Abstract
Little is known about the physiological roles of the M5 muscarinic receptor, the last member of the muscarinic receptor family (M1–M5) to be cloned. In the brain, the M5 receptor subtype is preferentially expressed by dopaminergic neurons of the substantia nigra and the ventral tegmental area. Dopaminergic neurons located in the ventral tegmental area are known to play important roles in mediating both the rewarding effects of opiates and other drugs of abuse and the manifestations of opiate/drug withdrawal symptoms. We therefore speculated that acetylcholine-dependent activation of M5 receptors might modulate the manifestations of opiate reward and withdrawal. This hypothesis was tested in a series of behavioral, biochemical, and neurochemical studies using M5 receptor-deficient mice (M5−/− mice) as novel experimental tools. We found that the rewarding effects of morphine, as measured in the conditioned place preference paradigm, were substantially reduced in M5−/− mice. Furthermore, both the somatic and affective components of naloxone-induced morphine withdrawal symptoms were significantly attenuated in M5−/− mice. In contrast, the analgesic efficacy of morphine and the development of tolerance to the analgesic effects of morphine remained unaltered by the lack of M5 receptors. The finding that M5 receptor activity modulates both morphine reward and withdrawal processes suggests that M5 receptors may represent a novel target for the treatment of opiate addiction.
The M5 muscarinic receptor is the most recent member of the muscarinic acetylcholine receptor family (M1–M5) to be cloned (1, 2). Owing to the lack of ligands that can selectively stimulate or inhibit the M5 receptor (3, 4), the physiological roles of this receptor subtype have remained obscure (5, 6). To address this issue, we recently used a gene-targeting approach to generate M5 receptor-deficient mice (M5−/− mice, ref. 7).
Immunoprecipitation studies have shown that M5 receptors are expressed at very low levels in the brain, representing less than 2% of the total muscarinic receptor population (M1–M5) (8). Interestingly, M5 receptor mRNA has been identified as the only muscarinic receptor mRNA in dopamine-containing neurons of the substantia nigra and the ventral tegmental area (VTA) (9, 10). It has therefore been proposed that M5 receptors may play a role in modulating dopamine release from midbrain dopaminergic neurons (9, 10). Consistent with this hypothesis, we recently demonstrated that muscarinic agonist-induced increases in striatal dopamine release were reduced in M5−/− mice (7). Moreover, Forster et al. (11) recently reported that the sustained increase in dopamine levels in the nucleus accumbens (Acb) observed after electrical stimulation of the laterodorsal tegmental nucleus (12) is absent in M5−/− mice. Laterodorsal tegmental nucleus neurons represent the major source of cholinergic input to the dopamine-containing neurons of the VTA (13, 14) that project to the Acb and other limbic areas (15–17). It is therefore likely that activation of M5 receptors expressed by VTA neurons (9, 10) is responsible for the prolonged efflux of dopamine in the mouse Acb following laterodorsal tegmental nucleus stimulation (11).
It is well known that opiates modulate the activity of dopaminergic neurons in the VTA and that this modulation plays an important role in mediating both the rewarding effects of opiates and the opiate withdrawal syndrome (15–17). Moreover, previous studies have shown that activation of muscarinic receptors stimulates the activity of dopaminergic VTA neurons (18–21). Given the known facilitatory effects of M5 receptor activation on dopamine release from midbrain dopaminergic neurons (7, 11), we hypothesized that M5 receptor activation might modulate the behavioral and neurochemical correlates of drug reward and withdrawal. To test this hypothesis, we initially examined whether inactivation of the M5 receptor gene affected the rewarding properties of morphine, the prototypical opiate analgesic, and the severity of morphine withdrawal symptoms.
Our data indicate that the rewarding effects of morphine were substantially reduced in M5−/− mice. Moreover, both the somatic and affective components of the morphine withdrawal syndrome were attenuated in M5−/− mice. In contrast, the analgesic efficacy of morphine was not affected by the lack of M5 receptors. We propose that pharmacological blockade of central M5 receptors may represent an approach for the treatment of opiate (drug) addiction and withdrawal.
Materials and Methods
Animals.
The M5 muscarinic receptor gene was disrupted in mouse TC1 (129SvEv) embryonic stem cells, and M5−/− mice were generated as described (7). The M5 receptor mutation was maintained on a mixed (129SvEv/CF1, 50%/50%) or an isogenic (129SvEv) background. M5+/+ and M5−/− mice of mixed genetic background (129SvEv/CF1) were obtained by either homozygous matings (intermating of M5+/+ or M5−/− mice, age-matched mice) or heterozygous matings (intermating of M5+/− mice, littermates). Isogenic M5+/+ and M5−/− 129SvEv mice were obtained by mating male germ-line chimeras with female 129SvEv mice (Taconic), followed by intermating of F1 offspring heterozygous for the M5 receptor mutation. The resulting F2 mice were maintained by homozygous matings. All experiments were carried out with male mice that were at least 8 weeks old.
