Abstract
Na+/H+ exchanger regulatory factor (NHERF)-1 and NHERF-2, two structurally related protein adapters containing tandem PSD-95/Discs large/ZO-1 (PDZ) domains, were identified as essential factors for protein kinase A-mediated inhibition of the sodium-hydrogen exchanger, NHE3. NHERF-1 and NHERF-2 also bound other cellular targets including the sodium-phosphate cotransporter type IIa encoded by the NPT2 gene. Targeted disruption of the mouse NHERF-1 gene eliminated NHERF-1 expression in kidney and other tissues of the mutant mice without altering NHERF-2 levels in these tissues. NHERF-1 (+/−) and (−/−) male mice maintained normal blood electrolytes but showed increased urinary excretion of phosphate when compared with wild-type (+/+) animals. Although the overall levels of renal NHERF-1 targets, NHE3 and Npt2, were unchanged in the mutant mice, immunocytochemistry showed that the Npt2 protein was aberrantly localized at internal sites in the renal proximal tubule cells. The mislocalization of Npt2 paralleled a reduction in the transporter protein in renal brush–border membranes isolated from the mutant mice. In contrast, NHE3 was appropriately localized at the apical surface of proximal tubules in both wild-type and mutant mice. These data suggested that NHERF-1 played a unique role in the apical targeting and/or trafficking of Npt2 in the mammalian kidney, a function not shared by NHERF-2 or other renal PDZ proteins. Phosphate wasting seen in the NHERF-1(−/−) null mice provided a new experimental system for defining the role of PDZ adapters in the hormonal control of ion transport and renal disease.
NHERF-1/EBP50 and NHERF-2/E3KARP/TKA1 are two members of the Na+/H+ exchanger regulatory factor (NHERF) protein family (1) that contain two tandem PSD-95/Discs large/ZO-1 (PDZ) protein interaction domains and a C-terminal ezrin-radixin-moesin-merlin–binding domain. Expression of either NHERF protein in cultured cells that expressed the Na+/H+ exchanger isoform, NHE3, promoted the formation of a multiprotein complex that included NHE3 and ezrin (2, 3). Ezrin tethered this complex to the actin cytoskeleton (4) and also functioned as an A-kinase-anchoring protein (AKAP) to recruit protein kinase A and promote NHE3 phosphorylation, which inhibited Na+/H+ exchange (2, 5), in response to hormones that elevated intracellular cAMP.
NHERF-1 and NHERF-2 bound numerous membrane and cytosolic proteins (6). Some of these, like the sodium-phosphate transporter, Npt2 (also termed Na-Pi IIa), not only bound both NHERF isoforms but also associated with several other PDZ proteins (7), which raised the question of which, if any, of these protein adapters participated in the hormone-induced internalization of Npt2. Some NHERF targets, like Taz (8) and the Ca2+-ATPase, PMCA2b (9), showed preferential in vitro binding to NHERF-2 over NHERF-1. The physiological relevance of this finding in tissues like kidney that expressed both NHERF proteins is unclear. NHERF-1 differed from NHERF-2 in being phosphorylated in mammalian cells by several protein kinases (10). NHERF-1 was also an estrogen-induced gene (11), and breast cancer cells lacking estrogen receptor failed to express NHERF-1 (12), which suggested that NHERF-1 played a unique role in transducing hormonal signals that regulate ion transport in the mammalian kidney. In both rat (13) and mouse (14) kidney, NHERF-1 was highly expressed in the proximal convoluted tubule, whereas NHERF-2 was principally expressed in the distal nephron. This finding also suggested that NHERF-1, and not NHERF-2, mediated the hormonal control of NHE3 and other ion transporters in the proximal tubules. To establish the physiological role of NHERF-1 in kidney and the potential redundancy in NHERF-1 and NHERF-2 functions in mammalian tissues, we undertook the targeted disruption of the mouse NHERF-1 gene. Our data suggested that NHERF-1 played a key role in targeting and/or trafficking Npt2 in the mouse kidney, and defects in apical localization of Npt2 contributed to the phosphate wasting seen in the NHERF-1-deficient mice.
