Abstract
By using a combination of genetic, pharmacological, and anatomical approaches, we show that the melanocortin 4 receptor (MC4R), implicated in the control of food intake and energy expenditure, also modulates erectile function and sexual behavior. Evidence supporting this notion is based on several findings: (i) a highly selective non-peptide MC4R agonist augments erectile activity initiated by electrical stimulation of the cavernous nerve in wild-type but not Mc4r-null mice; (ii) copulatory behavior is enhanced by administration of a selective MC4R agonist and is diminished in mice lacking Mc4r; (iii) reverse transcription (RT)-PCR and non-PCR based methods demonstrate MC4R expression in rat and human penis, and rat spinal cord, hypothalamus, brainstem, pelvic ganglion (major autonomic relay center to the penis), but not in rat primary corpus smooth muscle cavernosum cells; and (iv) in situ hybridization of glans tissue from the human and rat penis reveal MC4R expression in nerve fibers and mechanoreceptors in the glans of the penis. Collectively, these data implicate the MC4R in the modulation of penile erectile function and provide evidence that MC4R-mediated proerectile responses may be activated through neuronal circuitry in spinal cord erectile centers and somatosensory afferent nerve terminals of the penis. Our results provide a basis for the existence of MC4R-controlled neuronal pathways that control sexual function.
Our understanding of the physiology and anatomy of erectile function has advanced considerably in recent years (1–4). Penile erection is a highly coordinated reflex that is subject to modulation at many levels of the neuraxis. Relaxation of smooth muscle fibers of erectile tissue and concomitant dilatation of the arterial supply in the penis produce penile erection. Activation of neurons in the sacral spinal cord triggers activity in the pelvic nerve and, subsequently, the cavernous nerve, which can lead to the release of mediators of vasorelaxation, including nitric oxide. These mediators modulate cyclic nucleotide levels resulting in Ca2+ sequestration and relaxation of smooth muscle fibers of the corpora cavernosa and corpus spongiosum in the shaft of the penis to produce arterial dilatation, engorgement of the penis with blood, and tumescence. Erections can be triggered either by peripheral (tactile) or by central (visual, olfactory, auditory, or imaginative cues) activation of somatic pathways and, as such, are influenced by tonic and phasic activity in the lumbosacral spinal cord and the brain.
Five melanocortin heterotrimeric GTP-binding protein (G protein)-coupled receptors have been identified as expressed in different tissues (5, 6). The functional role of each of these five melanocortin receptors is being defined. Rodent and human genetic and pharmacological evidence indicates that activation of melanocortin 4 receptor (MC4R) results in a lean phenotype, whereas inactivation of the MC4R results in obesity (7–10). Recent studies have demonstrated that MTII, a cyclic analogue of α-melanocyte stimulating hormone (α-MSH) and a subnanomolar agonist of the MC1R, MC3R, MC4R, and MC5R can stimulate nonpainful intermittent penile erections in man (11). The receptor(s) responsible for the proerectile activity of MTII have not been defined. In the rat, the induction of grooming behavior by melanocortin agonists may also be related to sexual function (12). Intracerebroventricular injections of other melanocortin receptor agonists such as adrenocorticotropin (ACTH) and α-MSH induce episodes of stretching, yawning, and penile erection in rats (13). These behaviors are reproduced by microinjections of ACTH and α-MSH into specific hypothalamic regions, including the paraventricular nucleus (14). MC4R expressed in the hypothalamus (15) could mediate the erectogenic effects of α-MSH and its analogues. This notion is challenged by the finding that injection of a putative MC4R-specific antagonist, HS014, into the hypothalamus failed to block α-MSH-induced penile erections at doses that inhibited stretching and yawning (13, 14). Differential MC4R antagonist receptor occupancy at which stretching and yawning or penile erection can be blocked could provide an alternative explanation for these observations. We used pharmacological and genetic tools to unravel the melanocortin receptor involvement in erectile function and to characterize the mechanism(s) by which melanocortin receptors (MCRs) modulate sexual function.
Materials and Methods
All experiments were approved by the MRL-Rahway Institutional Animal Care and Use Committee.
Mouse Cavernous Nerve Stimulation.
A murine model was used to monitor direct and electrical stimulation-evoked changes in intracavernosal pressure (ICP; refs. 16 and 17). Mc4r knockout mice were generated from targeted 129SvEv embryonic stem (ES) cells, and knock out mice were subsequently backcrossed to F6 in C57BL/6J mice aged 11–18 weeks. Male C57BL/6J mice (The Jackson Laboratory) or Mc4r−/− mice and wild-type littermate controls were anesthetized with sodium pentobarbital (80–100 mg/kg, i.p.) and mean arterial pressure (MAP) and ICP, respectively) were monitored by means of pressure transducers. Responses to nerve stimulation (4.0 v, 16 Hz, 1 ms, for 30 s) in the presence and absence of compound or vehicle were compared, and electrical stimulation-evoked changes in ICP, MAP, and the ratio of ICP/MAP (to normalize for any blood pressure effects) were monitored 15, 45, and 60 min post compound or vehicle. These data were acquired, digitized at 1 Hz, visualized, stored, and analyzed by using the PONEMAH LIFE SCIENCE SUITE software (Gould, Cleveland). Two-tailed, paired t tests were conducted to compare responses in baseline vs. treatment groups. Repeated measures ANOVA and Dunnett's multiple comparison tests were used to compare treatments in individual treatment groups.
Copulatory Behavior.
