Abstract
In the current investigation, the neurophysiological organization of the neocortex was examined in adult animals that were bilaterally enucleated very early in life, before the retino-geniculo-cortical pathway was established. Our results indicate that some aspects of development of cortical fields are not mediated by specific sensory inputs. However, the current study also demonstrates that peripheral innervation plays a large role in the organization of the neocortex, as cortical territories normally involved in visual processing are completely captured by the auditory and somatosensory system. Thus, a large degree of phenotypic variability in cortical organization can be accomplished solely by removing or modifying sensory inputs.
Keywords: bilateral enucleations, electrophysiological recording, development, evolution
Until recently, the notion that humans with a congenital loss of one sensory system become better at making discriminations with the remaining sensory systems was mostly anecdotal. However, studies in congenitally blind individuals indicate that there is a shorter detection time for auditory discrimination tasks (1) and that blind individuals process language faster than sighted individuals (2). Thus, there is a compensatory adaptation of the auditory system in the congenitally blind, presumably because of a reorganization of the neocortex. This hypothesis is supported by recent neuroimaging studies of blind individuals demonstrating that both auditory localization tasks (3) and Braille reading (4–6) activate regions of cortex normally involved in visual processing. Similar types of cross-modal plasticity also have been demonstrated in congenitally deaf individuals (7–9). These results indicate that the amount of cortex devoted to a particular sensory system, and possibly the number and organization of cortical areas, is determined in large part by peripheral innervation and activity patterns generated with use. Indeed, a number of studies in the adult mammalian neocortex have demonstrated that cortical maps of sensory receptor arrays (areas) can be contracted or expanded by loss of peripheral inputs, or by enhanced use (10–24), and that cross-modal plasticity is possible when the modifications in peripheral activity patterns occur early in life (25). Yet, the extent to which peripheral innervation at early developmental stages can sculpt the architecture and function of entire sensory systems is not known.
The present investigation was prompted by two seemingly disparate observations related to this issue. First, developmental studies demonstrate that thalamocortical input is not required for the expression of molecules believed to be involved in some aspects of cortical field development (26, 27). This suggests that cortical arealization, or the emergence of cortical fields in development, is mediated by intrinsic genetic mechanisms that can operate independent of activity from peripheral receptors. This notion is difficult to reconcile with observations from comparative studies which demonstrate that the amount of cortical territory a sensory system assumes (sensory domains), and the individual subdivisions therein (cortical fields or areas), is highly dependent on peripheral innervation and the use of sensory receptor arrays (28, 29). In the current study, we examine the influence of extrinsic factors that contribute to neocortical organization by addressing two questions: What is the contribution of peripheral receptors, and ultimately the spontaneous and patterned activity they generate, to the establishment of major sensory domains in the neocortex? Can changes in peripheral innervation affect the amount of neocortex occupied by a particular sensory domain?
To address these questions, the functional organization and/or the cortical myelo- and cytoarchitecture were examined in six short-tailed opossums, Monodelphis domestica, that had been bilaterally enucleated early in development (postnatal day 4, P4), well before thalamocortical afferents enter the cortex at P7–P8 (30), and before ganglion cell axons enter the diencephalon at P9–P10.§ The Monodelphis is born at an extremely immature stage of development compared with most other mammals, so that manipulations can be done ex utero. Equally important, the neocortex of these mammals is relatively small, the number of fields is limited, and the organization and connections of much of the sensory cortex have already been described for normal animals (31, 32). Thus, the effects of the manipulations on all or most of the neocortex can be assessed in a single experiment. Multiunit activity was recorded at a number of closely spaced sites across most of the mediolateral and rostrocaudal extent of Monodelphis neocortex (see Methods and Fig. 1), and the type of sensory stimuli that elicited a response, as well as the receptive field for neurons at all somatosensory sites, was documented. By examining response patterns and matching electrophysiological recordings to histologically processed tissue, sensory domain maps could be established, and cortical areas within these domains could be identified and compared with normal animals (31, 32).
