Abstract
Caveolins are important components of caveolae, which have been implicated in vesicular trafficking and signal transduction. To investigate the in vivo significance of Caveolins in mammals, we generated mice deficient in the caveolin-1 (cav-1) gene and have shown that, in the absence of Cav-1, no caveolae structures were observed in several nonmuscle cell types. Although cav-1−/− mice are viable, histological examination and echocardiography identified a spectrum of characteristics of dilated cardiomyopathy in the left ventricular chamber of the cav-1-deficient hearts, including an enlarged ventricular chamber diameter, thin posterior wall, and decreased contractility. These animals also have marked right ventricular hypertrophy, suggesting a chronic increase in pulmonary artery pressure. Direct measurement of pulmonary artery pressure and histological analysis revealed that the cav-1−/− mice exhibit pulmonary hypertension, which may contribute to the right ventricle hypertrophy. In addition, the loss of Cav-1 leads to a dramatic increase in systemic NO levels. Our studies provided in vivo evidence that cav-1 is essential for the control of systemic NO levels and normal cardiopulmonary function.
Caveolae are 50–100 nm vesicular invaginations of the plasma membrane (1, 2), which have emerged in recent years as the site of the important dynamic events at the plasma membrane such as vesicular trafficking, as well as signal transduction (3–6). Three family members of caveolins, structural proteins of caveolae, were identified to date (7–9). Both caveolin-1 (cav-1), predominantly expressed in adipocytes, endothelial cells, and fibroblast cells, and caveolin-3 (cav-3), which is muscle-specific, are sufficient to drive the formation of caveolae in cells that do not have caveolins and caveolae (10–12). Caveolins were also shown to act as scaffolding proteins to organize and concentrate specific caveolin-interacting lipids and lipid-modified signaling molecules (13). Functional Caveolin-binding motifs have been deduced in G proteins, tyrosine and serine/threonine kinases, and endothelial nitric oxide synthase (eNOS) (14–21). It has been proposed that Caveolins could function as a “general kinase inhibitor” (22). In the resting endothelial cell, the formation of an inhibitory eNOS–Caveolin heteromeric complex may serve to ensure the latency of the NO signal until calcium-mobilizing extracellular stimuli destabilize this complex and activate the enzyme. It was found that agonist activation promotes the reversible, Ca2+-dependent dissociation of the eNOS-caveolin complex (23). Thus, transient changes in intracellular calcium ([Ca2+]i) consequent to agonist activation of endothelial cells are likely to be accompanied by cyclic changes in the interactions of eNOS with caveolin versus calmodulin. The physiological relevance of the inhibitory interaction of caveolar targeting on basal NO production was recently provided in a study on intact endothelial cells exposed to high levels of low-density lipoprotein (LDL) cholesterol (24). As originally identified (25), caveolae also participate in reverse cholesterol transportation by increasing Caveolin abundance to promote cholesterol trafficking and efflux. The consequence for eNOS function of this cholesterol-induced increase in Caveolin abundance is a marked decline in basal NO release, suggesting that the equilibrium between eNOS bound to Caveolin and Caveolin-free eNOS determines the basal component of eNOS-dependent NO release in endothelial cells. This interaction may be required to protect the cell from undesired, potentially cytoxic, or nonphysiological bursts of NO in response to small fluctuation in intracellular calcium (26, 27).
Abnormalities in caveolae and/or Caveolins have been found in various human diseases (28, 29). Cav-1 was found as a negative regulator of the ras-p42/p44 mitogen-activated protein kinase cascade and a potential tumor suppressor gene. The human cav-1 gene was mapped to a suspected tumor suppressor locus (7q31.1), which is often deleted in human cancer. Mutations in the cav-3 gene were found to cause autosomal dominant limb-girdle muscular dystrophy and mechanical hyperirritability of skeletal muscle in rippling muscle disease (30, 31), demonstrating the allelism of dystrophic and nondystrophic muscle diseases. With respect to vascular diseases, a drastic increase of caveolae was reported in spontaneous hypertensive rats in the arterial endothelium, along with abnormal intracellular Ca2+ homestasis (32). In addition, involvement of Caveolins in the pathophysiology of Alzheimer's disease was reported (33). To further investigate the in vivo function and the physiological significance of caveolae and Caveolins in mammals, several groups generated mice deficient in either cav-1 or cav-3 gene (34, 35). These authors reported that the ablation of cav-1 leads to the elimination of caveolae in nonmuscle cells. However, cav-1−/− mice are viable and display vascular abnormalities and pulmonary defects. The uptake and transport of macromolecules, such as albumin, was reported to be severely impaired in cav-1-deficient lung endothelial cells and the aortic segments (36). It has also been shown that cav-1 null mice have problems with lipid metabolism and adipocyte function, resulting in the severely elevated triglyceride and free fatty acid levels and resistance to diet-induced obesity (37).
We have independently generated cav-1 knockout mice, and, in addition to these aforementioned phenotypes, have uncovered evidence of dilated cardiomyopathy, pulmonary hypertension and resulting right ventricular hypertrophy, and elevated systemic NO levels. Taken together, these studies suggest that impairment of Caveolin function can lead to cardiac muscle injury and associated cardiomyopathy.
Materials and Methods
Generation of cav-1-Deficient Mice.