Materials and Methods
Generation of NHERF-1-Null Mice.
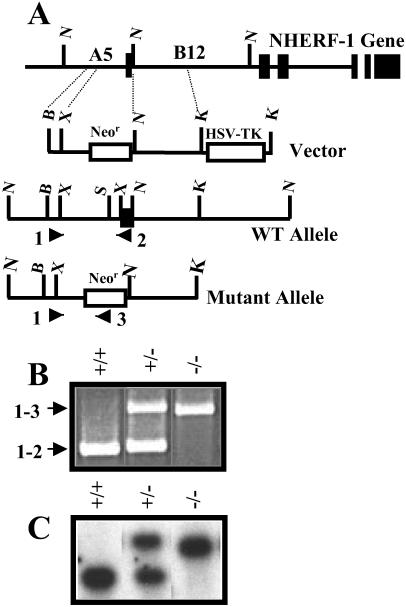
Two contiguous genomic fragments of the NHERF-1 gene located on mouse chromosome 11 (15), termed A5 (8 kb), containing sequences 5′ of the transcription start site and a portion of the first exon and B12 (10.5 kb), which contained the remainder of the first exon and a large portion of the first intron, were used to develop a gene-targeting vector (Fig. 1A). A fragment (0.7 kb) of A5 was obtained by using PCR with the primers, 5′-GGATCAGAGCCCACCGCGGTGGCTATATGC-3′ and 5′-GGTTGCGAATCTAGAAGGGAGCCGG-3′, inserted into pBluescript II SK(−) vector (Stratagene) and ligated to the neomycin resistance (Neor) gene obtained by PCR of the pBK-CMV vector with primers, 5′-GTATCCGCTGATCTAGAAATAACCCTGATAAATGC-3′ and 5′-GTATATATGGATCCCCTGAGGCTATGGC-3′. After the addition of an EcoRI–NotI linker, the NotI–KpnI fragment (4.5 kb) from B12 was inserted 3′ to the Neor gene. Finally, the BstXI fragment from pPNTK containing the Herpes simplex virus thymidylate kinase was ligated 3′ of the NotI/KpnI fragment from B12. A control vector containing the SacI/XbaI fragment from A5 (1.3 kb) ligated to an XbaI fragment containing the Neor gene was also constructed and used as positive control for PCR screening of genomic DNA from transfected R1 mouse embryonic stem cells that was serially diluted with mouse genomic DNA. The R1 cells were selected by resistance to G418 (positive selection) and growth on ganciclovir-containing media (negative selection) that excluded the random integration of the plasmid DNA. Isolated embryonic stem clones were analyzed by PCR to establish that a homologous recombination event that interrupted the NHERF-1 gene had occurred. The common primer 1, 5′-CTCTGTTTATTCCCAGAAGGA-3′ (a sequence at the 5′ end of the A5 fragment) was combined with either primer 2, 5′-CAAGAAGGCGATAGAAGGCGATG-3′, designed from the Neor gene or primer 3, 5′-GAGCCAGGTTCTACCAGACGGATAAACTGG-3′, from B12. Cells positive for the incorporation of Neor gene were injected into C57BL/6J blastocysts and implanted into pseudopregnant female B6SJLF1/J mice (The Jackson Laboratory). Chimeric animals, identified by their agouti coat color, were bred with wild-type C57BL/6 mice through six generations. All animals were genotyped by both PCR (described above) and Southern blotting of mouse genomic DNA extracted from tail samples and digested with BglII. After separation on a 1% agarose gel, the DNA fragments were transferred to a nitrocellulose membrane and probed with a 32P-labeled PCR fragment (0.7 kb) from A5 amplified by using the primers, 5′-GGACACTCGGAGGTGTGC-3′ and 5′-GACTATAGACAGAGGTTTGGGCTC-3′. The F6 heterozygous NHERF-1(+/−) male and female mice were bred to produce NHERF-1(−/−) mice.
