Abstract
The prefrontal cortex has been implicated in a variety of attentional, executive, and mnemonic mental operations, yet its functional organization is still highly debated. The present study used functional MRI to determine whether attentional and emotional functions are segregated into dissociable prefrontal networks in the human brain. Subjects discriminated infrequent and irregularly presented attentional targets (circles) from frequent standards (squares) while novel distracting scenes, parametrically varied for emotional arousal, were intermittently presented. Targets differentially activated middle frontal gyrus, posterior parietal cortex, and posterior cingulate gyrus. Novel distracters activated inferior frontal gyrus, amygdala, and fusiform gyrus, with significantly stronger activation evoked by the emotional scenes. The anterior cingulate gyrus was the only brain region with equivalent responses to attentional and emotional stimuli. These results show that attentional and emotional functions are segregated into parallel dorsal and ventral streams that extend into prefrontal cortex and are integrated in the anterior cingulate. These findings may have implications for understanding the neural dynamics underlying emotional distractibility on attentional tasks in affective disorders.
Keywords: novelty, prefrontal cortex, amygdala, cingulate gyrus
The prefrontal cortex (PFC) is a heterogeneous brain region whose expansion in primates contributes to increased flexibility and control of cognition and comportment. Whether the PFC is divided into domain-specific regions has come under close scrutiny. A traditional approach to this question has involved contrasting spatial versus object processing to determine whether the PFC is organized along a dorsal–ventral axis analogous to posterior visual neocortex (1). However, electrophysiological studies in monkeys (2, 3) and neuroimaging studies in humans (4–7) have produced conflicting evidence for such a functional parcellation.
An alternative organization of PFC has been proposed in recent neuroanatomical models. Mayberg (8) postulated that ventral regions of PFC are specialized for “vegetative–somatic” functions, whereas dorsal regions are specialized for “attentional–cognitive” functions. This model further posits that the rostral anterior cingulate gyrus acts as an interface between the two processing streams. Mood disorders are hypothesized to reflect failure of coordinated interaction among these PFC compartments. Other anatomical models support the distinction between a dorsal attentional control system and a ventral emotional arousal system that relay information from posterior parietal cortex and amygdala into dorsal and ventral sectors of the PFC, respectively (9, 10).
In the present study, functional MRI (fMRI) was used to test whether attentional and emotional functions are compartmentalized into distinct prefrontal systems in the human brain. An attention-demanding target detection task (“visual oddball” paradigm) was modified from our previous studies in which subjects discriminated rare targets embedded in a stream of frequent standard stimuli (11, 12). Responses to the attentional targets were segregated from responses to two categories of trial unique task-irrelevant distracters presented intermittently and distinguished by their emotional salience. The distracter categories were equated for presentation frequency and other aspects of stimulus novelty that could potentially drive activation of PFC.
Methods
Thirteen right-handed neurologically healthy subjects participated in the study. All subjects provided written informed consent for a protocol approved by the Duke University Institutional Review Board. Before analysis, data from three subjects were discarded because of excessive head movement. The remaining 10 subjects (four males) ranged in age from 20 to 22 yr.
Experimental Design.
An imaging session consisted of 10 runs, each containing 132 stimuli presented singly at the center of a back-projection screen with an onset-to-onset interval of 3,000 ms and a duration of 2,000 ms. A fixation cross was presented in the interval between stimuli. Standards consisted of squares of varying sizes and colors and were presented on 84% of trials. Targets consisted of circles of varying sizes and colors. Emotional distracters consisted of pictures selected primarily from the International Affective Picture System (IAPS; University of Florida, Gainesville, FL) and included unpleasant themes of human violence, mutilation, and disease. Neutral distracters consisted of pictures of ordinary activities and were equated to the emotional distracters with respect to mean luminance, chromatic features, and overall complexity of the scene. All distracters contained human figures and were chosen on the basis of 9-point arousal (1 = low/9 = high) and valence (1 = negative/9 = positive) scales provided in the IAPS norms and in a pilot group of undergraduate students. The range of arousal ratings for the distracters was as follows: emotional, 5–8; neutral, 1–3. The range of valence ratings was as follows: emotional, 1–3; neutral, 4–6. Thus, the ratings for the chosen pictures did not overlap across the stimulus categories. No individual distracter or target was repeated in an imaging session. Targets, emotional distracters, and neutral distracters comprised ≈8, 4, and 4%, respectively, of the stimuli in a given list. Successive targets and distracters were pseudorandomly distributed and separated by a 12- to 51-s interval (mean 18 s). A total of 106 targets and 50 each of the emotional and neutral stimuli were presented in a session.
