Abstract
The VirB/D4 type IV secretion system of Agrobacterium tumefaciens translocates virulence factors (VirE2, VirF, and the VirD2-T-DNA complex) to plant cells. The membrane-bound translocation machinery consists of 12 proteins (VirB1–11 and VirD4) required for substrate translocation. Protein–protein interactions in the membranes were analyzed after extraction with the mild detergent dodecyl-β-d-maltoside followed by separation under native conditions. Incubation of the membranes with increasing concentrations of the detergent differentially extracted virulence proteins. Separation of the solubilized proteins by blue native electrophoresis revealed cofractionation between two classes of protein complexes containing VirB7. The first class, consisting of major T-pilus component VirB2 and associated proteins VirB5 and VirB7, comigrated in the low molecular mass portion of the gel of about 100 kDa. The second class contains putative translocation complex core components VirB8, VirB9, and VirB10 in the high molecular mass portion of the gel larger than 232 kDa, as well as VirB7. Solubilized proteins were characterized further by gel filtration chromatography. This procedure separated T-pilus-associated proteins VirB2, VirB5, and VirB7 in the low molecular mass range from the other components of the translocation machinery and the substrates VirE2 and VirD2. Fractionation of VirB7-containing complexes (VirB7-VirB7 homodimers and VirB7-VirB9 heterodimers) suggested that they may link the T-pilus components to the core of the translocation machinery. Based on previously described VirB protein interactions and biochemical analysis of C58 wild type as well as of virB5 and virB6 deletion mutants, a model of T-pilus assembly in A. tumefaciens is suggested.
The discovery of sequence similarities between components of the DNA transfer machineries of Agrobacterium tumefaciens, the conjugative plasmids RP4 and R388, and secretion machineries from various pathogens suggested that they may share a common mechanism (1). Based on functional similarities and similar operon structure of the encoding genes, this group of macromolecule transporters was classified as type IV secretion systems (T4SS; refs. 2 and 3). Whereas conjugative transfer systems likely translocate DNA in a complex with relaxosome components or other DNA processing enzymes, the T4SS of pathogens may translocate proteinaceous virulence factors to modulate their host's defense response. This strategy is used by many important human and animal pathogens (Bartonella tribocorum, Bordetella pertussis, Brucella suis, Legionella pneumophila, Helicobacter pylori, Rickettsia prowazekii). With the exception of H. pylori and B. pertussis, the translocated substrates are unknown (4, 5).
Different model systems are under investigation to understand the mechanism of type IV secretion; the VirB/D4 system from A. tumefaciens is the most developed (6). A. tumefaciens translocates proteinaceous virulence factors (VirE2 and VirF) and the VirD2-DNA complex to plants (7–10). Twelve virulence proteins (VirB1–11 and VirD4) are required for efficient transfer and likely assemble into a membrane-spanning structure. VirB7, VirB8, VirB9, and VirB10 interact with each other, likely assemble in the periplasm, and may constitute the core of the complex (11–17). The core proteins stabilize other T4SS components, but so far, the nature of the channel for substrate translocation has not been revealed (18). Energy for substrate translocation and complex assembly may be provided by NTPases VirB4, VirB11, or VirD4 (17, 19–21). Crystal structure analyses of VirB11-like protein HP0525 from H. pylori and VirD4-like protein TrwB from plasmid R388 revealed central holes in the multimeric structures, suggesting a role as transporters (22, 23). VirD4-like proteins likely act as coupling proteins, which link the substrates to the translocation machinery (24).
In addition to substrate translocation, T4SS from A. tumefaciens and conjugative plasmids determine the production of surface-exposed pili (25, 26). These pili may initiate cell-to-cell contact before substrate translocation. VirB2 and TrbC were identified as major components of the A. tumefaciens T-pilus and of the RP4-determined conjugative pilus, respectively. The pilin precursors undergo proteolytic processing and cyclization before pilus assembly (25, 27). VirB5 and similar proteins from the IncN plasmid pKM101 (TraC) and the IncP plasmid pJP4 (TrbF) cofractionate with extracellular pili and may constitute minor components or factors required for pilus biogenesis (28–30). Homodimers of the outer membrane-localized lipoprotein VirB7 cofractionate with T-pili and may also contribute to extracellular polymerization of VirB2 (31, 32). T4SS-determined surface structures likely play a key role for the host recognition, which may trigger further transfer events.