Fig 1.
Targeted mutation of mouse NHERF-1 gene. (A) The NotI genomic fragments, A5 and B12, from the mouse NHERF-1 gene were used to design a targeting vector. PCR product encompassing the BstII/XbaI fragment from A5 and the NotI/KpnI fragment from B12 were ligated to Neor gene, which was combined with herpes simplex virus thymidylate kinase (HSV-TK; KpnI fragment) to construct a targeting vector. Primers based on the shared BstII/XbaI fragment (primer 1) and the Neor gene (primer 2) or the first exon of the mouse NHERF-1 gene (primer 3) were used to amplify the WT and mutant alleles by using the PCR. B, BstII; X, XbaI; K, KpnI; N, NotI; S, SacI (indicate the restriction sites). (B) Products generated by PCR amplification of genomic DNA from WT (+/+), heterozygous (+/−), and null (−/−) mice. (C) Southern blot of the BglII digest of mouse genomic DNA hybridized with the PCR product from the A5 region described in Materials and Methods.
Blood and Urine Analysis.
Mice were housed for 24 h in metabolic cages to habituate them to the new environment. Animals were maintained on a 12-h light-dark cycle and fed ad libitum, in accordance to National Institutes of Health guidelines. After 24 h, urine and feces samples were collected. After the collection of these samples, animals were lightly anesthetized by i.p. injections of Inactin (100 mg/kg body wt), and arterial blood pressure was measured by cannulating the femoral artery. Animals were euthanized by cardiac puncture to collect blood samples, and blood chemistry was analyzed by automated methods.
Other Techniques.
Brush–border membranes were isolated from mouse kidney by using described methods (5). In addition to the rabbit polyclonal antibodies generated against recombinant full-length NHERF-1 and C-terminal 100 aa of NHERF-2 described (13), new antipeptide antibodies were made against NHERF-1 (residues 298–314), NH2-CSQDSPKKEDSTAPSSTS-COOH, and NHERF-2 (amino acids 110–126), NH2-CRGLPPAHDPWEPKPDWA-COOH, in both chickens and rabbits. Western immunoblots (16, 17) and immunocytochemistry (13) used the monospecific antibodies against NHERF-1 and NHERF-2 and commercially available anti-NHE3 monoclonal antibodies (Chemicon) and an anti-Npt2 polyclonal (L697) antibody (18) provided by M. A. Knepper (National Heart, Lung, and Blood Institute, Bethesda). For immunocytochemistry, the appropriate species-specific secondary antibodies were coupled to Alexa 488 or 568 dyes (Molecular Probes) and used at a protein concentration of 10 μg/ml or 1:100 dilution. Histological tissue sections were stained with hematoxylin and eosin. Protein concentrations were determined by the method of Lowry et al. (19). Peritz ANOVA was used for statistical analyses of various groups.
Results
Inactivation of the Mouse NHERF-1 Gene.
Homologous recombination replaced the first exon containing the transcription start of the mouse NHERF-1 gene with Neor (Fig. 1A). By using PCR primers that hybridized to a common 5′ region of the NHERF-1 gene (primer 1) and either the first exon in the NHERF-1 gene (primer 2) or the Neor gene (primer 3), we established the presence of wild-type (WT) and mutant NHERF-1 alleles (Fig. 1B). Southern blotting of BglII-digested genomic DNA from WT and mutant mice yielded a 2.0-kb hybridizing fragment that represented the WT NHERF-1 gene and an ≈2.5-kb fragment indicating a mutant gene that conferred antibiotic resistance to the embryonic stem cells (Fig. 1C).