Subjects were required to press a button with the right index finger on detecting a circle (attentional target) and to press another button with the right middle finger for all other stimuli. Subjects were also required to silently count the number of targets presented during each list and to report that count at the list's conclusion. Stimuli were projected on a 10-in-wide screen located within the open magnet bore directly behind the subject's head. Subjects viewed the stimuli through mirrored glasses. Behavioral responses were acquired by using a fiber optic button box. Reaction times and accuracy were measured by customized experimental control software.
MRI Acquisition.
Images were acquired by using a 1.5-T General Electric Signa NVi scanner equipped with 41-mT/m gradients. The subject's head was immobilized by using a vacuum cushion and tape. The anterior (AC) and posterior commissures (PC) were identified in the midsagittal slice of a localizer series, and 34 contiguous slices were prescribed parallel to the AC-PC plane for high-resolution T1-weighted structural images [repetition time (TR) = 450 ms; echo time (TE) = 20 ms; field of view (FOV) = 24 cm; matrix = 2562; slice thickness = 3.75 mm] and gradient echo echoplanar images (TR = 3 s; TE = 40 ms; FOV = 24 cm; matrix = 642; flip angle = 90°; slice thickness = 3.75 mm; resulting in 3.75-mm3 isotropic voxels) sensitive to blood oxygenation-level-dependent contrast. An additional series of oblique T1-weighted structural images perpendicular to the AC-PC were also acquired by using the parameters specified above.
fMRI Data Analysis.
Head motion was detected by center of mass measurements, and the data of three subjects were discarded because of head motion greater than 3 mm. Compensation for the interleaved slice acquisition was performed by using cubic spline interpolation of each voxel's time course with realignment to the TR onset. Epochs synchronized to the onset of targets, emotional distracters, and neutral distracters were extracted from the continuous time series of image volumes following the method of Kirino et al. (12). Epochs containing two images preceding and five images following each stimulus type were segregated and averaged. The average MR signal values were converted to percent signal change relative to the 6-s prestimulus baseline.
The primary analysis was based on anatomical regions of interest (ROIs) drawn on each subject's high-resolution coronal structural images. These ROIs included the superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, cingulate gyrus, superior temporal gyrus, amygdala, hippocampus, intraparietal sulcus, supramarginal gyrus, and fusiform gyrus in each hemisphere. The group-averaged data showed no significant activation in the superior frontal gyrus, superior temporal gyrus, or hippocampus, so these ROIs were not considered further. Following the method of Jha and McCarthy (13) (see their figures 2 and 3), each ROI was drawn on a slice-by-slice basis, and each slice was indexed relative to the AC so that the distribution of activation within a ROI could be evaluated and summarized across subjects. For example, ROIs for the major gyri of the PFC were drawn on eight slices ranging from 7.50 to 33.75 mm anterior to the AC. Mean signal change for all voxels within each ROI was then computed for each time point and plotted to visualize the hemodynamic response profile for each ROI during each stimulus condition. The percent signal change at time points 3, 6, 9, 12, and 15 s poststimulus for each ROI was analyzed by repeated-measures ANOVA followed by post hoc analyses using the Student–Newman–Keuls test to further evaluate main effects due to stimulus category (target, emotional distracter, neutral distracter). An α level of 0.05 was used to determine significant activity in all contrasts.