In A. tumefaciens, VirB1 and VirB3–11 are required for extracellular assembly of the major T-pilus component VirB2. The interactions between these proteins were analyzed by extraction of the membrane-bound complex with the mild detergent dodecyl-β-d-maltoside (DDM); further analysis was carried out by blue native electrophoresis and gel filtration chromatography. This method was developed for the analysis of complexes from the mitochondrial respiratory chain and the mitochondrial protein uptake machinery (33–35). Adaptation of this technique to the A. tumefaciens VirB/D4 system allowed the dissection of two subassemblies of the T4SS complex. Whereas pilus-associated proteins VirB2, VirB5, and VirB7 cofractionated in a low molecular mass complex, most of the other virulence proteins, including the substrates VirD2 and VirE2, fractionated in a high molecular mass complex. The composition of these subassemblies suggests that the separation of functional units of the T4SS machinery may have been achieved.
Materials and Methods
Bacteria, Plasmid Construction, and Growth Conditions.
Experiments were performed with A. tumefaciens strain C58 (wild type), its derivatives CB1005 (ΔvirB5; ref. 28) or CB1006 (ΔvirB6; ref. 32) carrying plasmid pPZP300. The plasmid-encoded extra copies of virG led to moderately (2- to 3-fold) increased production of VirB proteins. For construction, vector pPZP200 (36) was cleaved with ScaI and treated with alkaline phosphatase followed by ligation with a 1.2-kb virG-containing PCR fragment amplified from chromosomal DNA. PCR amplification (oligonucleotides VirG5: 5′-GGGGGATATCAATGCCGCATGGCGCG-3′ and VirG3: 5′-GAAAGTACTCAACGAACAGCTGATCTCTC-3′), cleavage of the fragment with EcoRV and ScaI (sequence underlined above), cloning, and sequencing followed standard protocols (37). Overnight cultures of A. tumefaciens were grown in YEB medium (0.5% beef extract, 0.1% yeast extract, 0.5% peptone, 0.5% sucrose, 2 mM MgSO4) with spectinomycin (300 μg/ml) and streptomycin (100 μg/ml) for plasmid propagation. Cells were inoculated to an OD600 of 0.1 in liquid AB medium (1% glucose, 0.39% morpholinoethansulfonic acid, 1 mM Na-K phosphate, 0.1% NH4Cl, 0.03% MgSO4 × 7 H2O, 0.001% CaCl2, 0.00025% FeSO4 × 7 H2O, pH 5.5), grown for 5 h at 20°C, followed by plating of 1 ml per large (15-cm square) Petri dish with or without 200 μM acetosyringone (AS) for virulence gene induction, and further incubated at 20°C for 4 days (29).
Isolation of Membranes and Detergent Extraction.
Cells were washed from the plates with 10 ml of 50 mM Na-K-phosphate buffer (pH 5.5). Equal amounts of bacteria were obtained from 16 Petri dishes grown under virulence-inducing conditions (+AS) and eight Petri dishes grown under noninducing conditions (−AS) per experiment. The following steps were performed on ice or at 4°C in a cold room. Cells were suspended in 20 ml of 50 mM Na-K-phosphate buffer (pH 5.5), PMSF was added at 0.5 mM, followed by three passages through a French press at 18,000 psi. Cell debris was removed by centrifugation at 12,000 rpm in an SS-34 rotor (Sorvall) for 1 h, and the soluble and membrane proteins in the supernatant were separated by ultracentrifugation for 2 h at 40,000 rpm in a Beckman 50.2 Ti (Beckman Coulter) rotor. Membrane pellets were stored on ice for 12 h, 1 ml of ACA buffer [750 mM 6-amino-caproic acid (ACA), 50 mM Bis⋅Tris, pH 7] was added, and the membranes were suspended by sonication (4× 10 pulses, duty 40%, Branson B15 sonicator). For detergent solubilization, the protein concentration was adjusted to 10 mg/ml, and different amounts of DDM were added from a 10% stock solution in ACA buffer to give final concentrations of 0%, 0.5%, 1.5%, and 2% DDM, respectively, in a total volume of 1 ml. The samples were mildly shaken for 2 h at 4°C, followed by ultracentrifugation for 2 h at 40,000 rpm in a Beckman 70.1 Ti rotor (Beckman Coulter) to separate DDM-soluble and -insoluble proteins. Because of interference by the detergent, the protein concentrations in the soluble and insoluble fractions could not be determined at this point.
Blue Native Electrophoresis.
Proteins (25 μl) solubilized by 2% DDM were mixed with 2.5 μl of 5% (wt/vol) Coomassie blue G-250 (in 500 mM ACA) to confer negative electric charge for electrophoretic separation. Electrophoresis was performed for 48 h and 5 mA at 4°C in 18-cm long acrylamide gradient gels (7–20% or 7–14%), according to Schägger and von Jagow (33). Calibration was achieved by separation of reference proteins of known molecular masses: thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), lactate dehydrogenase (140 kDa), and BSA (67 kDa; Amersham Pharmacia High Molecular Weight Calibration kit for Electrophoresis).
