Ca2+-channel open probability, [Ca2+]i transient amplitude, and, most importantly, L-type Ca2+-channel current-induced NFAT activation, indicating that PKG I targets Ca2+-dependent steps upstream of calcineurin. Adenoviral expression of PKG I enhanced NO/cGMP inhibitory effects upstream of calcineurin, confirming that PKG I mediates NO/cGMP inhibition of calcineurin-NFAT signaling. In CM overexpressing PKG I, NO/cGMP also suppressed BNP induction and cell enlargement but not NFAT activation elicited by constitutively active calcineurin, which is consistent with additional, NFAT-independent inhibitory effect(s) of PKG I downstream of calcineurin. Inhibition of calcineurin-NFAT signaling by PKG I provides a framework for understanding how NO inhibits cardiac myocyte hypertrophy.
Cardiac hypertrophy has been viewed as a compensatory mechanism of the heart that helps to maintain cardiac output during pathological states with sustained increases in hemodynamic load. However, hypertrophy often heralds decompensation, transition to heart failure, and sudden death (1). To understand the molecular mechanisms of this ultimately maladaptive response, much investigation has focused on the signaling pathways controlling hypertrophy at the level of the single cardiac myocyte (CM). It has emerged that the hypertrophic response is orchestrated by growth factors and cytokines acting through several interdependent signaling cascades, including, for example, specific G protein isoforms, low-molecular-weight GTPases, mitogen-activated protein kinases, and protein kinase C (2, 3). In addition, recent studies have recognized the importance of Ca2+-sensitive signaling pathways in CM hypertrophy (4). One prominent Ca2+-dependent pathway involves the Ser/Thr protein phosphatase calcineurin. Activation of calcineurin by Ca2+ results in the dephosphorylation and nuclear translocation of cytoplasmic latent nuclear factor of activated T cells (NFAT) transcription factors (5). Many lines of evidence, including the antihypertrophic effects of endogenous calcineurin inhibitory peptides, have contributed to the conclusion that the calcineurin-NFAT pathway plays an essential role in the hypertrophic response to growth factor stimulation (6–9). Importantly, calcineurin activates and interacts with other hypertrophic signaling cascades (10–13), supporting the concept that CM hypertrophy is controlled by crosstalking signaling networks.
Adding yet another level of complexity, recent studies have identified signaling pathways opposing CM hypertrophy (14–16). In this context, the free radical gas nitric oxide (NO) has been recognized as a negative regulator of the hypertrophic response (17–20). Antihypertrophic effects of NO are mediated via the second messenger cGMP and its activation of cGMP-dependent protein kinase type I (PKG I) (19, 21), although the mechanisms involved have not been elucidated. Specifically, it is not known whether PKG I exerts antihypertrophic effects via an autonomous inhibitory pathway and/or via suppression of certain hypertrophic signaling cascades. Because PKG I regulates [Ca2+]i at multiple levels (22), we postulated that PKG I may suppress hypertrophy via inhibition of Ca2+-dependent signaling pathways.
In the present study, we demonstrate that NO and cGMP, acting via PKG I, inhibit the hypertrophic calcineurin-NFAT signaling pathway in CM. Our results reveal a complex interplay of the NO-cGMP-PKG I and calcineurin-NFAT signaling cascades and provide a framework for understanding how NO inhibits CM hypertrophy.
Materials and Methods
Materials.
The PKG-selective cGMP analog 8-para-chlorophenylthio-cGMP (8-pCPT-cGMP) was purchased from BioLog Life Science Institute (Bremen, Germany), the L-type Ca2+-channel (LTCC) blocker verapamil and α1-adrenoreceptor agonist phenylephrine (PE) from Sigma, and the NO donor S-nitroso-N-acetyl-d,l-penicillamine (SNAP) and LTCC agonist BAY K 8644 from Calbiochem.
Cell Culture.
Ventricular CM were isolated from 1- to 3-day-old Sprague–Dawley rats, as described (21). CM were plated overnight in gelatin-coated culture dishes (Nunc) in DMEM/medium 199 (4:1) supplemented with 10% (vol/vol) horse serum, 5% (vol/vol) FCS, glutamine, and antibiotics. The next morning, CM were switched to DMEM/medium 199 supplemented only with glutamine and antibiotics (maintenance medium).
Adenoviral Infection.
A replication-deficient adenovirus containing the cDNA of human PKG I (Ad.PKG I; ref. 23) was used to overexpress PKG I in CM. A replication-deficient adenovirus encoding a catalytically inactive form of human PKG I, generated by replacing the essential Lys in the ATP-binding site of PKG I with Ala (Ad.PKG I-K405A; ref. 24) was used as a control. To activate the calcineurin-NFAT signaling pathway in CM independently of Ca2+, CM were infected with a replication-deficient adenovirus encoding a Ca2+-independent, constitutively active, truncated form of mouse calcineurin Aα (Ad.Cn; ref. 25). Viruses were routinely used at a concentration of 1 × 104 viral particles per cell. At this concentration, infection with Ad.PKG I augments PKG activity 12-fold, as compared with noninfected or Ad.PKG I-K405A-infected CM (21).
Plasmid Constructs, Transfection, and Luciferase Assay.