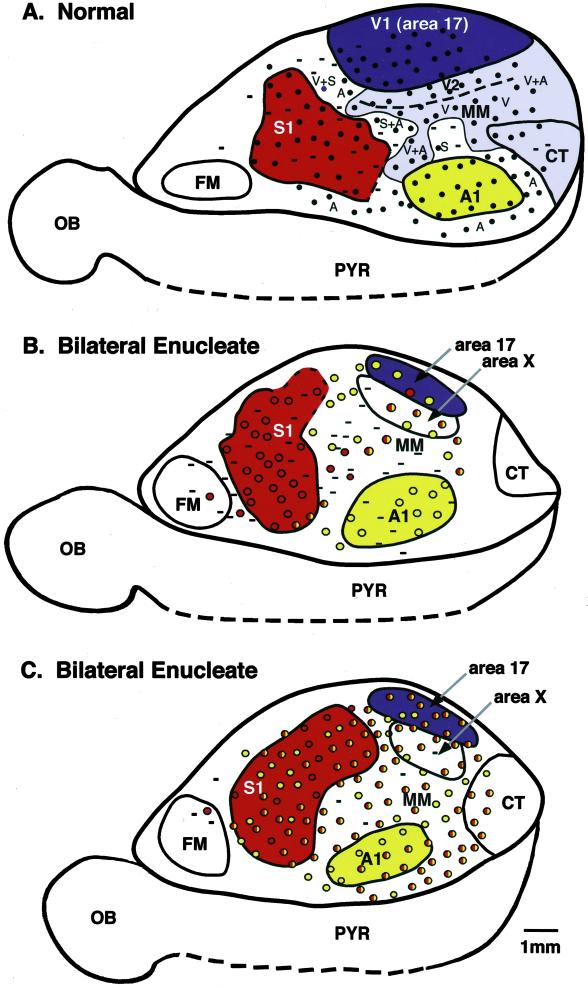
Fig 1.
A comprehensive reconstruction of electrophysiological and architectonic maps of the neocortex in normal (A, case 00-32) and bilaterally enucleated animals (B and C, cases 01-03 and 01-20). (A) The dark purple region indicates V1 as defined both electrophysiologically and architectonically. The light purple indicates extrastriate cortex in which neurons responded exclusively to visual, to visual and auditory, or to visual and somatosensory stimulation. The red and yellow areas correspond to S1 and A1, respectively. Black dots indicate electrode penetrations. (B and C) Red areas correspond to S1, yellow areas correspond to A1, and purple areas correspond to area 17 as architectonically defined. Each dot in A–C represents an electrode penetration. Red dots represent locations at which neurons responded exclusively to somatic stimulation, yellow dots indicate sites at which neurons responded exclusively to auditory stimulation, and red + yellow dots indicate sites at which neurons responded to both somatosensory and auditory stimulation. Recording sites in which neurons did not respond to any type of sensory stimulation are marked as minuses. Thick lines mark architectonic boundaries. Dashed lines represent a portion of the pyriform cortex, included for illustration purposes. In normal animals, S1 contains a complete representation of the contralateral body surface and is coextensive with a darkly myelinated region (Fig. 2). V1 has been well defined in Monodelphis as a complete representation of the contralateral visual hemifield coextensive with a moderately to darkly myelinated region. A1 contains neurons that respond almost exclusively to auditory stimulation, although the tonotopy of this region has not been described in detail in Monodelphis. In the bilaterally enucleated animals, an architectonic area 17 was observed (dark purple), but neurons in this region responded to auditory or auditory + somatosensory stimulation, and the field was substantially smaller than in normal animals (B and C). Also, in bilaterally enucleated animals, a new architectonic area (area X) emerged just lateral to area 17. Neurons in this region responded to auditory + somatosensory stimulation. In cortex lateral to area X, neurons responded to auditory and somatosensory stimulation as well. In the bilateral enucleates, receptive fields for neurons in areas 17, X, MM, A, and CT were mostly on the head, vibrissae, and snout. A1, primary auditory area; CT, caudotemporal area; FM, frontal myelinated area; MM, multimodal cortex; OB, olfactory bulb; PYR, pyriform cortex; S1, primary somatosensory area; V1, primary visual area; V2, second visual area; rostral is to the left and medial is up.
Methods
Surgery.
Bilateral enucleations were performed at postnatal day 4 (P4) in six M. domestica pups. The mother was anesthetized with alphaxalone (45 mg/kg) and alphadolone (15 mg/kg), administered intramuscularly. Body temperature was maintained, and heart rate and respiration were continuously monitored throughout the surgery. The pups were individually anesthetized by hypothermia while still attached to the mother. Once the pups were anesthetized, the eyes were manually excised under microscopic guidance. The skin surrounding the eyes was replaced over the exposed eye socket; in some cases, it was secured in place with surgical glue (Nexaband, Veterinary Products, Phoenix, AZ). The pups remained with the mother until postnatal week 4, at which time they were removed from the mother and hand-reared until postnatal week 7, and then housed separately for 8–12 months. Complete removal of the eyes was verified by dissecting the eye region after electrophysiological mapping and perfusion. All procedures used in these experiments were approved by the Animal Use and Care Administrative Advisory Committee of the University of California, Davis, and conform to National Institutes of Health guidelines.