In addition to the primary ROI analysis, a secondary voxel-based analysis was performed. After corrections for motion and temporal alignment, each subject's time series of whole-brain volumes was coregistered to a standard echoplanar template by using SPM99 (Wellcome Department of Neurology, London, U.K.). Epochs time-locked to stimulus onsets were excised and averaged in the manner specified above, such that each subject contributed a mean epoch of volumes for each of the target, emotional, and neutral categories. As the volumes for each subject were in a common spatial coordinate system, t tests were then applied to compare the signal change for each voxel over a collapsed 6- to 9-s poststimulus period. Contrasts were defined for targets versus emotional distracters and neutral versus emotional distracters. This secondary analysis was performed both as a check on the ROI analysis and to determine whether other brain regions not measured in our primary analysis were differentially influenced by the stimuli.
Results
Behavioral Performance.
A repeated-measures ANOVA revealed a main effect of stimulus type [F(3,27) = 43.12, P < 0.0001] on reaction time. Post hoc Student–Newman–Keuls analysis showed that reaction times to targets (691 ± 146 ms), neutral distracters (680 ± 153 ms), and emotional distracters (728 ± 156 ms) were significantly longer than to standards (536 ± 157 ms). Reaction times to emotional distracters were significantly longer than for all other stimulus types. None of the fMRI activation in our ROIs correlated with reaction times across subjects.
fMRI Results: Prefrontal Cortex.
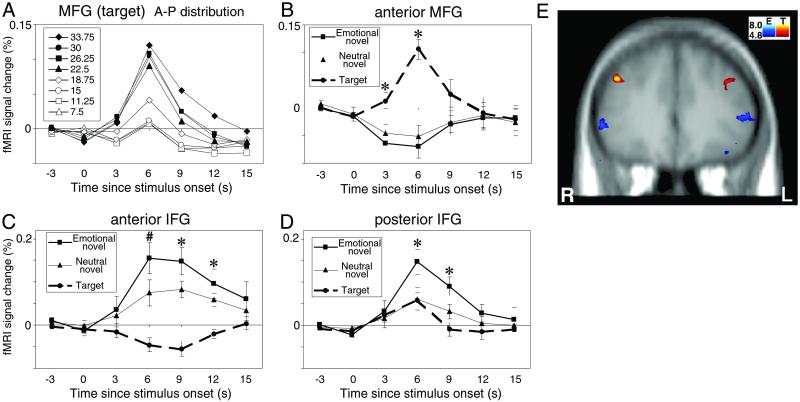
ROI analysis of the activation profile in the middle frontal gyrus (MFG) showed a main effect of stimulus type at 3 s [F(2,18) = 9.86, P < 0.0002] and 6 s [F(2,18) = 18.71, P < 0.0001] (Fig. 1B). Post hoc comparisons revealed that attentional targets elicited a larger signal than either emotional or neutral distracters, which did not differ significantly from each other. In 9 of 10 subjects, targets evoked greater activation in the right hemisphere. Fig. 1A shows the anterior–posterior distribution of MFG activation by targets. Targets produced the strongest response in the most anterior slices (22.50- to 33.75-mm anterior to the AC) with relatively little activation obtained more posteriorly. There was a trend for targets to evoke larger responses in the right hemisphere (P = 0.09).
Fig 1.
Anterior–posterior (A-P) distribution of prefrontal cortex activation. (A) MFG activation by attentional targets. Numbers in the box indicate relative distance from the anterior commissure in mm. (B–D) Mean fMRI signal change (±SEM) for the anterior MFG, anterior IFG, and posterior IFG, respectively. In A–D, data from the right and left hemispheres are collapsed. Note change in vertical scale across regions. Asterisks indicate time points where (B) targets evoked more activation than distracters, (C) distracters evoked more activation than targets, and (D) emotional distracters evoked more activation than neutral distracters or targets. The pound sign in C indicates the time point where emotional distracters evoked more activation than neutral distracters, which in turn evoked more activation than targets. (E) Group-averaged t test results (P < 0.001 uncorrected) for the contrast between emotional distracters (plotted in blue spectrum) and attentional targets (plotted in red spectrum). Attentional target activity was observed in left MFG (BA 9/46; Talairach coordinates –36, 35, 30) and right MFG (BA 9/46; 44, 35, 31). Emotional distracter activity was observed in left IFG (BA 45/47; −51, 33, 4) and right IFG (BA 45/47; 55, 33, 0). The coronal section in E shows the single prefrontal slice where differential activation between attentional targets and emotional distracters was most remarkable. However, peak activation to emotional distracters was located ≈1 cm more posteriorly within IFG. R, right hemisphere; L, left hemisphere.