Gel Filtration Chromatography.
Proteins (500 μl) solubilized by 2% DDM were applied to a Superdex 200 column (XK 16/70, Amersham Pharmacia), and chromatography in ACA buffer was performed at 4°C with a flow rate of 0.5 ml/min in an Äkta Purifier system (Amersham Pharmacia). Proteins in fractions of 2 ml were precipitated in 5% (vol/vol) trichloroacetic acid (TCA) and collected by centrifugation, and the sediment was suspended in Laemmli sample buffer. Reference proteins used for calibration were thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), and BSA (67 kDa; Amersham Pharmacia Gel Filtration Calibration Kit).
SDS/PAGE and Western Blotting.
Cell lysates and samples of chromatographic separation were incubated in Laemmli sample buffer for 30 min at 37°C to avoid aggregate formation of membrane proteins. Separation was conducted by SDS/PAGE (38, 39), followed by Western blotting in a tank blot apparatus. Proteins separated by blue native electrophoresis were transferred to poly(vinylidene difluoride) (PVDF) membranes by semidry blotting, and the membrane was incubated in destain solution (25% methanol, 10% acetic acid) for at least 3 h to remove the Coomassie dye. Alternatively, for second-dimension electrophoresis, lanes were excised from the blue native gel, incubated in Laemmli sample buffer without glycerol and bromophenol blue for 30 min at 50°C, and applied to the top of 12% tricine/SDS gels, followed by electrophoretic separation and silver staining or Western blotting. Detection was performed with a chemiluminescence-based system (NEN) with virulence protein-specific antisera (40).
Results
Differential Extraction of Virulence Proteins from the Membranes.
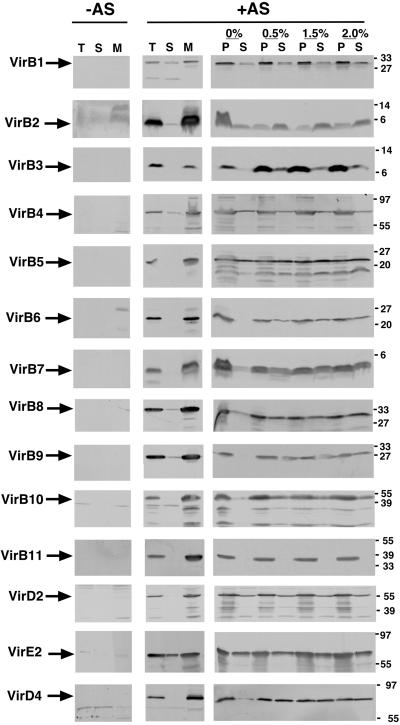
The goal of this study was the analysis of protein–protein interactions within the T4SS complex in the membranes of A. tumefaciens. To this end, subcellular fractions from virulence gene-induced and uninduced strain C58 were isolated; similarly to previous results, VirB proteins, VirD2 and VirD4, were detected predominantly in the membrane fraction (refs. 16 and 41–43; Fig. 1). In contrast, the substrate VirE2 was equally distributed between the membrane and the soluble fraction. Membranes were incubated with different concentrations of DDM (0%, 0.5%, 1.5%, and 2%) followed by separation of soluble and insoluble proteins by ultracentrifugation. Analysis of the susceptibility of virulence proteins to extraction revealed pronounced differences. VirB2 was solubilized even in the absence of DDM, and inclusion of the detergent further increased the extracted amount (Fig. 1). Similarly, VirB1, VirB4, VirB5, VirD2, and VirE2 were partially solubilized even without DDM, and with the exception of VirB4 and VirD2, addition of the detergent increased the extracted amounts. Most of the other virulence proteins were not solubilized in the absence of DDM, but inclusion of the detergent increased the extracted amount to at least 50% (VirB6, VirB7, VirB8, VirB9, VirB10, VirD4). Solubilization of VirB3 required the addition of DDM, but even in the presence of 2% DDM, only a small portion of the protein was solubilized. VirB11 was not noticeably solubilized under any of the conditions tested, which may be because of its negatively charged cytoplasmic domain (22). Differential extraction suggests that subassemblies of the T4SS machinery exist in the membranes, and this may allow their separation. To test this hypothesis, membranes were incubated with 2% DDM to give maximal extraction efficiency, and solubilized proteins were further separated under native conditions.
Fig 1.
DDM differentially extracts virulence proteins from the membranes. Virulence gene-induced (+AS) and uninduced C58 cells (−AS) were lysed, followed by fractionation of the total cell lysate (T) into soluble (S) and membrane fractions (M). Membranes were incubated with different concentrations of DDM; soluble proteins (S) were separated by ultracentrifugation from insoluble proteins (P). The different fractions were analyzed by SDS/PAGE and Western blotting with specific antisera. Molecular masses of reference proteins are shown on the right.