Electrophysiological Recordings.
Upon reaching adulthood, each animal was anesthetized with ketamine hydrochloride (40 mg/kg) and then isoflurane (1–2%) delivered via an endotracheal tube. The animal was then placed in a stereotaxic apparatus, the skin was cut, the temporal muscle over the left hemisphere was retracted, and the skull and dura were removed to expose the entire hemisphere. Subcutaneous injections of lactated Ringer's solution were administered every 3–4 h to maintain hydration. Body temperature was maintained, and heart rate and respiration were monitored continuously throughout the experiment. The exposed cortex was imaged by using a charge-coupled device camera (Optronics Engineering, Zeiss), and this image was used as a reference map to relate the electrode penetrations to cortical vasculature. An electrode designed to record from multiunit clusters (5 MΩ, 0.02 inch diameter) was lowered into the cortex, and recordings were made 200–400 μm from the pial surface, approximately in layer IV. Multiunit recordings were amplified, filtered (250 Hz to 4 kHz), viewed on an oscilloscope, and heard through a speaker. Auditory stimulation consisted of broad band clicks presented in a free field. Somatic stimuli consisted of light taps, displacement of hairs with brushes, light brushing of skin, hard taps, and manipulation of muscles and joints. Receptive fields were drawn onto pictures of the body. Descriptions of the receptive fields and the type of stimulus required to elicit a response were also documented. For the normal animals, visual stimulation consisted of full-field flashes of light and moving bars of light. Details of visual stimuli, receptive field progressions, and visual cortex organization have been previously documented for Monodelphis (32). Upon completion of the recording, probes were placed in the cortex to aid with reconstruction of the tissue.
Histology and Reconstructions.
Each animal was euthanized with an overdose of sodium pentobarbital and perfused transcardially with saline and then with a fixative of 3% paraformaldehyde in phosphate buffer (pH 7.4), followed by 10% sucrose in the fixative. After fixation, the brain was removed from the skull, the cortex was removed and flattened between two glass slides, and the thalamus and cortex were immersed in 30% sucrose overnight. The flattened cortex was sectioned at 30-μm thickness in a plane parallel to the cortical surface and stained for myelin (33). For two hemispheres, cortex was cut at 50-μm thickness in a parasagittal plane and stained for Nissl substance and myelin.
For each electrophysiological recording case, camera lucida drawings of individual myelin sections were made by using a stereomicroscope. Each drawing contained the outline of the section, blood vessels, tissue artifacts, probes, and myeloarchitectonic borders. All drawn sections were aligned by using these landmarks and compiled into one reconstruction. By matching blood vessel patterns obtained from the digital image of the exposed cortex with blood vessels, probes, and other artifacts in the histologically processed tissue, the electrode penetrations were plotted onto reconstructed myelin drawings to produce one comprehensive reconstruction of both architectonic boundaries and electrophysiological recordings.
Results
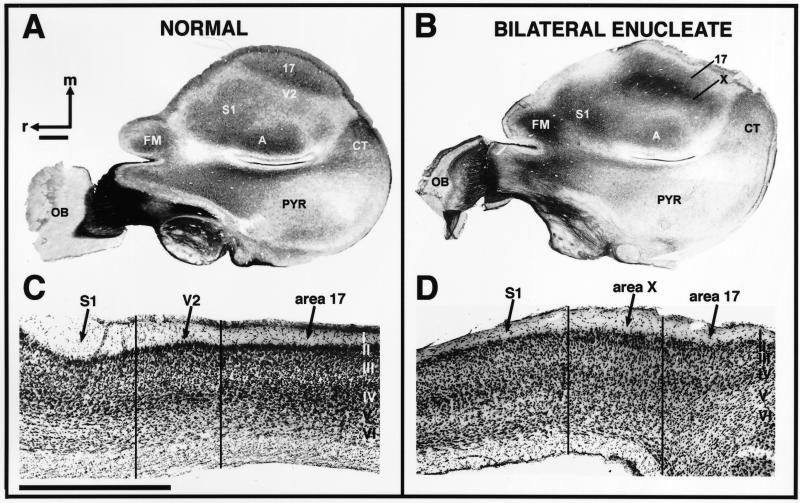
These experiments generated several results. First, based on architectonic examination, area 17 was still present, although substantially reduced in size compared with normal animals (Fig. 2). In normal animals, the architectonic field termed area 17 is coextensive with a complete map of the contralateral visual hemifield. This functional map is termed V1, and in normal animals the terms V1 and area 17 are often used interchangeably. However, in the bilaterally enucleated animal, no maps of the visual hemifield were present, so the field we term area 17 refers only to the architectonically defined region at the caudomedial pole of cortex. In tangentially sectioned tissue, area 17 in all of the bilaterally enucleated animals was readily identified as a moderate to darkly myelinated region (Fig. 2B). In parasagittally sectioned cortex that was stained for Nissl substance, area 17 was characterized by a densely packed, darkly staining granule cell layer (Fig. 2D). These descriptions are like those for area 17 in normal Monodelphis (Fig. 2 A and C) as well as a number of other mammals (28, 32, 34, 35).