Singly housed, 3–6-month-old male Mc4r−/− (or wild-type littermates) of strain C57BL/6J/129SJl were placed in a clear vivarium (38.5 × 26.5 × 30.7 cm) for a 15-min acclimation period, after which a wild-type female was introduced into the mating arena. Angled mirrors beneath the vivaria assisted in recording observations of the animals' sexual behavior. The following information was recorded: latency to begin mounting behavior, latency to first intromission, latency to first ejaculation, number of intromissions to ejaculation, number of mounts per ejaculation, intermount interval, interintromission interval, and the number of ejaculations during the test period. All behavioral scoring was done by two observers blinded to treatment, and a time-lapsed videotape backup was examined to corroborate the scoring. Each mating test with estrous females was conducted for 45 min. Operational definitions of the components of mating behavior have been described (18). Wild-type females were ovariectomized and received s.c. placements of estradiol Silastic capsules. Mating tests were started after a 2-week recuperation period. Before the mating tests (6–8 h prior to tests), the females were injected with progesterone (1 mg, suspended in sesame seed oil) to induce behavioral estrus. Mating tests were started at the onset of the dark period.
RNase Protection.
Fresh tissues were harvested from 2–4-month-old male Sprague–Dawley rats, snap-frozen in liquid nitrogen, and pulverized to a powder with a mortar and pestle. Poly(A) RNA was isolated from the powdered tissue after homogenization by using Ambion's (Austin, TX) Poly(A) pure kit following the manufacturer's instructions. The RNA underwent a single round of selection for mRNA. Primary rat corpus cavernosum cells were harvested at passage 2, and mRNA was purified as described above. The rat MC4R probe was a 409-bp fragment cloned into pBluescript II SK(+). The protected 313-bp fragment spanned the second intracellular loop to mid TM6 (bp 457 to bp 770 of the ORF). The internal standard was a 240-bp fragment. mRNA, probe, and internal standard were coprecipitated with Ambion's Hybspeed RPA kit, denatured, and hybridized. RNase A digestion was performed. The products were precipitated and size-separated by electrophoresis on denaturing PAGE gels. Bands observed per hybridization were the protected RNA fragment and internal standard to correct for any loading abnormalities.
Reverse Transcription (RT)-PCR.
Five degenerate primers were designed at highly conserved TM regions between MC1R, 3R, 4R, and 5R. These regions had been used previously by various groups (19, 20) for degenerate primer design on the melanocortin receptors. G protein-coupled receptors conservation also was taken into account in the design. In addition, a pair of exact match primers was designed to be highly specific for each MCR and chosen to have particular divergence at the 3′ ends when compared with the other subtypes at the same position. The forward primers were located in the first extracytoplasmic loop. The reverse primers were located in the third intracellular loop. mRNA was isolated from fresh tissues as described under RNase Protection. cDNA was prepared from the 0.5–1 μg mRNA by using the CLONTECH Advantage RT kit per manufacturer's instructions. The mRNA was treated with DNaseI (Ambion) before reverse transcription to remove any genomic DNA. Minus-RT controls were run for each cDNA preparation to rule out genomic contamination. Cycling conditions were 94°C for 1 min, 50°C (65°C for exact match PCR) for 2 min, and 72°C for 2 min for 30 cycles. PCR products were electrophoresed on 1% agarose TAE gels. Gels were transferred to membranes and hybridized sequentially with separate melanocortin probes, washed at high stringency, and exposed to film. Primer sequences are available upon request.
In Situ Hybridization.
A 315-bp human cDNA probe specific for the human MCR4 gene was ligated into the pBluescript II SK(+) vector. The plasmid was linearized with either EcoRI or NotI (Life Technologies, Rockville, MD) to create template DNA. T3 and T7 polymerases were used with the Fluorescein RNA Labeling Kit (NEN) to in vitro transcribe antisense and sense riboprobes. In situ probes specific for the rodent homologue were prepared in the same manner to digest the pBluescript II SK(+) vector containing a 300-bp fragment specific for rat MCR4 with NotI (Promega) and EcoRI (Promega). Eight micrometer sections of fresh frozen human penis were thaw mounted on SuperfrostPlus slides (Fisher Scientific) and fixed in paraformaldehyde for 35 min. In situ hybridization was carried out using an anti-fluorescein horseradish peroxidase conjugate (NEN Life Sciences) in combination with the TSA-Direct kit (NEN Life Sciences). Immunohistochemistry was carried out sequentially after in situ signal development, as described (21). Antibodies for tyrosine hydroxylase (Chemicon), Protein Gene Product 9.5 (Biomeda Corporation, Foster City, CA), nitric oxide synthase (Sigma), choline acetyltransferase (Chemicon), neurofilament protein (DAKO), and smooth muscle actin (DAKO) were used at the manufacturer's specified dilutions and incubated with sections for 2 h at room temperature. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Molecular Probes) to visualize cell nuclei, and images were digitally acquired and reassembled with a MicroMax charge-coupled device camera (Princeton Instruments, Trenton, NJ) and METAMORPH imaging program (Universal Imaging, Media, PA). Double-label colocalization images were obtained with a Leica TCS confocal system equipped with argon/krypton (red), HeNe (green), and Tsunami 2 photon (Spectra-Physics) lasers.
Microphysiometer Assay.
Primary cultures of rat smooth muscle cells from the corpus cavernosum of juvenile rats were prepared and maintained as described (22). Measurement of ligand-induced functional responses was performed in the microphysiometer (Cytosensor, Molecular Devices) which records the change in extracellular pH as a function of receptor activation. Prior (≈16 hr) to experiment initiation, rat smooth muscle cells (passage 2–4) were plated at 200,000 cells per microcapsule cup. Control Chinese hamster ovary (CHO) cells stably expressing the human MC4R or CHO wild-type cells were plated at a cell density of 300,000 cells per capsule cup. The cells were constantly washed with RPMI medium 1640 modified buffer (Molecular Devices) in the absence of BSA. After an equilibration time of ≈2 hr, the cells were exposed to ligands in a cumulative dose-response format for two pump cycles. Each pump cycle time was a total of 2 min with a pump-on time of 80 s and a pump-off time of 30 s. The ligand was applied to the cells at 20 s before the end of pump-on cycle.