In marked contrast to the results for the MFG, the ROI analysis of the inferior frontal gyrus (IFG) revealed strong activation by emotional distracters and lesser activation by neutral distracters at 6 s [F(2,18) = 18.55, P < 0.0001] and 9 s [F(2,18) = 14.71, P < 0.0002] (Fig. 1C). Post hoc tests showed that at the anterior IFG emotional distracters evoked more robust activity than neutral distracters that, in turn, evoked stronger activity than targets. Responses to emotional distracters were largest in the segment of the IFG located 18.75–22.50 mm anterior to the AC, i.e., more posteriorly than the maximum activity in MFG evoked by targets. At this more posterior IFG locus, targets and neutral distracters evoked little activity (Fig. 1D). The double dissociation between the role of MFG and IFG relative to attentional targets and emotional distracters was confirmed as an interaction in a two-way within-subjects ANOVA [F(2,18) = 87.60, P < 0.00001].
Qualitative inspection of the data from these PFC regions revealed a surprising feature. Namely, the MFG region activated by targets was deactivated by emotional distracters, and the anterior IFG region activated by distracters was relatively deactivated by targets (Fig. 1 B and C). Deactivations in these ROIs were confirmed by post hoc t tests computed to test negative deviations from zero signal change. Bilateral signal suppression in MFG by emotional distracters was significant at 6 s [(t(9) = −3.35, P = 0.009]. Bilateral signal suppression in IFG by targets was marginally significant at 9 s [t(9) = −2.20, P = 0.056], which was predominantly driven by the left hemisphere [t(9) = −2.76, P = 0.02]. A similar trend was observed in left IFG at 6 s [t(9) = −1.88, P = 0.093].
fMRI Results: Other Brain Regions.
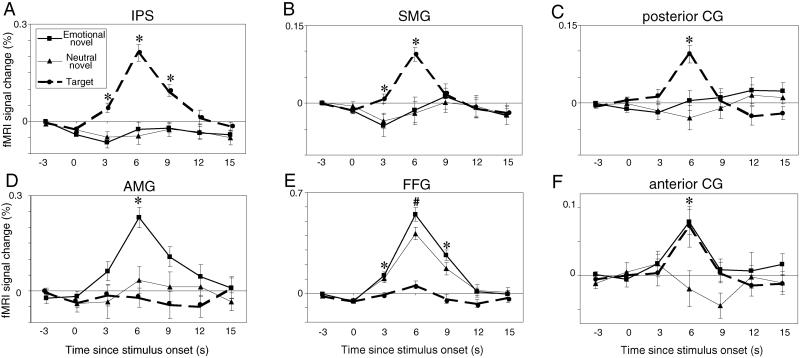
Strong and selective activation to targets was also observed at 6 s poststimulus in posterior parietal cortex, including the intraparietal sulcus [F(2,18) = 38.47, P < 0.0001] (Fig. 2A) and supramarginal gyrus [F(2,18) = 19.16, P < 0.0001] (Fig. 2B). The posterior cingulate gyrus was also strongly activated by targets (see Results) (Fig. 2C). None of these areas showed significant differences between emotional and neutral distracters.
Fig 2.
