Abstract
Conditioned taste aversion (CTA) is a learning paradigm in which an animal avoids a taste (conditioned stimulus) previously associated with visceral toxic effects [or unconditioned stimulus (US)]. Although many studies have implicated glutamate-mediated neurotransmission in memory consolidation of different types of learning tasks, including CTA, the exact role of this neurotransmitter system in memory formation is not known. Thus, we set out to determine whether glutamate mediates signaling of the US in CTA. We present evidence obtained by in vivo microdialysis that the US (i.p. injection of lithium chloride) induced a dramatic increase in glutamate release in the amygdala and a modest but significant release in the insular cortex. Moreover, CTA can be elicited by intra-amygdalar microinjections of glutamate; consequently, when glutamate is administered just before the presentation of a weak US, a clear CTA is induced. In contrast, the injection of glutamate alone or glutamate 2 h after the suboptimal US did not have any effect on the acquisition of CTA. These results indicate that glutamate activation of the amygdala can partially substitute the US in CTA, thus providing a clear indication that the amygdala conveys visceral information for this kind of memory.
A number of studies (1–5) have implicated glutamate-mediated transmission in consolidation of memory for different types of training, such as inhibitory avoidance, Morris water maze, and conditioned taste aversion (CTA). CTA is a learning paradigm in which the novel taste of food or drink (conditioned stimulus, CS) is paired with visceral signals of poisoning (unconditioned stimulus, US). Consequently, the animals avoid consuming the food or drink previously associated with toxic effects. CTA has unique properties; it is established after a single trial, permits long delays between stimuli presentation, and lasts for very long periods of time, even weeks. This feature makes it possible to separate the acquisition process into phases—CS presentation and US presentation—which can be studied independently under different experimental treatments (6).
CTA is established by the interaction of brainstem, limbic, and neocortical structures underlying different phases of the acquisition storage and retrieval of gustatory memory (7). Among the structures involved in the initial phases of taste memory formation are the gustatory neocortex and amygdala (8). Thus, damage to either the gustatory insular cortex (IC) or amygdala in adult rats leads to impaired acquisition of CTA (6, 7, 9–17). However, the functional roles of IC and amygdala seem to be different during the phases of taste memory formation. Functional blockade of IC before taste presentation, but not between taste presentation and lithium chloride (LiCl) injection, blocks CTA (16, 18), suggesting that the gustatory cortex is involved in taste processing and/or memory but is not necessary for processing the visceral signals of poisoning. Conversely, amygdala functional inactivation by tetrodotoxin before the gustatory stimulus presentation does not prevent CTA acquisition. However, tetrodotoxin inactivation of the amygdala after the gustatory stimulus, or before the visceral stimulus presentation, disrupts CTA memory formation (16). These results suggest that the amygdala does not play an important role in the initial processing of the taste signaling, but seems to be indispensable for processing the visceral stimulus (16, 19).
The finding that pharmacological manipulations, such as the injection of N-methyl-d-aspartate (NMDA) receptor antagonists into the IC or amygdala, disrupts CTA (2, 12, 20–23) suggests that glutamate release in these structures may be critically involved in taste aversion memory formation. However, these results provide only indirect evidence for the involvement of IC and amygdala glutamatergic activity in taste memory formation. Moreover, although previous reports indicate that the amygdala is critically involved in CTA, questions regarding the function of the amygdala and IC glutamate during the different stages of gustatory memory formation are still under debate.
The aim of the present work was to analyze, by using in vivo microdialysis, glutamate release in the IC and amygdala during the presentation of either gustatory (drinking saccharin) or the visceral stimulus (i.p. injections of LiCl). Additionally, to assess the role of glutamate during encoding of US in CTA acquisition, we evaluated the effects of bilateral injections of glutamate into the amygdala just before the injection of a low or high dose of LiCl.
Materials and Methods
Animals.
One hundred thirty-three male Wistar rats weighing 275–325 g at the time of surgery were used. They were housed under a 12-h light/12-h dark cycle, with food and water ad libitum, except during behavioral tests.
Guide Cannulae Implantation.