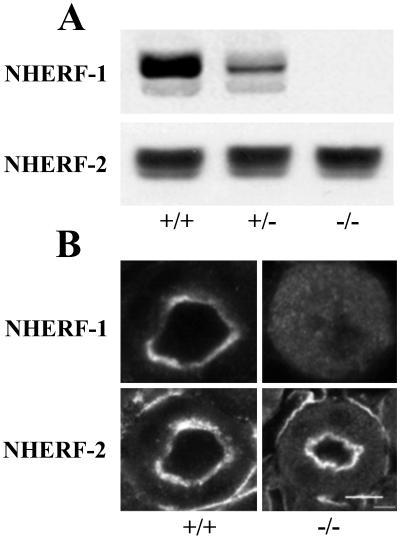
Immunoblotting mouse kidney extracts with two described (17, 20) as well as the two anti-NHERF-1 antibodies (described in Materials and Methods) established that a 55-kDa polypeptide representing NHERF-1 was expressed in WT (+/+) kidney tissue. NHERF-1 levels were significantly reduced in heterozygous (+/−) kidney and no NHERF-1 was detected in the kidney extracts from the NHERF-1(−/−) mice (Fig. 2A). Analysis of other mouse tissues, including liver, brain, and small intestine, confirmed these observations (data not shown). No significant difference in NHERF-2 protein levels was noted in the kidney (Fig. 2A) and other tissues from WT and mutant mice (n = 3, 104 ± 8%). Confocal microscopy of mouse kidney slices showed that NHERF-1 and NHERF-2 were both present at the apical surface of proximal tubule cells from WT (+/+) mice (Fig. 2B). Although the NHERF-2 staining was unchanged in the mutant NHERF-1(−/−) kidneys, no staining for NHERF-1 above background was observed in the kidneys of these NHERF-1-null mice. This observation established that the target disruption of the NHERF-1 gene abolished expression of NHERF-1 protein in the null animals (Fig. 2B).
Fig 2.
Expression of NHERF isoforms in WT and NHERF-1 mutant mice. (A) Immunoblots of kidney extracts from WT (+/+), heterozygous (+/−), and NHERF-1-null (−/−) mice by using the monospecific antibody against full-length NHERF-1 (1:1,000 dilution) and the anti-peptide antibody against NHERF-2 (1:100 dilution). The secondary antibody, anti-rabbit IgG, was used at a 1:5,000 dilution. (B) Immunostaining of renal proximal tubules from WT and NHERF-1-null mice by using the same anti-NHERF-1 and -NHERF-2 antibodies (both at 10 μg/ml protein concentration). (Bar = 100 μm.)
Characterization of the NHERF-1 Mutant Mice.
Attempts to generate an isogenic strain of NHERF-1(−/−) mutant mice in the F129/Svj strain yielded few, small litters (two to three animals). In contrast, litters of the C57BL/6 agouti mice through the first three generations were normal, but no homozygous null females were obtained through the F3 generation. NHERF-1(−/−) females obtained between F4 and F6 generations were generally smaller (30–50% reduced bodyweight) than their WT and heterozygous littermates and showed impaired mobility consistent with muscle weakness that was apparent in several performance/coordination tests. Most of the small NHERF-1(−/−) female animals died 30–35 days after birth. X-ray analysis showed that death was often accompanied by bone fractures. Preliminary analyses indicated that the smaller (−/−) females possessed a 25–30% reduced bone mineral density (e.g., 0.352 g/cm2 in null vs. 0.0442 g/cm2 in WT 30-day-old littermates by using a LUNAR PIXImus Densitometer) and approximately 40% decrease in bone mineral content (e.g., 0.229 g in null vs. 0.377 g in WT 30-day-old littermates). Autopsies of dead NHERF-1(−/−) females suggested that some developed hydrocephaly, which was not observed in any WT or heterozygous animals.
All NHERF-1(−/−) males and approximately 25% of the mutant NHERF-1(−/−) females resembled their WT and heterozygous littermates in body size and were fertile. Crosses using these null females yielded smaller litters (generally two to four pups) than those seen with either WT or heterozygous animals (typically eight to ten pups), but the distribution of sexes and the viability of these pups were normal. Histological analyses of kidney, brain, liver, spleen, and pancreas, all tissues that expressed NHERF-1, and ovary, lung, and heart, which expressed little or no NHERF-1, from WT and NHERF-1 mutant mice showed similar morphology.