[125I]MTII Binding.
Frozen whole penises from fifty rats (8 g, Pel Freez Biologicals, AK) were cut into pieces (≈2-mm length) and homogenized in 50 ml of ice-cold buffer containing 50 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 2 mM EDTA, and protease inhibitor mixture (leupeptin 5 μg/ml/aprotinin 5 μg/ml/bacitracin 40 μg/ml/pefabloc 25 μg/ml) with a large size Polytron (2 cm, PTA 20S) at low speed (set 5) for 30 s. The resulting coarse tissue solution was diluted to 300 ml with the same buffer and homogenized 5 × 30 s at high speed (set 6.5–7.0). The homogenates were centrifuged at 3,000 rpm for 8 min; the supernatants, after passage through three layers of cheese cloth, were centrifuged again at 12,500 rpm for 20 min (Beckman, JA21 rotor). Pellets were suspended in 30 ml of 50 mM Tris⋅HCl, pH 7.4/3 mM CaCl2/1.5 mM MgCl2 and stored at −80°C. Binding solution (0.5 ml) contained 50 mM Tris⋅HCl, pH 7.4, 3 mM CaCl2, 1.5 mM MgCl2, protease inhibitor mixture, 0.07 nM [125I]MTII [NEN; specific activity 2200 Ci/mmol (1 Ci = 37 GBq), 250,000 cpm], penile membranes (300 μg protein), and test compound as indicated. After incubation for 70 min at room temperature, aliquots were filtered through GF/C fiberglass filters in a Brandel 48-channel cell harvester. Filters were washed three times with 3 ml of ice-cold buffer (10 mM Tris⋅HCl, pH7.4/100 mM NaCl), and radioactivity on filters was measured in a Bio-Safe II scintillation counting mixture. Specific binding was defined as the difference between total binding and nonspecific binding assayed in 1,000 nM MTII. Specific binding was 40–50% of total binding. Assays were carried out in triplicate, and experiments were repeated three times.
cAMP Assays.
Agonist activity was measured by using CHO cells expressing the cloned melanocortin receptors as described (23). Compounds were evaluated in three independent experiments, and data were analyzed with PRISM curve-fitting software (GraphPad, San Diego).
Muscle Strip Assays.
Corpus cavernosum penile tissue muscle strips (partially free of the tunica albuginea) from juvenile (≈3 months old; ≈ 250 g) male Sprague–Dawley rats (3–5 per preparation) were prepared fresh (within 5 min of killing) as described (24). Tissues were mounted in a 10-ml organ bath chamber and a resting tension of 1.5 mN was applied. Chambers contained Krebs solution (pH = 7.4) and were maintained at 37°C with continuous infusion of 95% oxygen and 5% carbon dioxide. Measurement of relaxation of tissue precontracted with the α-adrenergic agonist phenylephrine (≈80% of maximum) was conducted by adding the MC4R selective tetrahydroisoquinoline (THIQ) agonist or the phosphodiesterase inhibitor papaverine in a cumulative dose-response format.
Results
Activation of MC4R Augments Electrical Stimulation-Induced Increases in ICP.
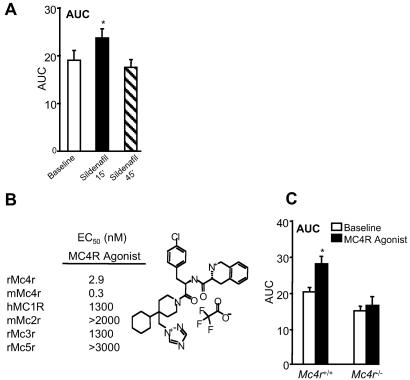
We initiated penile erection in anesthetized mice by electrically stimulating the cavernous nerve (for 30 s) and quantifying changes in ICP in the absence and presence of pharmacological treatment. Consistent with its ability to enhance erectile function in man (25), the phosphodiesterase V inhibitor sildenafil citrate (26, 27) augments erectile responses elicited by cavernous nerve stimulation in mice (Fig. 1A), as reflected by a statistically significant increase in the ICP/MAP ratio 15 min after i.v. administration (represented as an increase in area under the curve; AUC in Fig. 1A).
Fig 1.
Effects of sildenafil citrate (A) or THIQ MC4R agonist (C) in the mouse cavernous nerve-stimulated model of erectile function. Cavernous nerve stimulation (4.0 v, 16 Hz, 1 ms, for 30 s) was conducted in anesthetized male C57BL/6J mice [n = 6; Mc4r knock-out (−/−) mice were backcrossed six generations with C57BL/6J mice]. Mice were instrumented with corpus cavernosum, carotid artery, and jugular vein catheters for measurement of ICP, MAP, and i.v. compound administration (1 mg/kg for sildenafil, 10 mg/kg for MC4R agonist as a bolus), respectively. The ratio of ICP/MAP (to normalize for any effects on blood pressure) was calculated at −10 min (baseline) followed by 15 and 45 min postdose (sildenafil) or/and 15 min postdose (THIQ); the area under the curve during the 60 s of stimulation was then determined. *, P < 0.05. (B) MCR selectivity profile of THIQ MC4R agonist. EC50 values were determined in a functional activation assay in whole cells measuring cAMP production from cell lines expressing the appropriate MCR (rat Mc3r, 4r, 5r, murine Mc2r, 4r, and human MC1R; species specificity, affecting compound selectivity, has not been observed). The chemical structure of the MC4R agonist is shown.