The cav-1 targeting construct was generated from a 15-kb genomic fragment of cav-1 isolated from a 129SVJ mouse genomic library. Of 280 G418-resistant embryonic stem (ES) cell clones, two were identified as hologous recombinants, based on Southern blot hybridization analysis. Positive clones were microinjected into J1 1295SVJ blastocysts that were subsequently transferred to pseudopregnant females. Chimeric male mice were crossed with Black Swiss breeders.
Western Blot.
An equal amount of heart and/or lung lysates (15 μg) was separated in 4–20% gradient polyacrylamide gels. Proteins were transferred onto poly(vinylidene difluoride) membrane. The blots were incubated with primary antibodies at 4°C overnight (anti-Cav-1 and anti-Cav-3, monoclonal, 1:2000, Transduction Laboratories, Lexington, KY) and with secondary antibodies at room temperature for 1 h. An enhanced chemiluminescence kit was used for detection.
Quantitative Real Time Reverse Transcription (RT)-PCR.
Total RNA was isolated with the RNeasy Midi kit (Qiagen, Valencia, CA). In each reaction, 100 μg of total RNA isolated from the left ventricles (LVs) of 5-month-old wild-type mice and age- and gender-matched cav-1−/− mice was analyzed in a Model 7700 Sequence Detector (Applied Biosystems). RT-PCR conditions were 30 min at 48°C, 10 min at 95°C, and 40 cycles of 30 s at 95°C and 90 s at 60°C. Relative RNA equivalents for each sample were obtained by standardizing to cyclophilin levels. Each of the three samples per group was run in duplicates to determine sample reproducibility, and the average relative RNA equivalents per sample pair was used for further analysis. The primer/probe pairs used for RT-PCR analysis were: mouse skeletal α-actin, 5′(FAM)-AGGCTGGCCCCTCCATTGTGC-(TAMRA)p3′, forward primer 5′-TGTGGATCACCAAGCAGGAGTA-3′ and reverse primer 5′-TGCGCCTAGAAGCATTTGC-3′; mouse Glut-1, 5′(FAM)-CTGTGCACTGAGGGCCACACAA-(TAMRA)p3′, forward primer 5′-GGGCTGCCAGGTTCTAGTC-3′ and reverse primer 5′-CCTCCGAGGTCCTTCTCA-3′; mouse cyclophilin, 5′ (FAM)-CCACAATGTTCATGCCTTCTTTCACC-(TAMRA)p3′, forward primer 5′-CTTGTCCATGGCAAATGCTG-3′ and reverse primer 5′-GTGATCTTCTTGCTGGTCTTGC-3′ (FAM, 6-carboxyfluorescein; TAMRA, 6 carboxytetramethylrhodamine).
Transmission Electronic Microscope Examination.
The cav-1−/− or cav-1+/+ mice were perfused (10 min, RT) under anesthesia with oxygenated DMEM via the LV, followed by fixation by perfusion (10 min, RT) with 2.5% glutaraldehyde and 3% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.3). Specimens were taken from different tissues and trimmed into small blocks, which were immersed into fresh fixative (1 h, RT), washed (2 times, 15 min, RT) in 0.1 M cacodylate, postfixed in Palade's OsO4 (1 h, on ice), en bloc stained in Kellemberger's uranyl acetate (overnight, RT), dehydrated in graded ethanol and embedded in LX112 resin. Thin sections (≈50 nm) were cut on a Reichert microtome, stained with lead citrate, examined and photographed under either a JEOL 1200EX or a Philips CM10 electron microscope.
In Vivo Physiological Assays.
Transthoracic echocardiography (39), hemodynamic evaluation (43), and pulmonary artery pressure measurement (39) were performed as described.
Histochemical Analysis.
Hearts and lungs from four cav-1+/+ and six cav-1−/− mice at 6–12 months of age were fixed with 4% paraformaldehyde followed by dehydration and paraffin embedding. Serial of 2- to 5-μm sections were stained with hematoxylin/eosin or trichrome. The heart sections were also stained with primary antibodies against Cav-1 and Cav-3 and visualized with FITC-conjugated (for Cav-1 detection) or tetramethylrhodamine B isothiocyanate (TRITC)-conjugated (for Cav-3 detection) second antibodies.
Measurement of Plasma NO Metabolites.
Mouse blood was collected by retro-orbital bleeding, and plasma was prepared with plasma separator microtubes with Lithium Heparin (Becton Dickinson). The plasma total NO was quantitatively determined based on the enzymatic conversion of nitrate to nitrite by nitrate reductase, followed by the spectrophotometric quantitation of nitrite levels using Griess reagent (Calbiochem).
Results and Discussion
Targeted Disruption of the cav-1 Gene.