Fig 2.
Cortical myeloarchitecture and cytoarchitecture in normal (A and C, cases 97-41 and 00-30) and bilaterally enucleated (B and D, cases 01-09 and 01-20) Monodelphis. In cortex that has been flattened and stained for myelin, the primary areas are readily defined (A). V1 or area 17 is an area of moderately dense staining for myelin. The primary auditory cortex (A1) is a moderately dense oval just medial to the rhinal sulcus and pyriform cortex, and S1 is a moderately dense region rostral to A1. These regions correspond to electrophysiologically defined regions described in Fig. 1. V2 shares a common border with V1 at the representation of the vertical meridian and can be readily distinguished from V1 as a lightly myelinated region. In the bilaterally enucleated animal (B), S1 and A1 look very similar to the normal animal. Whereas an area 17 can be identified as a small oval at the caudomedial pole of the cortex, it is substantially reduced in size. In these animals, a lightly myelinated area 18 does not adjoin area 17. Rather, a very darkly myelinated area X shares a border with area 17. In normal animals (C), V1 or area 17 has a very densely packed, thickened layer IV. This is in contrast to V2 or area 18, which contains a reduced layer IV. At this mediolateral level, the caudomedial border of S1 is just rostral to V2 and can be identified by its dense granule cell layer. In the bilateral enucleate (D), a small area 17 can also be identified as containing a densely packed, thickened layer IV. The field immediately rostral to area 17, area X, does not look like area 18 in normal animals in that the granular and infragranular layers are indistinct. Cortex immediately rostral to area X at this mediolateral level corresponds to S1. Intensity of myelin stain varied between cases; however, the myeloarchitectonic patterns described here were clearly evident and were consistently observed in each case. In A and B, rostral is to the left and medial is to the top; in C and D, rostral is to the left and dorsal is to the top. OB, olfactory bulb; PYR, pyriform cortex. Other conventions are as in Fig. 1. (Scale bars = 1 mm.)
Our architectonic analysis consistently revealed the presence of a new cortical area. In cortex that was flattened and cut parallel to the cortical surface, a very darkly myelinated area was identified just rostrolateral to area 17 (Fig. 2B). This cortex is normally occupied by area 18 or V2, which is lightly myelinated (32, 34, 36). In cortex sectioned parasagittally and stained for Nissl substance, this area had a densely packed layer II, as in normal animals, and a slightly thickened layer III. However, the granular and infragranular layers were indistinct (compare Fig. 2 C with D). No area with this appearance has been observed in normal animals. Based on location and cortical architecture, this region is similar to area X described in bilaterally enucleated monkeys by Rakic and colleagues (37) (see below). In bilaterally enucleated animals, cortex lateral to area X was lightly myelinated (Fig. 2B), and in Nissl-stained sections it corresponded to extrastriate cortex. However, it was difficult to determine whether this region was a displaced area 18 or multimodal (MM) cortex, as described in normal animals. The architectonic appearance of the remaining sensory cortex, including the primary somatosensory area (S1) and the primary auditory area (A1), was normal as viewed in both Nissl and myelin-stained tissue (Fig. 2).