Given the evidence that nonsubtype selective MCR agonists enhance erectile function, we sought to determine whether selective activation of MC4R promotes erectile activity. We evaluated the proerectile effects of a highly selective THIQ MC4R agonist (28) in the mouse cavernous nerve stimulation model. This compound is a full, high-affinity agonist for MC4R (mouse Mc4r EC50 = 0.3 nM) and is >1,000-fold selective as compared with other MCR subtypes (Fig. 1B). Fig. 1C illustrates that activation of MC4R by the THIQ agonist (10 mg/kg, i.v.) results in potentiation of erectile responses in wild-type but not Mc4r-null mice. Control electrical stimulation in Mc4r−/− mice did not reveal any obvious deficiencies in their ability to respond to cavernous nerve stimulation (data not shown). Collectively, these data implicate the MC4R in the modulation of penile erectile function by melanocortin agonists.
MC4R Involvement in Mouse Copulatory Behavior.
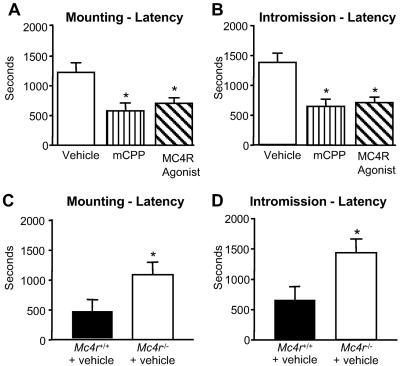
Because administration of an MC4R agonist enhances erectile activity, we next examined the extent to which activation of the MC4R system is an important determinant for copulatory behavior in male mice paired with estrous females. We examined latency to begin mounting behavior (reflecting motivation), latency to first intromission, latency to first ejaculation (performance parameters), number of intromissions to ejaculation, number of mounts per ejaculation, intermount interval, interintromission interval, and the number of ejaculations during the test period. Treatment with the THIQ MC4R agonist resulted in decreased mounting and intromission latencies (Fig. 2 A and B, respectively), the magnitudes of which were comparable to those produced by the known erectogenic agent methyl-chlorophenylpiperazine (mCPP; ref. 29), a nonselective 5HT2b,2c agonist. Next, we measured copulatory behavior in mice with a deletion of Mc4r. After pairing male Mc4r−/− and wild-type mice with estrous females, we found that Mc4r−/− mice showed an increased mounting and intromission latency compared with control littermate wild-type mice (Fig. 2 C and D). Moreover, Mc4r−/− mice exhibited a reduced ejaculatory efficiency in that none of the Mc4r−/− animals ejaculated during a 1 hr observation period, compared with 5 of 11 wild-type mice. Despite these impairments in copulatory behavior, an Mc4r−/− breeding colony could be established (8).
Fig 2.
(A and B) Copulatory behavior of male Mc4r−/− mice and wild-type mice treated with the THIQ MC4R agonist. (A) Male wild-type and Mc4r−/− mice (n = 10; strain C57Bl6J/129SJl) were paired with estrous females for 45 min. *, P < 0.05. (B) Male wild-type mice (n = 7 or 8; strain C57Bl6J) were injected i.p. with either vehicle, the nonselective 5HT2b, 2c agonist mCPP (0.75 mg/kg), or the THIQ MC4R agonist (2.5 mg/kg) 15 min before pairing them with an estrous female. *, P < 0.05 (please note that baseline values for mounting and intromission latencies differ between distinct genetic backgrounds; compare A and B with C and D).
Absence of Functional Melanocortin Receptors in the Corpus Cavernosum.
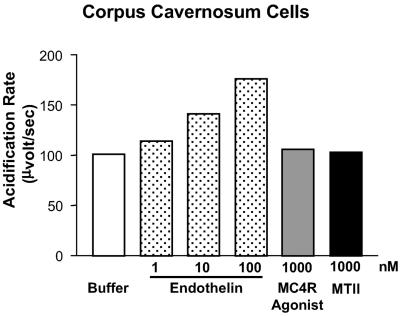
In vivo studies have shown that MCR agonists similarly can induce erectile activity in the rat (30, 31). Because the corpus cavernosum serves as the primary transducer of the functional erectile response, primary cultures of rat corpus cavernosum smooth muscle cells were prepared to determine whether MCR agonists directly affected signal transduction. Explant cultures were established 3–4 days after dissection of the penile tissue, and the resulting cells grew with a morphology (stellate, spindle-shaped) consistent with that of smooth muscle cells. Indices of cellular activation were studied by using a microphysiometer. Control conditions were first established to measure rat MC4R activation in CHO cells stably expressing the cloned MC4R. In these cells, low concentrations of αNDP-MSH (as little as 0.05 nM) evoked a substantial increase (40%) in the rate of change of extracellular pH, whereas control CHO cells (no MC4R) failed to respond to αNDP-MSH (≤10 nM; data not shown). In corpus cavernosum cells, which contain ET-a receptors, endothelin (1–100 nM) potently elicited robust functional responses in a concentration-dependent manner. By contrast, neither the nonselective MCR agonist MTII (≤1000 nM) nor the selective MC4R agonist, THIQ (≤1000 nM), produced any metabolic change (Fig. 3).
Fig 3.
Microphysiometric characterization of rat corpus cavernosum cells. Primary cultures of rat smooth muscle cells from rat corpus cavernosum (and CHO cells stably expressing the rat MC4R) were tested for ligand-stimulated metabolic activation. The acidification rate (μVolt/s) was monitored during the addition of the indicated concentrations of the melanocortin agonists MTII, α-NDP-MSH, and MC4R agonist. Endothelin-1, an agonist of ETA receptors present on smooth muscle cells, was used as a control. The values represent the rate of acidification compared with the unstimulated basal rate and are representative of three independent experiments.