Mineral and Electrolyte Analysis in the NHERF-1-Deficient Mice.
Blood, urine, and feces analyses were restricted to the male mice (30–50 days in age), because they were obtained in sufficiently high numbers to permit accurate statistical evaluation (Table 1) and the few viable females were saved for breeding. WT (+/+), heterozygous (+/−) and (−/−) null male mice displayed equivalent body weight, blood pressure, and urine and feces output. Blood chemistry was also identical in all animals with one exception. Serum phosphate concentration in the NHERF-1(−/−) mice was statistically lower than either the WT or heterozygous animals. Serum alkaline phosphatase, which progressively declines with the age of the mouse (18), was even lower in heterozygous (+/−) and (−/−) null mice than WT animals. The significance of this finding is currently unclear.
Table 1.
Blood and urine analysis of wild-type and NHERF-1 mutant mice
| NHERF-1(+/+) (n = 7) | NHERF-1(+/−) (n = 8) | NHERF-1(−/−) (n = 11) | |
|---|---|---|---|
| General properties | |||
| Body weight, g | 24 ± 0.6 | 29 ± 0.7 | 27 ± 0.7 |
| Blood pressure, mmHg | 99 ± 1 | 99 ± 2 | 97 ± 2 |
| Urine output, μl/min | 1.5 ± 0.3 | 1.8 ± 0.2 | 1.3 ± 0.2 |
| Feces output, g/day | 2.5 ± 0.2 | 2.6 ± 0.4 | 2.6 ± 0.3 |
| Blood analysis | |||
| Hematocrit, % | 49.4 ± 0.5 | 50.5 ± 0.3 | 48.3 ± 0.3 |
| Creatinine, mg/dl | 0.35 ± 0.02 | 0.44 ± 0.3 | 0.43 ± 0.03 |
| Sodium, meq/liter | 156 ± 3 | 162 ± 3 | 160 ± 2 |
| Potassium, meq/liter | 6.6 ± 0.4 | 6.3 ± 0.5 | 6.2 ± 0.4 |
| Chloride, meq/liter | 111 ± 4 | 111 ± 2 | 109 ± 1 |
| Calcium, mg/dl | 8.2 ± 0.4 | 7.8 ± 0.2 | 7.9 ± 0.2 |
| Magnesium, mg/dl | 3.2 ± 0.1 | 3.6 ± 0.2 | 3.4 ± 0.3 |
| Phosphate, mg/dl | 11.0 ± 1.0 | 10.8 ± 0.6 | 7.9 ± 0.2 |
| Alkaline phosphatase, units/liter | 102 ± 6 | 69 ± 12 | 48 ± 10 |
| Urine analysis | |||
| Creatinine clearance, μl/min | 110 ± 24 | 116 ± 13 | 113 ± 11 |
| UV sodium, μeq/day | 240 ± 30 | 285 ± 23 | 253 ± 21 |
| UV calcium, mg/day | 0.15 ± 0.3 | 0.23 ± 0.01 | 0.29 ± 0.03 |
| UV magnesium, mg/day | 0.72 ± 0.22 | 1.63 ± 0.23 | 1.82 ± 0.48 |
| UV phosphate, mg/day | 1.44 ± 0.36 | 3.75 ± 0.35 | 3.10 ± 0.29 |
| U phosphate/creatinine | 2.6 ± 0.5 | 5.5 ± 0.6 | 6.1 ± 0.3 |
| FE sodium, % | 0.81 ± 0.09 | 1.00 ± 0.09 | 0.93 ± 0.13 |
| FE phosphate, % | 10.8 ± 2.27 | 24.2 ± 2.4 | 32.4 ± 3.7 |
Blood and urine analyses were performed with WT (+/+), heterozygous (+/−), and null (−/−) male mice (30–50 days of age). Statistical analysis was undertaken with n indicating the number of mice by using Peritz analysis of variance.