A calcium phosphate procedure was used to transfect CM with luciferase reporter plasmids driven by three NFAT-binding sites (p3xNFAT-GL), three myocyte enhancer factor-2 (MEF2)-binding sites (p3xMEF2-GL), or by 2,501 bp of the rat brain natriuretic peptide (BNP) gene 5′ flanking region (pBNP2501-GL; refs. 26 and 27). Luciferase activities were measured by using a Lumat LB 9501 luminometer (Berthold, Nashua, NH).
NFAT3 Immunofluorescence.
The nuclear localization of NFAT3 in CM was determined by immunostaining (6). Antibodies directed against rat NFAT3 are not commercially available; therefore, CM were transfected with an expression plasmid encoding human NFAT3. An antibody against human NFAT3 and an FITC-conjugated secondary antibody were purchased from Santa Cruz Biotechnology and Dianova (Hamburg, Germany), respectively. Transfected CM were scored for nuclear NFAT3 staining.
Measurement of Single LTCC Activity.
Patch-clamp experiments were performed in the cell-attached configuration, and single LTCC open probability was calculated as described (28). Single-channel currents were elicited for 150 msec at 1.66 Hz by depolarizing pulses from −100 to +20 mV.
Measurement of [Ca2+]i Transients.
CM were loaded with the fluorescent Ca2+ indicator indo-1-AM (1.25 mM, Calbiochem) for 30 min at room temperature. Thereafter, the culture dish was mounted on the heated stage of an inverted microscope (Diaphot 300, Nikon), and CM were field-stimulated at 1 Hz and excited at a wavelength of 350 nm. [Ca2+]i transients were recorded at emission wavelengths of 405 nm and 490 nm with a photomultiplier (PTI 814, Photon Technology International, Lawrenceville, NJ; ref. 29).
Assessment of the Hypertrophic Response.
Brain natriuretic peptide mRNA expression was assessed by Northern blotting by using a 344-bp BNP cDNA probe (26). CM size was determined by planimetry by using phase contrast microscopy and a digital image analyzer (21).
Statistical Analysis.
Data are presented as mean ± SEM. Differences between groups were analyzed by one-way ANOVA followed by Student's t test with Bonferroni correction. A two-tailed P value <0.05 was considered to indicate statistical significance.
Results
PKG I Inhibits α1-Adrenergic Stimulation of NFAT Nuclear Translocation and Transcriptional Activity.
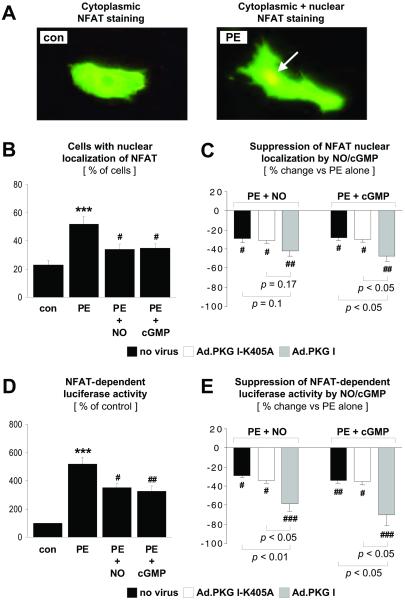
To start exploring whether PKG I interferes with the calcineurin-NFAT signal transduction pathway in CM, the subcellular localization of NFAT3 was examined (Fig. 1 A–C). α1-Adrenergic stimulation with PE, a known activator of calcineurin and cardiac hypertrophy (6), increased the percentage of CM positive for nuclear NFAT3 staining (Fig. 1B). The NO donor SNAP or direct activation of PKG I by the PKG-selective cGMP analog 8-pCPT-cGMP suppressed nuclear translocation of NFAT3 in response to PE stimulation (Fig. 1B). To determine whether reduced nuclear translocation resulted in decreased NFAT transcriptional activity, CM were transfected with a luciferase reporter plasmid driven by three NFAT consensus binding sites. As shown in Fig. 1D, PE increased NFAT transcriptional activity, whereas SNAP or 8-pCPT-cGMP reduced PE-stimulated NFAT activation. To examine PKG I as a potential mediator of NO/cGMP effects directly, CM were infected with adenoviral vectors expressing wild-type or catalytically inactive PKG I. Adenoviral expression of wild-type PKG I (1 × 104 viral particles per cell) augmented the inhibitory effects of SNAP and 8-pCPT-cGMP on nuclear translocation (Fig. 1C) and transcriptional activity of NFAT (Fig. 1E), supporting the concept that PKG I is a downstream mediator of these NO/cGMP effects. By contrast, adenoviral expression of the catalytically inactive PKG I mutant (1 × 104 viral particles per cell) did not affect the suppressive effects of NO/cGMP (Fig. 1 C and E). It should be noted that high concentrations of the catalytically inactive PKG I mutant (1 × 105 viral particles per cell) reversed the inhibitory effects of NO/cGMP on NFAT transcriptional activity (not shown), presumably by competing with endogenous PKG I for cGMP (24). In all subsequent experiments, PKG I adenoviruses were used at a concentration of 1 × 104 viral particles per cell.
Fig 1.