Muscle strips prepared from corpus cavernosum may be used as an assay system to evaluate compounds for their ability to alter the contractile state of penile smooth muscle (31, 33). Therefore, we also examined the effect of MC4R agonist treatment on rat corpus cavernosum strips before and after they were precontracted with the α-adrenergic agonist, phenylephrine. THIQ (10–10,000 nM) did not affect baseline tension and failed to relax tissue strips; in contrast, the control phosphodiesterase inhibitor, papaverine (100 μM), promoted relaxation back to baseline tension (data not shown).
MCRs Are Expressed in Tissues That Modulate Erectile Activity.
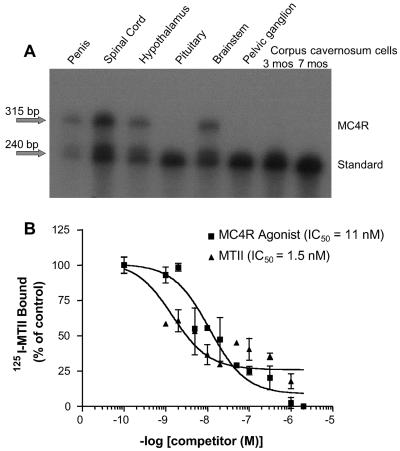
Because MC4R agonists do not promote direct relaxant effects in isolated erectile tissue preparations, we profiled the tissue expression pattern of MCRs by using multiple approaches. In the rat, RT-PCR and RNase protection was used to examine MCR expression in several tissues implicated in the control of erectile function, including penis, spinal cord, hypothalamus, brainstem, pelvic ganglion (major autonomic relay center to the penis), and primary corpus smooth muscle cavernosum cells. RT-PCR allowed the detection of MC4R mRNA in all of the above tissues (mouse penis also was positive for MC4R mRNA by RT-PCR; data not shown), except for primary corpus cavernosum cells. MC3R and MC5R were selectively expressed in hypothalamus and brainstem, consistent with published finding (6). An RNase A protection assay (RPA) was developed to detect rat MC4R mRNA by a non-PCR-based method. An ≈400-bp radiolabeled probe spanning TM-4 to TM-6 of the rat Mc4r gene was synthesized and hybridized to tissue poly(A)+ mRNA, digested with RNase A, and reaction products were size-separated followed by autoradiography. The appearance of an ≈300-bp band outlines the presence of MC4R RNA in the tissue (Fig. 4A). MC4R mRNA expression could be obtained in rat penis, spinal cord, brainstem, and after prolonged autoradiographic exposure, in pelvic ganglion and pituitary. For rat MC4R, PCR and RPA data agree in all tissues tested. Primary corpus cavernosum cells (from juvenile and older rats), by both RPA and PCR, did not express detectable transcripts encoding MC4R.
Fig 4.
(A) RNase A protection assay (RPA) of rat MC4R in penile tissues. Poly(A)+ RNA (6 μg) was hybridized with a rat MC4R cDNA probe (second intracellular loop to mid transmembrane-6) that, upon RNase digestion, would result in a radiolabeled fragment of 313 nucleotides, revealed by denaturing PAGE and autoradiography (as detailed in Materials and Methods). An internal standard rat MC4R fragment of 240 nucleotides is shown. (B) Binding of [125I]MTII to rat penile membranes. Inhibition of [125I]MTII binding by MTII and the THIQ MC4R agonist was performed by using 70 pM radiolabeled MTII for 70 min at 20°C; the results are expressed as percent of [125I]MTII specifically bound. IC50 values for MTII and MC4R agonist were 1.5 and 11 nM, respectively (n = 3 experiments).
To characterize the expression of rat penis MCRs by pharmacological means, crude cellular membranes from rat whole penis were evaluated and were shown to bind [125I]MTII with high affinity (IC50 MTII = 1.5 nM). A small, but reproducible, window of specific binding (Fig. 4B) was obtained by using large quantities of membrane protein, indicating the presence of a low-abundance MCR. The ability of the MC4R-selective THIQ agonist (IC50 = 11 nM) to completely block the binding suggests that the observed binding is caused by MC4R expression.
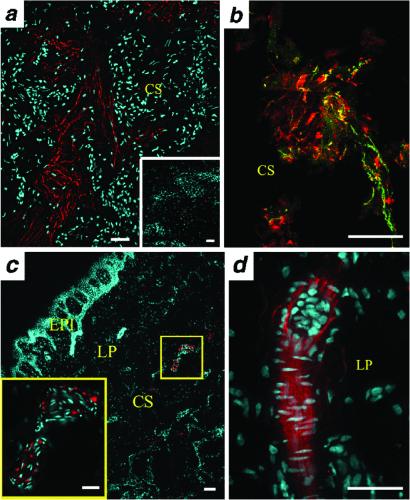
Based on the pharmacological determination of MC4R in whole rat penis, in situ hybridization and receptor autoradiography studies were carried out to map the distribution of MC4R in rat and human penis. In sagittal sections of the rat penis, localization of the MC4R mRNA signal was difficult to observe with low magnification microscopy and normal detection methodologies. A human penis sample from a 25 year-old accident victim with no known pathology was used to determine the localization of human MC4R. Specific RNA probes for the human MC4R were generated and hybridized to crosssections of human penis specimens, which contained both the corpus and glans. Tyramide-amplified in situ hybridization in combination with immunohistochemistry (using neuronal marker antibodies) was carried out using standard protocols. The lack of suitable MC4R antibodies [we failed to show specific binding in using the only previously published MC4R antibody (32) comparing staining patterns in wild type and Mc4r−/− brain samples] precludes a direct comparison or association of mRNA and protein in the identified cellular structures at this moment. Hybridization signals in both the corpus and glans penis illuminated nerve fibers. The sense control probe (Fig. 5a Inset) was devoid of signal. The association of MC4R with nerve fibers was confirmed by its colocalization with Pgp9.5 (Fig. 5b) and myelin-associated protein immunostaining, which highlights neuronal processes, and peripheral nerves (data not shown). Preliminary experiments using a nitric oxide synthase hybridization probe did not demonstrate colocalization of this neurotransmitter with MC4R (data not shown). Given the importance of glandular sensitivity in erection, we determined whether MC4R mRNA could be detected in afferent sensory receptor-like structures in the glans. In situ hybridization of glans tissue sections using the MC4R RNA probe identified two different sensory receptor types: an end bulb of Krause and Raffini nerve ending. Fig. 5 c (human) and d (rat) show the presence of MC4R mRNA in an encapsulated corpuscle, possibly a Raffini nerve ending. This nerve ending is known to be present in the glans and functions as a stretch receptor. It consists of a perineural cylinder positioned around dermal collagen strands into which one or two myelinated sensory nerve fibers enter, lose the myelin sheath, and branch into a “spraylike” appearance. Raffini endings, usually located in the deeper dermis and hypodermis, display a large receptive field and are the only type of cutaneous mechanoreceptor readily excited by dermal stretch.