, P < 0.05 compared with WT animals. FE defines fractional excretion or clearance of solutes shown as a percentage of the rate of creatinine clearance.
Urine volume and calculated creatinine clearance, a measure of glomerular filtration rate, did not differ between the three groups, indicating normal overall renal function in the absence of NHERF-1. Fractional excretions of sodium as well as absolute sodium, chloride, and potassium excretion were essentially the same in all mice. The most striking finding was an ≈3-fold increase in urinary phosphate excretion in the NHERF-1-null mice compared with WT animals, whether these findings were expressed as absolute excretion, fractional excretion, or urine phosphate to creatinine ratio. NHERF-1 heterozygous (+/−) animals exhibited an intermediate phenotype with phosphate excretion moderately exceeding that of WT animals. These data suggested a defect in renal tubular reabsorption of phosphate. As seen in several forms of hyperphosphaturia (21), urinary excretion of calcium was also increased in the NHERF-1 mutant mice despite comparable serum values for this cation. A modest increase in urinary excretion of magnesium also occurred in the NHERF-1 mutant mice.
Distribution of NHERF-1 Targets in the Proximal Tubules of WT and Mutant Mice.
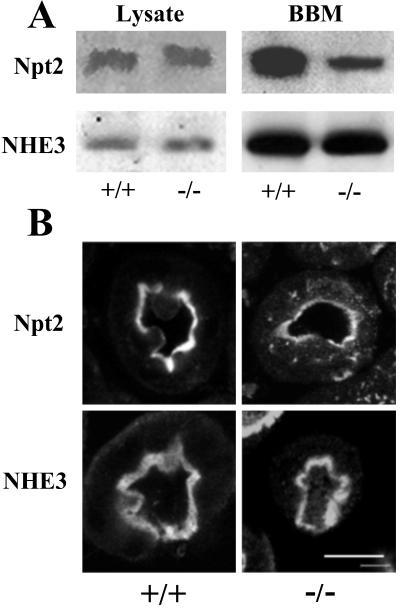
Phosphate homeostasis is principally controlled in the proximal tubule of the mammalian kidney where the bulk of the renal tubular phosphate reabsorption is mediated by the sodium-phosphate cotransporter type IIa or Npt2, localized at the apical membrane. Reabsorption of phosphate in response to hormones and dietary factors is controlled in the proximal tubules by the redistribution of Npt2 from internal vesicles to the apical membrane. Little evidence currently exists for hormone-induced changes in the intrinsic activity of Npt2 (22). By using a monospecific antibody, we examined the total renal content of Npt2 and found the levels of the cotransporter to be unchanged in kidney homogenates from WT and mutant mice (Fig. 3A). Immunoblotting kidney extracts for another NHERF-1 target, NHE3, showed that its levels were also unchanged in the absence of NHERF-1. Analysis of brush–border membranes purified from kidneys of WT and NHERF-1-null mice also showed equivalent levels of NHE3 in both WT and mutant mice (n = 3, 94 ± 11). In contrast, the reduction of the Npt2 protein in the renal brush–border membranes from NHERF-1(−/−) compared with WT mice was significant (50 ± 7%; Fig. 3A).
Fig 3.
Expression of NHERF-1 targets in renal tissue from WT and NHERF-1-null mice. (A) The immunoblots of kidney lysates and isolated brush-border membranes (BBM) from WT and NHERF-1-null mice by using antibodies (at 1:1,000 dilution) against Npt2 (L697) and NHE3 (Chemicon). (B) The immunostaining of kidney proximal tubules from WT and NHERF-1-null mice by using the same anti-Npt2 and anti-NHE3 antibodies. (Bar = 100 μm.)