Behavioral Testing.
All behavioral tests were performed between 9 a.m. and 5 p.m. The conditioned place preference (CPP) apparatus consisted of a rectangular Plexiglas box divided into two compartments (15 × 20 × 18 cm) by a wall with a door. One compartment was black with a wire grid for a floor, and the other one was white with a smooth Plexiglas floor. One week before pretesting, mice were subjected to a 20-min habituation session during which they could freely explore the CPP apparatus. During the pretesting session (20 min, day 1), mice had access to both compartments. The time mice spent in each compartment and the number of transitions between compartments were recorded, and side preference was determined for each animal. On conditioning days 2–9, mice were injected i.p. with either saline or morphine and immediately confined to either the preferred (saline, dark chamber) or the nonpreferred (morphine, white chamber) side, respectively (one 30-min session per day). M5+/+ and M5−/− mice were divided into two groups of similar size. One group received saline on days 3, 5, 7, and 9 and morphine on days 2, 4, 6, and 8. The other group received saline on days 2, 4, 6, and 8 and morphine on days 3, 5, 7, and 9. On day 10, side preference was assessed in a 20-min testing session during which mice had free access to both compartments. The time mice spent in each chamber and the number of transitions between the two compartments were recorded. The amount of time mice spent in the nonpreferred compartment of the apparatus during the pretesting (preconditioning) phase was as follows for the M5+/+ mice: 129SvEv/CF1, age-matched: 300 ± 33 s, n = 22; 129SvEv/CF1, littermates: 330 ± 25 s, n = 12; CF1: 200 ± 39 s, n = 12; and 129SvEv: 55 ± 3 s, n = 8. The values obtained with the corresponding M5−/− mice did not differ significantly from these numbers (data not shown).
For CPP studies assessing the conditioned response to antagonist-precipitated withdrawal, mice were implanted s.c. with a pellet containing morphine (75 mg) or placebo. Habituation and pretesting trials were conducted as described above. Mice were subjected to two conditioning sessions (one 30-min session per day) involving alternating naloxone and saline injections on days 7 and 8 after the implantation of pellets. Naloxone (1 mg/kg i.p.) was paired with the preferred chamber. Preference testing was carried out 72 h after the last conditioning session.
The times spent in the drug-paired compartment during pretest and testing phases were compared using multiway ANOVA and Bonferroni's post hoc comparison matrix. Place preference conditioning is presented as [(Tdrug − Tpretest)/(Ttotal)] × 100.
The number of occurrences of naloxone (1 mg/kg i.p.)-induced withdrawal behaviors were recorded over a 30-min period in M5+/+ and M5−/− mice implanted with morphine pellets (1 × 75 mg s.c., 7 days). We also examined the effect of concurrent, chronic atropine administration on naloxone-precipitated morphine withdrawal in M5+/+ mice implanted with an s.c. morphine pellet (75 mg) and an s.c. osmotic minipump (Alza) delivering atropine sulfate at a rate of 15 mg/kg per day for 7 days.
Analgesia measurements were performed using the hot-plate and tail-flick tests (22). Analgesic efficacy is expressed as the percentage of the maximum possible effect = 100 × [(Latencydrug − Latencycontrol)/(Timecutoff − Latencycontrol). The maximum cutoff times for the tail-flick and hot-plate tests were 10 s and 30 s, respectively. Acute tolerance to morphine analgesia was tested by administering a single high dose of morphine (100 mg/kg i.p.) and determining the analgesic potency of a smaller morphine dose (10 mg/kg i.p.) 24 h later.
Neurochemistry.
For in vivo microdialysis studies, mice were anesthetized with isoflurane, and guide cannulae were implanted above the Acb (+1.7 AP, −1.0 ML, −4.2 DV; ref. 23). Five days after surgery and 1 day before sampling, a microdialysis probe (CMA 11/14, 1-mm tip length) was inserted into the guide cannula and perfused with artificial cerebrospinal fluid at a rate of 0.6 μl/min while the mice ambulated on a tether. Samples were collected for 15 min, and dopamine concentrations quantified using HPLC with electrochemical detection (24). The overall effects of morphine and K+ on dialysate dopamine levels were analyzed by calculating area under the curve (AUC) values for each animal and stimulus condition relative to the last basal value. Time course data were analyzed using a two-factor (genotype × time) ANOVA with repeated measures over time and post hoc comparison matrices.