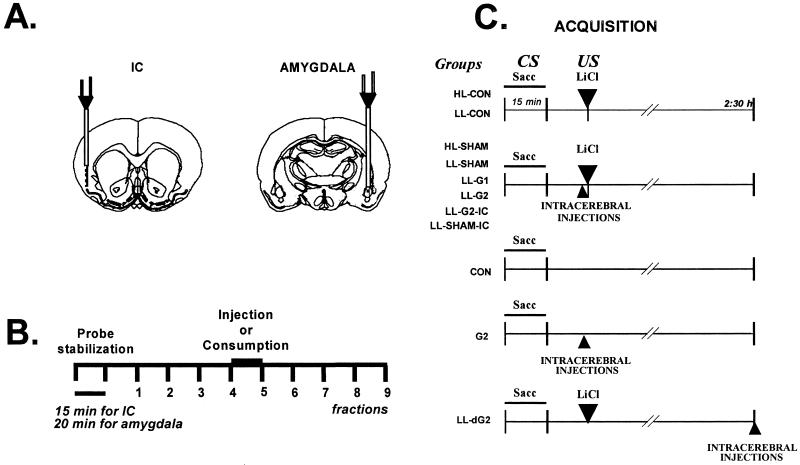
The animals for microdialysis experiment were anaesthetized with pentobarbital (50 mg/kg) and implanted with 2 microdialysis guide cannulae (BAS, West Lafayette, IN) by using standard stereotaxic procedures: one was implanted into the left basolateral amygdala (AP = −2.8 mm, L = −4.8 mm; V = −6.8 mm from Bregma), and the other was implanted into the right IC (AP = +1.2 mm; L = +5.5 mm; V = −3.5 mm from Bregma; Fig. 1A). The animals used for glutamate injections were implanted bilaterally to the amygdala (AP = −2.5 mm, L = ±4.8 mm, V = 5.5 mm from Bregma) and IC (see below, AP = +1.2 mm; L = ± 5.5 mm; V = −3.0 mm from Bregma) with 23-gauge stainless steel cannulae, by using standard stereotaxic procedures. The guide cannulae were kept in place with two skull screws and dental acrylic cement.
Fig 1.
(A) Schematic diagram of the location of the probes from the IC (Left) and amygdala (Right) during microdialysis. (B) General microdialysis procedure: the Ringer solution was set at a rate of 2 μl/min for IC and 1.5 μl/min for amygdala. (C) CTA acquisition procedure and groups used in amygdala bilateral injections. Intact animals: HL-CON, saccharin and 0.4 M LiCl; LL-CON, saccharin and 0.075 M LiCl. Sham groups with intra-amygdalar infusions of Ringer's solution: HL-SHAM, saccharin and 0.4 M LiCl; LL-SHAM, saccharin and 0.075 M LiCl, or different doses of glutamate; LL-G1, 1 μg; and LL-G2, 2 μg. LL-G2-IC and LL-SHAM-IC groups received 2 μg of glutamate or Ringer's in the IC with low doses of LiCl. G2, intra-amygdalar injection of glutamate (2 μg) 30 min after saccharin drinking without LiCl injection. CON, control group received only saccharin but never received the LiCl. LL-dG2, saccharin and low doses of LiCl and intra-amygdalar injections of glutamate 2 h and 30 min after saccharin drinking.
Microdialysis Procedure.
Two or 3 days after surgery, the animals were deprived of water for 24 h and then habituated to the microdialysis chamber once a day for 45-min trials. They were allowed to drink water from a graded bottle during 15-min periods for 5 days or until a stable water consumption baseline was reached. During the next day, the first microdialysis assay was performed concurrently in the IC and amygdala. The rats were randomly separated into the following groups: saccharin consumption (SAC; n = 10), water consumption (WAT; n = 7), i.p. injections of LiCl (LiCl 0.4; n = 8; 4 M, 7.5 ml/kg), and i.p. injections of sodium chloride (NaCl; n = 6; 0.15 M, 7.5 ml/kg). Groups SAC and WAT received 0.1% saccharin solutions or tap water, respectively, into the graded tube that was placed in the microdialysis chamber, whereas the animals of i.p. injection groups were injected with LiCl or NaCl, respectively. Dialysis was started by connecting the probe inlet (dialysis probes, BAS), with a total membrane length of 3 mm for the IC and 1 mm for the amygdala, to the microinfusion pump system (CMA/Microdialysis, West Lafayette, IN), which circulated the probe continuously at a rate of 2 μl/min for IC and 1.5 μl/min for the amygdala, with Ringer's solution (118 mM NaCl/4.7 mM KCl/2.5 mM CaCl2). Once the two probes were connected to the guide cannulae, the first 60-min sampling was discarded, and then samples were collected every 15 min for the IC or 20 min for the amygdala (30 μl/sample). The samples were immediately frozen at −80°C or analyzed by high-performance liquid chromatography (HPLC). The general microdialysis procedure is shown in Fig. 1B. Nine samples were obtained with the perfusion of Ringer's solution; in the fourth sample, stimulation was giving by drinking saccharin 0.1% or tap water (15 min), or i.p. injection of LiCl or NaCl was given. One extra group of animals with microdialysis guide cannulae aimed at the amygdala received the above-described water deprivation schedule; in the fourth sample, saccharin was presented, and 2 h 30 min later, they received an i.p. injection of LiCl (CS-US; n = 3), while the glutamate release was measured the whole time.