This finding suggested a defect in plasma membrane targeting of the Npt2 protein. We undertook immunohistochemistry of renal proximal tubules from WT and mutant mice to examine this possibility (Fig. 3B). Laser confocal microscopy established that NHE3 was localized exclusively to the apical brush–border membrane of proximal tubule cells in both WT and mutant mice with no significant immunostaining in the cytosol. Npt2 was similarly localized with the apical surface of proximal tubules in WT animals. However, in NHERF-1-null animals, Npt2 staining at the apical surface was noticeably weaker and more diffuse than in WT mice. The most striking result was that Npt2 was readily observed in internal vesicles in proximal tubules of the NHERF-1(−/−) mice, which suggested a critical role for NHERF-1 in the targeting and/or trafficking of Npt2 in the proximal tubules and regulation of renal phosphate reabsorption.
Discussion
The NHERF proteins link membrane targets, such as NHE3 (5) and cystic fibrosis transmembrane conductance regulator (23), to the underlying cytoskeleton to facilitate their regulation by hormones (24), their insertion into apical rather than basolateral membranes in polarized epithelial cells (25), and by scaffolding the transporters, coordinates their functions (26). Cystic fibrosis transmembrane conductance regulator (ΔF508), the major mutation associated with human cystic fibrosis, accumulates at intercellular sites (reviewed in ref. 27) and this in turn decreases the apical insertion of NHE3 (28). Unlike the constitutive association of NHERF with NHE3 (29), NHERF-1 binds to the β2-adrenergic receptor in an agonist-dependent manner, resulting in NHERF-1 sequestration by β2-adrenergic receptor and enhanced NHE3 activity (30). NHERF binding also facilitates the recycling of internalized β2-adrenergic receptor to the plasma membrane (31). Activation of the T cell receptor promotes NHERF association with Cbp/PAG, a protein adapter found in lipid rafts (32). This may facilitate the endocytosis of raft-associated proteins, such as NHE3 (33). Other membrane targets of NHERF include members of the Trp-4 family of ion channels (34), Npt2, the major renal Na+/PO4 cotransporter (7), H+-ATPase (35), and the platelet-derived growth factor receptor (36). NHERF also recruits soluble proteins, such as selected isoforms of PLCβ (37) and YAP65 (38), to the plasma membrane. These data suggest that NHERF proteins target proteins to the plasma membrane in kidney and other tissues (39).
Although some NHERF targets, such as the H+-ATPase (35) and Taz (8), showed a significant preference for NHERF-2 over NHERF-1, the two NHERF isoforms were functionally indistinguishable in most of the events described above. Recent studies showed that the NHERF proteins formed homo- and heterodimers (20, 40), suggesting the potential cooperation of the two NHERF proteins in some cellular functions. On the other hand, in rat kidney, NHERF-1 and NHERF-2 were expressed in distinct cells and regions of the nephron (13). Because NHERF-1 and not NHERF-2 was detected in the rat proximal tubule cells, we concluded that NHERF-1 acted alone to regulate NHERF-mediated events in the rat proximal tubule and NHERF-2 played a more significant role in the glomerulus, renal vasculature, and the distal nephron segments of the rat kidney. To establish the physiological importance of NHERF-1, we undertook the targeted inactivation of the mouse NHERF-1 gene. Immunological analysis of the mouse kidney showed that both NHERF isoforms were expressed in the proximal tubule cells in the WT mouse and highly localized at the apical surface, which was in contrast to other studies (14) that suggested that only NHERF-1 was expressed in mouse proximal tubules. Although we used several different anti-NHERF antibodies to validate our findings, potential ambiguities in these data were eliminated by immunocytochemistry of the NHERF-1(−/−) null mouse. Inactivating the mouse NHERF-1 gene eliminated all NHERF-1 expression in the kidney and definitively established the presence of NHERF-2 in the mouse proximal tubules. Reverse transcription–PCR of rabbit nephron segments (16) and preliminary analyses of human kidney also indicated that both NHERF isoforms are expressed in the proximal tubules in these species (J.B. and E.J.W., unpublished observations). These observations raised the possibility that NHERF-1 and NHERF-2 fulfilled overlapping functions in proximal tubules, and the gene disruption strategy that selectively eliminated one isoform was crucial for defining the unique functions of NHERF-1 (and NHERF–2) in the mouse kidney.