PKG I inhibition of NFAT nuclear translocation and transcriptional activity. Noninfected CM and CM infected with Ad.PKG I-K405A or Ad.PKG I were treated for 48 hr in the absence (con) or presence of PE (10 μM), SNAP (NO, 250 μM), or 8-pCPT-cGMP (cGMP, 500 μM). (A–C) For analysis of the subcellular localization of NFAT3, CM were transfected with an expression plasmid encoding NFAT3. Nuclear NFAT3 localization was assessed by immunostaining (arrow in A). (B) Data are given as % CM with nuclear localization of NFAT3. (C) Data are expressed as % suppression by NO or cGMP of PE-stimulated NFAT nuclear localization. (D and E) PKG I inhibition of NFAT transcriptional activity was demonstrated in CM transfected with p3xNFAT-GL, a luciferase reporter plasmid driven by three NFAT-binding sites. Control CM in D were defined as 100%. (E) Data are presented as % suppression by NO or cGMP of PE-stimulated NFAT-dependent luciferase activity. ***, P < 0.001 vs. con. #, P < 0.05, ##, P < 0.01, and ###, P < 0.001 vs. PE alone (n = 4–6 experiments). P values for other comparisons are shown directly in C and E.
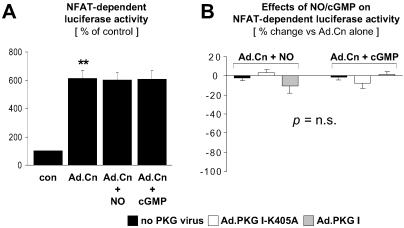
PKG I Does Not Inhibit NFAT Transcriptional Activity Induced by a Constitutively Active Calcineurin Mutant.
To distinguish whether PKG I inhibition of NFAT activation is upstream or downstream of calcineurin, CM were infected with an adenovirus encoding a constitutively active calcineurin mutant. Expression of the calcineurin mutant significantly enhanced NFAT transcriptional activity (Fig. 2A). In contrast to NFAT activation in response to PE treatment (Fig. 1 D and E), NFAT activation by the calcineurin mutant (Fig. 2 A and B) was not inhibited by activation of endogenous or adenovirally expressed PKG I by SNAP or 8-pCPT-cGMP. Taken together, the data presented in Figs. 1 and 2 indicate that NO and cGMP signal via PKG I to inhibit NFAT activation in CM by targeting the calcineurin-NFAT pathway upstream of calcineurin.
Fig 2.
Lack of PKG I inhibition of NFAT transcriptional activity induced by overexpression of constitutively active calcineurin. CM transfected with the luciferase reporter plasmid p3xNFAT-GL were either noninfected or infected with Ad.PKG I-K405A or Ad.PKG I, where indicated. NFAT activation was induced by adenoviral expression of a constitutively active calcineurin mutant (Ad.Cn). CM were treated for 48 hr in the absence (con) or presence of SNAP (NO, 250 μM) or 8-pCPT-cGMP (cGMP, 500 μM). (A) Control CM were defined as 100%. (B) Data are presented as % change of NFAT-dependent luciferase activity induced by NO or cGMP in Ad.Cn-infected CM. **, P < 0.01 vs. con (n = 4–5 experiments).
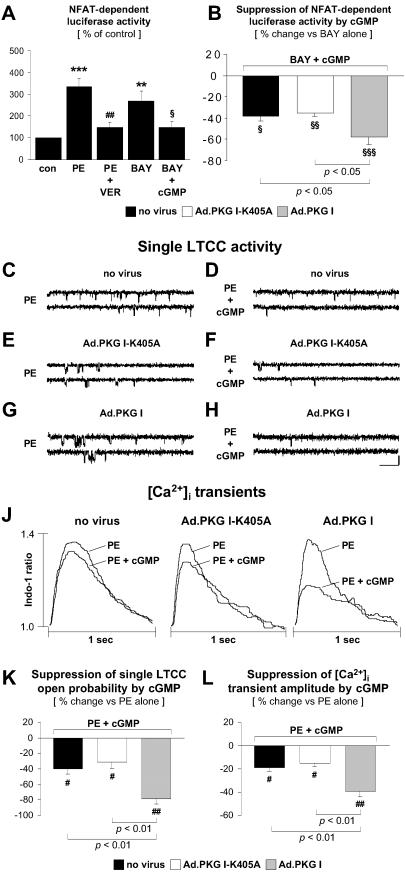
PKG I Suppresses BAY K 8644-Induced NFAT Activation, LTCC Open Probability, and [Ca2+]i Transients.