Fig 5.
MC4R expression in nerve fibers, nerve endings, and sensory corpuscles of the human glans penis. In situ hybridization was carried out by using riboprobes for human (a–c) or rat (d) MC4R. (a) MC4R mRNA in the human penis corpus spongiosum (CS) is seen in nerve fibers (shown in red); nuclei are seen in blue. Sense control probe produced no specific signal (Inset). (b) MC4R colocalized with the nerve fiber marker PGP9.5 in a CS mechanoreceptor. MC4R signal is shown in green, PGP9.5 immunoreactivity in red, and colocalization in yellow. (c) MC4R in situ hybridization signal in an encapsulated sensory corpuscle of the CS of the human glans penis. MC4R is shown in red, nuclei in blue. Layers of the human glans penis are labeled as epithelium (Epi), lamina propria (LP), and CS. High magnification view (Inset) suggests that this is a Pacinian-lamellated corpuscle. (d) MC4R localization in a Meissner-encapsulated corpuscle found in the LP of the rat glans penis. MC4R signal is shown in red, nuclei in blue. (Bars = 20 μm.)
Discussion
That melanocortins can influence sexual behavior and penile erection in animals has been known for almost 40 yr (33), and indices of erectile activity evoked by ACTH or αMSH have been documented in a range of species. These effects were manifest when test agents were administered intracerebroventricularly or by microinjection into specific hypothalamic regions, and melanocortin modulation of erectogenesis was assumed to be predominantly, if not exclusively, centrally mediated. The relevance of these observations in animals has been heightened by the studies of Wessells et al. (11), who demonstrated that parenteral (s.c.) administration of MTII could evoke spontaneous penile erections in men. Additionally, in double-blind, placebo-controlled, crossover studies, it was shown that parenteral MTII initiated erections in men with psychogenic (11) and organic erectile dysfunction. Because MTII does not discriminate among four of the five MCRs, its clinical efficacy in erectile dysfunction stimulated interest in clarifying the site(s) and receptor mechanism(s) of action of this agent. As in humans, MTII is proerectile following parenteral administration to rats and rabbits (30, 34).
By using a potent, selective MC4R agonist, we established that activation of MC4R enhanced electrically evoked increases in intracavernosal pressure in wild-type mice but not in Mc4r-null mice. Moreover, whereas copulatory behavior is diminished in Mc4r-null mice, the MC4R agonist improved sexual function in wild-type mice along both motivation (latency to begin mounting behavior) and performance (latency to first ejaculation) parameters. These results are consistent with the concept that activation of MC4R is an important determinant of erectile activity and copulatory behavior in mice.
Although studies in vivo in rats have shown that MC4R agonists and nonselective MCR agonists can elicit changes in erectile activity in rodents (30, 31), ex vivo studies yielded no evidence of MC4R agonist effects on isolated corpus cavernosum smooth muscle cells or rat corpus cavernosum muscle strips. These observations are consistent with reports that in vitro MTII fails to directly relax (rabbit) cavernosal strips, or alter electrically induced relaxation (34), and that, upon intracavernosal injection (to rats), MTII fails to alter ICP (31). Collectively, these data indicate that MC4R modulation of erectile activity is not mediated via a direct action on cavernosal smooth muscle.
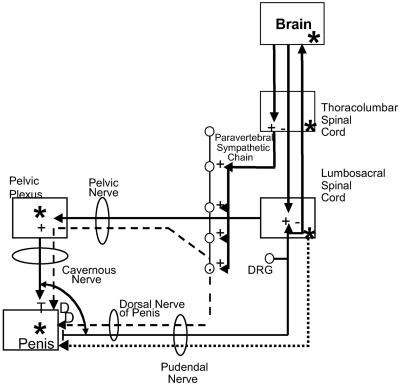
It is axiomatic that functional correlates of receptor activation may be inferred from tissue distribution of that receptor. By using protein and RNA indices, MC4R was shown to be expressed in mouse and rat brain and in the rat peripheral nervous system, including neurons of the human and rat penis (in Fig. 6, sites of MC4R expression are highlighted with an asterisk). More detailed mapping of the tissue distribution of MC4R using in situ hybridization was possible in the human and rat penis, and MC4R mRNA was found at free nerve endings and mechanoreceptors. The application of neurotransmitter markers can distinguish parasympathetic efferent from afferent sensory fibers. Our preliminary experiments using a nitric oxide synthase hybridization probe failed to demonstrate colocalization of this neurotransmitter with MC4R. Other putative proerectile neurotransmitters in the penis have not yet been compared. For example, besides acetylcholine, CGRP, VIP, and related peptides have been detected in the free nerve endings of the human corpus cavernosum and have been shown to be effective relaxants of human corpus cavernosum preparations and to increase cavernous arterial flow and smooth muscle relaxation when injected intercavernously in animals (1, 35). In situ hybridization of glans tissue sections using the MC4R RNA probe identified two different sensory receptor types: an end bulb of Krause and Raffini nerve ending. Typically the latter display a large receptive field and are the only type of cutaneous mechanoreceptor readily excited by dermal stretch. Activation of such stretch receptors triggers reflex activation of motor neurons that innervate the ischiocavernosus and bulbospongiosus muscles, resulting in sufficient rigidity for intromission and ejaculation. That the lower spinal cord may contain the neural networks responsible for reflexive erections elicited by stimulation of afferent receptors in the penis is indicated by the presence of erectile responses in animals or humans with suprasacral spinal lesions (36). One model for the mode of action of MC4R agonists, as depicted in Fig. 6, is consistent with evoked-activation of a spinal cord reflex loop, which relies on mechanosensory afferent input from the penis to the spinal cord. This reflex loop may be modulated by MC4R-controlled central nervous system input and by MC4R regulation of spinal networks that influence autonomic and somatic neural activity. Thus, MC4R agonist modulation of erectile and sexual function may derive from both peripheral and central actions.