Mean signal change (±SEM) in posterior brain regions. (Upper) Dorsal regions are presented in the (A) intraparietal sulcus (IPS), (B) supramarginal gyrus (SMG), and (C) posterior cingulate (CG). (Lower) Ventral regions are presented in the (D) amygdala (AMG), (E) fusiform gyrus (FFG), and (F) anterior CG (from 18.75 to 7.5 mm rostral to the AC). Data from the right and left hemispheres are collapsed. Note change in vertical scale across regions. Asterisks indicate time points where (A–C) targets evoked more activation than distracters, (D) emotional distracters evoked more activation than neutral distracters or targets, (E) distracters evoked more activation than targets, and (F) targets and emotional distracters evoked more activation then neutral distracters. The pound sign in E indicates where emotional distracters elicited stronger responses than neutral distracters, which in turn elicited stronger responses than targets.
In contrast to these dorsal areas, ventral brain regions did not respond to target stimuli but showed differential engagement to the distracters as a function of their emotional content. Emotional distracters evoked significant activity in the amygdala relative to neutral distracters and targets at 6 s [F(2,18) = 11.73, P < 0.0006] (Fig. 2D). Emotional distracters also evoked more activation in the fusiform gyrus than did targets at 3 s [F(2,18) = 14.27, P < 0.0002], 6 s [F(2,18) = 56.74, P < 0.0001] and 9 s [F(2,18) = 19.43, P < 0.0001] (Fig. 2E). Post hoc tests revealed significantly more fusiform activity to emotional than neutral distracters at 6 s. The ROI analysis did not show any hemispheric asymmetry effects in these structures.
To test Mayberg's (8) hypothesis regarding the integrative role of the anterior cingulate gyrus, cingulate ROIs were drawn by subdividing the gyrus into four sectors according to horizontal distance from the AC. Each cingulate region included four slices. In each region at 6 s, repeated-measures ANOVAs with two independent variables (stimulus condition and hemisphere) were computed. In range from 18.75 to 7.5 mm anterior to the AC, a main effect of stimulus type was found [F(2,18) = 6.66, P < 0.007] (Fig. 2F). Post hoc comparisons indicated that both emotional distracters and targets evoked larger activation than neutral distracters. In range from 3.75 mm anterior to 7.5 mm posterior to the AC, similar effects were observed [F(2,18) = 14.45, P < 0.0002]. Of all brain regions we examined, these portions of the cingulate gyrus [corresponding to Brodmann's area (BA) 24] were the only areas with equivalent responses to attentional and emotional stimuli. In range from 11.25 to 22.5 mm posterior to the AC, there were no significant results. In range from 26.25 to 37.5 mm posterior to the AC, a main effect of stimulus type was observed [F(2,18) = 14.17, P < 0.0003]. In contrast to anterior regions of the cingulate gyrus, here targets generated larger responses than either emotional or neutral distracters, similar to the pattern seen in MFG and posterior parietal cortex.
Discussion
Behavioral studies have long shown that emotional stimuli can modulate the allocation of attentional resources (14, 15). The neural systems mediating the interaction between emotional and attentional functions, though, have not been well characterized. Previous studies have compared brain activation during attentional tasks to task-relevant stimuli with different levels of emotional meaning, as in the emotional Stroop interference paradigm (16, 17). These studies have supported a role for the rostral anterior cingulate when a prepotent emotional reaction diverts processing resources away from a simultaneous competing task-appropriate response. The present study took an alternate approach to this topic. Here, subjects performed an attentional target detection task while novel stimuli, parametrically varied for emotional arousal levels, were intermittently presented. Making the emotional stimuli task irrelevant enabled a dissociation of attentional and emotional operations into their constituent networks, while simultaneously revealing where those networks intersected in the brain. Our results indicate that these faculties are segregated into dissociable dorsal and ventral processing streams that extend into the PFC and integrate in the anterior cingulate gyrus.
A Ventrolateral PFC Interface for Emotional Arousal.