The possibility that the inhibitory effects of PKG I upstream of calcineurin reflect alterations in Ca2+ handling via suppression of the LTCC current and [Ca2+]i transient amplitude was investigated. As shown in Fig. 3A, PE-induced NFAT activation was reduced by the LTCC blocker verapamil, indicating that NFAT activation in CM depends on Ca2+ influx via the LTCC. Conversely, the LTCC agonist BAY K 8644 induced NFAT transcriptional activity (Fig. 3A). BAY K 8644-induced NFAT activation was suppressed by 8-pCPT-cGMP (Fig. 3A), an effect that was significantly enhanced by adenoviral expression of wild-type but not catalytically inactive PKG I (Fig. 3B). To assess the effect of PKG I on LTCC activity directly, patch-clamp experiments were performed. Representative single LTCC recordings are presented in Fig. 3 C–H, and the effects of 8-pCPT-cGMP on single LTCC open probability are summarized in Fig. 3K. Activation of endogenous PKG I by 8-pCPT-cGMP suppressed single LTCC open probability in PE-treated CM (Fig. 3 C, D, and K). This effect was significantly enhanced by adenoviral expression of wild-type (Fig. 3 G, H, and K) but not catalytically inactive (Fig. 3 E, F, and K) PKG I. We next investigated whether PKG I suppression of LTCC activity caused a reduction of PE-stimulated [Ca2+]i transients in CM. Representative tracings are shown in Fig. 3J. Data from n = 6–7 experiments are summarized in Fig. 3L. Activation of endogenous PKG I suppressed the [Ca2+]i transient amplitude in PE-treated CM, and the inhibitory effects of endogenous PKG I were significantly enhanced by adenoviral expression of wild-type but not catalytically inactive PKG I.
Fig 3.
PKG I inhibition of BAY K 8644-induced NFAT activation, LTCC open probability, and [Ca2+]i transients. (A–L) Experiments were carried out in noninfected CM and in CM infected with Ad.PKG I-K405A or Ad.PKG I. NFAT transcriptional activity was determined in CM transfected with the luciferase reporter plasmid p3xNFAT-GL (A and B). CM were treated for 48 hr in the absence (con) or presence of PE (10 μM), verapamil (VER, 10 μM), BAY K 8644 (BAY, 0.1 μM), or 8-pCPT-cGMP (cGMP, 500 μM). Control CM in A were defined as 100%. (B) Data are presented as % suppression by cGMP of BAY K 8644-induced NFAT-dependent luciferase activity. **, P < 0.01 and ***, P < 0.001 vs. con. ##, P < 0.01 vs. PE alone. §, P < 0.05, §§, P < 0.01 and §§§, P < 0.001 vs. BAY alone (n = 4–5 experiments). P values for other comparisons are shown directly in B. (C–H) Representative single LTCC activity recordings. CM were treated with PE alone (10 μM; C, E, and G) or PE plus 8-pCPT-cGMP (cGMP, 500 μM; D, F, and H). In each panel are shown two consecutive traces elicited by 150-msec depolarizing pulses (H: bars = 20 msec and 1 pA). (K) Results summarized from n = 6–7 experiments. Data are expressed as % suppression by cGMP of PE-stimulated single LTCC open probability. [Ca2+]i transients were recorded in PE(10 μM)-stimulated CM before and after addition of 8-pCPT-cGMP (cGMP, 500 μM). (J) Superimposed tracings from PE and PE + 8-pCPT-cGMP-treated CM. (L) Data from n = 6–7 experiments are summarized. Data are expressed as % suppression by cGMP of PE-stimulated [Ca2+]i transient amplitudes. #, P < 0.05 and ##, P < 0.01 vs. PE alone. P values for other comparisons are shown directly in (K and L).
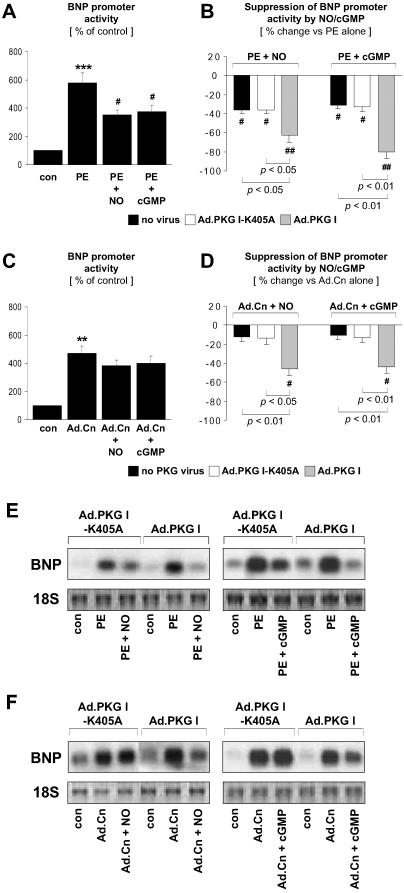
PKG I Inhibits the Promoter Activity and mRNA Expression of Brain Natriuretic Peptide.
α1-Adrenergic stimulation of both the promoter activity and mRNA expression of BNP, a primary response gene that is induced by NFAT-dependent mechanisms in hypertrophied CM (6), was inhibited by activation of endogenous PKG I with SNAP or 8-pCPT-cGMP (Fig. 4 A and E). The inhibitory effects were significantly enhanced by adenoviral expression of wild-type PKG I (Fig. 4 B and E). Similar to PE-stimulation, adenoviral expression of a constitutively active calcineurin mutant stimulated BNP promoter activity and mRNA expression; however, these effects were not significantly suppressed by activation of endogenous PKG I (Fig. 4 C and F). Remarkably, SNAP and 8-pCPT-cGMP significantly reduced calcineurin-induced BNP promoter activity and mRNA expression in CM overexpressing wild-type PKG I (Fig. 4 D and F), which is consistent with an inhibitory effect downstream of calcineurin, additional to the upstream site of inhibition concluded from Figs. 1 and 2.