No net flux determination of basal extracellular dopamine concentrations involved perfusion of dopamine (0, 5, 10, 20, and 40 nM) in random order through the dialysis probe (25). A linear plot of the dialysate dopamine concentrations (DAout) obtained after perfusion of the various dopamine concentrations (DAin) was constructed for each animal, (DAin − DAout) vs. DAin. The estimated extracellular dopamine concentration is defined as the concentration at which DAin − DAout = 0.
Τhe density of central μ-opioid receptors in membrane homogenates from selected brain regions was measured using [3H]DAMGO (5 nM) as a radioligand, essentially as described (26). D1 and D2 dopamine receptor levels were determined in the presence of 4 nM [3H]SCH23390 and 2 nM [3H]spiperone, respectively, following a published protocol (27). Data are presented as the mean ± SD of two independent experiments performed in triplicate, with each experiment using tissues pooled from four mice.
M4 muscarinic receptor levels were assessed using a previously described immunoprecipitation strategy (28). Membrane preparations were incubated with 2 nM [3H]quinuclidinyl benzilate, a non-subtype selective muscarinic receptor antagonist, and the labeled receptors were solubilized with 1% digitonin. These solubilized, radiolabeled receptors were then immunoprecipitated and quantified with an M4 receptor-specific antiserum (28). Data are presented as means ± SD of two independent experiments performed in triplicate, with each experiment using tissues pooled from four mice.
Fos-B expression in the Acb of M5+/+ and M5−/−mice was assessed by immunoblot analysis (29). Acb tissue was removed by frozen micropunch from mice implanted with either placebo or morphine pellets (75 mg/kg s.c., 7 days) alone or with a combination of pellets and osmotic minipumps delivering atropine sulfate (15 mg/kg per day s.c., 7 days). Rabbit anti-Fos-B (1:200, Santa Cruz Biotechnology) and rabbit anti-α-tubulin (1:10,000, Amersham Pharmacia) were used as the primary antibodies. Protein bands were visualized using horseradish peroxidase-conjugated goat anti-rabbit IgG, SuperSignal substrate (Pierce), and film exposure. Data are presented as ratios of calibrated optical densities of Fos-B and α-tubulin bands.
Results and Discussion
Morphine-Induced Conditioned Place Preference.
As described previously (7), M5−/− mice (129SvEv/CF1 hybrids) did not differ significantly from their wild-type littermates in body weight, body temperature, distance traveled in an open field, motor coordination, and several other physiological and behavioral parameters. To examine whether the lack of M5 receptors was associated with changes in the reinforcing properties of opiates, we used the CPP procedure (22, 30), a test useful for investigating pathways mediating the rewarding effects of drugs of abuse (30–32). In the CPP paradigm, mice learn to associate administration of a drug with one of two compartments which differ visually and texturally (30).
In M5+/+ mice, administration of morphine (2.5, 5, 10, and 25 mg/kg i.p.) significantly increased the amount of time the animals spent in the morphine-associated chamber (Fig. 1A). In contrast, morphine doses of 2.5, 5, or 10 mg/kg i.p. failed to induce place preference in M5−/− mice (Fig. 1A). Only the highest morphine dose used (25 mg/kg i.p.) was effective in producing significant place conditioning in M5−/− mice (Fig. 1A). However, the magnitude of this effect was significantly smaller than the corresponding response of M5+/+ mice.
Fig 1.
Morphine-induced CPP in M5+/+ and M5−/− mice. (A) CPP studies were carried out as described under Materials and Methods. Morphine-associated place conditioning is substantially reduced in M5−/− mice (129SvEv/CF1 hybrids resulting from homozygous matings, age-matched mice). *, P < 0.05, **, P < 0.01. Significantly different from the corresponding M5+/+ morphine-treated groups (ANOVA, Bonferroni's test, n = 10–13). a, significantly different from the corresponding saline groups (P < 0.05). (B) The reduction in preference for the morphine (5 mg/kg i.p.)-paired chamber is also observed in M5−/− mice generated by intermating heterozygous M5+/− mice (129SvEv/CF1 hybrids, littermates) or in isogenic M5−/− mice (129SvEv). The CPP responses to 5 mg/kg morphine shown in A (129SvEv/CF1 hybrids, age-matched) are included for comparison. Data obtained using the two parental strains (CF1 and 129SvEv) are presented as controls. **, P < 0.01. Significantly different from the two parental strains and the corresponding M5+/+ morphine-treated groups (ANOVA, Bonferroni's test, n = 8, 12).