Separation of DDM-Soluble Proteins by Blue Native Electrophoresis.
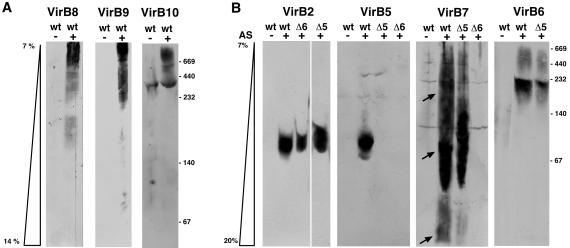
DDM-solubilized proteins were mixed with Coomassie blue G-250 to confer negative charge and separated by electrophoresis by using 7–14% or 7–20% acrylamide native gradient gels. This method allows separation according to molecular mass, because migration stops after the protein-lipid-dye complexes reach a certain density of the acrylamide mesh, which precludes further migration. Analysis with VirB8-, VirB9-, and VirB10-specific antisera identified the proteins isolated from the wild-type C58 in complexes of apparent molecular masses larger than 232 kDa (Fig. 2A). This finding is in accord with previous results, suggesting the association of VirB8, VirB9, and VirB10 in a high molecular mass complex in the periplasm (12). No difference was observed when similar analyses were performed with extracts from strains CB1005 (ΔvirB5) or CB1006 (ΔvirB6; not shown). In contrast, VirB2 and VirB5 were exclusively detected in the low molecular mass range between 67 and 140 kDa in the wild type (Fig. 2B). Whereas the chromatographic behavior of VirB2 was similar in all strains, VirB5 was not detected in DDM extracts from CB1005 and CB1006. Analysis with VirB7-specific antiserum showed a broad distribution and three major fractions were detected in the wild type and in strain CB1005 (Fig. 2B). In contrast, VirB7 was rarely detected in different experiments performed with extracts from strain CB1006, and the amounts were always strongly reduced. The lowest molecular mass fraction less than 67 kDa, which was the least prominent one in the wild type and strain CB1005, did not colocalize with any of the other virulence proteins. In contrast, the highest amount of VirB7 was detected in the 67- to 140-kDa range, similar to VirB2 and VirB5. A significant amount of VirB7 also was detected in the molecular mass range larger than 140 kDa. VirB6 was detected in a region between 140 and 232 kDa, between the high and low molecular mass complexes in the wild type and strain CB1005. Thus, VirB6 partly cofractionated with VirB7 but not with VirB2 and VirB5 (Fig. 2B). Other virulence proteins were not detected, which may be explained by the small amounts extracted with DDM. Because analysis by one-dimensional native PAGE did not allow the identification of all proteins associated with the different complexes, further resolution was attempted by second-dimension SDS/PAGE.
Fig 2.
Blue native gel electrophoresis separates virulence protein complexes of different molecular masses. Membranes from virulence gene-induced (+AS) and uninduced cells (−AS; C58, wt; CB1005, Δ5; CB1006, Δ6) were incubated with 2% (wt/vol) DDM, and soluble proteins were mixed with Coomassie blue G-250. Separation was achieved by electrophoresis in native acrylamide gradient gels, followed by Western blotting with specific antisera. VirB7-specific signals in different molecular mass ranges are indicated by arrows. Molecular masses of reference proteins are shown on the right.
Analysis by Two-Dimensional Electrophoresis.
After first-dimension blue native PAGE, lanes containing proteins from virulence-induced and uninduced C58 cells were excised from the gel, applied to tricine/SDS gels, followed by electrophoretic separation. Analysis of silver-stained gels identified a major protein species of 5.5 kDa from virulence-induced cells in a region corresponding to the 100-kDa molecular mass range of blue native PAGE (Fig. 3A). Western blot analysis identified the 5.5-kDa protein as VirB2, and VirB5 was shown to cofractionate in the 100-kDa range (Fig. 3B). Analysis with VirB9- and VirB7-specific antisera showed that VirB9-VirB7 heterodimers of 36 kDa and VirB7 homodimers of 9 kDa were present in the fractions larger than 232 kDa of the blue native gel. The lower molecular mass VirB9-crossreactive proteins probably correspond to degradation products, and similar observations have been reported (14, 44). In contrast, VirB7-VirB7 homodimers as well as VirB7 monomers were found to comigrate with VirB2 and VirB5 in the 100-kDa range. The low molecular mass complex of VirB7 detected in the first dimension obviously consisted of VirB7 homodimers and monomers. The results of the analysis by first-dimension blue native and second-dimension SDS/PAGE suggest that different subassemblies were isolated from the membranes.
Fig 3.