Fig 6.
The neural control of penile erection (2) summarizing putative sites of erectogenesis of MC4R agonists (modified from ref. 41). *, distribution of MC4R.
A potential shortcoming of the current study relates to the genetic experiments performed in mice, whereas most RNA, protein-binding, and cell-based studies have been performed with rat (and human) tissues. The issues presented by working with the inherently smaller quantities of tissue that can be obtained from the mouse, when compared with the rat, forced us to focus on rat tissues for several of our experiments. Because melanocortin agonists are effective in modulating mouse, rat, and human erectile function, while the MC4R is expressed in mouse, rat and human penis, we infer that this potential issue does not invalidate our conclusions, which define a role for the MC4R in rodent and possibly human sexual function.
Previously published work and observations presented in this report indicate a dual action of MC4R agonists in modulating appetite and sexual function. Research should be undertaken to determine for which of these indications MC4R agonists will be most beneficial. It is interesting to note that several examples may exist where satiety and sexual function may possibly be modulated by the same neurotransmitter. Examples include the modulation of 5HT2c receptor activity by mCPP (37), the possible action of melanin concentrating hormone on appetite and sexual function (38, 39), and the role of the satiety hormone leptin in reproductive function (40). It is, therefore, possible that energy homeostasis and reproductive function are broadly linked in mammals.
Acknowledgments
We thank Ann LaTourette and Kimberly Likowski for administrative assistance in finalizing this manuscript, Akio Kanatani and Takehiro Fukami (Banyu Research Laboratories) for assay support, and Francois Giuliano for helpful discussion.
Abbreviations
MC4R, melanocortin 4 receptor
α-MSH, α-melanocyte stimulating hormone
MCR, melanocortin receptor
ICP, intracavernosal pressure
ES cell, embryonic stem cell
MAP, mean arterial pressure
RT-PCR, reverse transcription–PCR
CHO, Chinese hamster ovary
THIQ, tetrahydroisoquinoline
References
- 1.Andersson K. E. & Wagner, G. (1995) Physiol. Rev. 75, 191-236. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano F. A., Rampin, O., Benoit, G. & Jardin, A. (1995) Urol. Clin. North Am. 22, 747-766. [PubMed] [Google Scholar]
- 3.Lue T. F. (2000) N. Engl. J. Med. 342, 1802-1813. [DOI] [PubMed] [Google Scholar]
- 4.Moreland R. B., Hsieh, G., Nakane, M. & Brioni, J. D. (2001) J. Pharmacol. Exp. Ther. 296, 225-234. [PubMed] [Google Scholar]
- 5.Adan R. A. H. & Gispen, W. H. (1997) Peptides 18, 1279-1287. [DOI] [PubMed] [Google Scholar]
- 6.Cone R. D., Lu, D., Koppula, S., Vage, D. I., Klungland, H., Boston, B., Chen, W., Orth, D. N., Pouton, C. & Kesterson, R. A. (1996) Recent Prog. Horm. Res. 51, 287-317. [PubMed] [Google Scholar]
- 7.Lu D., Willard, D., Patel, I. R., Kadwell, S., Overton, L., Kost, T., Luther, M., Chen, W., Woychik, R. P., Wilkison, W. O., et al. (1994) Nature (London) 371, 799-802. [DOI] [PubMed] [Google Scholar]
- 8.Fong T. M., Mao, C., MacNeil, T., Kalyami, R., Smith, T., Weinberg, D., Tota, M. R. & Van der Pleeg, L. H. (1997) Biochem. Biophys. Res. 237, 629-631. [DOI] [PubMed] [Google Scholar]
- 9.Farooqi I. S., Yeo, G. S., Keogh, J. M., Aminian, S., Jebb, S. A., Butler, G., Cheetham, T. & O'Rahilly, S. (2000) J. Clin. Invest. 106, 271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zemel M. B. & Shi, H. (2000) Nutr. Rev. 58, 177-180. [DOI] [PubMed] [Google Scholar]
- 11.Wessells H., Gralnek, D., Dorr, R., Hruby, V. J., Hadley, M. E. & Levine, N. (2000) Urology 56, 641-646. [DOI] [PubMed] [Google Scholar]
- 12.Adan R. A., Szklarczyk, A. W., Oosterom, J., Brakkee, J. H., Nijenhuis, W. A., Schaaper, W. M., Meloen, R. H. & Gispen, W. H. (1999) Eur. J. Pharmacol. 378, 249-258. [DOI] [PubMed] [Google Scholar]