Fig 4.
PKG I inhibition of BNP promoter activity and mRNA expression. Noninfected CM and CM infected with Ad.PKG I-K405A or Ad.PKG I were treated for 48 hr in the absence (con) or presence of SNAP (NO, 250 μM) or 8-pCPT-cGMP (cGMP, 500 μM). BNP promoter activity and mRNA expression were induced by PE (10 μM) stimulation or adenoviral expression of a constitutively active calcineurin mutant (Ad.Cn). BNP promoter activity was assessed in CM transfected with the luciferase reporter plasmid pBNP2501-GL. (A–D) Results from n = 4–6 experiments. Control CM in (A and C) were defined as 100%. (B and D) Data are presented as % suppression by NO or cGMP of PE-stimulated (B) or Ad.Cn-induced (D) BNP promoter activity. **, P < 0.01 and ***, P < 0.001 vs. con. #, P < 0.05 and ##, P < 0.01 vs. PE alone (A and B) or Ad.Cn alone (D). P values for other comparisons are shown directly in (B and D). Assessment of BNP mRNA/18S expression by Northern blotting is shown (E and F) and is representative of results from n = 3 experiments.
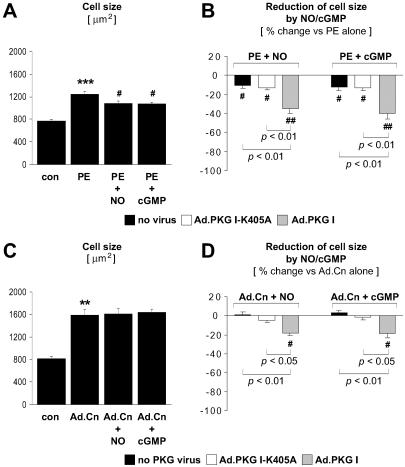
PKG I Activation Reduces Cardiac Myocyte Size.
An increase in CM size is the hallmark of CM hypertrophy. As previously shown by our group (21), activation of endogenous PKG I by SNAP or 8-pCPT-cGMP reduced the α1-adrenergic increase in CM size (Fig. 5A). Adenoviral expression of wild-type but not catalytically inactive PKG I significantly enhanced the inhibitory effects of SNAP and 8-pCPT-cGMP (Fig. 5B). An increase in CM size also was induced by adenoviral expression of the constitutively active calcineurin mutant (Fig. 5C). Activation of endogenous PKG I, however, did not reduce CM size in calcineurin-overexpressing CM (Fig. 5C), which is consistent with PKG I inhibitory effects upstream of calcineurin. By contrast, adenoviral expression and NO/cGMP activation of wild-type PKG I suppressed calcineurin-induced increases in CM size (Fig. 5D), further confirming the additional inhibitory effect of overexpressed PKG I downstream of calcineurin.
Fig 5.
PKG I inhibition of the increase in cell size induced by α1-adrenergic stimulation or expression of constitutively active calcineurin. Noninfected CM and CM infected with Ad.PKG I-K405A or Ad.PKG I were treated for 48 hr in the absence (con) or presence of PE (10 μM), SNAP (NO, 250 μM), or 8-pCPT-cGMP (cGMP, 500 μM). (C and D) CM were infected with Ad.Cn for expression of a constitutively active calcineurin mutant. CM size was determined by planimetry (n = 4–5 experiments). (B and D) Data are presented as % reduction by NO or cGMP of CM size in PE-stimulated (B) or Ad.Cn-infected (D) CM. **, P < 0.01 and ***, P < 0.001 vs. con. #, P < 0.05 and ##, P < 0.01 vs. PE alone (A and B) or Ad.Cn alone (D). P values for other comparisons are shown directly in B and D.
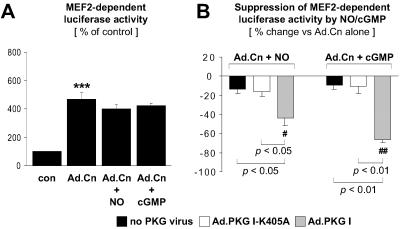
Inhibition of Calcineurin-Induced MEF2 Transcriptional Activity by Overexpressed PKG I.
Intriguingly, overexpressed PKG I inhibited calcineurin-induced hypertrophy markers (Figs. 4 and 5) but not NFAT activation (Fig. 2), suggesting that overexpressed PKG I can target NFAT-independent pathway(s). Therefore, we investigated whether overexpressed PKG I interferes with calcineurin activation of the transcription factor MEF2. As shown in Fig. 6A, expression of the constitutively active calcineurin mutant enhanced MEF2 transcriptional activity in CM. Activation of endogenous PKG I by SNAP or 8-pCPT-cGMP did not significantly reduce calcineurin-induced MEF2 activation (Fig. 6A). However, in CM overexpressing wild-type PKG I, SNAP and 8-pCPT-cGMP exerted a significant inhibitory effect on calcineurin-induced MEF2 activation (Fig. 6B).
Fig 6.