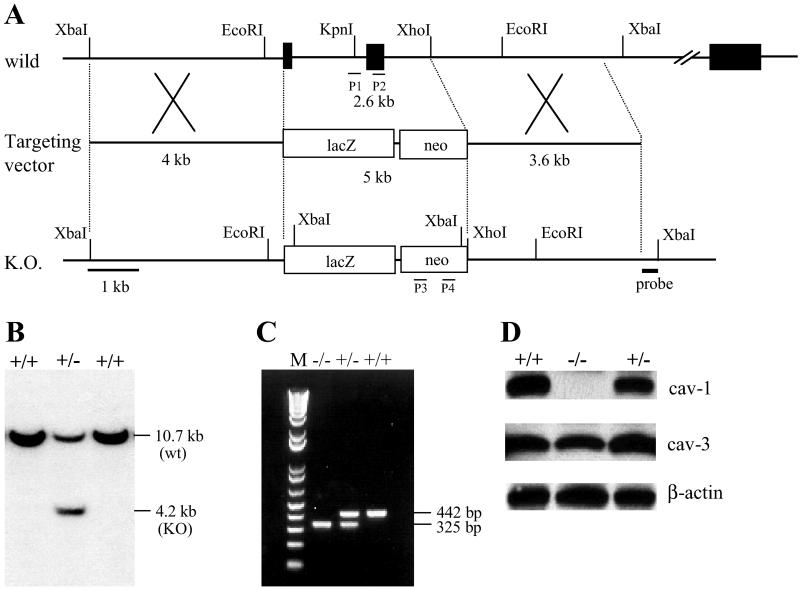
The murine cav-1 gene is encoded by three exons within 15 kb of genomic DNA (38). We constructed a targeting vector in which exon 1 and 2 were replaced with the lacZ and neo genes (Fig. 1A). The vector was introduced into J1 mouse ES cells, and G-418-resistant transformants containing the mutation in one allele of the cav-1 gene were established (Fig. 1B). The ES clones were aggregated with 8-cell embryos, and the embryos were implanted into foster mothers. Chimeric mice derived from two clones transmitted the mutation to their offspring (F1). Heterozygous (cav-1+/−) offspring appeared normal in all respects and were crossed to generate cav-1 homozygous mice (cav-1−/−). Mice of all three genotypes from the progeny (F2) of the cav-1+/− intercrosses are present at the expected Mendelian frequency, indicating no embryonic lethality.
Fig 1.
Generation of cav-1 null mutation mice. (A) Schematic representation of the wild-type and mutant loci of the cav-1 gene together with the targeting vector. Exons for the gene encoding cav-1 are represented by a black box. The size of intron 2 is approximately 10 kb. The targeting vector carries the lacZ gene and the neomycin resistance gene (neo). (B) A representative Southern blot analysis of XbaI-digested DNA isolated from ES clones. Hybridization was carried out with a 0.4-kb DNA fragment located 5 kb downstream of exon 2. (C) PCR analysis of tail genomic DNA from littermates generated by heterozygous pairs. Lane M, molecular size marker DNA. The locations of PCR primers (P1 to P4) are shown in A. (D). Western blotting analysis of cav-1 and cav-3 expression in the hearts from littermates. Ventricle lysates were immunoblotted with a polyclonal antibody against Cav-1. The same blot was reprobed with a monoclonal antibody against Cav-3. Equal loading amounts were confirmed by β-actin immunoblotting. The experiment was repeated twice with similar results.
Characterization of the cav-1 Mutant Mice.
Quantitative real time RT-PCR failed to detect cav-1 mRNA in the heart and lung tissues from the cav-1−/− mice (data not shown). In addition, no Cav-1 protein was detected by Western blotting, whereas the cav-3 expression remained unaffected (Fig. 1D). Immunostaining of ventricular semithin frozen sections with an antibody against Cav-1 revealed that the cav-1 expression was restricted to endothelial cells, and was not present in cardiac muscle cells in cav-1+/+ mice hearts, confirming the cell-specific expression pattern of the cav-1 gene (data not shown). In agreement with the Western blotting analysis, no Cav-1 was detected in the ventricle of the cav-1−/− mouse. In contrast, a similar intensity of Cav-3 staining was readily detectable in cardiac muscle cells, but not in endothelial cells in both cav-1+/+ and cav-1−/− mice, eliminating the possibility of a compensatory elevation of cav-3 expression in nonmuscle cells (data not shown).
The Essential Role of Cav-1 in Caveolae Formation in Nonmuscle Cells.
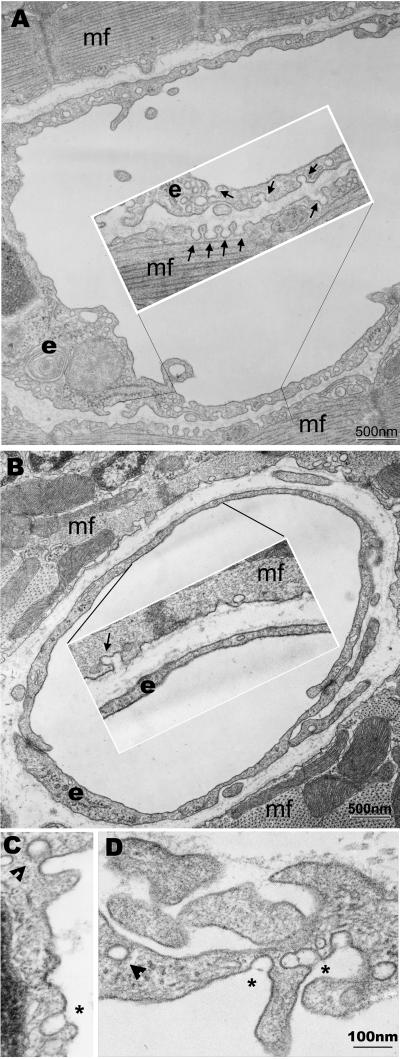
Previous studies have shown that transfection of either cav-1 or cav-3 in cell lines lacking endogenous caveolins was sufficient to form caveolae (10, 13, 14). Transmission electron microscopy analysis of specimens from different organs and tissues obtained from the cav-1−/− animals, confirmed the structural role of Cav-1 in caveolae formation. There were no caveolae invaginations in cell types such as the microvascular endothelium, smooth muscle cells, lung type I epithelium, or fibroblasts in the cav-1−/− mice. However, similar distribution of caveolae was found in the cardiac muscle cell plasma membrane in both cav-1+/+ and cav-1−/− mice (Fig. 2 A and B, and data not shown). Thus, Cav-1 is essential for caveolae formation in nonmuscle cells.