Second-dimension SDS/PAGE resolves virulence proteins from complexes of the first-dimension native gel. Lanes containing DDM-extracted proteins from virulence gene-induced (+AS) and uninduced strain C58 (−AS) resolved by first-dimension blue native PAGE (1 D) were excised from the gel and incubated in Laemmli sample buffer. Lanes were applied on top of a 12% tricine gel, and proteins were separated by electrophoresis (2 D), followed by silver staining (A). Alternatively, proteins were transferred to poly(vinylidene difluoride) membranes followed by detection with VirB2- and VirB5-specific (B), or VirB7- and VirB9-specific antisera (C). Arrowhead indicates VirB2 in silver-stained gel. Molecular masses of reference proteins are shown on the right.
Separation of DDM-Soluble Proteins by Gel Filtration Chromatography.
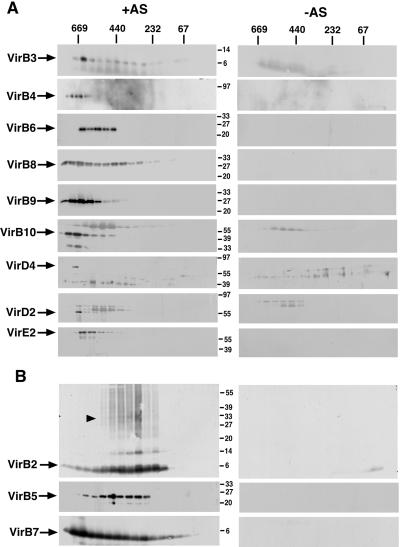
Gel filtration chromatography was chosen as an alternative approach to separate different T4SS subassemblies. DDM-solubilized proteins were applied to a gel filtration column, and the fractions of the eluate were analyzed with virulence protein-specific antisera. Most VirB proteins (VirB3, VirB4, VirB6, VirB8, VirB9, and VirB10) eluted predominantly in fractions corresponding to high molecular mass complexes larger than 500 kDa (Fig. 4A). The substrates VirD2 and VirE2 and coupling protein VirD4 eluted in similar fractions. Significant amounts of VirB8 also were detected in fractions corresponding to proteins smaller than 440 kDa. In contrast, VirB2 and VirB5 predominantly eluted in fractions corresponding to proteins of apparent molecular masses between 232 and 440 kDa (Fig. 4B). After SDS/PAGE, we consistently detected VirB2-crossreactive protein species of higher molecular mass. This finding may indicate that incubation in Laemmli sample buffer at 37°C and SDS/PAGE did not totally dissociate the multimeric forms of the protein. Similar to the results of blue native electrophoresis, VirB7 eluted in a broad range from the column, suggesting that it may be part of different subassemblies (Fig. 4B). The different complexes of VirB7 and VirB9 were analyzed further by Western blotting after SDS/PAGE under nonreducing conditions. This result showed that VirB9 monomers and VirB7-VirB9 heterodimers were present exclusively in the high molecular mass fraction (Fig. 5). In contrast, VirB7-VirB7 homodimers and VirB7 monomers were detected mostly in the low molecular mass fractions, but both also were detected in the region between the high and the low molecular mass fractions (Fig. 5).
Fig 4.
Gel filtration chromatography separates virulence protein complexes of different molecular masses. Membranes from virulence gene-induced (+AS) and uninduced strain C58 (−AS) were incubated with 2% DDM, and solubilized proteins were applied to a Superdex 200 column. Eluted proteins were collected by TCA precipitation, subjected to SDS/PAGE under reducing conditions, followed by Western blotting with specific antisera. (A) Proteins eluting in high molecular mass complexes. (B) Proteins eluting in low molecular mass complexes. Higher molecular mass forms of VirB2 are indicated by an arrowhead. Molecular masses of reference proteins are shown on the right.
Fig 5.
Analysis of complexes of VirB7 and VirB9 under nonreducing conditions. Membranes from virulence gene-induced (+AS) and uninduced strain C58 (−AS) were extracted with 2% (wt/vol) DDM, and solubilized proteins were applied to chromatography on a Superdex 200 column. Eluted proteins were collected by TCA precipitation, subjected to SDS/PAGE under nonreducing conditions, followed by Western blotting with specific antisera. Molecular masses of reference proteins are shown on the right.
Discussion
The results of a biochemical approach to characterize protein–protein interactions in the VirB/D4 T4SS machinery from A. tumefaciens are presented. Mild detergent treatment was used for membrane extraction, followed by separation of different subassemblies under native conditions. Virulence protein composition indicates that we may have separated functional units of the T-pilus assembly complex and of the core complex, respectively.
