Abstract
Cervix cancer is a major health concern globally, being the fourth most prevalent type of cancer among females across the globe. Despite existing preventive measures like cytology and HPV testing, more precise diagnostic and treatment strategies are needed. This study leverages single-cell sequencing and transcriptome analysis to identify LLPS-linked therapeutic targets in cervical cancer. Utilizing data from the GEO database, we characterized six main cell types in cervical cancer and calculated LLPS scores for each. Analysis of LLPS-related genes revealed seven prognostic genes, and we constructed a predictive model demonstrating promising stability and accuracy. Furthermore, high-risk patients exhibited higher LLPS scores and lower survival probabilities, which may be influenced by differences in the tumor immune microenvironment, particularly involving immune cell types such as CD8+ T cells, M0 macrophages and regulatory T cells. Exploring the expression patterns of model genes, we found PDIA6, PGK1, ASPH, and FNDC3B to be involved in immune infiltration during tumorigenesis. Finally, the signature genes that related to immune microenvironment was confirmed by immunohistochemistry staining. PGK1 was found to be most closely associated with the prognosis of cervical cancer. The correlation analysis of PGK1 with pathways in cervical cancer revealed that the expression level of PGK1 is associated with lipid peroxidation. Furthermore, our analysis using immunofluorescence and flow cytometry showed that lipid peroxidation increased after PGK1 knockdown. We further employed the proliferation experiment and cell-derived xenograft (CDX) mouse model to verify that downregulating PGK1 suppresses proliferation of cervical cancer in vitro and in vivo. Taken together, our study provides comprehensive insights into cervical cancer prognosis, identifying PGK1 as a potential therapeutic target among LLPS-related genes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-14637-4.
Keywords: Liquid-liquid phase separation, Transcriptome analysis, Single-cell sequencing, PGK1, Cervical cancer
Introduction
The cellular interior is compartmentalized into numerous membrane-bound organelles and membrane-free regions, facilitating the precise spatial and temporal regulation of diverse cellular functions [1]. The cellular interior is compartmentalized into numerous membrane-bound organelles and membrane-free regions, facilitating the precise spatial and temporal regulation of diverse cellular functions [2]. Liquid-liquid phase separation (LLPS) plays a crucial role in this compartmentalization by forming biomolecular condensates, which are essential for various cellular functions such as chromatin organization, genomic stability, DNA damage response and repair, transcription, and signal transduction [2]. Recent reviews have highlighted the molecular mechanisms and biophysical properties of LLPS in cancer. For instance, it has been shown that LLPS can drive the assembly of membrane-free condensates, thereby promoting oncogenic processes such as epigenetic changes, metabolic regulation, and immune evasion [3]. Additionally, LLPS is involved in the formation of stress granules and other membrane-free organelles that contribute to cancer cell survival and proliferation [3]. The dynamics of membrane-bound organelles, including processes like fusion and fission, are well-documented and are often facilitated through vesicle transport mechanism. However, inside eukaryotic cells, many “membraneless” structures exist and perform critical functions [4, 5]. The intricacies of how membraneless compartments assemble and perform their functions are not yet fully understood [1]. Recently, these membraneless structures are proved to be formed through LLPS, which also called “membraneless condensates” or “biological condensates” [1, 4–6]. Phase separation is driven by molecular concentration and is influenced by environmental factors like temperature, pH, and ionic strength [7]. In essence, LLPS is a fundamental cellular mechanism. Typically, it operates in a well-regulated dynamic equilibrium. However, dysregulation of LLPS can result in pathological outcomes, such as the development of cancer [8].
Genomic mutations in malignant tumor cells can disrupt the coordinated dynamic balance of LLPS, leading to pathological outcomes such as tumorigenesis. These mutations impact various biological processes that are mediated by LLPS, including changes in chromatin structure, transcription regulation, DNA damage response, and the mechanisms of tumor suppression [2]. These mutations can result in abnormal cellular functions, including promoting uncontrolled cell division and replication, inducing blood vessel formation, allowing cancer cells to bypass growth inhibitors, resisting cell death, and enabling invasion and spread to other tissues [9]. Disruptions to the normal LLPS process, due to genetic or epigenetic alterations, can lead to the formation of abnormal biomolecular condensates, which may contribute to the initiation of cancer and foster its progression [2]. For example, aberrant LLPS promotes tumorigenesis by interfering with anti-tumor signaling pathways [2, 10]. Merlin, also known as NF2 or schwannomin, functions as a tumor suppressor. It coordinates and modulates intracellular signaling and the extracellular matrix, while also enhancing the body’s natural defenses against cancer [10, 11]. While, genetic inactivation and mutations of the NF2 gene are observed across a spectrum of cancers, including colorectal cancer, type 2 neurofibromatosis, and skin cancers, among others [12]. Recent studies have indicated that a mutation in the FERM domain of NF2 can significantly inhibit the cGAS-STING signaling pathway by forming phase-separated condensates with IRF3, thereby hindering the antitumor immunity triggered by STING. This discovery highlights the role of NF2 mutations in regulating immune responses and suggests potential therapeutic targets for NF2-related tumors [10]. Currently, a lot of studies on LLPS have been completed, but there are still few articles on the relationship between cervical cancer and LLPS.
Cervical cancer ranks as one of the leading types of cancer globally and is the fourth most prevalent among women. According to the latest data from the American Cancer Society’s “Cancer Statistics, 2024,” it is projected that there will be 2,001,140 new cancer cases and 611,720 cancer deaths in the United States in 2024 [13]. The 5-year relative survival rate for ovarian cancer, another specific cancer type, is reported to be 51.6%, with significant variations based on the stage at diagnosis [13]. Cervical cancer is known to be caused by persistent infection of the lower reproductive tract by about 15 high-risk types of human papillomavirus (hrHPV) [14]. In most cases, the disease is preventable [15]. In additon, when cervical cancer is detected at an early stage, it is still considered curable and can be effectively treated with surgery and concurrent chemotherapy and radiation therapy. However, in both developed and developing countries, cervical cancer is a significant public health problem, which means improved diagnosis and treatment options are needed [16]. Therefore, it is very important to depend the knowledge of the molecular pathogenesis of Cervical Cancer and put forward some new therapeutic targets or new methods for diagnosis and treatment in its early stages.
Currently, three main methods of diagnosing cervical cancer are cytologic testing, human papillomavirus (HPV) testing, and visual examination [17]. In clinical practice, these methods are often used in combination [18]. For females exhibiting signs of cervical cancer, a comprehensive approach is typically employed, which includes a pelvic exam, visual inspection of the cervical and vaginal tissues, and a cervical cytology test [15]. With the multiple screening protocols available, current screening guidelines uniformly use HPV testing as the primary test, or in conjunction with cytology, as the preferred test [19]. For these examination methods, it is even more important to develop precise tests to reduce overtreatment and improve specificity. Currently, chemotherapy is a supplement to definite local regional treatment (surgery or radiotherapy) to improve their prognosis, as well as in the treatment of recurrent or nascent metastatic disease patients. Cancer treatment has evolved significantly over the past few decades, transitioning from conventional methods such as surgery, chemotherapy, and radiotherapy to more advanced and targeted therapies [20]. The field of cancer therapy is rapidly advancing, with immunotherapy and targeted therapies offering new hope for patients. Future directions, including combination therapies and the use of next-generation sequencing, hold promise for more effective and personalized treatments [21]. However, the application of these therapeutic modalities is independent of the histology of the disease and may affect prognosis and therapeutic response [16].
The progression of cervical cancer is largely attributed to the activation of gene mutations that are linked to various signaling pathways [22, 23]. Research indicates that the immune microenvironment could facilitate tumor expansion and spread by suppressing the infiltration of lymphocytes and by altering the tumor extracellular matrix. Consequently, we need to delve deeper into cervical cancer and the tumor microenvironment.
Currently, we have gained some insight into the involvement of LLPS in the genomics and proteomics of cancer. However, its specific role in cervical cancer remains to be elucidated. In this research, we aim to investigate the function of LLPS in cervical cancer using single-cell sequencing and transcriptome analysis. We analyzed the heterogeneity of LLPS in cervical cancer through single-cell sequencing and employed weighted gene co-expression network analysis (WGCNA) along with Lasso regression to develop a prognostic model related to LLPS. This model is capable of precisely forecasting the prognosis and immune status of cervical cancer patients. Our findings may offer potential therapeutic targets for precision medicine in cervical cancer and contribute to the understanding of LLPS’s role in this disease.