Fig 2.
Transmission electron microscopy analysis. (A) Representative micrograph of heart ventricular endothelial and muscle cells from cav-1 wild-type mice. Caveolae are indicated in both endothelial and muscle cells by arrows. (B) Micrograph of heart ventricular endothelial and muscle cells from cav-1 knockout mice. Caveolae were only found in the muscle cells not in the endothelial cells. (C and D) Micrographs of the endothelial cells from larger blood vessels in the cav-1 knockout mice. Clathrin coated vesicles were indicated (arrowheads) for size comparison. Interestingly, in both the heart and diaphragm muscle of cav-1−/− mice, larger caveolae-like structures could be occasionally observed in the vessels with diameters larger than capillaries (>10 μm) not surrounded by pericytes or smooth muscle cells. These structures appear to be provided with stomatal diaphragms, and were also reported by Drab et al. (35). e, endothelial cells; mf, cardiac muscle cells.
Dilated Cardiomyopathy in the cav-1−/− Mice.
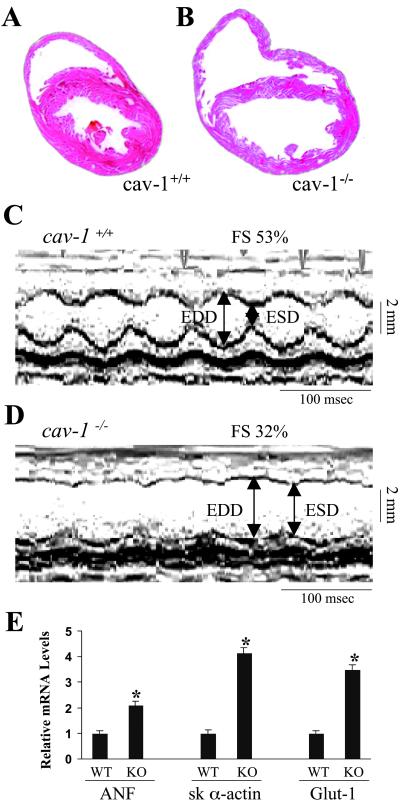
Despite the loss of caveolae structure, the cav-1−/− mice are fertile and indistinguishable from the wild-type and heterozygous littermates in appearance, gross phenotype, or routine behavior. More than 90% of the null mutation mice can survive at least 12 months (n = 61). However, histological examination of the cav-1−/− mice hearts revealed dilated ventricular chambers, and thin posterior wall and septum at the age of 5 months (Fig. 3 A and B). The cav-1−/− mice displayed a marked increase in LV/body weight ratio (3.28 ± 0.17 mg/g, n = 8) versus age- and gender-matched wild-type mice (2.73 ± 0.32 mg/g, n = 8; P < 0.01). Echocardiography (39) in anesthetized mice was used to evaluate in vivo cardiac function of age-matched littermates. The cav-1−/− mice had enlarged cardiac chambers, as revealed by increased left ventricular end-diastolic dimensions (LVEDD) and end-systolic dimensions (LVESD), whereas the cav-1+/+ mice had LVEDD and LVESD in normal range (Fig. 3 C and D). Both the fractional shortening percentage and mean velocity of circumferential fiber shortening, indicators of systolic cardiac function, were significantly decreased in the cav-1−/− mice (Table 1), indicating depressed cardiac contractility. Human heart failure and physiologically relevant experimental models of murine cardiac hypertrophy and failure exhibit increased expression of embryonic markers (40). An approximately 2- and 4-fold increase of atrial natriuretic factor (ANF) and skeletal α-actin expression, respectively, was identified in cav-1−/− mice LVs by quantitative real time RT-PCR (Fig. 3E). Notably, the expression of Glut-1 glucose transporter was also significantly increased in cav-1−/− mice, consistent with a switch in myocardial energy metabolism. Taken together, these findings indicated that cav-1−/− mice exhibit dilated cardiomyopathy, as seen in the clinical setting and in analogous mouse model systems (40–42).
Fig 3.
Pathological analysis of the cav-1 knockout hearts. (A and B) Histological sections of hearts from 5-month-old littermates. Hearts were fixed in paraformaldehyde and stained with hematoxylin and eosin. Data are representative of three independent experiments with nearly identical results. (C and D) Transthoracic M-mode echocardiographic tracings in a wild-type mouse (C) and cav-1 knockout mouse (D). LV dimensions are indicated by the double-sided arrows. EDD, end diastolic dimension; ESD, end systolic dimension. Cav-1 knockout mice have chamber dilation with reduced wall motion, indicating depressed cardiac function and increased wall stress. (E) Quantitative analysis of the expression levels of ANF, skeletal α-actin and glut-1 genes in the heart by real time RT-PCR. The data were expressed as mean ± SEM. *, P < 0.05. ANF, proatrial natriuretic factor; sk α-actin, skeletal α-actin; Glut-1, glucose transporter-1.