- 13.Vergoni A. V., Bertolini, A., Mutulis, F., Wikberg, J. E. & Schioth, H. B. (1998) Eur. J. Pharmacol. 362, 95-101. [DOI] [PubMed] [Google Scholar]
- 14.Argiolas A., Melis, M. R., Murgia, S. & Schioth, H. B. (2000) Brain Res. Bull. 51, 425-431. [DOI] [PubMed] [Google Scholar]
- 15.Mountjoy K. G., Mortrud, M. T., Low, M. J., Simerly, R. B. & Cone, R. D. (1994) Mol. Endocrinol. 8, 1298-1308. [DOI] [PubMed] [Google Scholar]
- 16.Mizusawa H., Hedlund, P., Hakansson, A., Alm, P. & Andersson, K. E. (2001) Br. J. Pharmacol. 132, 1333-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cashen D. E., MacIntyre, D. E. & Martin, W. J. (2002) Br. J. Pharmacol. 136, 693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson R. J., Demas, G. E., Huang, P. L., Fishman, M. C., Dawson, V. L., Dawson, T. M. & Snyder, S. H. (1995) Nature (London) 378, 383-386. [DOI] [PubMed] [Google Scholar]
- 19.Chhajlani V. & Wikberg, J. E. (1996) FEBS Lett. 390, 238. [DOI] [PubMed] [Google Scholar]
- 20.Libert F., Parmentier, M., Lefort, A., Dinsart, C., Van Sande, J., Maenhaut, C., Simons, M. J., Dumont, J. E. & Vassart, G. (1989) Science 244, 569-572. [DOI] [PubMed] [Google Scholar]
- 21.Figueroa D. J., Hess, J. F., Ky, B., Brown, S. D., Sandig, V., Hermanowski-Vosatka, A., Twells, R. C., Todd, J. A. & Austin, C. P. (2000) J. Histochem. Cytochem. 48, 1357-1368. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W. & Christ, G. J. (1995) J. Urol. 154, 1571-1579. [PubMed] [Google Scholar]
- 23.Bednarek M. A., MacNeil, T., Kalyani, R. N., Tan, R., Van der Ploeg, L. H. T. & Weinberg, D. H. (2001) J. Med. Chem. 44, 3665-3672. [DOI] [PubMed] [Google Scholar]
- 24.Italiano G., Calabro, A. & Pagano, F. (1994) Pharmacol. Res. 30, 325-334. [DOI] [PubMed] [Google Scholar]
- 25.Lue T. F. & Tanagho, E. A. (1987) J. Urol. 137, 829-836. [DOI] [PubMed] [Google Scholar]
- 26.Boolell M., Allen, M. J., Ballard, S. A., Gepi-Attee, S., Muirhead, G. J., Naylor, A. M., Osterloh, I. H. & Gingell, C. (1996) Int. J. Impot. Res. 8, 47-52. [PubMed] [Google Scholar]
- 27.Corbin J. D. & Francis, S. H. (1999) J. Biol. Chem. 274, 13729-13732. [DOI] [PubMed] [Google Scholar]
- 28.Bakshi R., Barakat, K., Nargund, R., Palucki, B., Patchett, A., Sebhat, I., Ye, Z. & Van der Ploeg, L., (2000) PCT (Merck, Rahway, NJ).
- 29.Millan M. J., Peglion, J. L., Lavielle, G. & Perrin-Monneyron, S. (1997) Eur. J. Pharmacol. 325, 9-12. [DOI] [PubMed] [Google Scholar]
- 30.Lindia J. A., Nargund, R., Sebhat, I., Weinberg, D., Van der Ploeg, L. H. T., MacIntyre, D. E. & Martin, W. J. (2001) Int. J. Imp. Res. 13, S55. [Google Scholar]
- 31.Wessells H. (2000) Int. J. Imp. Res. 12, S74-S77. [Google Scholar]
- 32.Cowley M. A., Pronchuk, N., Fan, W., Dinulescu, D. M., Colmers, W. F. & Cone, R. D. (1999) Neuron 24, 155-163. [DOI] [PubMed] [Google Scholar]
- 33.Argiolas A. (1999) Neurosci. Biobehav. Rev. 8, 1127-1142. [DOI] [PubMed] [Google Scholar]
- 34.Vemulapalli R., Kurowski, S., Salisbury, B., Parker, E. & Davis, H. (2001) Br. J. Pharmacol. 134, 1705-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Argiolas A. & Melis, M. (1995) Prog. Neurobiol. 47, 235-255. [PubMed] [Google Scholar]
- 36.Rampin O., Bernabe, J. & Giuliano, F. (1997) World J. Urol. 15, 2-13. [DOI] [PubMed] [Google Scholar]
- 37.Tecott L. H., Sun, L. M., Akana, S. F., Strack, A. M., Lowenstein, D. H., Dallman, M. F. & Julius, D. (1995) Nature (London) 374, 542-546. [DOI] [PubMed] [Google Scholar]
- 38.Murray J. F., Mercer, J. G., Adan, R. A., Datta, J. J., Aldairy, C., Moar, K. M., Baker, B. I., Stock, M. J. & Wilson, C. A. (2000) J. Neuroendocrinol. 12, 1133-1139. [DOI] [PubMed] [Google Scholar]
- 39.Shimada M., Tritos, N. A., Lowell, B. B., Flier, J. S. & Maratos-Flier, E. (1998) Nature (London) 396, 670-674. [DOI] [PubMed] [Google Scholar]
- 40.Chehab F. F., Mounzih, K., Lu, R. & Lim, M. E. (1997) Science 275, 88-90. [DOI] [PubMed] [Google Scholar]
- 41.Steers W. D. (2000) Neurosci. Biobehav. Rev. 24, 507-516. [DOI] [PubMed] [Google Scholar]