Results
Expression of LLPS-related genes in single cell sequencing data
The original 14,220 cells were screened and 3,073 cells were left through the quality control standard (Fig. 1A). A robust positive association was identified between the aggregate gene expression levels and the count of genes involved, with a correlation coefficient (cor) of 0.92. Mitochondrial genes accounted for less than 10% of the total (Fig. 1B). Figure 1C shows the 2,000 genes with the most significant expression changes in these cell populations of cervical cancer tissue, which are the 2,000 high-variation genes we screened out. Then, based on the gene expression profiles, these cells were clustered to generate 9 distinct clusters (Fig. 1D). Therefore, the cells were annotated according to their expression through cell clusters and marker genes of each cluster. Ultimately, the cells were separated into six classes (Fig. 1E), which are basal cell, epithelial cell, B cells, macrophage, monocyte, and T cells. Besides, the results showed that basal cell and epithelial cell accounted for significant high proportion in all cells. Figure 1F depicted the expression levels of the two most significant marker genes from each cluster. Subsequently, cells were categorized into low and high LLPS groups according to the enrichment scores of genes associated with LLPS. And low-LLPS was primarily expressed in Monocyte and Basal cell, while high-LLPS was primarily expressed in T-cells, Macrophage, B-cell, and Epithelial cell (Fig. 1G). By intersecting LLPS-related genes with those in the cervical cancer cell database (GSM5155196), 3,667 genes were obtained. This result indicates that the gene set in cervical cancer cells may be associated with the phenomenon of LLPS.
Fig. 1.
Single cell sequencing analysis. A, B Quality control of single cell sequencing data. C 2000 hypervariable genes screened out. D All cells are clustered into 9 clusters in total. E Cell annotation. All cells were annotated as basal cell, epithelial cell, B cell, macrophage, monocyte, and T cells. F The expression levels of the first two marker genes in each cell. G According to the LLPS-related genes, the cells were divided into high-LLPS group and low-LLPS group, and the differentially expressed genes between the two groups were obtained
Weighted genes correlation network analysis in transcriptome data
We firstly calculated the enrichment scores of LLPS and MLO phenotypes of each sample using the single-sample gene set enrichment analysis (ssGSEA) algorithm in the TCGA cohort. Subsequently, based on the median value, the cells were bifurcated into two groups: those with high LLPS and MLO levels, and those with low LLPS and MLO levels. Figure 2A illustrates that, according to Kaplan-Meier survival analysis, individuals in the high LLPS and MLO group experienced poorer survival outcomes. Then, WGCNA was further applied to recognize genes related with the LLPS and MLO phenotypes in cervical cancer. When the soft domain value went to 6, the resulting R^2 value was above 0.8, indicating that the data followed a scale-free distribution, thus fit for WGCNA analysis. Moreover, the average connectivity also about to be stable (Fig. 2B). As shown in Fig. 2C, all genes were classified into 72 non-grey modules. We found the turquoise module exhibited the highest correlation with LLPS scores. A substantial positive correlation was identified between the genes within the turquoise module and their membership within it (cor = 0.72, p < 1e-200) (Fig. 2D). At the same time, the pale turquoise module had the highest scores when correlated with MLO (p < 0.05) (Fig. S1). Therefore, 2810 genes in the module were screened. These genes could provide new insights into the molecular mechanisms of cervical cancer and may reveal potential therapeutic targets or biomarkers.
Fig. 2.
Weighted genes correlation network analysis (WGCNA). A K-M survival analysis. The high LLPS and MLO group had a worse survival prognosis. B When the soft domain value is set to 6, data followed a scale-free distribution and the average connectivity becomes stable, which is suitable for WGCNA. C All genes were clustered into 72 non-grey modules. Turquoise module had the strongest correlation with LLPS scores. D a strong positive correlation between the importance of genes in the turquoise module and the membership of the module (cor = 0.72, p < 1e-200)
Construction of the prognostic model and validation in external dataset
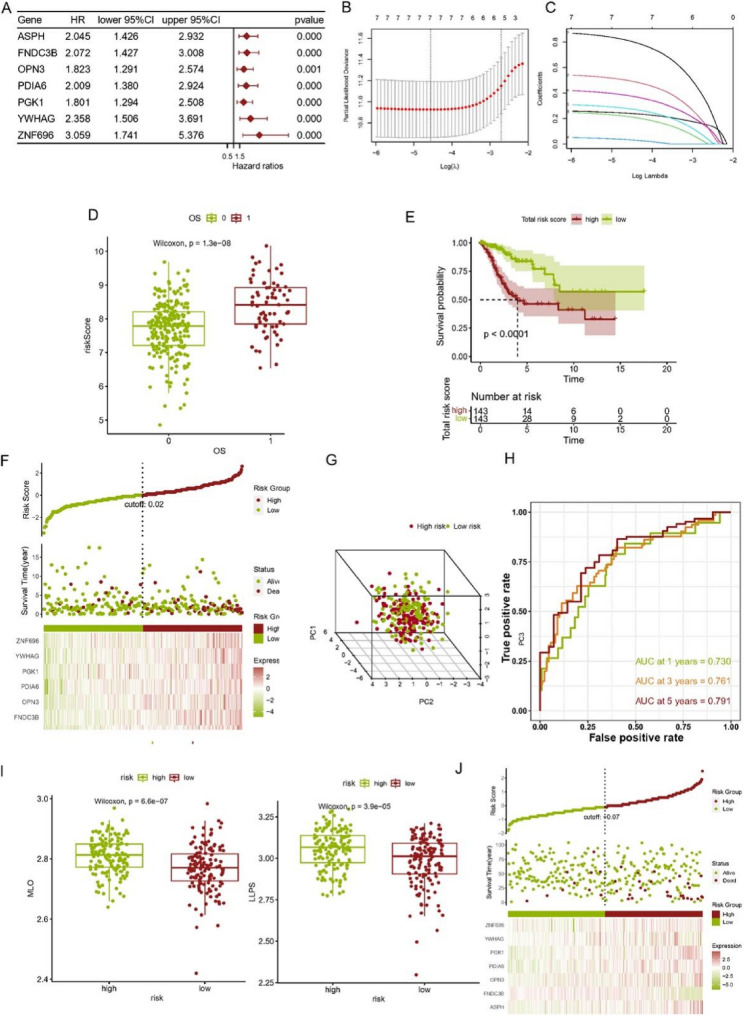
To pinpoint the precise gene set, we merged 3667 genes identified through single-cell analysis with 2810 genes from WGCNA, resulting in a set of 856 genes for constructing the prognostic model. Within the TCGA cohort, we performed a preliminary screen for prognostic gene candidates using Univariate Cox analysis. When p value was set as 0.001, 7 candidate genes were obtained. Figure 3A shows these genes along with their (Hazard Ratio) HR and p-values. Then, as shown in Fig. 3B and C, LASSO regression was used with a random seed set to 3. The lambda.min value was selected, retaining the signature composed of 7 genes, including ASPH, FNDC3B, OPN3, PDIA6, PGK1, YWHAG, ZNF696. The risk value of the model was evaluated by Risk Score = 0.240 * ASPH + 0.495 * FNDC3B + 0.213 * OPN3 + 0.032 * PDIA6 + 0.275 * PGK1 + 0.378 * YWHAG + 0.808 * ZNF696. Subsequently, each patient in the cohort was given a risk score through the formula and based on the median value, all cervical cancer patients were categorized into two groups: a high-risk group and a low-risk group. Further, prognoses of the two subgroups were compared. Figure 3D and F showed the risk scores of deceased and surviving patients were different, with deceased patients (p < 0.0001) (Fig. 3D) having higher LLPS scores. Additionally, Kaplan-Meier survival analysis indicated that the group with a high risk score had a lower likelihood of survival (p < 0.0001) (Fig. 3E). Figure 3F revealed that the expression levels of seven marker genes were elevated in deceased patients, consequently resulting in a higher LLPS score. In addition, PCA analysis revealed that the prognostic model could effectively differentiate between different cervical cancer patients (Fig. 3G). Figure 3H illustrates the prognostic model’s diagnostic capability in predicting patient survival at the 1-year, 3-year, and 5-year marks through ROC curve analysis. The AUC values for these time points were all above 0.7, with specific values of 0.730, 0.761, and 0.791, respectively, suggesting that the model possesses significant predictive value for survival outcomes. We used boxplots to further explore the relationship between risk sore and LLPS (MLO) score. In Fig. 3I, high risk score showed a higher LLPS or MLO score in TCGA cohort(p < 0.0001). In the external cohort (GSE44001), we also calculated risk score for each patient and divided them into two group. The expression of seven tag genes in dead patients were higher also with higher risk scores. And the expression levels of 6 genes corresponds with TCGA cohort except for FNDC3B (Fig. 3J). The observed discrepancy in FNDC3B expression between the TCGA and GSE44001 cohorts can be attributed to several factors, including batch effects, cohort heterogeneity, and technical variability. By validating the prognostic model on an external dataset, we can draw conclusions about the model’s generalizability, clinical application value, and reliability. The results showed that the model can be applied in clinical practice.
Fig. 3.