Inhibition of calcineurin-induced MEF2 activation by overexpressed PKG I. CM transfected with p3xMEF2-GL, a luciferase reporter plasmid driven by three MEF2-binding sites, were either noninfected or infected with Ad.PKG I-K405A or Ad.PKG I, where indicated. MEF2 activation was induced by adenoviral expression of a constitutively active calcineurin mutant (Ad.Cn). CM were treated for 48 hr in the absence (con) or presence of SNAP (NO, 250 μM) or 8-pCPT-cGMP (cGMP, 500 μM). (A) Control CM were defined as 100%. (B) Data are presented as % suppression by NO or cGMP of Ad.Cn-induced MEF2-dependent luciferase activity. ***, P < 0.001 vs. con. #, P < 0.05 and ##, P < 0.01 vs. Ad.Cn alone (n = 7 experiments). P values for other comparisons are shown directly in B.
Discussion
NO, signaling via cGMP and PKG I, has been recognized as a suppressor of CM hypertrophy (17–21); however, there has been little elucidation of the molecular mechanisms involved. The present study shows that NO/cGMP activation of PKG I inhibits the hypertrophic calcineurin-NFAT pathway in CM by targeting critical, Ca2+-dependent steps upstream of calcineurin. Adenoviral expression of PKG I enhances the inhibition upstream of calcineurin and unmasks an additional blockade downstream of calcineurin.
Blockade of NFAT-Dependent Hypertrophy Signaling Upstream of Calcineurin.
Calcineurin phosphatase activity is critically dependent on intracellular Ca2+, and our results indicate that inhibitory effects of PKG I upstream of calcineurin are mediated by interfering with Ca2+ entry via the LTCC. The source of intracellular Ca2+ for stimulating calcineurin is somewhat cell-type specific (5). In neuronal cells, for example, calcineurin activation is triggered by Ca2+ influx through the LTCC (27). It has been proposed that calcineurin activation in CM depends on Ca2+ entry via the LTCC as well (8, 9), and that the LTCC plays a critical role in the regulation of CM hypertrophy after stimulation of G protein-coupled receptors (19, 30, 31). Consistent with this, our data demonstrate that the LTCC blocker verapamil inhibits α1-adrenoreceptor-induced NFAT activation, whereas the LTCC agonist BAY K 8644 induces NFAT transcriptional activity. PKG I has been shown to suppress LTCC current and [Ca2+]i transients in CM (28, 32, 33). In agreement with these studies, single LTCC open probability and [Ca2+]i transient amplitude were reduced by endogenous PKG I in our experimental setting, effects that could be enhanced further by adenoviral expression of PKG I. Importantly, NO/cGMP activation of PKG I suppressed BAY K 8644-induced NFAT activation, providing direct evidence that PKG I inhibits NFAT activation triggered by Ca2+ entry via the LTCC. In vascular smooth muscle cells, PKG I reduces [Ca2+]i by several mechanisms, including decreasing Ca2+ release from the endoplasmic reticulum by phosphorylating the Ins(1,4,5)P3-receptor-associated PKG I substrate (34). Although cardiac muscle function, unlike that of smooth muscle, depends more on Ca2+ influx than Ca2+ release from internal stores, it is possible that the LTCC may not be the only target mediating PKG I effects on [Ca2+]i and calcineurin-NFAT activation.
Blockade of NFAT-Independent Hypertrophy Signaling Downstream of Calcineurin.
Whereas endogenous PKG I inhibited NFAT-dependent hypertrophy signaling upstream of calcineurin, overexpressed PKG I not only enhanced inhibition at this site but also exposed another site of inhibition downstream of calcineurin which was independent of NFAT. Both endogenous PKG I and adenovirally expressed PKG I could inhibit PE activation of NFAT, but neither could inhibit NFAT activation after expression of a Ca2+-independent, constitutively active calcineurin mutant. However, adenovirally expressed PKG I inhibited other indicators of hypertrophy elicited by constitutively active calcineurin, such as BNP transcription and expression and CM size, suggesting that adenovirally expressed PKG I inhibits NFAT-independent steps downstream of calcineurin. Importantly, adenoviral expression of a point-mutated, catalytically inactive form of PKG I did not promote antihypertrophic effects downstream of calcineurin. Therefore, spurious effects possibly related to the overexpression of a foreign protein are very unlikely. Calcineurin activates protein kinase C, c-Jun NH2-terminal kinase, and the transcription factor MEF2 in CM, indicating that hypertrophic effects of calcineurin are not exclusively mediated by NFAT (10, 11). In our system, expression of constitutively active calcineurin induced transcription from an MEF2-luciferase reporter plasmid, an effect that was significantly reduced by activation of overexpressed but not endogenous PKG I. Because MEF2 controls the expression of a number of hypertrophy-associated genes, including BNP (35), PKG I inhibitory effects downstream of calcineurin may relate, at least in part, to an inhibition of MEF2 activation.
Antihypertrophic Signaling of NO-cGMP-PKG I via Inhibition of Calcineurin-NFAT.