Table 1.
Analysis of in vivo cardiac size and function by echocardiography in wild-type and cav-1 knockout mice
| LVEDD, mm | LVESD, mm | % FS | SEPth, mm | PWth, mm | Mean VCF, cir/s | BW, g | Age, day | |
|---|---|---|---|---|---|---|---|---|
| cav-1+/+ (n = 6) | 3.25 ± 0.07 | 1.68 ± 0.13 | 48.5 ± 3.6 | 0.71 ± 0.04 | 0.73 ± 0.03 | 9.71 ± 1.14 | 30.4 ± 1.6 | 143 ± 3 |
| cav-1−/− (n = 7) | 3.97 ± 0.19 | 2.63 ± 0.24 | 34.6 ± 3.2 | 0.60 ± 0.03 | 0.61 ± 0.02 | 6.23 ± 0.51 | 29.5 ± 1.6 | 140 ± 4 |
Echocardiography was performed in anesthetized age- and gender-matched mice. LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; %FS, percent fractional shortening calculated as %FS = [(EDD − ESD)/EDD] × 100; SEPth, intraventricular septal wall thickness; PWth, left ventricular posterior wall thickness; mean VCF, heart-rate-corrected mean velocity of circumferential fiber shortening; BW, body weight. Data are expressed as mean ± SEM.
, P < 0.05.
Cav-1 Deficiency Causes Pulmonary Hypertension and Resulting Right Ventricular Hypertrophy.
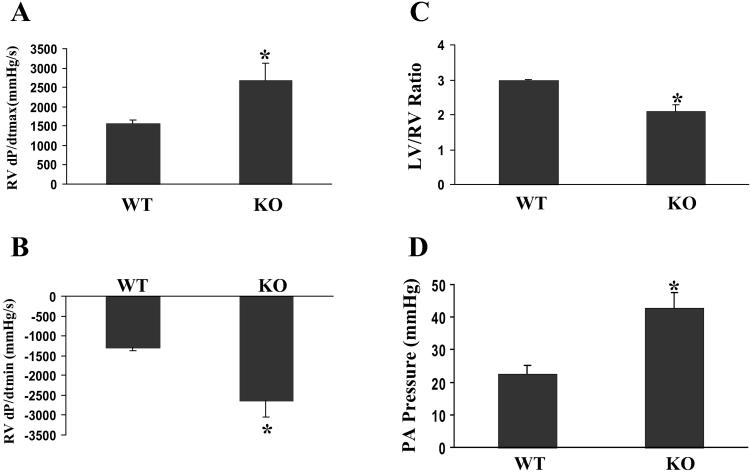
The cav-1−/− mice also displayed markedly hypertrophied and dilated right ventricles (RVs). Hemodynamic data revealed significant increases of RV contractility (assessed by RV dp/dtmax) and diastolic function (assessed by RV dp/dtmin) at baseline in cav-1−/− mice compared with those in age- and gender-matched wild-type mice (Fig. 4 A and B) (43). The RV/body weight ratio (1.61 ± 0.36, n = 8) was significantly increased compared with that of cav-1+/+ mice (0.92 ± 0.15, n = 8; P = 0.001). Interestingly, the LV/RV weight ratio of cav-1−/− mice was dramatically decreased and similar to that seen in pulmonary hypertension animal models (Fig. 4C) (44). To directly measure the pulmonary artery pressure, pulmonary artery catheterization in anesthetized mice was performed. The pulmonary artery pressure of cav-1−/− mice was 90% higher than that of cav-1+/+ mice (Fig. 4D). The RV pressure at baseline was similar to the pulmonary artery pressure either in wild-type or mutant mice, indicating no defects in outflow tracts (i.e., pulmonary valvular defects) of the cav-1−/− mice. This finding was also confirmed by angiography (data not shown). In addition, both the lung/body weight ratio and liver/body weight ratio are dramatically increased in the cav-1−/− mice (8.0 ± 2.1 mg/g; 51.1 ± 6 .0 mg/g, respectively, n = 7) versus that in the age- and gender-matched wild-type mice (5.1 ± 0.4 mg/g; 43.2 ± 4.2 mg/g, respectively, n = 8, P < 0.01). Therefore, the loss of cav-1 and caveolae in the lung parenchyma results in pulmonary hypertension, which, in turn, causes marked RV hypertrophy that is distinct from the dilated cardiomyopathy seen in the LV chamber. Pulmonary hypertension could be caused by an intrinsic defect in the pulmonary vasculature due to the lack of Cav-1. It could also be secondary to the pulmonary fibrosis (ref. 35, and our unpublished observation) or caused by LV dysfunction. However, the LV pressure, LV end diastolic pressure, and τ, an indicator of LV relaxation and diastolic function, at baseline in cav-1−/− mice were similar to those in wild-type mice (data not shown). Therefore, pulmonary hypertension in observed cav-1−/− mice is unlikely caused by LV dysfunction.
Fig 4.