The purification strategy relies on mild detergent treatment of the membranes with DDM, which causes minimal disruption of protein–protein interactions (33, 34). Significant amounts of VirB2 and of a few other virulence proteins were extracted in the absence of DDM. This effect may rely on the detergent properties of ACA in the extraction buffer, which may solubilize proteins in loose association with the membranes. At least 50% of most of the virulence proteins were solubilized by the addition of DDM, but most of VirB3, VirB4, and VirB11 remained insoluble. This result is in accord with the fact that VirB4 and VirB11 do not contain strongly hydrophobic regions. Their membrane association likely relies on protein–protein interactions or electrostatic interactions with the lipid bilayer.
The NTPases VirB4 and VirB11 may provide energy for substrate translocation and/or assembly of the transmembrane complex. Separation by DDM suggests that their interaction with the T4SS complex can easily be disrupted, and it will be interesting to identify their binding partners. VirB3 is located in the outer membrane in a VirB4-dependent manner (45). Inefficient extraction with DDM suggests that VirB3 may associate with VirB4 more closely than anticipated, and it may play a role in VirB4-mediated transmission of energy for pilus assembly. In contrast, the third NTPase VirD4 was extracted with DDM to a similar extent as the other virulence proteins. Crystal structure analysis of VirD4-like TrwB from plasmid R388 (23) and biochemical analysis of VirD4 (46) revealed that, in contrast to VirB4 and VirB11, VirD4 contains an N-terminal membrane-spanning region. This fact may explain its extraction with DDM. An alternative explanation would be close association of VirD4 with other virulence proteins. Interestingly, the substrates VirE2 and VirD2 predominantly associated with the membranes and were solubilized with DDM similarly to the other virulence proteins. Potentially, their association with the membranes and with the T4SS may be mediated by coupling protein VirD4 (21, 23, 24).
DDM extraction likely led to the formation of soluble micelles containing proteins, phospholipids, and detergent. Proteins were separated further by blue native electrophoresis and gel filtration chromatography. The limited resolution of the blue native gels had the consequence that only those proteins strongly represented in the solubilized fraction were detected. In contrast, after separation by gel filtration chromatography, most of the virulence proteins were detected. Both methods gave comparable results for virulence proteins in complexes of different molecular masses. Coomassie dye binds to the proteins to confer negative charge for electrophoresis and thereby replaces DDM (33, 34). This fact may explain the differences of the apparent molecular masses obtained by both methods. The putative core components VirB8, VirB9, and VirB10 were detected in a high molecular mass complex together with most of the other virulence proteins, including VirD2 and VirE2. Thus, the high molecular mass species may correspond to the core complex with associated proteins (4, 5, 12). VirB2 and VirB5 cofractionated in a low molecular mass complex, and these proteins have previously been shown to colocalize in extracellular T-pili (29, 31). However, the present procedure is not suited for the isolation of T-pili, which are present in small amounts on the cells and would be subjected to fragmentation because of the strong shearing forces imposed in the French press and by sonication. The VirB2-VirB5 complex isolated here may, therefore, constitute a cell-bound pilus assembly element.
Analysis by blue native electrophoresis as well as by gel filtration chromatography showed that VirB7 is present in complexes of different molecular masses. The VirB7-VirB9 heterodimer was detected in the high molecular mass fraction, in accord with its role in stabilizing the T4SS machinery (13–15). VirB7-VirB7 homodimers and VirB7 monomers colocalized with VirB2 and VirB5 and also were present in higher and lower molecular mass species. This result agrees with the observation that VirB7 cofractionates with extracellular T-pili (31). That VirB7 associates with complexes containing core as well as pilus components leads to the hypothesis that VirB7 may actually link the T-pilus to the core of the T4SS machinery.
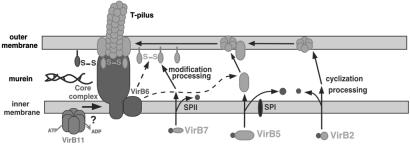
Interestingly, VirB6 was detected in an intermediate molecular mass range between the high molecular mass core and the pilus components. VirB6 stabilizes VirB5, and mediates the formation of VirB7-VirB7 homodimers (32). In the absence of VirB6, fractionation of VirB2 was not affected; however, VirB5 complexes were eliminated and those of VirB7 were strongly reduced. This result suggests that VirB6 influences the membrane association of VirB5 and VirB7. Future studies should address the question whether this association relies on direct interactions or whether other VirB proteins are required. Thus, VirB6 likely plays a key role in T-pilus assembly. Based on current knowledge, we propose a model for this process (Fig. 6). After removal of signal peptides from VirB2 and VirB5, and cyclization of VirB2, association of VirB2 with VirB5 may occur in the membranes forming a pilus assembly complex. VirB7 undergoes N-terminal lipoprotein processing and VirB6-assisted formation of VirB7 homodimers. VirB7 homodimers and monomers may link the pilus assembly complex to the membrane-spanning T4SS complex. VirB7 may even play a direct role in T-pilus assembly, e.g., by stabilizing VirB5 or by mediating the VirB2–VirB5 interaction. The fractionation and cellular levels of VirB6, VirB7, VirB8, VirB9, and VirB10 were not affected in the absence of VirB5. This observation suggests that the VirB6-mediated incorporation of VirB5 into the pilus assembly element may be a key signal for extracellular assembly of VirB2. VirB5 may directly interact with VirB2 or confer its effect by means of other proteins, e.g., VirB7.