Neuropsychological reports have revealed dissociations across patient populations regarding the role of dorsal and ventral regions of PFC for cognitive and emotional functions, respectively (18, 19). Our findings provide evidence for this double dissociation in the healthy human brain. However, in the present study, activation to emotional distracters was strong in ventrolateral rather than ventromedial PFC, an area that has been emphasized in recent neuropsychological work. Neuroanatomical studies of the limbic forebrain have identified two parallel pathways by which emotionally arousing stimuli processed in the amygdala potentially interface with PFC (9). The first pathway is the canonical medial circuit linking the basal amygdala with ventromedial orbitofrontal cortex (BA 11), rostral insula, and subgenual portions of the anterior cingulated gyrus (BA 25). A second lateral pathway interconnects inferotemporal cortex and basal amygdala with ventrolateral PFC (BA 10/47) and rostral anterior cingulate (BA 24/32). The distracters in our task engaged the components of this latter circuit, with increasing levels of activation as a function of stimulus arousal (Figs. 1 and 2).
Two accounts have been generated to explain the differential engagement of medial versus lateral sectors of ventral PFC during emotional tasks. The first hypothesis is that negatively valent stimuli engage medial sectors, whereas positively valent stimuli engage lateral sectors (20). An alternative hypothesis is that internally generated emotional states and motivated behaviors preferentially elicit ventromedial PFC, whereas externally triggered ones depend on lateral regions (21, 22). The results of the present experiment and our prior study using auditory cues (23) are consistent with the latter interpretation. One must keep in mind, though, that fMRI susceptibility artifacts have precluded observation of a reliable signal in the medial circuit, and a direct test of these two accounts remains to be undertaken.
Defining the Role of PFC in Stimulus Novelty and Memory Encoding.
A number of electrophysiological studies conducted in normal subjects (24, 25), patients with prefrontal lesions (26–28), and epilepsy patients with implanted electrodes (29) have implicated the involvement of PFC in novelty detection. Little attention, however, has been paid to the properties of novel stimuli that are critical for engaging PFC. Our findings suggest that IFG activation to novel distracters depends on their emotional salience, particularly in more posterior regions of the IFG (Fig. 1D). Trial unique task-irrelevant novels that were neutral in emotional content also activated the anterior IFG, but the signal change was approximately 50% of that evoked by emotionally arousing novels (Fig. 1C). The neutral and emotional distracters were equated for at least four aspects of novelty: presentation history (habituation or repetition effects), presentation frequency (rarity of occurrence relative to other task events), stimulus complexity (including presence of human figures), and lower-order perceptual features (distinguishing colors, luminance, size, etc.). Therefore, these stimulus properties could not account for the differential engagement of IFG across novel categories. The anterior–posterior distribution of IFG activation to novel scenes—≈2 cm anterior to the AC—was the same as that seen in our previous study using alerting novel sounds (23). In combination, these results argue for a multimodal representation of sensory cues with high emotional salience in IFG.
The foregoing discussion has implications for understanding which features of novel sensory events make them particularly memorable. Several neuroimaging experiments using “subsequent memory” paradigms have shown that stimuli engaging IFG during their initial encoding are selectively retained over time (30, 31). The qualities of the stimuli coded in IFG that facilitate memory retention are unknown. The region of IFG whose activity predicts subsequent recollection overlaps with that observed to novel stimuli in the present report. Thus, the engagement of IFG may reflect an encoding mechanism that promotes stimuli into long-term storage as a function of their arousal value to the individual. However, we note that the same posterior IFG region (Fig. 1D) was also strongly activated by arousing environmental sounds (23), such as gun shots and breaking glass, suggesting that emotional arousal may be the critical factor in evoking activity in this region.
Role of Dorsolateral PFC in Attention and Cognition.
Attentional targets evoked significant activation in MFG, in consort with the parietal cortex and posterior cingulate gyrus, but novel stimuli did not, consistent with our prior work (11, 12). The MFG activation was maximal in a region more than 3 cm anterior to the AC, similar to that observed in our auditory study (23). Thus, both the IFG region activated by novels and the MFG region activated by targets appear to be multimodal in nature. The specific task-relevant computations performed within MFG remain unclear. We have shown activity in this area regardless of whether subjects mentally count the targets or respond to them with a button-press response (12). Thus, a specific task requirement to remember particular stimuli is not a necessary prerequisite for engaging this area.
A Clinical Model of Emotional Distraction on Attention-Demanding Tasks.