Measurement of Glutamate Release.
The microdialysis samples collected were assayed for glutamate content by using HPLC (Beckman Coulter) with electrochemical detection (BAS). First, the samples were automatically derivatized (Sample Sentinel, BAS) with o-phthaldehyde in the presence of tert-butythiol (amino acid analysis standard bore kit, BAS) and then injected into a loaded phase II octadecylsilyl 3-μm column (BAS); glutamate and aspartate were detected by using a solvent program designed to allow rapid determination of only these two amino acids. It is based on isocratic elution for the derivatives of interest followed by a step to a higher solvent so as to strip the more strongly retained materials. The time between injections was 6–7 min. The mobile phase consisted of 90% of 0.1 M acetate-buffer (pH 6.5) and 10% of HPLC/UV grade acetonitrile. Five different concentrations were used to generate a calibration curve, and, periodically, control standards were injected between samples to verify good detection. The detection limit was approximately 20 nM. All of the results of HPLC-glutamate analysis were converted into percentage of baseline (% BL = fraction × 100/mean of the three first samples). ANOVA with repeated measures were performed with the percentage BL glutamate release of fractions 4–9.
Bilateral Glutamate Microinjections.
Two or 3 days after surgery, the animals were deprived of water for 24 h and then habituated to drink water from a graded bottle during 15 min for 5 days, or until a stable water consumption baseline was reached. During the next day, animals were randomly separated into groups (see Fig. 1C), and acquisition of CTA was performed. For the CTA acquisition, water in the graded tube was substituted by 0.1% saccharin solution, and 30 min later the animals were injected i.p. with a high dose of LiCl (HL, 0.4 M), which induces a clear CTA, or a low dose of lithium (LL, 0.075 M), which does not produce a clear taste aversion. For the next 3 days, water baselines were recorded and, on the tenth day, retrieval (test) of CTA was carried out: the water was substituted for 0.1% of saccharin solution to test taste aversion. The saccharin consumption volume was taken as the taste aversion score. To compare groups, simple ANOVA were performed with the percentage of acquisition consumption (% of acquisition = consumption test × 100/consumption acquisition), followed by post hoc pairwise Fisher tests, where appropriate.
The microinjections were made through the intracerebral cannulae by using dental needles (30 gauge, which protrude from the tip of the guide cannulae 3 mm for amygdala and 2.5 mm for IC) attached to a microinfusion pump. Microinjections were performed in a 0.5-μl volume delivered over 60 s per hemisphere of the drug corresponding to each group. The injection cannulae were left in position for an additional 60 s to minimize dragging of the injection liquid along the injection tract and then withdrawn.
During acquisition day (see Fig. 1C) two noncannulated control groups received saccharin and high (HL-CON; n = 5) or low (LL-CON; n = 5) doses of LiCl. Four cannulated groups of animals received intra-amygdalar microinjections of vehicle (Ringer's solution; HL-SHAM; n = 7, LL-SHAM; n = 6) or different doses of glutamate (1 μg, LL-G1; n = 7, 2 μg, LL-G2; n = 12) immediately before i.p. administration of the low or high doses of LiCl. Doses of glutamate were chosen according to previous data (24–26). To test whether glutamate can enhance taste aversion by itself, another cannulated group (G2; n = 8) received only an intra-amygdalar injection of glutamate (2 μg) 30 min after saccharin drinking, without LiCl injection. For comparison, one control group received only saccharin but never received the LiCl injection (CON; n = 8). To test whether microinjections of glutamate might have effects 2 h after LiCl injections, at the time that microdialysis showed near-to-baseline glutamate release, another group of animals receiving saccharin and low doses of LiCl was given intra-amygdalar injections of glutamate (LL-dG2; n = 8) 2 h and 30 min after saccharin drinking. In addition, for comparison of IC effects of glutamate, two extra groups cannulated into the IC received either glutamate 2 μg (LL-G2-IC; n = 8) or Ringer's solution (LL-SHAM-IC; n = 8) immediately before the i.p. injection of low doses of LiCl.