The reduction in morphine place preference was observed independent of whether M5−/− mice (129SvEv/CF1 hybrids, 50%/50%) were produced by homozygous matings (age-matched mice, Fig. 1A) or by crosses of heterozygous M5+/− mice (littermates, Fig. 1B). In addition, the two parental mouse strains (CF1 and 129SvEv) did not differ significantly in the degree of morphine-induced place preference (Fig. 1B), excluding the possibility that the behavioral deficit displayed by the M5−/− mice was caused by differences in morphine sensitivity between the CF1 and 129SvEv strains. Consistent with this notion, isogenic M5−/− mice containing the M5 receptor mutation on a pure genetic background (129SvEv) also exhibited an attenuated conditioned response to morphine (Fig. 1B). Together, these observations demonstrate that the reduction in morphine-associated place preference displayed by the M5−/− mice is because of the lack of functional M5 receptors and is not an artifact associated with a particular genetic background or mating scheme (33).
Morphine-Stimulated Dopamine Release in the Acb.
The activity of midbrain dopamine neurons projecting to the Acb has been implicated in the process of incentive salience and in mediating the conditioned rewarding effects of opiates (15–17, 34). We therefore used in vivo microdialysis to quantify basal and morphine-evoked dopamine levels in the Acb of M5+/+ and M5−/− mice (129SvEv/CF1 hybrids). The lack of M5 receptors did not alter basal extracellular dopamine concentrations (M5+/+: 8.7 ± 1.2 nM, n = 12; M5−/−: 8.2 ± 1.1 nM, n = 10) or depolarization (60 mM KCl)-induced dopamine release (M5+/+: 260 ± 90 AUC units, n = 8; M5−/−: 280 ± 71 AUC units, n = 8). Morphine administration (25 mg/kg i.p.) significantly increased dialysate concentrations of dopamine in the Acb of M5+/+ mice (Fig. 2). However, consistent with the reduced efficacy of morphine in producing conditioned rewarding effects, this response was substantially reduced in M5−/− mice (M5+/+: 170 ± 31 AUC units, n = 13; M5−/−: 21 ± 28 AUC units, n = 16, P < 0.01, t test).
Fig 2.
Morphine-induced increases in dopamine levels in the Acb of M5+/+ and M5−/− mice. Dialysate dopamine concentrations in the Acb of M5−/− mice (129SvEv/CF1 hybrids, age-matched) are greatly reduced following acute morphine administration (arrow, 25 mg/kg i.p.). Each point represents the mean ± SEM of dopamine concentrations in individual samples accumulated over 15 min of perfusion (0.6 μl/min). *, P < 0.05, **, P < 0.01. Significantly different from corresponding time point in M5−/− mice (repeated measures ANOVA, Tukey's test, n = 13, 16).
Central μ-Opioid, D1 and D2 Dopamine, and M4 Muscarinic Receptor Densities.
The rewarding effects of morphine result from the activation of μ-opioid receptors in the mesocorticolimbic system associated with an increase in dopamine release in the Acb (35). To exclude the possibility that the deficits in morphine-induced place preference and dopamine release displayed by the M5−/− mice were because of a reduction in μ-opioid receptor densities, we quantified central μ-receptor levels using [3H]DAMGO (5 nM) as a radioligand. M5+/+ and M5−/− mice (129SvEv/CF1 hybrids) displayed similar densities of μ-opioid receptors (P > 0.05, unpaired two-tailed Student's t test) in all analyzed brain regions (fmol/mg protein, M5+/+ vs. M5−/−): cortex, 58 ± 3.3 vs. 74 ± 18; hippocampus, 70 ± 1.9 vs. 61 ± 6.4; striatum, 92 ± 4.2 vs. 100 ± 1.6; brainstem, 63 ± 2.4 vs. 78 ± 0.4; cerebellum, 67 ± 6.1 vs. 68 ± 11; hypothalamus, 73 ± 1.9 vs. 61 ± 6.4; spinal cord, 100 ± 6.2 vs. 94 ± 8.5.
To exclude the possibility that the lack of M5 receptors led to secondary changes in dopamine receptor densities, we also investigated the binding of D1 ([3H]SCH23390, 4 nM) and D2 ([3H]spiperone, 2 nM) receptor-selective radioligands in striatum and midbrain, the two brain regions in which M5 receptors are preferentially expressed (9, 10). We found that D1 and D2 dopamine receptor levels in these two brain regions were not significantly affected (P > 0.05, unpaired two-tailed Student's t test) by the absence of functional M5 receptors (129SvEv/CF1 hybrid mice). Specifically, the following receptor densities were obtained: fmol/mg protein, M5+/+ vs. M5−/−; [3H]SCH23390: striatum, 2,100 ± 160 vs. 2000 ± 63; midbrain, 1,000 ± 130 vs. 1,200 ± 240; [3H]spiperone: striatum, 970 ± 80 vs. 900 ± 19; midbrain, 500 ± 103 vs. 500 ± 46.