An unexpected outcome of this study was that the fMRI signal from the MFG region sensitive to attentional targets was suppressed or deactivated in response to emotional distracters. Similarly, the fMRI signal from the IFG region sensitive to emotional distracters was suppressed in response to attentional targets (Fig. 1C). Analogous deactivations in posterior ROIs were not observed. This pattern supports Drevets and Raichle's (32) observation that neural activity is reduced in some areas required for emotional processing during higher cognitive processes and vice versa.
A reciprocal relationship between dorsal and ventral PFC may provide a neural substrate for cognitive–emotional interactions and their dysregulation in mental illness. A hallmark of many affective disorders is the inability to maintain attentional focus on task-relevant operations in the face of prepotent distracting stimuli. Some subjects in our sample showed delayed reaction times to standards immediately after the emotional distracters, indicating a more protracted period of distraction than that seen to the emotional stimuli themselves. This pattern was less prominent to standards after neutral distracters. Thus, both the behavioral and neural effects of task-irrelevant stimulation were modulated by the arousing quality of the distracting material rather than to distraction or novelty per se. Our results provide support for Mayberg's (8) dual-stream theory of mood regulation, at least in healthy subjects. Failure to coordinate the PFC compartments mediating attention, emotion, and their interaction may provide a neural substrate underlying emotional distractibility in clinical populations.
Acknowledgments
This work was supported by Department of Veterans Affairs, National Institute of Mental Health Grants MH-05286 and MH-60451; the National Alliance for Research on Schizophrenia and Depression (K.S.L.); and the Japan Foundation for Aging and Health (H.Y.).
Abbreviations
PFC, prefrontal cortex
fMRI, functional MRI
AC, anterior commissure
PC, posterior commissure
ROI, region of interest
MFG, middle frontal gyrus
IFG, inferior frontal gyrus
BA, Brodmann's area
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ungerleider L. G. & Mishkin, M. (1982) in Analysis of Visual Behavior, eds. Ingle, D. J., Goodale, M. A. & Mansfield, R. J. W. (MIT Press, Cambridge, MA), pp. 549–586.
- 2.Wilson F. A. & Rolls, E. T. (1993) Exp. Brain Res. 93, 367-382. [DOI] [PubMed] [Google Scholar]
- 3.Rao S. C., Rainer, G. & Miller, E. K. (1997) Science 276, 821-824. [DOI] [PubMed] [Google Scholar]
- 4.Smith E. E., Jonides, J., Koeppe, R. A., Awh, E., Schumacher, E. H. & Minoshima, S. (1995) J. Cognit. Neurosci. 7, 337-356. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy G., Puce, A., Constable, R. T., Krystal, J. H., Gore, J. C. & Goldman-Rakic, P. (1996) Cereb. Cortex 6, 600-611. [DOI] [PubMed] [Google Scholar]
- 6.Owen A. M., Stern, C. E., Look, R. B., Tracey, I., Rosen, B. R. & Petrides, M. (1998) Proc. Natl. Acad. Sci. USA 95, 7721-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Esposito M., Postle, B. R., Ballard, D. & Lease, J. (1999) Brain Cognit. 41, 66-86. [DOI] [PubMed] [Google Scholar]
- 8.Mayberg H. S. (1997) J. Neuropsychiatr. 9, 471-481. [DOI] [PubMed] [Google Scholar]
- 9.Mega M. S., Cummings, J. L., Salloway, S. & Malloy, P. (1997) J. Neuropsychiatr. Clin. Neurosci. 9, 315-330. [DOI] [PubMed] [Google Scholar]
- 10.Mesulam M.-M., (2000) Principles of Behavioral and Cognitive Neurology (Oxford Univ. Press, New York), pp. 1–120.