Electrophysiological recording results were superimposed on architectonic boundaries to generate a comprehensive reconstruction of the neocortex. These results demonstrated that in bilaterally enucleated animals, much of the cortex that would normally receive visual inputs, including architectonically defined visual areas, contained neurons that responded to sensory stimulation from other modalities. Although there were a few islands of cortex in one of the bilaterally enucleated animals where no response could be elicited (Fig. 1B), this was not substantially different from the normal animals. Thus, cortex that would normally contain neurons that respond to visual stimulation, such as the primary and second visual areas (V1 and V2) (32), the caudotemporal visual area (CT), and portions of multimodal cortex contained neurons that responded to either auditory or auditory + cutaneous somatosensory stimulation. For example, in one case there was an island of cortex located in area 17, which contained neurons that responded solely to auditory stimulation (Fig. 1B). In another case (Fig. 1C), only a few sites in area 17 contained neurons that responded solely to auditory stimulation; the remaining portion of area 17 was occupied by neurons that responded to both auditory and somatosensory stimulation.
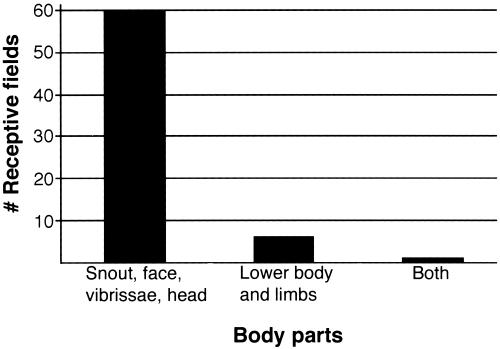
The low density of recordings in area 17 made it difficult to determine whether a complete representation of the contralateral body surface was present, but in one case that had nine recording sites in area 17, neurons at these sites had receptive fields on the snout, head, and vibrissae (Figs. 1C and 3). In area X, neurons at rostral recording sites tended to have receptive fields on the head, snout, face, and vibrissae, and neurons at caudal recording sites tended to have receptive fields on the forepaw and forelimb (and in one case on the trunk). However, the head, vibrissae, and face representation dominated the field. In the remaining cortex that would normally contain neurons that respond to visual stimulation, including MM and CT, receptive fields for neurons were mostly on the face, snout, vibrissae, and head (Fig. 3). There were a few sites in CT in which neurons had receptive fields on the trunk. The precise topographic organization within an architectonic area, like that found in the primary areas of normal animals, was not observed. The observation that most of the multimodal recording sites in areas 17, X, MM, and CT contained neurons with cutaneous receptive fields on the face, head, and vibrissae rather than on the limbs or trunk (Fig. 3) suggests that inputs from particular body parts have priority in acquiring cortical territory.
Fig 3.
The distribution of somatosensory receptive fields in areas 17, X, MM, CT, and A1 in the bilaterally enucleated Monodelphis. Most receptive fields are on the snout, face, head, and vibrissae. The number of recording sites in these areas varied across cases, but the preponderance of receptive fields on these body parts was consistent.
An unexpected result was that visual cortex was not the only region of cortex affected by bilateral enucleations. Auditory and somatosensory cortex were affected as well, particularly in one case (Fig. 1C). For instance, in A1 somatosensory receptive fields for neurons at the multimodal sites were on the head, vibrissae, face, and chin (Fig. 1C). In this same case S1 contained a number of sites in which neurons responded to both somatosensory and auditory stimulation (Fig. 1C). However, receptive field progression in S1 was similar to that described for normal animals with caudal body parts represented medially and rostral body parts represented laterally.
Discussion
The present results demonstrate that massive changes in cortical organization and architecture can be accomplished by changing patterns of peripheral innervation. The contribution of peripheral innervation to some aspects of cortical organization has been demonstrated previously in macaque monkeys (37–40) and mice and rats (41–44) that underwent bilateral enucleations relatively early in development, but at a later stage than in the current study. These previous studies also report that area 17 could be recognized by its architectonic appearance, but that it was smaller than in normal animals (37, 38) and that callosal connectivity was relatively normal (38, 41, 43, 44). Further, as in the present investigation, a new architectonic zone (area X), interposed between area 17 and 18, had emerged (37). We add here that area X is processing auditory inputs as well as somatic inputs primarily from the head, vibrissae, and snout.
The finding in the present study that neurons in “visual” cortex are responsive to stimulation of other sensory modalities is also consistent with previous studies in ferrets, which demonstrate that auditory cortex can be transformed into visual cortex by surgically “rewiring” a number of subcortical circuits (45). This new cortex contained a map of visual space and even had orientation modules within this map (46).
A question that arises from our results is what is the source of somatic and auditory input to “visual” cortex. Studies of connections in anophthalmic (eyeless) mice indicate that auditory and somatic activation of visual cortex may be the result of subcortical rerouting of connections. In these animals, the lateral geniculate nucleus receives novel input from the inferior colliculus¶ and the cuneate nucleus (47). Reports of interhemispheric connections in these animals indicate that callosal connectivity was relatively normal (44). Further, studies in mammals with a highly reduced visual system, such as the blind mole rat, demonstrate that the lateral geniculate nucleus is activated by auditory inputs (48). Thus, a similar type of rewiring seems to occur naturally in evolution.