Although no signal transduction cascade regulates hypertrophy in isolation, a single pathway that operates as an integral part of crosstalking networks can have far-reaching effects (2, 3). Such an integrated model of hypertrophic signaling predicts that specific inhibition of one central regulatory pathway will diminish activation of other interdependent pathways and will suffice to inhibit the hypertrophic response. Previous studies have shown that pharmacological (6, 10) or genetic (9, 14) inhibition of the calcineurin-NFAT pathway effectively blocks agonist-induced CM hypertrophy, suggesting that PKG I inhibition of calcineurin-NFAT signaling could serve as a central mechanism of the antihypertrophic effects of NO/cGMP in CM. In vascular smooth cells and other cell types, PKG I inhibits signaling via low-molecular-weight GTPases Ras (36) and RhoA (37), mediators which have also been implicated in the regulation of CM hypertrophy. It is feasible that PKG I inhibition of such additional signaling pathways also could promote NO/cGMP antihypertrophic effects in CM. It is noteworthy that NO and cGMP, signaling via PKG I, also suppressed basal NFAT-transcriptional activity and BNP-promoter activity in CM (not shown), suggesting that the NO-cGMP-PKG I pathway can modulate gene transcription in nonhypertrophied CM via inhibition of calcineurin-NFAT signaling.
The calcineurin-NFAT signaling cascade is a potent inducer of CM hypertrophy. Thus, cellular defenses involving counterregulatory pathways such as calcineurin-inhibitory peptides (9, 38) and kinases that promote the nuclear export of NFAT (14) are clearly very important. As shown here, the NO-cGMP-PKG I pathway constitutes an additional and distinct mechanism to suppress calcineurin-NFAT hypertrophy signaling in CM.
Acknowledgments
We thank Dr. G. Crabtree for providing us with p3xNFAT-GL and Dr. C. Glembotski for pBNP2501-GL and BNP cDNA. This work was supported by Grant WO 552/2–1 from the Deutsche Forschungsgemeinschaft (to K.C.W.) and Grant SFB 355 (to S.M.L.).
Abbreviations
CM, cardiac myocyte
NFAT, nuclear factor of activated T cells
PKG I, cGMP-dependent protein kinase type I
8-pCPT-cGMP, 8-para-chlorophenylthio-cGMP
LTCC, L-type Ca2+-channel
PE, phenylephrine
SNAP, S-nitroso-N-acetyl-d,l-penicillamine
MEF2, myocyte enhancer factor-2
BNP, rat brain natriuretic peptide
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mann D. L. (1999) Circulation 100, 999-1008. [DOI] [PubMed] [Google Scholar]
- 2.Hunter J. J. & Chien, K. R. (1999) N. Engl. J. Med. 341, 1276-1283. [DOI] [PubMed] [Google Scholar]
- 3.Molkentin J. D. & Dorn, I. G. (2001) Annu. Rev. Physiol. 63, 391-426. [DOI] [PubMed] [Google Scholar]
- 4.Frey N., McKinsey, T. A. & Olson, E. N. (2000) Nat. Med. 6, 1221-1227. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree G. R. (2001) J. Biol. Chem. 276, 2313-2316. [DOI] [PubMed] [Google Scholar]
- 6.Molkentin J. D., Lu, J. R., Antos, C. L., Markham, B., Richardson, J., Robbins, J., Grant, S. R. & Olson, E. N. (1998) Cell 93, 215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W., Zou, Y., Shiojima, I., Kudoh, S., Aikawa, R., Hayashi, D., Mizukami, M., Toko, H., Shibasaki, F., Yazaki, Y., et al. (2000) J. Biol. Chem. 275, 15239-15245. [DOI] [PubMed] [Google Scholar]
- 8.Kato T., Sano, M., Miyoshi, S., Sato, T., Hakuno, D., Ishida, H., Kinoshita-Nakazawa, H., Fukuda, K. & Ogawa, S. (2000) Circ. Res. 87, 937-945. [DOI] [PubMed] [Google Scholar]
- 9.Taigen T., De Windt, L. J., Lim, H. W. & Molkentin, J. D. (2000) Proc. Natl. Acad. Sci. USA 97, 1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Windt L. J., Lim, H. W., Haq, S., Force, T. & Molkentin, J. D. (2000) J. Biol. Chem. 275, 13571-13579. [DOI] [PubMed] [Google Scholar]
- 11.Passier R., Zeng, H., Frey, N., Naya, F. J., Nicol, R. L., McKinsey, T. A., Overbeek, P., Richardson, J. A., Grant, S. R. & Olson, E. N. (2000) J. Clin. Invest. 105, 1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichida M. & Finkel, T. (2001) J. Biol. Chem. 276, 3524-3530. [DOI] [PubMed] [Google Scholar]
- 13.Lim H. W., New, L., Han, J. & Molkentin, J. D. (2001) J. Biol. Chem. 276, 15913-15919. [DOI] [PubMed] [Google Scholar]