- 11.McCarthy G., Luby, M., Gore, J. & Goldman-Rakic, P. (1997) J. Neurophysiol. 77, 1630-1634. [DOI] [PubMed] [Google Scholar]
- 12.Kirino E., Belger, A., Goldman-Rakic, P. & McCarthy, G. (2000) J. Neurosci. 20, 6612-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jha A. P. & McCarthy, G. (2000) J. Cognit. Neurosci. 12, 1-16. [DOI] [PubMed] [Google Scholar]
- 14.Easterbrook J. A. (1959) Psychol. Rev. 66, 183-201. [DOI] [PubMed] [Google Scholar]
- 15.LaBar K. S., Mesulam, M.-M., Gitelman, D. R. & Weintraub, S. (2000) Neuropsychologia 38, 1734-1740. [DOI] [PubMed] [Google Scholar]
- 16.George M. S., Ketter, T. A., Parekh, P. I., Rosinsky, N., Ring, H., Casey, B. J., Trimble, M. R., Horwitz, B., Herscovitch, P. & Post, R. M. (1994) Hum. Brain Mapp. 1, 194-209. [DOI] [PubMed] [Google Scholar]
- 17.Whalen P. J., Rauch, S. L., Etcoff, N. L., McInerney, S. C., Lee, M. B. & Jenike, M. A. (1998) J. Neurosci. 18, 411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bechara A., Damasio, H., Tranel, D. & Anderson, S. W. (1998) J. Neurosci. 18, 428-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuss D. T., Levine, B., Alexander, M. P., Hong, J., Palumbo, C., Hamer, L., Murphy, K. J. & Izukawa, D. (2000) Neuropsychologia 38, 388-402. [DOI] [PubMed] [Google Scholar]
- 20.Northoff G., Richter, A., Gessner, M., Schlagenhauf, F., Fell, J., Baumgart, F., Kaulisch, T., Kötter, R., Stephan, K. E., Leschinger, A., et al. (2000) Cereb. Cortex 10, 93-107. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y.-C., Thaler, D., Nixon, P. D., Stern, C. E. & Passingham, R. E. (1995) Exp. Brain Res. 102, 461-473. [DOI] [PubMed] [Google Scholar]
- 22.Lane R. D., Reiman, E. M., Axelrod, B., Yun, L.-S., Holmes, A. & Schwartz, G. E. (1998) J. Cognit. Neurosci. 10, 525-535. [DOI] [PubMed] [Google Scholar]
- 23.Hinton S. C., MacFall, J. R. & McCarthy, G. (1999) Neuroimage 9, S793. [Google Scholar]
- 24.Daffner K. R., Mesulam, M. M., Scinto, L. F. M., Cohen, L. G., Kennedy, B. P., West, W. C. & Holcomb, P. J. (1998) NeuroReport 9, 787-791. [DOI] [PubMed] [Google Scholar]
- 25.Opitz B., Mecklinger, A., Friederici, A. D. & von Cramon, D. Y. (1999) Cereb. Cortex 9, 379-391. [DOI] [PubMed] [Google Scholar]
- 26.Knight R. T. (1984) Electroencephalogr. Clin. Neurophysiol. 59, 9-20. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi S. & Knight, R. T. (1991) Electroencephalogr. Clin. Neurophysiol. 78, 50-55. [DOI] [PubMed] [Google Scholar]
- 28.Daffner K. R., Mesulam, M. M., Holcomb, P. J., Calvo, V., Acar, D., Chabrerie, A., Kikinis, R., Jolesz, F. A., Rentz, D. M. & Scinto, L. F. M. (2000) J. Neurol. Neurosurg. Psychiatr. 68, 18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baudena P., Halgren, E., Heit, G. & Clarke, J. M. (1995) Electroencephalogr. Clin. Neurophysiol. 94, 251-264. [DOI] [PubMed] [Google Scholar]
- 30.Brewer J. B., Zhao, Z., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. E. (1998) Science 281, 1185-1187. [DOI] [PubMed] [Google Scholar]
- 31.Wagner A. D., Schacter, D. L., Rotte, M., Koutstaal, W., Maril, A., Dale, A. M., Rosen, B. R. & Buckner, R. L. (1998) Science 281, 1188-1191. [DOI] [PubMed] [Google Scholar]
- 32.Drevets W. C. & Raichle, M. E. (1998) Cognit. Emot. 12, 353-385. [Google Scholar]