Fig 6.
Hypothetical model for the mechanism of T-pilus assembly. After removal of signal peptides from VirB2 and VirB5 and cyclization of VirB2, the major T-pilus component associates with VirB5 in the membranes. VirB6 is essential for stabilization of VirB5, and this effect may rely on direct binding or necessitate other proteins. N-terminal lipoprotein processing of VirB7 is followed by VirB6-assisted formation of VirB7 homodimers, which may link the T-pilus to the core complex. Energy for T-pilus assembly may be provided by VirB11 (20), and it may be transduced to the pilus assembly complex in the outer membrane via VirB6 and VirB7. SPI and SPII, signal peptidases.
Future work will purify the different subassemblies of the T4SS machinery further, and develop assays for its functional units. A more detailed analysis of the A. tumefaciens VirB/D4 system will likely advance the understanding of T4SS in other organisms. As VirB5-like proteins cofractionate with extracellular high molecular mass structures determined by different T4SS, e.g., from the IncN plasmid pKM101 and the IncP plasmid pJP4 (28, 30), they also may function in the assembly of pilus-like structures. In contrast, the T4SS of B. pertussis does not encode a VirB5-like protein, and there is no evidence for a pilus-like extracellular structure (47). Thus, VirB5-like proteins may not be required for secretion of the pertussis toxin and possibly for secreted substrates of other T4SS.
Acknowledgments
We thank August Böck for continued support, and Peter J. Christie and Anath Das for discussions and the communication of results before publication. This work was supported by the Deutsche Forschungsgemeinschaft via Sonderforschungsbereich 369/C9.
Abbreviations
ACA, 6-amino-caproic acid
AS, acetosyringone
DDM, dodecyl-β-d-maltoside
T4SS, type IV secretion systems
References
- 1.Lessl M. & Lanka, E. (1994) Cell 77, 321-324. [DOI] [PubMed] [Google Scholar]
- 2.Salmond G. P. C. (1994) Annu. Rev. Phytopathol. 32, 181-200. [Google Scholar]
- 3.Covacci A., Telford, J. L., Del Giudice, G., Parsonnet, J. & Rappuoli, R. (1999) Science 284, 1328-1333. [DOI] [PubMed] [Google Scholar]
- 4.Christie P. J. (2001) Mol. Microbiol. 40, 294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron C., O'Callaghan, D. & Lanka, E. (2002) Mol. Microbiol. 43, 1359-1366. [DOI] [PubMed] [Google Scholar]
- 6.Zupan J., Muth, T. R., Draper, O. & Zambryski, P. C. (2000) Plant J. 23, 11-28. [DOI] [PubMed] [Google Scholar]
- 7.Vergunst A. C., Schrammeijer, B., den Dulk-Ras, A., de Vlaam, C. M., Regensburg-Tuink, T. J. & Hooykaas, P. J. (2000) Science 290, 979-982. [DOI] [PubMed] [Google Scholar]
- 8.Simone M., McCullen, C. A., Stahl, L. E. & Binns, A. N. (2001) Mol. Microbiol. 41, 1283-1293. [DOI] [PubMed] [Google Scholar]
- 9.Ziemienowicz A., Merkle, T., Schoumacher, F., Hohn, B. & Rossi, L. (2001) Plant Cell 13, 369-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z., Sagulenko, E., Ding, Z. & Christie, P. J. (2001) J. Bacteriol. 183, 3855-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaupre C. E., Bohne, J., Dale, E. M. & Binns, A. N. (1997) J. Bacteriol. 179, 78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das A. & Xie, Y.-H. (2000) J. Bacteriol. 182, 758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spudich G. M., Fernandez, D., Zhou, X.-R. & Christie, P. J. (1996) Proc. Natl. Acad. Sci. USA 93, 7512-7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron C., Thorstenson, Y. R. & Zambryski, P. C. (1997) J. Bacteriol. 179, 1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson L. B., Vogel Hertzel, A. & Das, A. (1996) Proc. Natl. Acad. Sci. USA 93, 8889-8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finberg K. E., Muth, T. R., Young, S. P., Maken, J. B., Heitritter, S. M., Binns, A. N. & Banta, L. M. (1995) J. Bacteriol. 177, 4881-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward D. V., Draper, O., Zupan, J. R. & Zambryski, P. C. (2002) Proc. Natl. Acad. Sci. USA 99, 11493-11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez D., Spudich, G. M., Zhou, X.-R. & Christie, P. J. (1996) J. Bacteriol. 178, 3168-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang T. A., Zhou, X.-R., Graf, B. & Christie, P. J. (1999) Mol. Microbiol. 32, 1239-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagulenko E., Sagulenko, V., Chen, J. & Christie, P. J. (2001) J. Bacteriol. 183, 5813-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R. B. & Das, A. (2002) Mol. Microbiol. 43, 1523-1532. [DOI] [PubMed] [Google Scholar]