- 14.Haq S., Choukroun, G., Kang, Z. B., Ranu, H., Matsui, T., Rosenzweig, A., Molkentin, J. D., Alessandrini, A., Woodgett, J., Hajjar, R., et al. (2000) J. Cell. Biol. 151, 117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto K., Ohki, R., Lee, R. T., Ikeda, U. & Shimada, K. (2001) Circulation 104, 1670-1675. [DOI] [PubMed] [Google Scholar]
- 16.Bueno O. F., De Windt, L. J., Lim, H. W., Tymitz, K. M., Witt, S. A., Kimball, T. R. & Molkentin, J. D. (2001) Circ. Res. 88, 88-96. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka H., Nakata, M., Kohno, K., Koga, Y., Nomura, G., Toshima, H. & Imaizumi, T. (1996) Hypertension 27, 14-18. [DOI] [PubMed] [Google Scholar]
- 18.Ishigai Y., Mori, T., Ikeda, T., Fukuzawa, A. & Shibano, T. (1997) Am. J. Physiol. 273, H2659-H2663. [DOI] [PubMed] [Google Scholar]
- 19.Calderone A., Thaik, C. M., Takahashi, N., Chang, D. L. & Colucci, W. S. (1998) J. Clin. Invest. 101, 812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherrer-Crosbie M., Ullrich, R., Bloch, K. D., Nakajima, H., Nasseri, B., Aretz, H. T., Lindsey, M. L., Vancon, A. C., Huang, P. L., Lee, R. T., et al. (2001) Circulation 104, 1286-1291. [DOI] [PubMed] [Google Scholar]
- 21.Wollert K. C., Fiedler, B., Gambaryan, S., Smolenski, A., Heineke, J., Butt, E., Trautwein, C., Lohmann, S. M. & Drexler, H. (2002) Hypertension 39, 87-92. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann F., Ammendola, A. & Schlossmann, J. (2000) J. Cell. Sci. 113, 1671-1676. [DOI] [PubMed] [Google Scholar]
- 23.Begum N., Sandu, O. A., Ito, M., Lohmann, S. M. & Smolenski, A. (2002) J. Biol. Chem. 277, 6214-6222. [DOI] [PubMed] [Google Scholar]
- 24.Smolenski A., Poller, W., Walter, U. & Lohmann, S. M. (2000) J. Biol. Chem. 275, 25723-25732. [DOI] [PubMed] [Google Scholar]
- 25.De Windt L. J., Lim, H. W., Taigen, T., Wencker, D., Condorelli, G., Dorn, G. W., Kitsis, R. N. & Molkentin, J. D. (2000) Circ. Res. 86, 255-263. [DOI] [PubMed] [Google Scholar]
- 26.Hanford D. S., Thuerauf, D. J., Murray, S. F. & Glembotski, C. C. (1994) J. Biol. Chem. 269, 26227-26233. [PubMed] [Google Scholar]
- 27.Graef I. A., Mermelstein, P. G., Stankunas, K., Neilson, J. R., Deisseroth, K., Tsien, R. W. & Crabtree, G. R. (1999) Nature (London) 401, 703-708. [DOI] [PubMed] [Google Scholar]
- 28.Klein G., Drexler, H. & Schroder, F. (2000) Cardiovasc. Res. 48, 367-374. [DOI] [PubMed] [Google Scholar]
- 29.Baudet S., Weisser, J., Janssen, A. P., Beulich, K., Bieligk, U., Pieske, B., Noireaud, J., Janssen, P. M., Hasenfuss, G. & Prestle, J. (2001) Cardiovasc. Res. 50, 486-494. [DOI] [PubMed] [Google Scholar]
- 30.Sei C. A., Irons, C. E., Sprenkle, A. B., McDonough, P. M., Brown, J. H. & Glembotski, C. C. (1991) J. Biol. Chem. 266, 15910-15916. [PubMed] [Google Scholar]
- 31.Zhang S., Hiraoka, M. & Hirano, Y. (1998) J. Mol. Cell. Cardiol. 30, 1955-1965. [DOI] [PubMed] [Google Scholar]
- 32.Mery P. F., Lohmann, S. M., Walter, U. & Fischmeister, R. (1991) Proc. Natl. Acad. Sci. USA 88, 1197-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumii K. & Sperelakis, N. (1995) Circ. Res. 77, 803-812. [DOI] [PubMed] [Google Scholar]
- 34.Schlossmann J., Ammendola, A., Ashman, K., Zong, X., Huber, A., Neubauer, G., Wang, G. X., Allescher, H. D., Korth, M., Wilm, M., et al. (2000) Nature (London) 404, 197-201. [DOI] [PubMed] [Google Scholar]
- 35.Morin S., Charron, F., Robitaille, L. & Nemer, M. (2000) EMBO J. 19, 2046-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suhasini M., Li, H., Lohmann, S. M., Boss, G. R. & Pilz, R. B. (1998) Mol. Cell. Biol. 18, 6983-6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauzeau V., Le Jeune, H., Cario-Toumaniantz, C., Smolenski, A., Lohmann, S. M., Bertoglio, J., Chardin, P., Pacaud, P. & Loirand, G. (2000) J. Biol. Chem. 275, 21722-21729. [DOI] [PubMed] [Google Scholar]
- 38.Rothermel B. A., McKinsey, T. A., Vega, R. B., Nicol, R. L., Mammen, P., Yang, J., Antos, C. L., Shelton, J. M., Bassel-Duby, R., Olson, E. N. & Williams, R. S. (2001) Proc. Natl. Acad. Sci. USA 98, 3328-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]