Because M5 receptors are localized on midbrain dopaminergic neurons (9, 10) and M4 receptors are thought to modulate dopaminergic neurotransmission (28), we also tested the possibility that disruption of the M5 receptor gene might have caused secondary changes in M4 receptor levels. For these studies, membranes derived from the striatum and midbrain of M5+/+ and M5−/− mice (129SvEv/CF1 hybrids) were first incubated with the non-subtype-selective muscarinic radioligand, [3H]quinuclidinyl benzilate (2 nM), followed by immunoprecipitation of the solubilized, radiolabeled receptors with an M4 receptor-specific antiserum (28). This analysis showed that disruption of the M5 receptor gene did not lead to altered M4 receptor densities (P > 0.05, unpaired two-tailed Student's t test) in the two analyzed brain areas (fmol/mg protein, M5+/+ vs. M5−/−; striatum: 380 ± 5 vs. 390 ± 37; midbrain: 360 ± 27 vs. 310 ± 18).
Morphine-Induced Analgesic Effects.
The sensitivity of M5+/+ and M5−/− mice to the anti-nociceptive effects of morphine was evaluated using the tail-flick and hot-plate tests (22). In M5+/+ mice (129SvEv/CF1 hybrids), morphine (2.5, 5, and 10 mg/kg i.p.) produced dose-related analgesic effects in both assays (Fig. 3A). The magnitude of these responses remained essentially unaltered in M5−/− mice (Fig. 3A). Moreover, M5+/+ and M5−/− mice developed a similar degree of tolerance to morphine-induced analgesia. This is illustrated in Figs. 3B and 4, which show the development of morphine tolerance 24 h after administration of a single high dose of morphine (100 mg/kg i.p.) or during chronic morphine administration (75 mg s.c. pellet, 14 days), respectively.
Fig 3.
Morphine-induced analgesia and development of acute morphine tolerance in M5+/+ and M5−/− mice. (A) The lack of M5 receptors has no significant effect on morphine (i.p.) analgesia. HP, hot-plate; TF, tail-flick (n = 9–12). (B) M5+/+ and M5−/− mice do not differ in the development of acute tolerance to morphine analgesia. Tolerance was assessed by determining the analgesic potency of morphine (10 mg/kg i.p.) 24 hr after administration of a single dose of morphine (100 mg/kg i.p.). a, Significantly different from the corresponding naive groups, P < 0.01, n = 9–14. All experiments were carried out with 129SvEv/CF1 hybrid mice.
Fig 4.
Development of tolerance to the analgesic actions of morphine chronically administered to M5+/+ and M5−/− mice. Disruption of the M5 receptor gene has no significant effect on the development of tolerance to the analgesic effects of chronically administered morphine (75 mg s.c. pellet). Analgesia measurements were carried out at the indicated time points (M, morphine pellet; P, placebo pellet) (n = 7–16, 129SvEv/CF1 hybrids).
Fos-B Expression in the Acb After Chronic Administration of Morphine.
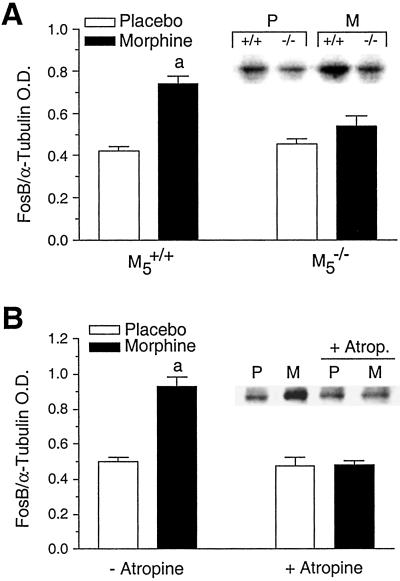
The long-term synaptic changes that develop after chronic morphine administration are associated with elevated expression levels of Fos-related immediate early gene products including Fos-B (29). Consistent with this finding, immunoblot analysis showed that chronic administration of morphine (75 mg s.c. pellet, 7 days) led to a significant increase in Fos-B expression in the Acb of M5+/+ mice (Fig. 5A, 129SvEv/CF1 hybrids). Strikingly, the magnitude of this response was greatly reduced in M5−/− mice (Fig. 5A). In agreement with this observation, the morphine-induced increase in Fos-B expression observed in M5+/+ mice was blocked by coadministration of the centrally active muscarinic antagonist, atropine (administered s.c. via osmotic minipumps at a dose of 15 mg/kg per day for 7 days, Fig. 5B).
Fig 5.