- 22.Yeo H. J., Savvides, S. N., Herr, A. B., Lanka, E. & Waksman, G. (2000) Mol. Cell 6, 1461-1472. [DOI] [PubMed] [Google Scholar]
- 23.Gomis-Ruth F. X., Moncalian, G., Perez-Luque, R., Gonzalez, A., Cabezon, E., de la Cruz, F. & Coll, M. (2001) Nature (London) 409, 637-641. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton C. M., Lee, H., Li, P. L., Cook, D. M., Piper, K. R., von Bodman, S. B., Lanka, E., Ream, W. R. & Farrand, S. K. (2001) J. Bacteriol. 182, 1541-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai E.-M. & Kado, C. I. (1998) J. Bacteriol. 180, 2711-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fullner K. J., Lara, J. L. & Nester, E. W. (1996) Science 273, 1107-1109. [DOI] [PubMed] [Google Scholar]
- 27.Eisenbrandt R., Kalkum, M., Lai, E. M., Lurz, R., Kado, C. I. & Lanka, E. (1999) J. Biol. Chem. 274, 22548-22555. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Eisenlohr H., Domke, N. & Baron, C. (1999) J. Bacteriol. 181, 5563-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt-Eisenlohr H., Domke, N., Angerer, C., Wanner, G., Zambryski, P. C. & Baron, C. (1999) J. Bacteriol. 181, 7485-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt-Eisenlohr H., Rittig, M., Preithner, S. & Baron, C. (2001) Environ. Microbiol. 3, 720-730. [DOI] [PubMed] [Google Scholar]
- 31.Sagulenko V., Sagulenko, E., Jakubowski, S., Spudich, E. & Christie, P. J. (2001) J. Bacteriol. 183, 3642-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hapfelmeier S., Domke, N., Zambryski, P. C. & Baron, C. (2000) J. Bacteriol. 182, 4505-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schägger H. & von Jagow, G. (1991) Anal. Biochem. 199, 223-231. [DOI] [PubMed] [Google Scholar]
- 34.Schägger H., Cramer, W. A. & von Jagow, G. (1994) Anal. Biochem. 217, 220-230. [DOI] [PubMed] [Google Scholar]
- 35.Cruciat C. M., Brunner, S., Baumann, F., Neupert, W. & Stuart, R. A. (2000) J. Biol. Chem. 275, 18093-18098. [DOI] [PubMed] [Google Scholar]
- 36.Hajdukiewicz P., Svab, Z. & Maliga, P. (1994) Plant Mol. Biol. 25, 989-994. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J., Fritsch, E. F. & Maniatis, T., (1982) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 38.Schägger H. & von Jagow, G. (1987) Anal. Biochem. 166, 368-379. [DOI] [PubMed] [Google Scholar]
- 39.Laemmli U. K. (1970) Nature (London) 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 40.Baron C., Domke, N., Hapfelmeier, S. & Beinhofer, M. (2001) J. Bacteriol. 183, 6852-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorstenson Y. R., Kuldau, G. A. & Zambryski, P. C. (1993) J. Bacteriol. 175, 5233-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rashkova S., Spudich, G. M. & Christie, P. J. (1997) J. Bacteriol. 79, 583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirasu K. & Kado, C. I. (1993) FEMS Microbiol. Lett. 111, 287-294. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez D., Dang, T. A. T., Spudich, G. M., Zhou, X.-R., Berger, B. R. & Christie, P. J. (1996) J. Bacteriol. 178, 3156-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones A. L., Shirasu, K. & Kado, C. I. (1994) J. Bacteriol. 176, 5255-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das A. & Xie, Y.-H. (1998) Mol. Microbiol. 27, 405-414. [DOI] [PubMed] [Google Scholar]
- 47.Weiss A. A., Johnson, F. D. & Burns, D. L. (1993) Proc. Natl. Acad. Sci. USA 90, 2970-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]