Construction of the prognostic model and validation in external dataset. A Univariate Cox analysis of OS for each gene, and 7 genes with P < 0.001. B LASSO coefficient profiles of 7 genes. C Plots of the ten-fold cross-validation error rates. D box plot. LLPS score was higher in dead patients of TCGA cohort. E Survival analysis curve of TCGA cohort. The high LLPS group had a worse prognosis (p < 0.0001). F Distribution of risk score, survival status (G) The 3d-PCA of patients in two subgroups. H ROC curve of measuring the predictive value. I High risk score showed a higher LLPS or MLO score in TCGA cohort(p < 0.0001). J External cohort verification. The expression of seven tag genes in dead patients was higher also with higher risk score. And the expression level of 6 genes corresponds with TCGA cohort except for FNDC3B
Development of a predictive nomogram
To further assess the model’s predictive power, we initially investigated the correlation between the FIGO stage and the risk score. We found less significant difference of the variation in risk scores as FIGO stage varies from 1 to 3. But there is a significant rise when FIGO stage went to 4 (p < 0.025) (Fig. 4A). Additionally, to delve into the link between survival outcomes and clinical aspects of cervical cancer, a multivariate Cox regression analysis was conducted. This analysis demonstrated that the FIGO stage and the Risk Score are independent predictors significantly associated with the prognosis of cervical cancer (Fig. 4B). So, we constructed a nomogram to specifically quantified and evaluated prognostic effect. Moreover, the Calibration curve indicated that the nomogram was fairly accurate in predicting the survival probabilities at the 1-year, 3-year, and 5-year marks (Fig. 4C, D). In summary, the nomogram not only demonstrates good predictive performance statistically but also holds practical value in clinical applications. It is capable of providing personalized prognostic information for patients and assisting physicians in making more precise treatment decisions.
Fig. 4.
Development of a predictive nomogram. A box plot. Correlation between risk score and Figo stage. B Multivariate cox regression analysis of Figo stage and risk score were independent prognostic factors. C, D Calibration curve showed that the nomogram could well predict the probability of survival at 1, 3, and 5 years
Differential expression genes (DEGs) and functional enrichment in cervical cancer risk groups
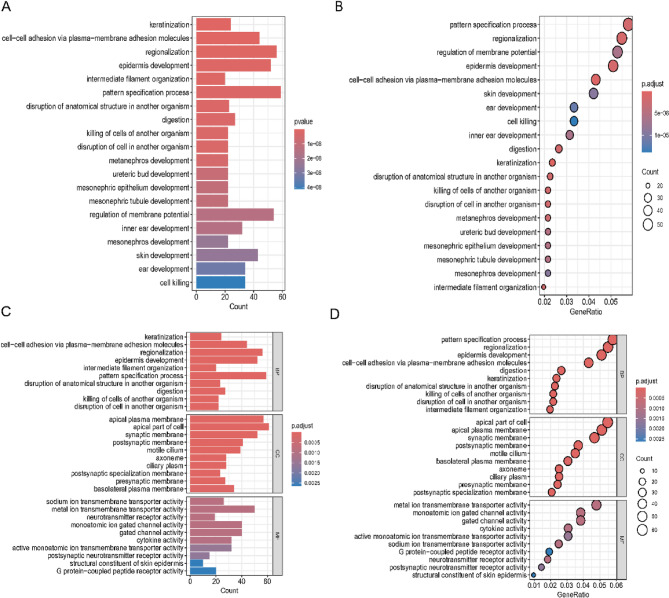
To explore functional pathway differences between high- and low-risk groups in the prognostic model, we first used the “limma” R package to identify DEGs with a P value threshold of < 0.01 and a Log2(|Fold Change|) > 1.5. A total of 1188 DEGs were then subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. The GO analysis revealed that these genes were predominantly associated with processes such as pattern formation, regional specification, membrane potential regulation, epidermal development, and the involvement of adhesion molecules in cell-cell adhesion through plasma membrane adhesion molecules (Fig. 5A, B). These findings indicate that the distinct expression patterns of liquid-liquid phase separation-related genes between high and low-risk groups could be involved in and influence the functional processes within tumor cells. Moreover, KEGG analysis showed that in these functional categories, a considerable proportion of differential genes constituted the apical plasma membrane of cells and participated in transmembrane ion transport (Fig. 5C-D). This functional enrichment analysis provides us with crucial information for gaining a deeper understanding of the molecular mechanisms of diseases.
Fig. 5.
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. A Barplot graph of LLSP and cervical cancer related gene for the enriched item in gene ontology analysis. B Dotplot graph of LLPS and cervical cancer related gene for the enriched item in GO analysis. C Barplot graph for the enriched item in KEGG analysis. The length of bar represented the number of genes enriched. BP biological process, CC cellular component, MF molecular function (D) Dotplot graph for the enriched item. The count of Bubble represented the number of genes enriched
Immunoinfiltration analysis of cervical cancer
To better understand the divergent outcomes between the low- and high-risk groups, we conducted ssGSEA analysis. This analysis revealed that in the high-risk group, the expression levels of 28 types of immune cells were comparatively reduced. Notable differences were observed in the expression levels of activated B cells, activated CD8 T cells, activated dendritic cells, MDSCs, eosinophils, effector memory CD8 T cells, immature B cells, macrophages, monocytes, regulatory T cells, and Type 1 T helper cells between the two groups (Fig. 6A). Moreover, we conducted the Cibersort algorithm to calculate proportion of immune cells (22 types) in high and low- risk groups of cervical cancer patients. The high-risk groups had decreased expression levels of regulatory T cells, CD8 T cells, and resting mast cells, while the proportion of M0 macrophages increased (Fig. 6B, C). To delve deeper into the link between prognostic genes and immune cells, we determined the gene scores within various immune cell types. We discovered that OPN3, PDIA6. PGK1, YWHAG, ZNF696 were positively related with M0 Macrophages (r = 0.19; r = 0.12; r = 0.21; r = 0.19, r = 0.13). And ASPH, FNDC3B, OPN3, PDIA6, PGK1 and YWHAG was negatively related with CD8 T cells (r = −0.21; r =−0.18; r = −0.15; r =- 0.12, r=−0.19, r=−0.11) and regulatory T cells (r = −0.19; r =−0.18; r = −0.22; r = −0.25, r=−0.13, r=−0.21) (Fig. 6D). This result indicated that these genes may exert different role in tumor immune related microenvironment.
Fig. 6.
Immune infiltration analysis. A ssGSEA. The expression levels of 28 immune cells were relatively low in the high-risk group. B, C CIBERSORT algorithm calculated the proportion of 22 kinds of immune cells in high-risk and low-risk subgroups of CC patients. D The relationship between prognostic genes and immune cells was investigated, and the score of each gene in each immune cell was calculated
Analysis of gene and protein expression level
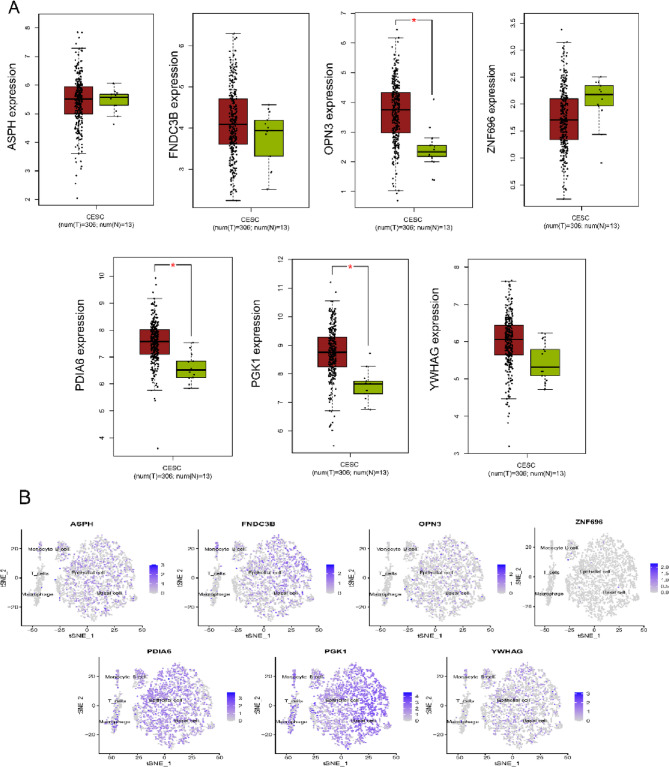
Among 7 model genes, only OPN3, PDIA6, PGK1 showed a significant higher expression in tumor tissues (p < 0.05) (Fig. 7A). To further explore where the model genes are expressed, we analyzed their expression at the single-cell level (Fig. 7B). Our analysis revealed that ASPH, FNDC3B, OPN3, and YWHAG were predominantly expressed in basal and epithelial cells, whereas PGK1 and PDIA6 showed high expression in monocytes, T cells, B cells, macrophages, as well as in epithelial and basal cells. This suggests that PDIA6 and PGK1 might be involved in immune cell infiltration during tumor formation and progression. In addition, ASPH and FNDC3B showed moderate expression level in Monocyte and B cells. ZNF696 exhibited low expression level across all cells, aligning with the finding from the transcriptome analysis.