Immunoblot analysis of morphine-induced Fos-B expression in the Acb of M5+/+ and M5−/− mice. (A) The magnitude of Fos-B expression in the Acb of M5−/− mice chronically treated with morphine pellets (75 mg s.c., 7 days) is reduced relative to M5+/+ mice. (Inset) Immunoblot of Fos-B expression in M5+/+ (+/+) and M5−/− (−/−) mice implanted with placebo (P) or morphine (M) pellets. a, significantly different from all other groups, P < 0.01, ANOVA, Bonferroni's test, n = 7 or 8. (B) Concurrent treatment of M5+/+ mice with morphine (75 mg s.c. pellet, 7 days) and atropine (15 mg/kg per day, s.c., 7 days) mimics the effect of the M5 receptor mutation on Fos-B expression in the Acb. (Inset) Immunoblot of Fos-B expression in M5+/+ mice implanted with placebo (P) or morphine (M) pellets alone or combined with atropine administered via osmotic minipumps. a, significantly different from all other groups, P < 0.01, ANOVA, Bonferroni's test, n = 4 or 5. All experiments were carried out with 129SvEv/CF1 hybrid mice.
Naloxone-Induced Withdrawal Symptoms and Conditioned Place Aversion in Mice Chronically Treated with Morphine.
Naloxone (1 mg/kg i.p.) induced a characteristic spectrum of somatic withdrawal signs in M5+/+ and M5−/− mice chronically treated with morphine (75 mg s.c. pellet, 7 days; Fig. 6). However, the severity of withdrawal symptoms was significantly reduced in M5−/− mice (Fig. 6). These differences were observed independent of whether M5−/− mice (129SvEv/CF1 hybrids, 50%/50%) were obtained by homozygous matings (age-matched mice) or by crosses of heterozygous M5+/− mice (littermates) (Fig. 6). As observed with the M5−/− hybrid mice, isogenic M5−/− mice carrying the M5 receptor mutation on a pure genetic background (129SvEv) showed similar reductions in the severity of morphine withdrawal symptoms (Fig. 6). These findings rule out the possibility that the observed differences in the severity of withdrawal symptoms were an artifact caused by a particular genetic background or mating scheme (33).
Fig 6.
Naloxone-induced morphine withdrawal symptoms in M5+/+ and M5−/− mice. The number of occurrences of the indicated withdrawal behaviors (A, jumps; B, wet-dog shakes; C, paw tremors; D, teeth chattering) were recorded over a 30-min period following administration of naloxone (1 mg/kg i.p.) to mice chronically treated with morphine (75 mg s.c. pellet, 7 days). The severity of morphine withdrawal symptoms was less pronounced in M5−/− mice, independent of whether M5−/− mice were generated by homozygous (129SvEv/CF1 hybrids, age-matched mice) or heterozygous matings (129SvEv/CF1 hybrids, littermates). Similar results were obtained with isogenic M5+/+ and M5−/− mice (129SvEv). Data obtained using the two parental strains (CF1 and 129SvEv) are also included for control purposes. *, P < 0.05; **, P < 0.01. Significantly different from the corresponding M5+/+ groups, ANOVA, Bonferroni's post hoc test, n = 8–26.
In addition to the above somatic manifestations of naloxone-precipitated morphine withdrawal, M5−/− mice (129SvEv/CF1 hybrids) showed significantly less weight loss 4 h after naloxone administration than the corresponding M5+/+ mice (0.4 ± 0.2 vs. 1.6 ± 0.2 g, respectively, n = 8, P < 0.01). However, there were no significant differences between the two genotypes in the number of fecal boli excreted (per hour: M5−/−, 10 ± 0.8; M5+/+, 10 ± 0.6, n = 8), a peripheral manifestation of opiate withdrawal.
In agreement with the results obtained with M5−/− mutant mice, studies with M5+/+ mice (129SvEv/CF1 hybrids) showed that coadministration of morphine (75 mg s.c. pellet, 7 days) with atropine (15 mg/kg per day s.c. for 7 days via osmotic minipump) strongly suppressed the naloxone-precipitated withdrawal symptoms (observation period: 30 min). Specifically, the number of episodes of jumping (morphine alone vs. morphine + atropine: 50.2 ± 4.1 vs. 10.4 ± 2.7, n = 10, 9), wet-dog shakes (13.1 ± 1.3 vs. 4.0 ± 0.7), paw tremors (4.4 ± 0.9 vs. 1.1 ± 0.4), and teeth chattering (1.4 ± 0.3 vs. 0.3 ± 0.2) was significantly reduced in atropine-treated mice (P < 0.01 in all cases, t test).
Finally, the conditioned aversive effects of morphine withdrawal were investigated using the CPP paradigm (23, 30). In M5+/+ mice (129SvEv mice) chronically treated with morphine (75 mg s.c. pellet, 7 days), the opioid antagonist, naloxone (1 mg/kg i.p.), induced a pronounced conditioned place aversion response, as indicated by a significant reduction in the amount of time M5+/+ mice spent in the naloxone-paired chamber (Fig. 7). The magnitude of this effect was significantly reduced (by ≈50%) in M5−/− mice (Fig. 7).
Fig 7.