Histology.
One day after microdialysis or after behavioral testing in microinjection experiments the animals were deeply anaesthetized with pentobarbital and perfused transcardially with a 4% (vol/vol) solution of paraformaldehyde in phosphate buffer (0.15 M, pH 7.4). The brains were placed overnight in paraformaldehyde and then transferred to a 20% buffered sucrose solution and stored at 4°C until they were cut. Coronal sections (50 μM thick) were taken through the areas of the probe. The slide sections were stained for cresyl violet.
Results
Verification of Probe Placement.
Fig. 1A shows a schematic diagram of the location of the probes from the IC and amygdala during microdialysis and injections of glutamate. In all groups, the location of the guide cannulae and probes was within the granular and dysgranular part of the IC and in the basolateral amygdala. The 1-mm probe exceeds the vertical limits of the basolateral amygdala, and the dialysis could extract solutes from central amygdala medial division and capsular part, as well as part of the intra-amygdaloid division of the bed nucleus of the stria terminalis. The selection criteria included all of the needle tips located into the basolateral amygdala nucleus, including the basolateral anterior and posterior amygdala nuclei; in some cases (15%), the cannula tips were located in the border of central and lateral amygdala nuclei. Six animals from amygdala microdialysis and seven animals from glutamate microinjections were discarded from further analysis because of a cannula misplaced away from the amygdala.
Glutamate Release Increases Significantly During Presentation of Visceral but Not Gustatory Stimuli.
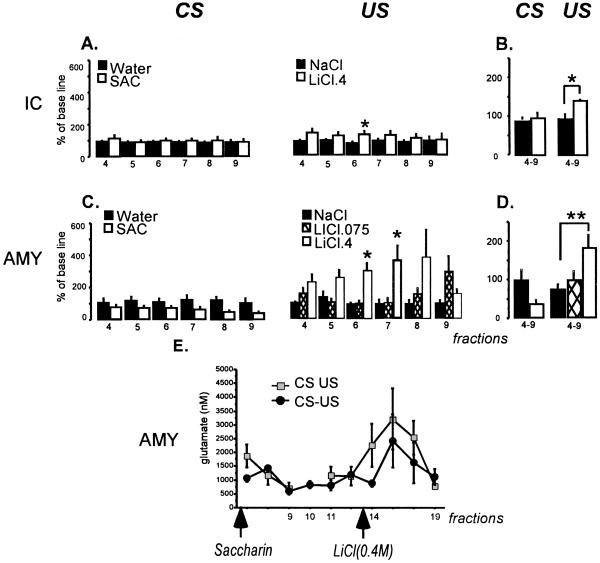
Baseline water consumption in the dialysis chamber was not significantly different among groups, and the animals drank saccharin or water during the microdialysis assay in quantities similar to the baseline consumption (average, 12 ml). Fig. 2 shows glutamate release in the amygdala and IC during presentation of saccharin or water, and during the i.p. injection of LiCl or NaCl. Regarding the gustatory CS, statistical analysis revealed no significant differences between groups (SAC or WAT) in the release of glutamate in IC or amygdala in any fraction, and no significant changes between fractions (see Fig. 2 A and C). However, there was a tendency to glutamate release decrements after presentation of saccharin which did not reach statistical significance.
Fig 2.
Glutamate release in the insular cortex (A) and amygdala (C) during the CS (drinking saccharin presentation, 0.1%) or US (i.p. injection of 0.4 M LiCl, 0.074 M LiCl, or 0.15 M NaCl). (A and C) Glutamate release concentrations that were converted into percentage of baseline (% BL = fraction × 100/mean of the three first samples). (B and D) Mean of the four to nine fractions. (E) Comparison between groups that were presented the CS and US in the same session (CS-US) or separately (CS or US). In CS, each fraction represents the average of two fractions. *, P < 0.05; **, P < 0.01.