Pulmonary hypertension in cav-1-deficient mice. (A and B) Maximal in vivo cardiac contractility. Cardiac catheterization was performed at basal conditions in intact, anesthetized mice of wild-type (WT) (n = 8) and cav-1 knockout (KO) (n = 9) background. (A) Maximal first derivative of RV pressure, RV dP/dt max; (B) minimal first derivative of RV pressure, RV dP/dt min. (C) Comparison of LV/RV ratios between age- and gender-matched WT (n = 6) and cav-1 KO (n = 6) mice. (D) Direct measurements of pulmonary artery (PA) pressure. Values are mean ± SEM. *, P < 0.01. WT, cav-1+/+; KO, cav-1−/−.
Increased Systemic NO in cav-1−/− Mice.
Previous studies have shown that Cav-1 can bind and negatively regulate eNOS activity (27, 45, 46). To determine whether the loss of cav-1/caveolae could lead to a high NO levels, we assayed the systemic NO levels in littermates of cav-1−/− and wild-type mice (47). In the plasma prepared from the cav-1−/− mice, the NO concentration was about 5-fold higher than that in the wild-type mice (1.73 ± 0.37 μM in cav-1+/+ mice, n = 12; vs. 9.7 ± 2.3 μM in cav-1−/− mice, n = 12, P < 0.01). Western blots revealed no overt change in the expression levels of NO synthases (i.e., eNOS and inducible NOS) in the heart and lung from the cav-1−/− mouse (data not shown), suggesting that the high NO level was most likely caused by an elevation in eNOS activity resulting in massive NO production. This finding is in agreement with recent in vitro studies that hypercholesterolemia decreased NO production by promoting the interaction of Cav-1 and eNOS and a decrease in Cav-1 abundance through atorvastatin, an inhibitor of hydroxy-methylglutaryl-CoA reductase, can promote eNOS activation (26, 27).
In conclusion, the present study reiterates the essential roles of Cav-1 in caveolae formation in nonmuscle cell types (34, 35). Besides these findings, we provide the first evidence that defects in cav-1 can be associated with dilated cardiomyopathy in the left ventricular chamber, as well as chronic pulmonary hypertension and secondary right ventricular hypertrophy. There are several possibilities why the lack of Cav-1 in noncardiomyocytes can lead to dilated cardiomyopathy. First, the loss of Cav-1 in endothelium may cause reduced endothelial cell permeability (ref. 36, and our unpublished observation), thus limiting nutrients to the myocardium. Second, the loss of Cav-1 may cause abnormal expression of endothelium-derived factors such as tumor necrosis factor (TNF)-α, interleukin-6, and interleukin-2, which lead to adverse effect on the myocardium. Third, endothelium-derived excessive NO provides adverse effect to the myocardium. There is increasing evidence, both in vitro and in vivo, that NO has negative inotropic effects on the myocardium. Endogenous NO reduces myocardial contractility and myocardial lactate formation during myocardium isochemia in dogs (48). NO may also mediate the negative inotropic effects of other cytokines on the myocardium (49). Combined with previous extensive in vitro studies on the inhibitory role of Cav-1/caveolae on eNOS activity, our study suggests that Cav-1 inhibition of eNOS may be critical in preventing the dysregulation of systemic NO levels. Because high NO levels have been reported in cardiomyopathy and pulmonary hypertension in both human and animal models (44, 50–57), it is likely that chronic and dramatic elevations in systemic NO levels caused by the loss of Cav-1 and caveolae may contribute the cardiopulmonary defects. It will become of interest to see whether defects in the cav-1 gene are represented in patient populations with idiopathic dilated cardiomyopathy.
Acknowledgments
We are grateful to Dr. Ralph A. Kelly at Harvard Medical School (Boston) and Dr. Ju Chen at University of California at San Diego for fruitful discussion. We thank Dr. Eric Blomme at Pharmacia Co. (Skokie, IL) for his expertise in lung pathology. This work was supported by the National Institutes of Health, the Jean Le Ducq Foundation, and an endowed Chair of the American Heart Association (to K.R.C.), by National Institutes of Health grants (to J.R.), and by National Institutes of Health and American Heart Association grants (to R.-V.S.). P.-H.C. was supported by National Health Research Institute Grant NHRI-EX91-9108SC.