Naloxone-induced conditioned place aversion in M5+/+ and M5−/− mice. M5−/− mice (129SvEv mice) chronically treated with morphine (75 mg s.c. pellet, 7 days) show a reduction in the magnitude of naloxone (1 mg/kg i.p.)-induced conditioned place aversion. a, significantly different from M5+/+ pretest and naloxone/morphine-treated M5−/− test groups, P < 0.01; b, significantly different from M5−/− pretest group, P < 0.05; ANOVA, Bonferroni's test, n = 10–12.
In summary, we demonstrate that M5−/− mice displayed a significant reduction in morphine-induced CPP and the severity of the naloxone-precipitated withdrawal syndrome. These data are consistent with the concept that M5 receptor activation stimulates midbrain dopaminergic neurons (7, 9–11), thus modulating the behavioral manifestations of both morphine reward and withdrawal. Because at least some of the reinforcing properties of other drugs of abuse, including nicotine, alcohol, and cocaine, also involve mesocorticolimbic dopaminergic pathways (15–17), it is possible that M5 receptors have a more general role in modulating drug-seeking behavior. In agreement with this hypothesis, preliminary studies showed that cocaine-induced CPP was also significantly reduced in M5−/− mice (I.F., J.W., and A.S.B., unpublished results).
Yeomans et al. (36) recently showed that central administration of an M5 receptor antisense oligonucleotide decreased the sensitivity to rewarding hypothalamic stimulation in rats, as assessed in a bar-pressing paradigm. These data further support the concept that M5 receptors play a key role in modulating behavioral processes that are dependent on the activity of midbrain dopaminergic neurons.
Whereas the rewarding effects of morphine were substantially reduced in M5−/− mice, the analgesic efficacy of morphine remained unaffected by the lack of M5 receptors. Morphine and other opiate analgesics are widely used for the control of severe pain, but they have a high potential of abuse and dependence (37). Our data therefore suggest that disruption of M5 receptor function, e.g., by administration of centrally active, selective M5 receptor antagonists, may represent a strategy to reduce the abuse liability of opiates without affecting their analgesic properties. Because M5 receptors show a relatively restricted localization in the brain (9, 10) and the lack of M5 receptors does not lead to significant peripheral muscarinic side effects (7), it is likely that such agents will be well tolerated.
Our results underscore the usefulness of M5−/− mice as experimental tools for delineating the behavioral and pharmacological roles of the M5 muscarinic receptor subtype.
Acknowledgments
We thank Yinghong Cui for her expert technical assistance. This research was supported by a Cooperative Research and Development Agreement between the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and the Eli Lilly Research Laboratories.
Abbreviations
Acb, nucleus accumbens
AUC, area under the curve
CPP, conditioned place preference
VTA, ventral tegmental area
References
- 1.Bonner T. I., Young, A. C., Brann, M. R. & Buckley, N. J. (1988) Neuron 1, 403-410. [DOI] [PubMed] [Google Scholar]
- 2.Liao C. F., Themmen, A. P., Joho, R., Barberis, C., Birnbaumer, M. & Birnbaumer, L. (1989) J. Biol. Chem. 264, 7328-7337. [PubMed] [Google Scholar]
- 3.Wess J. (1996) Crit. Rev. Neurobiol. 10, 69-99. [DOI] [PubMed] [Google Scholar]
- 4.Caulfield M. P. & Birdsall, N. J. M. (1998) Pharmacol. Rev. 50, 279-290. [PubMed] [Google Scholar]
- 5.Reever C. M., Ferrari-DiLeo, G. & Flynn, D. D. (1997) Life Sci. 60, 1105-1112. [DOI] [PubMed] [Google Scholar]
- 6.Eglen R. M. & Nahorski, S. R. (2000) Br. J. Pharmacol. 130, 13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada M., Lamping, K. G., Duttaroy, A., Zhang, W., Cui, Y., Bymaster, F. P., McKinzie, D. L., Felder, C. C., Deng, C. X., Faraci, F. M. & Wess, J. (2001) Proc. Natl. Acad. Sci. USA 98, 14096-14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuda R. P., Ciesla, W., Flores, L. R., Wall, S. J., Li, M., Satkus, S. A., Weisstein, J. S., Spagnola, B. V. & Wolfe, B. B. (1993) Mol. Pharmacol. 43, 149-157. [PubMed] [Google Scholar]