Fig. 7.
Analysis of gene and protein expression level. A Gene expression of 7 model genes in GEPIA. B Gene expression distribution of 7 model genes in cervical cancer cell
To further explore protein levels of the signature genes that related to immune microenvironment (PDIA6, PGK1, ASPH and FNDC3B), we obtained immunohistochemistry staining from the HPA database (Fig. 8A-D). Consistent with previous result PDIA6, PGK1, ASPH and FNDC3B showed higher protein levels in CC patients. Among 4 genes in tumor tissues, protein PGK1 mostly expresses in nuclear while PDIA6, ASPH and FNDC3B express in cytoplasm or cytoplasmic membrane. However, the correlation of these genes with the prognosis of cervical cancer and their functions within cervical cancer still need further exploration.
Fig. 8.
Analysis of Genes and protein expression level. A-D Four gene’s protein levels between normal and cancer tissues from the HPA database
Downregulation of PGK1 promotes lipid peroxidation and suppresses proliferation in cervical cancer cells
To analyze the function of these genes in cervical cancer, we further examined the prognostic significance of differentially expressed genes PGK1, PDIA6, and OPN3 in cervical cancer. The results indicated that PGK1 was most closely associated with the prognosis of cervical cancer (p = 0.024) (Fig. 9A-C). To verify the function of PGK1 in cervical cancer, we downloaded STAR-counts data and corresponding clinical information for cervical cancer from the TCGA database. We studied the correlation between PGK1 expression and pathway scores through Spearman correlation analysis. The results showed that PGK1 expression is related with biosynthesis of unsaturated fatty acid and ferroptosis (Fig. 9D-E). To further investigate the function of PGK1 with ferroptosis in cervical cancer, we selected effective siRNA to knock down PGK1. Subsequently, utilizing the fluorescent probe BODIPY 581/591 C11 to assess lipid peroxidation, our analysis revealed that PGK1 knockdown significantly increased lipid peroxidation levels (Fig. 9F-G). Additionally, we utilized the BD FACSAria II flow cytometer with the BODIPY™ 581/591 C11 probe to evaluate lipid peroxidation in cells subjected to PGK1 knockdown. The flow cytometry histograms (Fig. 9H) illustrate an observed increase in the FITC/Texas Red fluorescence ratio (%) in the PGK1 knockdown groups, which is further confirmed by the statistical graph (Fig. 9I). The analysis revealed that the PGK1 knockdown groups displayed a significant increase in FITC fluorescence intensity compared to the Texas Red fluorescence in the mock group, indicative of enhanced lipid peroxidation.
Fig. 9.
Downregulation of PGK1 promotes lipid peroxidation and suppresses proliferation in cervical cancer cells. A-C The prognostic significance of PGK1, PDIA6, and OPN3 in cervical cancer plotted by the Kaplan-Meier method using TCGA data; (D) The correlation between PGK1 and the biosynthesis of unsaturated fatty acids, data derived from TCGA. Spearman correlation analysis plot is used to show the correlation between the biosynthesis of unsaturated fatty acids pathway score and the expression of gene PGK1. E The correlation between PGK1 and ferroptosis, data derived from TCGA. Spearman correlation analysis plot is used to show the correlation between ferroptosis score and the expression of gene PGK1. F, G Detection of lipid peroxidation in PGK1 knockdown cells using the C11-BODIPY fluorescent probe. Scale bar, 20 μm. H, I Assessment of lipid peroxidation in PGK1 knockdown SiHa cells using the BD FACSAria II flow cytometer with the BODIPY™ 581/591 C11 probe. J, K Colony formation assays assessing the proliferation of CaSKi and SiHa cells when PGK1 is knocked down; (L) Cell proliferation of CaSKi and SiHa cells measured using CCK-8 assays at 24 h, 48 h, and 72 h after knock down PGK1; (M, N) Tumor size (M) and volume (N) measurements in the CDX model following PGK1 inhibitor Z57346765 treatment (5 mg/kg, 15 mg/kg and 45 mg/kg). O Body weights of mice across different treatment groups. Data presented are the mean ± S.D. of three independent experiments. Significance levels are denoted by asterisks (*): *p < 0.05, **p < 0.01, ***p < 0.001
To verify the effect of PGK1 on the proliferation of cervical cancer, we conducted a plate colony formation experiment, and the results indicated that the cloning ability of cell lines CaSKi and SiHa was diminished after PGK1 knockdown (Fig. 9J, K). Furthermore, we used the CCK8 assay to determine the impact of PGK1 knockdown on the proliferation of these two cell lines, and the results showed that the proliferation ability of cell lines CaSKi and SiHa was reduced after PGK1 knockdown (Fig. 9L). To further analyzed the effects of PGK1 on cervical cancer, we utilized a cell-derived xenograft (CDX) mouse model treated with PGK1 inhibitor Z57346765 to assess its anti-tumor efficacy in vivo. Compared to the control group, Z57346765 treatment resulted in a significant reduction in tumor volume (Fig. 9M-N), with no substantial change in the body weights of mice across the different treatment groups (Fig. 9O). These findings suggest that PGK1 plays a significant role in the occurrence and development of cervical cancer and can serve as a potential therapeutic target.
Discussion
Cervical cancer ranks as the fourth most common cancer among women worldwide and represents a significant public health challenge, particularly in low- and middle-income countries. Despite being largely preventable and curable when detected early, improved diagnostic and therapeutic strategies are needed. Diagnostic options include cytology, HPV testing, and visual inspection, yet more precise tests are necessary to reduce overtreatment [16, 17]. Chemotherapy, often used alongside local treatments, improves outcomes, though its efficacy varies. Various biological processes involving gene mutation and tumor microenvironment [24], particularly LLPS which may play crucial roles in cancer progression, but their exact impact remains unclear. To address this gap, our study employed single-cell sequencing and transcriptome analyses to explore LLPS in cervical cancer, uncovering its heterogeneity and developing a prognostic model. Our findings offer potential targets for precise treatment and shed light on LLPS in cervical cancer biology.
Tumor heterogeneity is manifested by morphological differences or different karyotype patterns between cells, proteins and expression levels of biomarker [25, 26]. Tumors are complex systems which is consist of malignant cells and non-malignant stroma including endothelial cells, fibroblasts, and immunoinfiltration cells [27–29]. The diverse genetic and phenotypic characteristics play distinct roles in facilitating tumor progression, metastasis, and the development of drug resistance. Single cell analysis serves as a novel strategy to discover a single kind of cell within tumors and their roles in above processes [30]. Based on dataset in GEO database, we identified six main cells comprised of cervical cancer, including Epithelial cell, Basal cell, T cells, B cells, Macrophage and Monocyte and we calculated LLPS score for each cell. Our analysis revealed that the high LLPS score group exhibited a significantly higher proportion of various immune cell types, including T cells, B cells, and macrophages. Previous studies have demonstrated that LLPS plays a critical role in the activation of T and B cells [31], which is also closely related to T cell signal pathway [29]. Our result provided rough bioinformatics support for these findings. However, in vivo experiment should be conducted to further clarify this phenomenon in cervical cancer.
In recent years, the identification of biomarkers for cancer diagnosis and prognosis has become a crucial area of research. The work of Liu’s Lab in TCGA biomarker studies has been pioneering in this field. Their research has developed various strategies to identify and validate potential biomarkers, contributing significantly to our understanding of cancer biology [32]. For instance, their studies have explored the potential roles of several genes in different types of cancer, such as SCN3B in glioma [33], CDK2 in glioma [34], AIMP1 in head and neck squamous cell carcinoma [35], CNIH4 in head and neck squamous cell carcinoma [36], and RAD50 in breast cancer [37]. These studies highlight the importance of comprehensive bioinformatics analysis in identifying biomarkers that can improve cancer diagnosis and prognosis. In our study, we firstly identified the modules most corelated with LLPS in cervical cancer using WGCNA. 2810 genes were found having a correlation with LLPS. After filtering and taking intersection, we selected 856 genes for the development of the prognostic model. We adopted univariate cox regression analysis to seek the correlation between LLPS-related genes and prognosis of CC. 7 prognostic genes (ASPH, FNDC3B, OPN3, PDIA6, PGK1, YWHAG, ZNF696) were found negative for survival possibility of CC patient(p < 0.001). Among them, ZNF696 have the highest hazard ratio (HR = 3,059,95% CI = 1,741 ~ 5.376). Then we performed Lasso cox regression and the model showed relatively good stability and predictive ability. In addition, we constructed nomogram considering to combine our model and clinical features. It showed a relatively good predictive ability for 1, 3, 5- year survival possibility as calibration curve.