There are several implications regarding cortical development and evolution that arise from the current study as well as previous investigations. First, some aspects of arealization do not depend on specific sensory inputs, but are intrinsically regulated. This has been shown previously in developmental studies in knock-out mice that fail to develop thalamocortical afferents (26, 27). These mice still express graded and abrupt patterns of gene expression, and some of these expression boundaries seem to be related to the boundaries of cortical fields. Further, several of the genes examined have been implicated in some aspects of development, such as axon guidance, fasciculation, and target finding. Thus, it is possible that genes regulate some features of cortical lamination, thalamocortical and corticocortical connectivity. This is supported by the finding in the present study that an architectonic area 17 is still present and that thalamocortical afferents to area 17 are essentially normal∥ despite the complete and very early absence of visual input. Both of these observations are consistent with comparative studies in mammals which demonstrate that despite the absence or near absence of use of the visual system and the concomitant reduction of the eyes, it is not possible to abolish the architectonic structure or thalamocortical afferent patterning associated with area 17 (49–52, **). Thus, genetically regulated, intrinsic mechanisms that shape some aspects of cortical field development must constrain the evolution of the neocortex.
A second implication is that a high degree of phenotypic variability in cortical organization across species, specifically the amount of cortex assumed by a particular sensory system, need not be orchestrated by intrinsic cortical mechanisms. This implication is supported by observations in mammals that naturally have a reduced visual system, but have evolved morphological specializations associated with other sensory systems, such as the duck-billed platypus, the naked mole rat, the star-nosed mole, and the blind mole rat (49–52, **). As in the bilaterally enucleated Monodelphis, these animals have a small area 17 and little if any cortex in which neurons respond to purely visual stimulation. Indeed, much of cortex that would be visual in animals with a well developed visual system, including area 17, is taken over by other sensory modalities (49). Thus, both naturally and experimentally modified peripheral morphology results in a dramatic reassignment of sensory modalities to the cortical sheet within the life of an individual and across species over time.
The final implication is that peripheral influences contribute to the emergence of cortical fields within sensory domains. Whereas area 17 in the bilaterally enucleated Monodelphis is functionally different from and substantially smaller than its normal counterpart, the architectonic appearance and thalamocortical connections of area 17 are essentially like those in normal animals.∥ For this reason, we do not consider this as a new field. On the other hand, the region of cortex termed area X has emerged as a new architectonic area and is like nothing previously described for the Monodelphis or for other mammals. Area X seems to meet some of the criteria used to define a valid subdivision of the neocortex (53). It has a distinct architectonic appearance coextensive with neurons that respond to tactile and auditory stimulation. Although connections of this field have yet to be investigated, we hypothesize that it will have some unique pattern of interconnections and possibly function to enhance stimulus localization in immediate body space by integrating somatic inputs primarily from the head, vibrissae, and face with auditory inputs. Thus, if one defines a cortical field as possessing these attributes, then we should consider area X as a new cortical field that may underlie some novel (enhanced) sensory processing capabilities such as those described in congenitally blind humans.
Acknowledgments
We thank Martin Wilson, Marie Burns, Kim McAllister, Bruno Olshausen, Elizabeth Disbrow, and Deborah Hunt for helpful comments on this manuscript.
Abbreviations
MM, multimodal
CT, caudotemporal
Catania, K. C. & Remple, M. (2001) Soc. Neurosci. Abstr. 27, 127.
Bronchti, G., Molnár, Z., Welker, E., Croquelois, A. & Krubitzer, L. (2000) Soc. Neurosci. Abstr. 26, 2193.
Kahn, D. M. & Krubitzer, L. (2001) Soc. Neurosci. Abstr. 27, 1250.
Dunn, C. A., Kahn, D. M. & Krubitzer, L. (2001) Soc. Neurosci. Abstr. 27, 1523.