- 9.Vilaro M. T., Palacios, J. M. & Mengod, G. (1990) Neurosci. Lett. 114, 154-159. [DOI] [PubMed] [Google Scholar]
- 10.Weiner D. M., Levey, A. I. & Brann, M. R. (1990) Proc. Natl. Acad. Sci. USA 87, 7050-7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forster G. L., Yeomans, J. S., Takeuchi, J. & Blaha, C. D. (2002) J. Neurosci. 22, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forster G. L. & Blaha, C. D. (2000) Eur. J. Neurosci. 12, 3596-3604. [DOI] [PubMed] [Google Scholar]
- 13.Oakman S. A., Faris, P. L., Kerr, P. E., Cozzari, C. & Hartman, B. K. (1995) J. Neurosci. 15, 5859-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaha C. D., Allen, L. F., Das, S., Inglis, W. L., Latimer, M. P., Vincent, S. R. & Winn, P. (1996) J. Neurosci. 16, 714-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koob G. F. (1992) Trends Pharmacol. Sci. 13, 177-184. [DOI] [PubMed] [Google Scholar]
- 16.Wise R. A. (1996) Curr. Opin. Neurobiol. 6, 243-251. [DOI] [PubMed] [Google Scholar]
- 17.Koob G. F., Sanna, P. P. & Bloom, F. E. (1998) Neuron 21, 467-476. [DOI] [PubMed] [Google Scholar]
- 18.Lacey M. G., Calabresi, P. & North, R. A. J. (1990) J. Pharmacol. Exp. Ther. 253, 395-400. [PubMed] [Google Scholar]
- 19.Yeomans J. & Baptista, M. (1997) Pharmacol. Biochem. Behav. 57, 915-921. [DOI] [PubMed] [Google Scholar]
- 20.Gronier B. & Rasmussen, K. (1999) Neuropharmacology 38, 1903-1912. [DOI] [PubMed] [Google Scholar]
- 21.Gronier B., Perry, K. W. & Rasmussen, K. (2000) Psychopharmacology 147, 347-355. [DOI] [PubMed] [Google Scholar]
- 22.Fedorova I., Hashimoto, A., Fecik, R. A., Hedrick, M. P., Hanus, L. O., Boger, D. L., Rice, K. C. & Basile, A. S. (2001) J. Pharmacol. Exp. Ther. 299, 322-342. [PubMed] [Google Scholar]
- 23.Paxinos G. & Franklin, K. B. J., (2001) The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego).
- 24.Pani A. K., Shippenberg, T. S., Heidebreder, C. & Espey, M. G. (1997) in Current Protocols in Neuroscience, eds. Crawley, J. N., Gerfen, C. R., Rogawski, M. A., Sibley, D. R. & Skolnick, P. (Wiley, New York).
- 25.Justice J. B. (1993) J. Neurosci. Methods 48, 263-276. [DOI] [PubMed] [Google Scholar]
- 26.Duttaroy A. & Yoburn, B. C. (2000) Synapse 37, 118-124. [DOI] [PubMed] [Google Scholar]
- 27.Farrell C. B., Lawlor, M., Dunne, A. & O'Boyle, K. M. (1995) J. Neurochem. 65, 1124-1130. [DOI] [PubMed] [Google Scholar]
- 28.Gomeza J., Zhang, L., Kostenis, E., Felder, C., Bymaster, F., Brodkin, J., Shannon, H., Xia, B., Deng, C. & Weiss, J. (1999) Proc. Natl. Acad. Sci. USA 96, 10483-10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nye H. E. & Nestler, E. J. (1996) Mol. Pharmacol. 49, 636-645. [PubMed] [Google Scholar]
- 30.Tzschentke T. M. (1998) Prog. Neurobiol. 56, 613-672. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado R., Saiardi, A., Valverde, O., Samad, T. A., Roques, B. P. & Borrelli, E. (1997) Nature (London) 388, 586-589. [DOI] [PubMed] [Google Scholar]
- 32.Murtra P., Sheasby, A. M., Hunt, S. P. & De Felipe, C. (2000) Nature (London) 405, 180-183. [DOI] [PubMed] [Google Scholar]
- 33.Gerlai R. (1996) Trends Neurosci. 19, 177-181. [DOI] [PubMed] [Google Scholar]
- 34.Shippenberg T. S. & Elmer, G. I. (1998) Crit. Rev. Neurobiol. 12, 267-303. [DOI] [PubMed] [Google Scholar]
- 35.Matthes H. W., Maldonado, R., Simonin, F., Valverde, O., Slowe, S., Kitchen, I., Befort, K., Dierich, A., Le Meur, M., Dolle, P., et al. (1996) Nature (London) 383, 819-823. [DOI] [PubMed] [Google Scholar]
- 36.Yeomans J. S., Takeuchi, J., Baptista, M., Flynn, D. D., Lepik, K., Nobrega, J., Fulton, J. & Ralph, M. R. (2000) J. Neurosci. 20, 8861-8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien C. P. (2001) in The Pharmacological Basis of Therapeutics, eds. Hardman, J. G. & Limbird, L. E. (McGraw-Hill, New York), pp. 621–642.