Based on the expression levels of model genes, risk scores were calculated for each patient within the TCGA dataset. These patients were subsequently classified into high-risk and low-risk groups. The correlation analysis and K-M survival analysis demonstrated a significant relationship between the high-risk group and an increased LLPS score, as well as a diminished probability of survival (p < 0.0001). To explain this discrepancy, cibersort and ssGSEA were performed to explore whether it was differences in the tumor immune microenvironment that caused the different results. A comparative analysis revealed that a panel of 28 immune cell types—such as activated B cells, CD8 T cells, dendritic cells, effector memory CD8 T cells, eosinophils, immature B cells, macrophages, MDSC, monocytes, regulatory T cells, and type 1 helper T cells—exhibited similarly low expression levels across both study groups. In the high-risk group, Cibersort analysis showed decreased expression among CD8 T cells, regulatory T cells, and resting Mast cells, alongside an elevated proportion of M0 macrophages. In different types of cancer, the composition of tumor immune microenvironment is dynamic and complex, with immune cells playing a relevant role in eliminating tumor cells, but in some cases, they contribute to tumor development [38]. So we speculate high level of LLPS weakens the anti-tumor immune response which result in progress of tumor and poor prognosis. Additionally, there is a complex relationship between LLPS dysregulation and immune evasion, with LLPS dysregulation potentially leading to the suppression and exhaustion of CD8 + T cells in high-risk groups, thereby facilitating tumor immune evasion [39]. Future research needs to further explore the specific mechanisms of LLPS dysregulation and how to intervene in LLPS dysregulation to restore T-cell function, thereby enhancing the efficacy of cancer immunotherapy.
To further identify the influence of 7 model genes on the tumor development and prognosis, we investigate the expression of genes and proteins. Significant upregulation of OPN3, PDIA6, and PGK1 was observed in the expression levels within tumor tissues. Significantly, the elevated expression of PDIA6 and PGK1 in immune and epithelial cell types, including Monocytes, T cells, B cells, Macrophages, Epithelial cells, and Basal cells, implies a possible participation in the immune response and tumor progression. Additionally, ASPH and FNDC3B demonstrated moderate expression specifically in Monocytes and B cells, suggesting their potential role in immune infiltration as well. PDIA6, a protein belongs to PDI protein family, which consists of more than 21 members [40]. A number of researches have proved that in several cancers, such as ovarian cancer [41] and primary ductal breast cancer [42], the high expression of PID family members could lead to poor prognosis while increase tumor metastasis and drug-resistance [43]. PDIA6 have four different domains including 2 catalytically active domains a, a’ and two inactive domains b, b’ [44]. Recent studies have found that PDIA6 is up-regulated in non-small cell lung cancer (NSCLC), which suppresses cisplatin-induced apoptosis by interacting with MAP4K1 to inhibiting JNK/c-Jun signaling and inhibiting caspase-9 and 3 activation, leading to the growth of NSCLC cells [45]. Our earlier research demonstrated that caspase-8 (CASP8) and caspase-3 (CASP3), key enzymes in the caspase family, act as protective factors in ovarian cancer and significantly influence tumor progression and patient prognosis. Herein, we speculate that PDIA6 may play it role as an upstream protein in tumor cell growth and apoptosis in ovarian cancer and cervical cancer. However, to substantiate this hypothesis regarding the two types of gynecological tumors, both in vivo and in vitro experiments are necessary.
Phosphoglycerate kinase (PGK), the main enzyme that catalyzes ATP formation in the aerobic glycolysis pathway, exists in all organisms and has a high degree of sequence conservation throughout evolution [46]. PGK1 is one of the PGK isoforms, located on the X-chromosome and expressed in all cells [47], involving the first ATP generation step of the glycolytic pathway [48]. More importantly, PGK1 mediates glycolysis, produces ATP for tumor cells, especially under hypoxia conditions, and has been implicated in the development and progression of various cancers [49]. Elevated expression of PGK1 is associated with intensified aerobic glycolysis, as well as heightened migration and invasion capabilities of cancer cells in cervical cancer [50]. However, transcription factor HOXA1 directly regulates PGK1, which induces glycolysis and promots cancer growth [51]. PGK1 is a key glycolytic enzyme that regulates lipid peroxidation and ferroptosis by catalyzing ATP production [52]. PGK1 also acts as a protein kinase, influencing apoptosis, autophagy, and ion channels [53]. Recent studies show that PGK1 is involved in the HIF-1 signaling pathway, which is crucial for cellular responses to hypoxia and oxidative stress. PGK1 may regulate lipid peroxidation through this pathway, affecting ferroptosis [54]. Additionally, PGK1 activation enhances ATP production but can also generate reactive oxygen species (ROS) under oxidative stress, highlighting its dual role in energy metabolism and oxidative stress management [55]. This dual role of PGK1 highlights its importance in balancing energy production and oxidative stress management. PGK1 has been shown to play a role in DNA repair mechanisms [56]. In addition to cell metabolism regulation, PGK1 is involved in multiple biological activities, including angiogenesis, autophagy and DNA repair [32].
The potential of PGK1 as a biomarker is supported by its significant expression and prognostic value across various cancers, including breast cancer. In breast cancer, PGK1 is highly expressed and associated with poor prognosis, making it a potential biomarker for diagnosis and treatment [57]. However, its role in HPV + vs. HPV − subtypes remains unclear and requires further investigation. Compared to existing biomarkers like PD-L1, PGK1 may offer additional insights into cancer prognosis and treatment response. While PD-L1 is a well-established biomarker for immunotherapy response, PGK1’s involvement in metabolic pathways and its correlation with immune cell infiltration suggest it could complement PD-L1 in personalized therapy strategies [58]. Further validation through multi-cohort analyses and functional assays is necessary to confirm PGK1’s broader applicability as a pan-cancer biomarker. CDX models used in this study originate from established cell lines, which lack the genetic diversity found in patient tumors. patient-derived xenograft (PDX) models offer a more comprehensive and clinically relevant platform for cancer research. Future studies should leverage PDX models to validate findings and explore new therapeutic strategies. Additionally, as TCGA provides a wealth of data for cancer research, it is crucial to recognize and address the technical and biological biases inherent in the data. Researchers should employ robust normalization and batch correction techniques, and be cautious in interpreting bulk sequencing data. Future studies should aim to validate findings in larger, more diverse cohorts and consider using single-cell sequencing technologies to better capture tumor heterogeneity.
The ASPH gene, which encodes aspartate beta-hydroxylase, is an enzyme that is widely preserved and occurs in cells stemming from the initial stages of embryonic artery formation in mammals [59]. High expression of ASPH may contribute to the cell formation, proliferation and invasion in cancer [59]. Pathological analyses of intermediate to advanced cervical squamous cell carcinoma have demonstrated that patients exhibiting radiation resistance tend to have increased ASPH expression levels compared to those who are radiation-sensitive [60]. This result could explain the reason that patients with lower survival rates had higher ASPH expression. Recent researches revealed that FNDC3B potentially plays a role in mediating endoplasmic reticulum (ER) stress through its involvement in stress granule formation [61–63], promoting tumor growth, as it raises cell viability in conditions of hypoxia and nutrient exhaustion, facilitating metastatic dissemination through the promotion of epithelial-mesenchymal transition (EMT), sustaining tumor cell dormancy and the function of tumor-initiating cells, thereby inducing angiogenesis [64]. However, this phenomenon requires further validation through both in vitro and in vivo experiments.
To conclude, our research performed a comprehensive bioinformatics analysis both at single cell level and transcriptome level in cervical cancer. Our research group has crafted a model involving 7 LLPS-associated genes that could significantly contribute to the betterment of cervical cancer prognosis. Among 7 genes, the expression of PDIA6, PGK1, ASPH and FNDC3B are heterogeneous in tumor cells, which may act as potential therapeutic targets for cervical patients.
Methodology
Collection and preprocessing of transcriptome data
UCSC XENA (http://xena.ucsc.edu/) is a detailed and comprehensive website that stores RNA sequence data for gene expression of multiple cancer patients and clinical data of patients with various cancers. Standardized transcriptome data of 309 patients with cervical cancer (HTSeq-FPKM and Counts) were downloaded from GDC TCGA cervical cancer in this database. Using the “cbind” function in R, we matched the gene expression data with the clinical signature data to obtain a total of 296 patients. The expression data of HTSeq- Counts were transformed by“ceiling(2^(counts1)−1)” and the genes were renamed as Gene symbol for subsequent analysis. And in GEO database, we obtained 300 cervical cancer patients from GSE44001 for external validation for the prognostic model. The prognosis of OPN3, PDIA6, and PGK1 was assessed utilizing data from The Cancer Genome Atlas (TCGA), which includes 252 samples of female cervical squamous cell carcinoma. This dataset encompasses detailed clinical information and survival data of the samples. The relationship between gene expression and survival time and status in TCGA data is depicted through KM survival curves, and the gene’s impact on survival is statistically tested using the log-rank test.