To see whether a lower LiCl (0.075 M) dose produces reliable glutamate increments, an extra group (n = 4) of amygdala microdialysis during injections of LiCl was added. Repeated-measures ANOVA of the glutamate levels in amygdala fractions showed significant differences between groups (F2,55 = 8.94, P < 0.01) and no significant differences between fractions; however, there was a tendency to interaction (F10,55 = 1.90, P < 0.06). Post hoc analysis showed significant differences between fractions 6 and 7. The high-LiCl dose group released significantly more glutamate than the low-dose LiCl or NaCl control groups (P < 0.05; Fig. 2 C and D), with these latter groups showing similar releases. After fraction 9, the glutamate levels in all groups reached values similar to the base line (data not show). The glutamate release in amygdala in the high-LiCl group increased dramatically after LiCl i.p. injection and began to decrease 2 h after injection. Repeated-measures ANOVA of IC fractions showed significant differences between groups (F1,20 = 11.38, P ≤ 0.02), with no significant differences between fractions and no interactions. Post hoc analysis revealed significant differences between groups only in fraction 6 (P < 0.05), indicating a small but reliable increase in the release after LiCl i.p. injection when compared with the release produced by i.p. injection of NaCl (Fig. 2A). To facilitate the analyses, we used an experimental design with independent groups of animals. Thus, we also compared a group of animals that received saccharin or LiCl in the same session (CS-US) with the groups of animals that received the stimuli in separate sessions (CS US). As can be seen in Fig. 2E, there were significant differences between fractions (F14,28 = 4.80; P < 0.01), and there were no significant differences between groups and no interaction.
From these results, it is clear that injections of the high dose of LiCl produced a significant increment of glutamate release in the amygdala. To assess the role of glutamate during encoding of US in CTA acquisition, we evaluated the effects of bilateral injections of glutamate into the amygdala and IC just before the injection of a low dose of LiCl.
Glutamate in the Amygdala Enhances Visceral Input During Taste Aversion Acquisition.
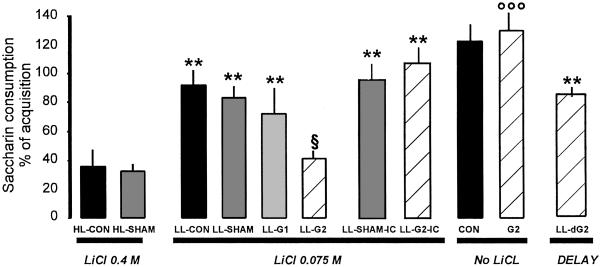
No significant differences among groups were found in baseline water intake or during acquisition saccharin consumption. There was, however, a significant difference between groups in saccharin consumption on the test day (Fig. 3), as revealed by ANOVA (F10,81 = 13.50; P < 0.001). Post hoc analysis showed that i.p. injection of a high dose of LiCl (HL-CON and HL-SHAM) significantly reduced the saccharin consumption when compared with injection of a low dose of LiCl in the control groups (LL-CON, LL-SHAM), as well as in the groups receiving 1 μg of glutamate (LL-G1, P < 0.01). It is noteworthy that the group receiving 2 μg of glutamate in the amygdala (LL-G2) in association with low doses of LiCl showed clearly reduced saccharin consumption and was significantly different from control groups with low doses of LiCl (LL-CON, and LL-SHAM; P < 0.01), and with the IC groups (LL-SHAM-IC and LL-G2-IC; P < 0.01). However, the LL-G2 group did not differ from the taste aversion of groups receiving high doses of LiCl (HL-SHAM or HL-CON). These results indicate that only the dose of 2 μg of glutamate in the amygdala, but not in the IC, was effective to produce CTA memory when a low LiCl dose was used as a US.
Fig 3.
CTA retrieval expressed as a percentage of consumption of acquisition during the test day. **, differences from both HL-CON and HL-SHAM (P < 0.01). §, differences from both LL-CON and LL-SHAM (P < 0.05). ooo, differences from all of the other groups (P < 0.01).
The group that received 2 μg (G2) of glutamate in amygdala without LiCl injection showed no rejection of saccharin and did not differ from the control group (CON) not receiving any LiCl injections. Moreover, saccharin consumption in these groups (CON, G2) was significantly higher than all of the other groups (P < 0.01, Fig. 3). This result indicates that 2 μg of glutamate by itself was not sufficient to induce aversion but does enhance it in combination with low LiCl doses (LL-G2 group). Finally, when injection of glutamate (2 μg, LL-dG2) was delayed 2 h 30 min after low-lithium injection, it did not produce a reliable taste aversion and was not different from the control groups (LL-CON, LL-SHAM), indicating that glutamate injection in amygdala must be contingent upon LiCl injection to induce reliable CTA memory formation.