Abbreviations
cav-1, caveolin-1
cav-3, caveolin-3, LV, left ventricle
RV, right ventricle
eNOS, endothelial nitric oxide synthase
ANF, atrial natriuretic factor
RT, reverse transcription
References
- 1.Palade G. E. (1953) J. Appl. Phys. 24, 1424. [Google Scholar]
- 2.Rothberg K. G., Heuser, J. E., Donzell, W. C., Ying, Y. S., Glenney, J. R. & Anderson, R. G. (1992) Cell 68, 673-682. [DOI] [PubMed] [Google Scholar]
- 3.Palade G. E. (1960) Anat. Rec. 136, 254. [Google Scholar]
- 4.Anderson R. G., Kamen, B. A., Rothberg, K. G. & Lacey, S. W. (1992) Science 255, 410-411. [DOI] [PubMed] [Google Scholar]
- 5.Anderson R. G. (1998) Annu. Rev. Biochem. 67, 199-225. [DOI] [PubMed] [Google Scholar]
- 6.Galbiati F., Razani, B. & Lisanti, M. P. (2001) Cell 106, 403-411. [DOI] [PubMed] [Google Scholar]
- 7.Glenney J. R. (1989) J. Biol. Chem. 264, 20163-20166. [PubMed] [Google Scholar]
- 8.Way M. & Parton, R. G. (1995) FEBS Lett. 376, 108-112. [DOI] [PubMed] [Google Scholar]
- 9.Scherer P. E., Okamoto, T., Chun, M., Nishimoto, I., Lodish, H. F. & Lisanti, M. P. (1996) Proc. Natl. Acad. Sci. USA 93, 131-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fra A. M., Williamson, E., Simons, K. & Parton, R. G. (1995) Proc. Natl. Acad. Sci. USA 92, 8655-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S., Okamoto, T., Chun, M., Sargiacomo, M., Casanova, J. E., Hansen, S. H., Nishimoto, I. & Lisanti, M. P. (1995) J. Biol. Chem. 270, 15693-15701. [DOI] [PubMed] [Google Scholar]
- 12.Li S., Couet, J. & Lisanti, M. P. (1996) J. Biol. Chem. 271, 29182-29190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurzchalia T. V. & Parton, R. G. (1999) Curr. Opin. Cell. Biol. 11, 424-431. [DOI] [PubMed] [Google Scholar]
- 14.Li S., Song, K. S., Koh, S. S., Kikuchi, A. & Lisanti, M. P. (1996) J. Biol. Chem. 271, 28647-28654. [DOI] [PubMed] [Google Scholar]
- 15.Shaul P. W., Smart, E. J., Robinson, L. J., German, Z., Yuhanna, I. S., Ying, Y., Anderson, R. G. & Michel, T. (1996) J. Biol. Chem. 271, 6518-6522. [DOI] [PubMed] [Google Scholar]
- 16.Feron O., Belhassen, L., Kobzik, L., Smith, T. W., Kelly, R. A. & Michel, T. (1996) J. Biol. Chem. 271, 22810-22814. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Cardena G., Martasek, P., Masters, B. S., Skidd, P. M., Couet, J., Li, S., Lisanti, M. P. & Sessa, W. C. (1997) J. Biol. Chem. 272, 25437-25440. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y. Y., Feron, O., Dessy, C., Han, X., Marchionni, M. A. & Kelly, R. A. (1999) Circ. Res. 84, 1380-1387. [DOI] [PubMed] [Google Scholar]
- 19.Feron O., Zhao, Y. Y. & Kelly, R. A. (1999) Ann. N.Y. Acad. Sci. 874, 11-19. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Cardena G., Fan, R., Stern, D. F., Liu, J. & Sessa, W. C. (1996) J. Biol. Chem. 271, 27237-27240. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Cardena G., Oh, P., Liu, J., Schnitzer, J. E. & Sessa, W. C. (1996) Proc. Natl. Acad. Sci. USA 93, 6448-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto T., Schlegel, A., Scherer, P. E. & Lisanti, M. P. (1998) J. Biol. Chem. 273, 5419-5422. [DOI] [PubMed] [Google Scholar]
- 23.Feron O., Saldana, F., Michel, J. B. & Michel, T. (1998) J. Biol. Chem. 273, 3125-3128. [DOI] [PubMed] [Google Scholar]
- 24.Feron O., Dessy, C., Moniotte, S., Desager, J. P. & Balligand, J. L. (1999) J. Clin. Invest. 103, 897-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fielding C. J. & Fielding, P. E. (2000) Biochim. Biophys. Acta 1529, 210-222. [DOI] [PubMed] [Google Scholar]
- 26.Feron O., Dessy, C., Desager, J. P. & Balligand, J. L. (2001) Circulation 103, 113-118. [DOI] [PubMed] [Google Scholar]
- 27.Feron O. & Kelly, R. A. (2001) Circ. Res. 88, 129-131. [DOI] [PubMed] [Google Scholar]
- 28.Engelman J. A., Zhang, X., Galbiati, F., Volonte, D., Sotgia, F., Pestell, R. G., Minetti, C., Scherer, P. E., Okamoto, T. & Lisanti, M. P. (1998) Am. J. Hum. Genet. 63, 1578-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimoto T. (2000) Nagoya J. Med. Sci. 63, 9-18. [PubMed] [Google Scholar]
- 30.Minetti C., Sotgia, F., Bruno, C., Scartezzini, P., Broda, P., Bado, M., Masetti, E., Mazzocco, M., Egeo, A., Donati, M. A., et al. (1998) Nat. Genet. 18, 365-368. [DOI] [PubMed] [Google Scholar]