Acquisition of liquid-liquid phase separation related genes
A new version of the protein isolate data, PhaSepDB, was released (http://db.phasep.pro/). This new database includes phase isolated protein studies as of April 1 st, 2022, providing annotated information on phase separation droplet status, phase separation regulation, etc [65]. From this database, we download phasepdbv2_data, and through the intersection process with transcriptome and single-cell data, we obtained 286 LLPS-related genes and 4329 MLO-related genes for the following analysis.
Single cell sequencing data analysis
In our paper, GSE168652 was attained which is a single cell dataset from the GEO database. The following shows the standard of quality control. 1) Genes expressed in more than or equal to three cells were remained. 2) Cells with a mitochondrial gene percentage exceeding 10% were excluded. Only cells with gene expression counts between 200 and 2,500 were retained. Next, LogNormalize method was used in the NormalizeData function to standardize the data. Then, we used the FindVariableFeatures function to confirm gene features with high intercellular differences using normalized data, and adopted the ScaleData function to scale the data for the top 2000 highly variable genes. Afterthat, we reduced the dimension of the data using Principal Component Analysis (PCA). Continuously we used the FindNeighbors and FindClusters functions for graph-based clustering and to find the optimal clustering resolution. And we applied the RunTSNE function for appropriate visualization. Cells were annotated through Single R’ s method based on “Human Primary Cell Atlas Data” database.
Weighted genes correlation network analysis (WGCNA)
WGCNA is a widespread used method mining data in the field of systems biology, which can describe the correlation patterns between genes across different samples, and is particularly useful for identifying highly correlated gene sets. The power index range is set from 1 to 20. Next, Using the pickSoftThreshold function from the WGCNA package, we determined that the optimal soft threshold value (β) is 6. This value was selected based on the function’s calculation, which ensures the network’s scale-free topology fit. This approach provides a robust basis for our network analysis. This research identified candidate genes associated with LLPS and MLO through WGCNA analysis.
Establishment of the prognostic model and validation in external dataset
LLPS related genes in tumor tissues obtained from single cell sequencing were intersected with module genes obtained from WGCNA. Then, univariate Cox analysis was conducted for the above genes, and preliminary prognostic genes were obtained using a threshold of p < 0.001. The following analysis was then performed using the minimum absolute contraction and selection operator (LASSO), randomly set to 3 seeds. The selection of 7 genes was based on LASSO regression, with the optimal regularization parameter (λ) chosen using cross-validation. Specifically, the λ value was selected to minimize the cross-validation error (λmin). This approach ensures that the model is both predictive and parsimonious. To assess the robustness of the model, we conducted sensitivity analyses by varying the λ values around λmin and evaluating the model’s performance using the area under the ROC curve (AUC) for overall survival (OS) at different time points (1, 3, and 5 years). The model demonstrated consistent predictive accuracy across a range of λ values. Regression coefficients were compressed and the optimal prognostic model was ultimately obtained by constructing the penalty function. R packages “glmnet” “survival” “ggplot2” “forestplot” “rms” “survivalROC” “plotROC” were used to visualize research results.
Development of a predictive nomogram
To further indentify the correlation between FIGO stages and risk score, boxplot was applied and Kruskal-Wallis test was performed. Considering the clinical stages and other features, we applied multivariate cox regression. Further, a nomogram predicting the likelihood of overall survival at 1,3, and 5 years was developed, and the nomogram was assessed by Calibration curves.
Differential expression genes (DEGs) between low- and high- risk group and functional enrichment analysis
We applied “Limma” R package to explore different expression level of genes (P valus < 0.01 and log2|FC| >1.5) in low- risk and high- risk groups which were separated by median risk score. We performed the Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis to pinpoint associated metabolic pathways and functional categorizations among differentially expressed genes (DEGs). The analysis utilized the “org.Hs.eg.db” and “clusterProfiler” packages, with visualization achieved through the"ggplot2”, “GOplot,” and “raster” packages within the R software environment.
Immunoinfiltration analysis of cervical cancer
We applied “Limma” R package to explore different expression level of genes (P valus < 0.01 and log2|FC| >1.5) in low- risk and high- risk groups which were separated by median risk score. We performed the Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis to pinpoint associated metabolic pathways and functional categorizations among differentially expressed genes (DEGs). The analysis utilized the “org.Hs.eg.db” and “clusterProfiler” packages, with visualization achieved through the"ggplot2”, “GOplot,” and “raster” packages within the R software environment.
Analysis of genes and protein expression level
At the transcriptome level, we use GEPIA (http://gepia.cancer-pku.cn/) to identify the differential expression of model genes in tumor(N = 306) and normal tissues(N = 13). And at the single cell level, “FeaturePlot” package was applied to locate gene expression. The Human Protein Atlas 5(HPA, http://www.proteinatlas.org/) provides an open-access resource for human proteins. Morever, we utilized the HPA database to detect the protein expression level that related to the immune microenvironment by immunohistochemistry (IHC). IHC images are also available from the database.
Analysis of association between PGK1 and pathway
We downloaded STAR-counts data and corresponding clinical information for cervical cancer from the TCGA database. We then extracted data in TPM format and performed normalization using the log2(TPM + 1) transformation. We collected the genes included in the corresponding pathways and then analyzed them using the GSVA package in R software, choosing the parameter method=‘ssgsea’ for single-sample gene set enrichment analysis (ssGSEA). Finally, We studied the correlation between gene expression and ferroptosis pathway scores through Spearman correlation analysis. Statistical analysis was conducted using R software, version v4.0.3. Results were considered statistically significant when the p-value was less than 0.05.
Cell lines
The human cervical cancer cell lines CaSKi (CL-0048) and SiHa (CL-0210) were obtained from the Wuhan Pricella Biotechnology Co., Ltd. (Wuhan, China). All cells were cultured at 37℃ in a humidified atmosphere with 5% CO2. RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Biological Industries) and penicillin (100 U/ml) and streptomycin (100 ng/ml) (Meilunbio, Dalian, China) was used as the culture medium for CaSKi cell line. SiHa cells are cultured in MEM (with NEAA) supplemented with 10% FBS and 1% P/S.
siRNA synthesis and screening
The siRNAs were synthesized by GenePharma Company, comprising two PGK1 siRNA sequences: siRNA-1-S: 5’-GCU GUC CCA AGC AUC AAA UTT-3’, siRNA-1-AS: 5’-AUU UGA UGC UUG GGA CAG CTT-3’, siRNA-2-S: 5’-GAG CUA AAG UUG CAG ACA ATT-3’, and siRNA-2-AS: 5’-UUG UCU GCA ACU UUA GCU CTT-3’. Additionally, the negative control FAM sense sequence is 5’-UUC UCC GAA CGU GUC ACG UTT-3’, and the negative control FAM antisense sequence is 5’-ACG UGA CAC GUU CGG AGA ATT-3’. The GAPDH positive control sense sequence is 5’-UGA CCU CAA CUA CAU GGU UTT-3’, and the GAPDH positive control antisense sequence is 5’-AAC CAU GUA GUU GAG GUC ATT-3’. The primers for PGK1 are PGK1-F: 5’-TGG GAA CAA GGT TAA AGC CGA-3’ and PGK1-R: 5’-AAA ACC CAC CAG CCT TCT GT-3’. The housekeeping gene primers are GAPDH-F: 5’-AAG CTC ATT TCC TGG TAT GAC AA-3’ and GAPDH-R: 5’-CTT ACT CCT TGG AGG CCA TGT-3’. On the day before transfection, 2 × 105 cells were seeded into a 10 cm cell culture dish to achieve a cell density of 70% on the day of transfection. We took 10 µl Lipofectamine 2000 and diluted it with 500 µl serum-free medium, mixed well, and let it stand at room temperature for 5 minutes. We then took 20 µl siRNA and diluted it with 500 µl serum-free medium, mixed well, and after a 5-minute incubation of the diluted Lipofectamine 2000, it was gently mixed with the siRNA. The mixture was left to stand at room temperature for 20 min to form the siRNA-Lipofectamine 2000 complex, which was then added to the culture dish. After 48 h, cells were harvested and the mRNA levels of PGK1 were detected using the aforementioned primers.
Cell counting kit-8 (CCK-8) assay
A CCK-8 (Meilunbio, Dalian, China) was employed in accordance with the manufacturer’s guidelines to evaluate cellular viability. In summary, the cell lines were inoculated into a 96-well plate at a concentration of 1000 cells per well. This plate was incubated at 37℃ for a duration ranging from 24 to 72 h. Subsequently, the culture medium was substituted with a CCK-8 working solution, which comprised 10% CCK-8 reagent. The cells were then incubated at 37℃ for an additional 30 min. Ultimately, the optical density at 450 nm for each well was quantified utilizing a microplate reader.