References
- 1.Röder B., Rosler, F. & Neville, H. J. (1999) Neurosci. Lett. 264, 53-56. [DOI] [PubMed] [Google Scholar]
- 2.Röder B., Rosler, F. & Neville, H. J. (2000) Neuropsychologia 38, 1482-1502. [DOI] [PubMed] [Google Scholar]
- 3.Weeks R., Horwitz, B., Aziz-Sultan, A., Tian, B., Wessinger, C. M., Cohen, L. G., Hallett, M. & Rauschecker, J. P. (2000) J. Neurosci. 20, 2664-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadato N., Pascualleone, A., Grafman, J., Ibanez, V., Deiber, M. P., Dold, G. & Hallett, M. (1996) Nature (London) 380, 526-528. [DOI] [PubMed] [Google Scholar]
- 5.Cohen L. G., Celnik, P., PascualLeone, A., Corwell, B., Faiz, L., Dambrosia, J., Honda, M., Sadato, N., Gerloff, C., Catala, M. D. & Hallett, M. (1997) Nature (London) 389, 180-183. [DOI] [PubMed] [Google Scholar]
- 6.Büchel C., Price, C., Frackowiak, R. S. J. & Friston, K. (1998) Brain 121, 409-419. [DOI] [PubMed] [Google Scholar]
- 7.Neville H. J. & Lawson, D. (1987) Brain Res. 405, 268-283. [DOI] [PubMed] [Google Scholar]
- 8.Neville H. J., Schmidt, A. & Kutas, M. (1983) Brain Res. 266, 127-132. [DOI] [PubMed] [Google Scholar]
- 9.Neville H. J. (1989) Ann. N.Y. Acad. Sci. 608, 71-87. [DOI] [PubMed] [Google Scholar]
- 10.Merzenich M. M., Kaas, J. H., Wall, J. T., Sur, M., Nelson, R. J. & Felleman, D. J. (1983) Neuroscience 10, 639-665. [DOI] [PubMed] [Google Scholar]
- 11.Merzenich M. M., Nelson, R. J., Stryker, M. P., Cynader, M. S., Schoppmann, A. & Zook, J. M. (1984) J. Comp. Neurol. 224, 591-605. [DOI] [PubMed] [Google Scholar]
- 12.Wall J. T., Kaas, J. H., Sur, M., Nelson, R. J., Felleman, D. J. & Merzenich, M. M. (1986) J. Neurosci. 6, 218-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calford M. B. & Tweedale, R. (1990) in Neurology and Neurobiology, Information Processing in Mammalian Auditory and Tactile Systems, eds. Rowe, M. & Aitkin, L. (Liss, New York), pp. 221–236.
- 14.Kaas J. H., Krubitzer, L. A., Chino, Y. M., Langston, A. L., Polley, E. H. & Blair, N. (1990) Science 248, 229-231. [DOI] [PubMed] [Google Scholar]
- 15.Donoghue J. P., Suñer, S. & Sanes, J. N. (1990) Exp. Brain Res. 79, 492-503. [DOI] [PubMed] [Google Scholar]
- 16.Recanzone G. H., Jenkins, W. M., Hradek, G. T. & Merzenich, M. M. (1992) J. Neurophysiol. 67, 1015-1030. [DOI] [PubMed] [Google Scholar]
- 17.Recanzone G. H., Merzenich, M. M. & Jenkins, W. M. (1992) J. Neurophysiol. 67, 1057-1070. [DOI] [PubMed] [Google Scholar]
- 18.Recanzone G. H., Merzenich, M. M. & Schreiner, G. E. (1992) J. Neurophysiol. 67, 1071-1091. [DOI] [PubMed] [Google Scholar]
- 19.Recanzone G. H., Schreiner, G. E. & Merzenich, M. M. (1993) J. Neurophysiol. 13, 87-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajan R., Irvine, D. R. F., Wise, L. Z. & Heil, P. (1993) J. Comp. Neurol. 338, 17-49. [DOI] [PubMed] [Google Scholar]
- 21.Darian-Smith C. & Gilbert, C. D. (1995) J. Neurosci. 15, 1631-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nudo R. J., Milliken, G. W., Jenkins, W. M. & Merzenich, M. M. (1996) J. Neurosci. 16, 785-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaas J. (2000) in The New Cognitive Neurosciences, ed. Gazzaniga, M. (MIT Press, Cambridge, MA), pp. 223–236.
- 24.Recanzone G. (2000) in The New Cognitive Neurosciences, ed. Gazzaniga, M. (MIT Press, Cambridge, MA), pp. 237–247.