- 31.Betz R. C., Schoser, B. G., Kasper, D., Ricker, K., Ramirez, A., Stein, V., Torbergsen, T., Lee, Y. A., Nothen, M. M., Wienker, T. F., et al. (2001) Nat. Genet. 28, 218-219. [DOI] [PubMed] [Google Scholar]
- 32.Goto Y., Yoshikane, H., Honda, M., Morioka, S., Yamori, Y. & Moriyama, K. (1990) J. Submicrosc. Cytol. Pathol. 22, 535-542. [PubMed] [Google Scholar]
- 33.Nishiyama K., Trapp, B. D., Ikezu, T., Ransohoff, R. M., Tomita, T., Iwatsubo, T., Kanazawa, I., Hsiao, K. K., Lisanti, M. P. & Okamoto, T. (1999) J. Neurosci. 19, 6538-6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Razani B., Engelman, J. A., Wang, X. B., Schubert, W., Zhang, X. L., Marks, C. B., Macaluso, F., Russell, R. G., Li, M., Pestell, R. G., et al. (2001) J. Biol. Chem. 276, 38121-38138. [DOI] [PubMed] [Google Scholar]
- 35.Drab M., Verkade, P., Elger, M., Kasper, M., Lohn, M., Lauterbach, B., Menne, J., Lindschau, C., Mende, F., Luft, F. C., et al. (2001) Science 293, 2449-2452. [DOI] [PubMed] [Google Scholar]
- 36.Schubert W., Frank, P. G., Razani, B., Park, D. S., Chow, C. W. & Lisanti, M. P. (2001) J. Biol. Chem. 276, 48619-48622. [DOI] [PubMed] [Google Scholar]
- 37.Razani B., Combs, T. P., Wang, X. B., Frank, P. G., Park, D. S., Russell, R. G., Li, M., Tang, B., Jelicks, L. A., Scherer, P. E. & Lisanti, M. P. (2002) J. Biol. Chem. 277, 8635-8647. [DOI] [PubMed] [Google Scholar]
- 38.Engelman J. A., Zhang, X. L., Galbiati, F. & Lisanti, M. P. (1998) FEBS Lett. 429, 330-336. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka N., Ryoke, T., Hongo, M., Mao, L., Rockman, H. A., Clark, R. G. & Ross, J., Jr. (1998) Am. J. Physiol. 275, H393-H399. [DOI] [PubMed] [Google Scholar]
- 40.Chien K. R. (1999) Cell 98, 555-558. [DOI] [PubMed] [Google Scholar]
- 41.Minamisawa S., Hoshijima, M., Chu, G., Ward, C. A., Frank, K., Gu, Y., Martone, M. E., Wang, Y., Ross, J., Jr., Kranias, E. G., et al. (1999) Cell 99, 313-322. [DOI] [PubMed] [Google Scholar]
- 42.Chien K. R. (2000) Nature (London) 407, 227-232. [DOI] [PubMed] [Google Scholar]
- 43.Palakodeti V., Oh, S., Oh, B. H., Mao, L., Hongo, M., Peterson, K. L. & Ross, J., Jr. (1997) Am. J. Physiol. 273, H1283-H1290. [DOI] [PubMed] [Google Scholar]
- 44.Tyler R. C., Muramatsu, M., Abman, S. H., Stelzner, T. J., Rodman, D. M., Bloch, K. D. & McMurtry, I. F. (1999) Am. J. Physiol. 276, L297-L303. [DOI] [PubMed] [Google Scholar]
- 45.Michel T. & Feron, O. (1997) J. Clin. Invest. 100, 2146-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bucci M., Gratton, J. P., Rudic, R. D., Acevedo, L., Roviezzo, F., Cirino, G. & Sessa, W. C. (2000) Nat. Med. 6, 1362-1367. [DOI] [PubMed] [Google Scholar]
- 47.Zhang C., Reiter, C., Eiserich, J. P., Boersma, B., Parks, D. A., Beckman, J. S., Barnes, S., Kirk, M., Baldus, S., Darley-Usmar, V. M. & White, C. R. (2001) J. Biol. Chem. 276, 27159-27165. [DOI] [PubMed] [Google Scholar]
- 48.Node K., Kitakaze, M., Kosaka, H., Komamura, K., Minamino, T., Inoue, M., Tada, M., Hori, M. & Kamada, T. (1996) Circulation 93, 356-364. [DOI] [PubMed] [Google Scholar]
- 49.Finkel M. S., Oddis, C. V., Jacob, T. D., Watkins, S. C., Hattler, B. G. & Simmons, R. L. (1992) Science 257, 387-389. [DOI] [PubMed] [Google Scholar]
- 50.Heymes C., Vanderheyden, M., Bronzwaer, J. G., Shah, A. M. & Paulus, W. J. (1999) Circulation 99, 3009-3016. [DOI] [PubMed] [Google Scholar]
- 51.Huang P. L., Huang, Z., Mashimo, H., Bloch, K. D., Moskowitz, M. A., Bevan, J. A. & Fishman, M. C. (1995) Nature (London) 377, 239-242. [DOI] [PubMed] [Google Scholar]
- 52.Paulus W. J. (2001) Heart Fail. Rev. 6, 105-118. [DOI] [PubMed] [Google Scholar]
- 53.Shah A. M. & MacCarthy, P. A. (2000) Pharmacol. Ther. 86, 49-86. [DOI] [PubMed] [Google Scholar]
- 54.Singal P. K., Khaper, N., Farahmand, F. & Bello-Klein, A. (2000) Curr. Cardiol. Rep. 2, 206-211. [DOI] [PubMed] [Google Scholar]
- 55.Xue C., Regasamy, A., Lecras, T. D., Koberna, P. A., Dailey, G. C. & Johns, R. A. (1994) Am. J. Physiol. 267, L667-L678. [DOI] [PubMed] [Google Scholar]
- 56.Madden J. A., Keller, P. A., Choy, J. S., Alvarez, T. A. & Hacker, A. D. (1995) J. Appl. Physiol. 79, 1589-1593. [Google Scholar]
- 57.Xue C. & Johns, R. A. (1995) N. Engl. J. Med. 333, 1642-1644. [DOI] [PubMed] [Google Scholar]