Colony formation assay
Cell lines were inoculated into a 6-well plate at a density of 400 cells per well, followed by exposure to varying concentrations of nobiletin. The plates were incubated at 37℃ in an atmosphere containing 5% CO2. Cultivation was halted upon the emergence of discernible colonies within the culture dish. The culture medium was then removed, and the cells were gently rinsed 2–3 times with PBS. Following this, 1 mL of methanol was introduced to each well for fixation, with the fixing solution being discarded after a duration of 15 min. To visualize the colonies, 1 mL of Giemsa staining solution was applied to each well, with staining conducted for 10 min. After the staining process, the staining solution was carefully washed away with running water, and the plates were allowed to air-dry. Ultimately, the colonies containing over 50 cells were counted using a microscope, and images were captured for record-keeping.
C11-BODIPY assay
We prepared the C11-BODIPY staining solution as per the manufacturer’s protocol. After treatment, the culture medium was aspirated, and the cells were rinsed gently with pre-warmed PBS. The C11-BODIPY staining solution was then added to each well containing the cells. The cells were incubated with the staining solution for 30 min at room temperature in the dark. Following incubation, the cells were washed with warm PBS to eliminate any residual stain. The stained cells were visualized using a fluorescence microscope or a fluorescence plate reader with the appropriate excitation and emission filters for C11-BODIPY. The intensity of immunofluorescence was quantified using Image Pro Plus software.
Detection of lipid peroxidation by flow cytometry
To measure lipid peroxidation, we utilized the BODIPY™ 581/591 C11 fluorescent probe, which selectively binds to lipid peroxides, in combination with the BD FACSAria II flow cytometer. The BODIPY™ 581/591 C11 probe was purchased from Maokangbio, China, with the product code MX5211-1MG. Cells were grown under standard culture conditions until they reached 70–80% confluence. Lipid peroxidation was induced by treating cells with Nobiletin or by knocking down ZIP8 for 24 h. After treatment, cells were collected via trypsinization, washed twice with cold PBS, and resuspended in 500 µL of PBS. Next, 5 µL of the BODIPY™ 581/591 C11 stock solution (2 mM in DMSO) was added to the cell suspension and incubated for 30 min at 37℃ in the dark to facilitate probe binding. The stained cells were then analyzed using the BD FACSAria II flow cytometer, configured with excitation at 488 nm and emission detection through a 515–545 nm bandpass filter (FL1 and FL2 channels). Data analysis was performed using BD FACSDiva software, where gates were set to exclude debris and doublets, and the mean fluorescence intensity of C11-positive cells was calculated. To ensure accurate fluorescence detection, the BD FACSAria II was calibrated daily according to the manufacturer’s instructions.
Xenograft mice tumor model
Six-week-old female Balb/c-nu mice, with a body weight of around 20 g were sourced from Changsheng Biotechnology and housed under a regulated 12-hour light and 12-hour dark cycle, with unrestricted access to food and water. Each subject received an intracranial injection of 5 × 106 ES-2 cells. A total of eight mice were allocated to each experimental group. Once the mean tumor volume hit 100mm3, the mice were randomly assigned into three cohorts. The first cohort received a 0.9% saline solution, the second was administered PGK1 inhibitor Z57346765 at 5 mg/kg, the third received Z57346765 at 15 mg/kg, and the fourth received Z57346765 at 45 mg/kg. After a two-week period, the mice were humanely euthanized by direct cervical dislocation, a method chosen due to their small body weight (approximately 20 g) and the proficiency of the operator. Subsequently, their tumors were excised for further examination.
Statistical analysis
R4.3.2 (https://www.r-project.org/) and corresponding programs were used for statistical analysis. Except for special cases, p < 0.05 was considered statistically significant. GraphPad Prism 9.5 was utilized for the graphical representation of the experimental data, and the quantitative data were expressed as the mean ± standard deviation (SD). To compare significant differences, unpaired t-test and non-parametric comparisons method was used. For each experiment, *p < 0.05, **p < 0.01, and ***p < 0.001 were chosen to indicate significance.
Supplementary Information
Acknowledgements
None.
Authors’ contributions
Conceptualization: Beilei Zhang; Methodology: Beilei Zhang, Zhanghang Li, Zhaojie Yang, Yidan Yin; Formal analysis and investigation: Zhaojie Yang, Mingke Duan, Lei Wang, Qirui Zhan; Writing - original draft preparation: Zhanghang Li, Zhaojie Yang; Writing - review and editing: Beilei Zhang; Funding acquisition: Fu Wang; Supervision: Ruifang An. All the authors reviewed the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (No. 32271512), Natural Science Basic Research Program of Shaanxi (No. 2023-JC-ZD-43).
Data availability
All data and software used in this study are publicly available. They are included in the manuscript or supporting information. RNA sequence data and clinical data from patients with a variety of cancers are obtained from UCSC XENA (http://xena.ucsc.edu/). Liquid-liquid phase separation related genes are gained from PhaSepDB (http://db.phasep.pro/). GSE168652 data was from GEO database (https://www.ncbi.nlm.nih.gov/gds). No special code was used in this study.
Declarations
Ethics approval and consent to participate
Animal studies were performed and sanctioned by the Biomedical Ethics Committee of the Health Science Center at Xi’an Jiaotong University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang H, Ji X, Li P et al. Liquid-liquid phase separation in biology: mechanisms, physiological functions and human diseases. SCI China Life Sci. 2020;63(7):953–85. [DOI] [PubMed]
- 2.Tong X, Tang R, Xu J, et al. Liquid-liquid phase separation in tumor biology. Signal Transduct Target Ther. 2022;7:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z, Liu Z, Chen L, et al. Liquid-liquid phase separation in cell physiology and cancer biology: recent advances and therapeutic implications. Front Oncol. 2025;15:1540427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin Y, Brangwynne C. Liquid phase condensation in cell physiology and disease. Science. 2017;357(6357):eaaf4382. [DOI] [PubMed]
- 5.Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017;18(5):285–98. [DOI] [PMC free article] [PubMed]
- 6.Nozawa RS, Yamamoto T, Takahashi M et al. Nuclear microenvironment in cancer: Control through liquid-liquid phase separation. Cancer Sci. 2020;111(9):3155–63. [DOI] [PMC free article] [PubMed]
- 7.Gao Z, Zhang W, Chang R, Zhang S, Yang G, Zhao G. Liquid-Liquid phase separation: Unraveling the Enigma of Biomolecular Condensates in Microbial Cells. Front Microbiol. 2021;12:751880. [DOI] [PMC free article] [PubMed]
- 8.Mehta S, Zhang J. Liquid-liquid phase separation drives cellular function and dysfunction in cancer. Nat Rev Cancer. 2022;22:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradner JE, Hnisz D, Young RA. Transcriptional Addiction in Cancer. Cell. 2017;168(4):629–43. [DOI] [PMC free article] [PubMed]
- 10.Meng F, Yu Z, Zhang D et al. Induced phase separation of mutant NF2 imprisons the cGAS-STING machinery to abrogate antitumor immunity. Mol Cell. 2021;81(20):4147–64. [DOI] [PubMed]
- 11.Stamenkovic I, Yu Q. Merlin, a "magic" linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci. 2010;11(6):471–84. [DOI] [PMC free article] [PubMed]
- 12.Ardern-Holmes S, Fisher G, North K. Neurofibromatosis Type 2. J Child Neurol. 2017;32(1):9–22. [DOI] [PubMed]
- 13.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. [DOI] [PubMed] [Google Scholar]
- 14.Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. 2021 update. Int J Gynaecol Obstet. 2021;155 Suppl 1(Suppl 1):28–44. [DOI] [PMC free article] [PubMed]
- 15.Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169–82. [DOI] [PubMed]
- 16.Liontos M, Kyriazoglou A, Dimitriadis I, Dimopoulos MA, Bamias A. Systemic therapy in cervical cancer: 30 years in review. Crit Rev Oncol Hematol. 2019;137:9–17. [DOI] [PubMed]
- 17.Stumbar SE, Stevens M, Feld Z. Cervical Cancer and Its Precursors: A Preventative Approach to Screening, Diagnosis, and Management. Prim Care. 2019;46(1):117–34. [DOI] [PubMed]
- 18.WHO Guidelines for Screening and Treatment of Precancerous Lesions for Cervical Cancer Prevention. Geneva: World Health Organization; 2013. https://pubmed.ncbi.nlm.nih.gov/24716265/. [PubMed]
- 19.Rajaram S, Gupta B. Screening for cervical cancer: Choices & dilemmas. Indian J Med Res. 2021;154(2):210–20. [DOI] [PMC free article] [PubMed]
- 20.Sonkin D, Thomas A, Teicher BA. Cancer treatments: past, present, and future. Cancer Genet. 2024;286–287:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi RM, Telang B, Soni G, Khalife A. Overview of perspectives on cancer, newer therapies, and future directions. Oncol Translational Med. 2024;10:105–9. [Google Scholar]