- 25.Rauschecker J. (1995) Trends Neurosci. 18, 36-43. [DOI] [PubMed] [Google Scholar]
- 26.Miyashita-Lin E. M., Hevner, R., Wassarman, K. M., Martinez, S. & Rubenstein, J. L. (1999) Science 285, 906-909. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa Y., Johnson, J. E. & O'Leary, D. D. (1999) J. Neurosci. 19, 10877-10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krubitzer L. (1995) Trends Neurosci. 18, 408-417. [DOI] [PubMed] [Google Scholar]
- 29.Krubitzer L. & Huffman, K. J. (2000) Brain Behav. Evol. 55, 322-335. [DOI] [PubMed] [Google Scholar]
- 30.Molnár Z., Knott, G. W., Blakemore, C. & Saunders, N. R. (1998) J. Comp. Neurol. 398, 491-514. [PubMed] [Google Scholar]
- 31.Huffman K. J., Nelson, J., Clarey, J. & Krubitzer, L. (1999) J. Comp. Neurol. 403, 5-32. [DOI] [PubMed] [Google Scholar]
- 32.Kahn D. M., Huffman, K. J. & Krubitzer, L. (2000) J. Comp. Neurol. 428, 337-354. [PubMed] [Google Scholar]
- 33.Gallyas F. (1979) Neurology 1, 203-209. [Google Scholar]
- 34.Rosa M. G. P. & Krubitzer, L. A. (1999) Trends Neurosci. 22, 242-248. [DOI] [PubMed] [Google Scholar]
- 35.Kaas J. H. & Collins, C. E. (2001) Curr. Opin. Neurobiol. 11, 498-504. [DOI] [PubMed] [Google Scholar]
- 36.Rosa M. G. P., Krubitzer, L. A., Molnár, Z. & Nelson, J. E. (1998) Eur. J. Neurosci. 11, 907-915. [DOI] [PubMed] [Google Scholar]
- 37.Rakic P., Suñer, I. & Williams, R. W. (1991) Proc. Natl. Acad. Sci. USA 88, 2083-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehay C., Horsburgh, G., Berland, M., Killackey, H. & Kennedy, H. (1989) Nature (London) 337, 265-267. [DOI] [PubMed] [Google Scholar]
- 39.Dehay C., Horsburgh, G., Berland, M., Killackey, H. & Kennedy, H. (1991) Dev. Brain Res. 62, 137-141. [DOI] [PubMed] [Google Scholar]
- 40.Dehay C., Giroud, P., Berland, M., Killackey, H. & Kennedy, H. (1996) J. Comp. Neurol. 367, 70-89. [DOI] [PubMed] [Google Scholar]
- 41.Olavarria J., Malach, R. & Van Sluyters, R. C. (1987) J. Comp. Neurol. 260, 321-348. [DOI] [PubMed] [Google Scholar]
- 42.Robertson R., Fogolin, R., Tijerina, A. A. & Yu, J. (1987) Dev. Brain Res. 33, 185-198. [DOI] [PubMed] [Google Scholar]
- 43.Olavarria J. F. & Li, C.-P. (1995) J. Comp. Neurol. 361, 138-151. [DOI] [PubMed] [Google Scholar]
- 44.Olavarria J. & Van Sluyters, R. C. (1984) J. Comp. Neurol. 230, 249-268. [DOI] [PubMed] [Google Scholar]
- 45.Roe A. W., Pallas, S. L., Hahm, J. O. & Sur, M. (1990) Science 250, 818-820. [DOI] [PubMed] [Google Scholar]
- 46.Sharma J., Angelucci, A. & Sur, M. (2000) Nature (London) 404, 841-847. [DOI] [PubMed] [Google Scholar]
- 47.Asanuma C. & Stanfield, B. B. (1990) Neuroscience 39, 533-545. [DOI] [PubMed] [Google Scholar]
- 48.Bronchti G. P., Heil, P., Scheich, H. & Wollberg, Z. (1989) J. Comp. Neurol. 284, 253-274. [DOI] [PubMed] [Google Scholar]
- 49.Heil P., Bronchti, G., Wollberg, Z. & Scheich, H. (1991) NeuroReport 2, 735-738. [DOI] [PubMed] [Google Scholar]
- 50.Krubitzer L. (1998) Philos. Trans. R. Soc. London 353, 1127-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catania K. C., Northcutt, R. G., Kaas, J. H. & Beck, P. D. (1993) Nature (London) 364, 493. (lett.). [DOI] [PubMed] [Google Scholar]
- 52.Catania K. C. & Kaas, J. H. (1995) J. Comp. Neurol. 351, 549-567. [DOI] [PubMed] [Google Scholar]
- 53.Kaas J. H. (1982) Contrib. Sens. Physiol. 7, 201-240. [Google Scholar]