Discussion
The findings of these experiments suggest that glutamate transmission in the basolateral amygdala signals a visceral US input during taste aversion memory formation. Accordingly, after visceral stimulation (induced by LiCl), a dramatic glutamate release in the amygdala is produced, compared with that induced by lower LiCl doses or isotonic NaCl injections. This signaling seems to be specific to the visceral (irritation) stimulation, because injections of lower doses of LiCl or NaCl or consumption of saccharin or water did not significantly affect glutamate release. Although there is also a significant increase in the IC glutamate release, the signaling seems to be more specific to the amygdala, because the cortical glutamate levels are lower and extinguish more rapidly. Furthermore, consistent with this conclusion, we demonstrated by using intra-amygdalar, but not intra-cortical, injections of glutamate just before the injection of suboptimal LiCl doses that glutamate could improve the input signal of the visceral stimulus, thus inducing a strong CTA.
In contrast with other learning models, the CTA protocols used in the present study allow the separation of the presentations of the CS and the US up to 4 h (27) and analysis of the molecular signals involved in each event in the same animal. Thus, by using in vivo microdialysis, we continuously analyzed the glutamate release after presentation of gustatory and visceral stimuli in the same animal with a separation of almost 2 h 30 min between stimuli presentations. The results are very similar to our results obtained with independent groups (each stimulus measured in different animals); i.e., an increase in glutamate release only after the visceral stimulation (injection of LiCl) but not after saccharin drinking presentation in the two structures analyzed. These data are in agreement with other reports which suggest that the amygdala seems to be important during the latest associative phase between the gustatory and visceral stimulus in CTA (12, 20–23, 28). For instance, amygdala inactivation by tetrodotoxin before the gustatory stimulus presentation failed to prevent CTA acquisition, but its inactivation after the gustatory stimulus, or before the visceral stimulus presentation, disrupted the CTA (16). Our results not only confirm these possibilities, but assign a more specific role to glutamate during learning, namely US signaling.
It has been demonstrated that after training, intrahippocampal or intracaudate injection of glutamate enhanced memory in hidden-platform or visible-platform water maze tasks (24). However, the mechanism for such improvements in memory consolidation is not clear. One possibility is that exogenous glutamate mimics the input information carried out by the US, making it more relevant and, therefore, enhancing the memory consolidation. Accordingly, when we made bilateral amygdala injections of glutamate, followed by a low suboptimal dose of LiCl, they produced reliable taste aversion. However, when similar treatments were made into the IC, there were no effects on CTA, suggesting a specific role of glutamate in the amygdala in US processing. In addition, the dose (2 μg) of glutamate was effective, whereas a lower one (1 μg) did not affect retention. Preliminary results in our laboratory showed that, when applied in combination with low doses of LiCl, higher doses of glutamate (5 μg) did not improve taste aversion, similar to an inverted-U dose-response curve reported for memory consolidation (25). Furthermore, the glutamate injection (2 μg) 2 h after the low-LiCl injection did not improve formation of CTA. These findings suggest that an adequate dosage, specific loci, and time activation of glutamate receptors are necessary to achieve an important functional effect, and that glutamatergic activation in the amygdala is essential during the initial phases of US processing.
As mentioned, the role of glutamate transmission and its receptors has been implied for a long time in the synaptic plasticity (29) underlying the acquisition and consolidation processes of different kinds of learning paradigms, such as spatial learning (1, 2), contextual Pavlovian fear conditioning (3), and inhibitory avoidance (4). Intra-amygdalar infusions of ionotropic (D-APV; ref. 30), MK-801 (23), or metabotropic (MCPG; ref. 22) glutamate receptor antagonists into the basolateral amygdala affected CTA memory formation. If glutamate signals the visceral input, then the best disruptive effects on memory formation by NMDA-receptor antagonists will be attained just before the US presentation. Accordingly, the amygdala infusion of D-APV, MCPG, or CNQX after saccharin presentation, and just before LiCl injection blockade taste aversion memory formation (22), has been demonstrated. These and our results are consistent with the hypothesis that amygdala glutamate activation plays a role in the representation of the US (LiCl-induced gastric irritation) in the brain.