- 22.Ciccarese FA-O, Zulato EA-O, Indraccolo SA-O. LKB1/AMPK pathway and drug response in Cancer: A Therapeutic Perspective. Oxid Med Cell Longev. 2019;2019:8730816. [DOI] [PMC free article] [PubMed]
- 23.Bahrami A, Hasanzadeh M, ShahidSales S et al. Clinical significance and prognosis value of Wnt signaling pathway in cervical Cancer. J Cell Biochem. 2017;118(10):3028–33. [DOI] [PubMed]
- 24.Ou ZA-O, Lin S, Qiu J et al. Single-Nucleus RNA sequencing and Spatial transcriptomics reveal the Immunological Microenvironment of Cervical Squamous Cell Carcinoma. Adv Sci. 2022;9(29):e2203040. [DOI] [PMC free article] [PubMed]
- 25.Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–65. [PubMed] [Google Scholar]
- 26.Welch DR. Tumor Heterogeneity–A ‘contemporary concept’ founded on historical insights and predictions. Cancer Res. 2016;76:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mroz EA, Tward AD, Pickering CR, Myers JN, Ferris RL, Rocco JW. High intratumor genetic heterogeneity is related to worse outcome in patients with head and neck squamous cell carcinoma. Cancer. 2013;119:3034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–34. [DOI] [PubMed] [Google Scholar]
- 29.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint Blockade. Science. 2016;351:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N, Werb Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat Cell Biol. 2018;20:1349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone MB, Shelby SA, Nunez MF, Wisser K, Veatch SL. Protein sorting by lipid phase-like domains supports emergent signaling function in B lymphocyte plasma membranes. Elife. 2017;6:e19891. [DOI] [PMC free article] [PubMed]
- 32.He Y, Luo Y, Zhang D, et al. PGK1-mediated cancer progression and drug resistance. Am J Cancer Res. 2019;9:2280–302. [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Weng J, Huang CL, Jackson AP. Is the voltage-gated sodium channel β3 subunit (SCN3B) a biomarker for glioma? Funct Integr Genomics. 2024;24:162. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Weng J. A comprehensive bioinformatic analysis of cyclin-dependent kinase 2 (CDK2) in glioma. Gene. 2022;822:146325. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Liu H. Clinical powers of aminoacyl tRNA synthetase complex interacting multifunctional protein 1 (AIMP1) for head-neck squamous cell carcinoma. Cancer Biomark. 2022;34:359–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H, Li Y. Potential roles of Cornichon family AMPA receptor auxiliary protein 4 (CNIH4) in head and neck squamous cell carcinoma. Cancer Biomark. 2022;35:439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chhatwal KS, Liu H. RAD50 is a potential biomarker for breast cancer diagnosis and prognosis. bioRxiv. 2024. 10.1101/2024.09.07.611821.
- 38.Trujillo-Cirilo L, Weiss-Steider B, Vargas-Angeles CA, Corona-Ortega MT, Rangel-Corona R. Immune microenvironment of cervical cancer and the role of IL-2 in tumor promotion. Cytokine. 2023;170:156334. [DOI] [PubMed] [Google Scholar]
- 39.Zhang B, Liu J, Mo Y, Zhang K, Huang B, Shang D. CD8(+) T cell exhaustion and its regulatory mechanisms in the tumor microenvironment: key to the success of immunotherapy. Front Immunol. 2024;15:1476904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galligan JJ, Petersen DR. The human protein disulfide isomerase gene family. Hum Genomics. 2012;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samanta S, Tamura S, Dubeau L, et al. Expression of protein disulfide isomerase family members correlates with tumor progression and patient survival in ovarian cancer. Oncotarget. 2017;8:103543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramos FS, Serino LT, Carvalho CM, et al. PDIA3 and PDIA6 gene expression as an aggressiveness marker in primary ductal breast cancer. Genet Mol Res. 2015;14:6960–7. [DOI] [PubMed] [Google Scholar]
- 43.Han TS, Kim DS, Son MY, Cho HS. SMYD family in cancer: epigenetic regulation and molecular mechanisms of cancer proliferation, metastasis, and drug resistance. Exp Mol Med. 2024;56:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozlov G, Maattanen P, Thomas DY, Gehring K. A structural overview of the PDI family of proteins. FEBS J. 2010;277:3924–36. [DOI] [PubMed] [Google Scholar]
- 45.Bai Y, Liu X, Qi X, et al. PDIA6 modulates apoptosis and autophagy of non-small cell lung cancer cells via the MAP4K1/JNK signaling pathway. EBioMedicine. 2019;42:311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarrey JR, Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987;326:501–5. [DOI] [PubMed] [Google Scholar]
- 47.Danshina PV, Geyer CB, Dai Q, et al. Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice. Biol Reprod. 2010;82:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowler MW. Conformational dynamics in phosphoglycerate kinase, an open and shut case? FEBS Lett. 2013;587:1878–83. [DOI] [PubMed] [Google Scholar]
- 49.Daly EB, Wind T, Jiang XM, Sun L, Hogg PJ. Secretion of phosphoglycerate kinase from tumour cells is controlled by oxygen-sensing hydroxylases. Biochim Biophys Acta. 2004;1691:17–22. [DOI] [PubMed] [Google Scholar]
- 50.Liu S, Song L, Yao H, Zhang L. HPV16 E6/E7 stabilize PGK1 protein by reducing its poly-ubiquitination in cervical cancer. Cell Biol Int. 2022;46:370–80. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, Peng J, Li B, et al. HOXA1 promotes aerobic Glycolysis and cancer progression in cervical cancer. Cell Signal. 2023;109:110747. [DOI] [PubMed] [Google Scholar]
- 52.Hou R, Denisenko E, Ong HT, Ramilowski JA, Forrest ARR. Predicting cell-to-cell communication networks using NATMI. Nat Commun. 2020;11:5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu F, He H, Yang W, et al. Novel energy optimizer, meldonium, rapidly restores acute hypobaric hypoxia-induced brain injury by targeting phosphoglycerate kinase 1. Cell Commun Signal. 2024;22:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Tian J, Wang L, Yan H, Feng S, Zhang Y. PGK1 is involved in the HIF-1 signaling pathway as a hub gene for ferroptosis after traumatic brain injury. Mol Neurobiol. 2025;62:233–45. [DOI] [PubMed] [Google Scholar]
- 55.Hahn KR, Kwon HJ, Yoon YS, Kim DW, Hwang IK. Phosphoglycerate kinase 1 protects against ischemic damage in the gerbil hippocampus. Aging. 2022;14:8886–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu H, Wang X, Shen P, Ni Y, Han X. The basic functions of phosphoglycerate kinase 1 and its roles in cancer and other diseases. Eur J Pharmacol. 2022;920:174835. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Wang S, Zhang X, et al. Expression characteristics and significant prognostic values of PGK1 in breast Cancer. Front Mol Biosci. 2021;8:695420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel JJ, Levy DA, Nguyen SA, Knochelmann HM, Day TA. Impact of PD-L1 expression and human papillomavirus status in anti-PD1/PDL1 immunotherapy for head and neck squamous cell carcinoma-Systematic review and meta-analysis. Head Neck. 2020;42:774–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ince N, de la Monte SM, Wands JR. Overexpression of human aspartyl (asparaginyl) beta-hydroxylase is associated with malignant transformation. Cancer Res. 2000;60:1261–6. [PubMed] [Google Scholar]
- 60.Zhang Z, Xiang K, Tan L, et al. Identification of critical genes associated with radiotherapy resistance in cervical cancer by bioinformatics. Front Oncol. 2022;12:967386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R. ATPase-Modulated stress granules contain a diverse proteome and substructure. Cell. 2016;164:487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Namkoong S, Ho A, Woo YM, Kwak H, Lee JH. Systematic characterization of Stress-Induced RNA granulation. Mol Cell. 2018;70:175–e187178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kedersha N, Ivanov P, Anderson P. Stress granules and cell signaling: more than just a passing phase? Trends Biochem Sci. 2013;38:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of Endoplasmic reticulum stress in Cancer. Cell. 2017;168:692–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hou C, Wang X, Xie H, et al. PhaSepDB in 2022: annotating phase separation-related proteins with droplet states, co-phase separation partners and other experimental information. Nucleic Acids Res. 2023;51:D460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and software used in this study are publicly available. They are included in the manuscript or supporting information. RNA sequence data and clinical data from patients with a variety of cancers are obtained from UCSC XENA (http://xena.ucsc.edu/). Liquid-liquid phase separation related genes are gained from PhaSepDB (http://db.phasep.pro/). GSE168652 data was from GEO database (https://www.ncbi.nlm.nih.gov/gds). No special code was used in this study.