In a previous work (28, 31), we have demonstrated that the signaling of the CS (novel taste) is related to cholinergic activity coming from the nucleus basalis magnocellularis to the IC and amygdala during taste aversion memory formation. As noted, significant effects have been found when glutamatergic receptors were blocked before and after CTA acquisition in both the amygdala and IC (20–22, 32, 33). The interaction of ACh and glutamate receptors is of special importance, considering that their activities may converge, as demonstrated by the study of the multiple signal-transduction pathways mediated by those receptors (34). Therefore, it has been demonstrated that one subunit of the NMDA receptor, the NR2B, undergoes phosphorylation by novel taste presentation or muscarinic activation (35). In this regard, we recently investigated the possible differential involvement of cholinergic and glutamatergic receptors in short- and long-term memory formation of CTA. Intracortical microinjection of the muscarinic antagonist scopolamine before, but not after, the presentation of the novel taste abolished both short- and long-term memory, whereas the blockade of the NMDA receptor by AP5 impaired only long-term memory (36). These results suggest that cholinergic activity is involved in the acquisition of taste memory, whereas glutamatergic activity participates in taste memory consolidation.
Two major roles for amygdala, and especially basolateral amygdala, in aversive memories have been proposed. One is that the basolateral amygdala plays a critical role in modulating memory consolidation processes in other brain regions (37); the other role is a storage site for aversive events (38). Previous works in our laboratory and others have demonstrated the interaction between amygdala and insular cortex during taste aversive memory formation. Accordingly, high-frequency stimulation in the basolateral amygdala induced long term potentiation in the IC that enhances CTA consolidation (20, 39, 40), and these effects were completely blocked by NMDA antagonists (20). These results suggest that the functional connection between the basolateral amygdala and IC during taste aversion memory has a modulatory action on memory formation (40). Moreover, the results presented herein imply that exogenous glutamate administration in amygdala partially mimics the visceral entrance of gastric irritation. Altogether, these results suggest an interaction between IC and amygdala in which cholinergic activity has the function of signaling the CS (novel taste) that would eventually converge with the glutamatergic visceral input (US) signal during acquisition and consolidation of taste aversive memory formation.
Acknowledgments
We thank Oreste Carbajal and Federico Jandete for their assistance, Drs. Ricardo Tapia and Juan Carlos Lopez for their comments on earlier drafts of this manuscript, Shaun Harris for his text review, and Yolanda Díaz de Castro for preparing the manuscript. This research was supported by Milenio-CONACyT Grants 2000–2001 35806-N, 31842-N, and DGAPA-UNAM IN-215001.
Abbreviations
CTA, conditioned taste aversion
CS, conditioned stimulus
US, unconditioned stimulus
IC, insular cortex
NMDA, N-methyl-d-aspartate
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Davis S., Butcher, S. P. & Morris, R. G. (1992) J. Neurosci. 12, 21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutierrez H., Hernandez, E., Ramirez, A. & Bermúdez-Rattoni, F. (1999) Neuroscience 89, 751-758. [DOI] [PubMed] [Google Scholar]
- 3.Fanselow M. S. & Kim, J. J. (1994) Behav. Neurosci. 108, 210-212. [DOI] [PubMed] [Google Scholar]
- 4.Kim M. & McGaugh, J. L. (1992) Brain Res. 585, 35-48. [DOI] [PubMed] [Google Scholar]
- 5.Castro A. & Borrel, J. (1995) Neuroscience 68, 793-805. [DOI] [PubMed] [Google Scholar]
- 6.Miranda M. I. & Bermúdez-Rattoni, F. (1999) Proc. Natl. Acad. Sci. USA 96, 6478-6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermúdez-Rattoni F. & Yamamoto, T., (1998) Conditioned Taste Aversion: Memory of a Special Kind (Oxford Univ. Press, New York), pp. 28–44.
- 8.Lasiter P. S. & Glanzman, D. L. (1985) Behav. Neurosci. 99, 257-276. [DOI] [PubMed] [Google Scholar]
- 9.Aggleton J. P., Petrides, M. & Iversen, S. D. (1981) Physiol. Behav. 27, 397-400. [DOI] [PubMed] [Google Scholar]
- 10.Kiefer S. W. & Braun, J. J. (1977) J. Comp. Physiol. Psychol. 91, 498-507. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez C. L., Miranda, M. I., Gutierrez, H., Ormsby, C. & Bermúdez-Rattoni, F. (2000) Behav. Brain Res. 116, 89-98. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T., Shimura, T., Sako, N., Yasoshima, Y. & Sakai, N. (1994) Behav. Brain Res. 65, 123-137. [DOI] [PubMed] [Google Scholar]
- 13.Bermúdez-Rattoni F., Grijalva, C. V., Kiefer, S. W. & Garcia, J. (1986) Physiol. Behav. 38, 503-508. [DOI] [PubMed] [Google Scholar]
- 14.Kesner R. P., Berman, R. F., Burton, B. & Hankins, W. G. (1975) Behav. Biol. 13, 349-358. [DOI] [PubMed] [Google Scholar]
- 15.Nachman M. & Ashe, J. H. (1974) J. Comp. Physiol. Psychol. 87, 622-643. [DOI] [PubMed] [Google Scholar]
- 16.Gallo M., Roldan, G. & Bures, J. (1992) Behav. Brain Res. 52, 91-97. [DOI] [PubMed] [Google Scholar]
- 17.Lasiter P. S., Glanzman, D. L. & Mensah, P. A. (1982) Brain Res. 234, 111-121. [DOI] [PubMed] [Google Scholar]
- 18.Buresova O. & Bures, J. (1973) Acta Neurobiol. Exp. (Warsz) 33, 689-698. [PubMed] [Google Scholar]
- 19.Roldan G. & Bures, J. (1994) Behav. Brain Res. 65, 213-219. [DOI] [PubMed] [Google Scholar]
- 20.Escobar M. L., Chao, V. & Bermúdez-Rattoni, F. (1998) Brain Res. 779, 314-319. [DOI] [PubMed] [Google Scholar]
- 21.Rosenblum K., Berman, D. E., Hazvi, S., Lamprecht, R. & Dudai, Y. (1997) J. Neurosci. 17, 5129-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasoshima Y., Morimoto, T. & Yamamoto, T. (2000) Brain Res. 869, 15-24. [DOI] [PubMed] [Google Scholar]
- 23.Tucci S., Rada, P. & Hernandez, L. (1998) Brain Res. 813, 44-49. [DOI] [PubMed] [Google Scholar]
- 24.Packard M. G. (1999) Proc. Natl. Acad. Sci. USA 96, 12881-12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Packard M. G. & Teather, L. A. (1999) Psychobiology 27, 40-50. [Google Scholar]
- 26.Izquierdo I., da Cunha, C., Rosat, R., Jerusalinsky, D., Ferreira, M. B. & Medina, J. H. (1992) Behav. Neural Biol. 58, 16-26. [DOI] [PubMed] [Google Scholar]
- 27.Bures J., Bermúdez-Rattoni, F. & Yamamoto, T., (1998) Conditioned Taste Aversion: Memory of a Special Kind (Oxford Univ. Press, New York).
- 28.Gutierrez H., Gutierrez, R., Ramirez, T., Silva, G., Ormsby, C. E., Miranda, M. I. & Bermúdez-Rattoni, F. (1999) J. Neurosci. 19, 7661-7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris R. G. (1989) J. Neurosci. 9, 3040-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto T. & Fujimoto, Y. (1991) Brain Res. Bull. 27, 403-406. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez H., Gutierrez, R., Silva, G., Estrada, J., Miranda, M. I. & Bermúdez-Rattoni, F. (1999) Brain Res. 834, 136-141. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez H., Miranda, M. I. & Bermúdez-Rattoni, F. (1997) J. Neurosci. 17, 3796-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otawa S., Takagi, K. & Ogawa, H. (1995) Exp. Brain Res. 106, 391-402. [DOI] [PubMed] [Google Scholar]
- 34.Woolf N. J. (1998) Prog. Neurobiol. 55, 59-77. [DOI] [PubMed] [Google Scholar]
- 35.Rosenblum K., Futter, M., Jones, M., Hulme, E. C. & Bliss, T. V. (2000) J. Neurosci. 20, 977-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira, G., Gutiérrez, R., De la Cruz, V. & Bermúdez-Rattoni, F. (2002) Eur. J. Neurosci., in press. [DOI] [PubMed]
- 37.McGaugh J. L. (2000) Science 287, 248-251. [DOI] [PubMed] [Google Scholar]
- 38.LeDoux J. E. (2000) Annu. Rev. Neurosci. 23, 155-184. [DOI] [PubMed] [Google Scholar]
- 39.Escobar M. L. & Bermúdez-Rattoni, F. (2000) Brain Res. 852, 208-212. [DOI] [PubMed] [Google Scholar]
- 40.Escobar M. L., Alcocer, I. & Bermúdez-Rattoni, F. (2002) Behav. Brain Res. 129, 101-106. [DOI] [PubMed] [Google Scholar]