Our earlier studies (2) in cultured cells pointed to a preformed complex of NHERF-1 with NHE3 and ezrin. These studies suggested that NHERF-1 tethered NHE3 at the apical membrane and allowed ezrin-bound protein kinase A to phosphorylate NHE3 and inhibit transport activity effectively. Protein kinase A-mediated phosphorylation of NHE3 was also implicated in the internalization of NHE3 after the chronic exposure of cells to hormones that elevate cAMP (3), which suggested that, in the absence of NHERF-1, NHE3 localization may be modified. However, no difference was discernable in the apical localization of NHE3 in proximal tubules from WT and NHERF-1(−/−) mice, which could reflect a low rate of NHE3 recycling under basal or resting conditions (41) or functional compensation by NHERF-2 to maintain NHE3 at the apical membrane. Recent studies have also suggested a direct link between NHE3 and the actin cytoskeleton (41), which may also play a role in the membrane localization and activity of the antiporter. Given that NHE3 is the major transporter of sodium and hydrogen ions in the proximal tubule, any misregulation of NHE3 might be predicted to alter in extracellular fluid volume or acid/base status in the mutant mice. This alteration, however, was not observed and the blood pressure, hematocrit, serum chloride concentration, and calculated anion gap were essentially identical in WT and mutant mice. A more detailed examination of the function and regulation of NHE3 in the NHERF-1-null mice will be necessary to define the contribution of the two NHERF proteins and the actin cytoskeleton in basal and hormone-regulated NHE3 function in the mouse kidney.
The most striking abnormality in the NHERF-1 mutant mice was the defect in renal tubular reabsorption of inorganic phosphate. In the presence of normal or slightly decreased serum phosphate concentrations, the absolute and fractional excretion of phosphate was increased in both heterozygous and null mice. Moreover, the apical membrane localization of Npt2, the major hormone-regulated sodium-phosphate cotransporter in the renal proximal tubule, was decreased with a significant portion of the Npt2 protein found in internal sites in the proximal tubule cells. Like NHE3, Npt2 function is inhibited by parathyroid hormone and cAMP. Despite the presence of multiple phosphorylation sites in the Npt2 protein (22), the predominant mechanism by which hormones regulate Npt2 activity is by internalization and subsequent lysosomal degradation of the transporter protein (42). At least four different PDZ proteins, including NHERF-1 and NHERF-2, bound Npt2 and may play a role in endocytosis and sorting of the renal phosphate transporter (7). Two-hybrid assays indicated that two of these PDZ proteins, NaPi-Cap-1 and NaPi-Cap-2, which are also expressed in proximal tubules of the mouse kidney, were significantly preferred by Npt2 over either NHERF isoform. Our data, however, suggested that NHERF-1 played a critical role, not duplicated by other PDZ proteins, in the trafficking and/or retention of Npt2 at the apical surface of renal proximal tubules. In the absence of NHERF-1, Npt2 was aberrantly targeted with a significant portion of the transporter found in internal compartments. As a consequence, the renal tubular reabsorption of phosphate was decreased in the NHERF-1 mutant mice. The increased calcium excretion observed in the NHERF-1 knockout animals is most likely secondary to the reduced phosphate reabsorption, because calcium is reabsorbed in the thick ascending limb and the distal convoluted tubule, and neither of these sites (13, 14) expressed detectable NHERF-1 or NHERF-2, so that a direct association between NHERF and renal calcium transport seemed unlikely. Although urinary excretion of calcium was seen in both male and female NHERF-1-null animals, the reduced bone mineralization was most striking in female mice and further work is needed to elucidate the underlying mechanism.
Human X-linked hypophosphatemia and autosomal dominant hypophosphatemic rickets are disorders of renal phosphate wasting and bone mineralization. Decreased renal phosphate reabsorption in the diseased humans and in various mouse models of the human diseases resulted from reduced Npt2 mRNA and protein expression and decreased insertion of Npt2 in that brush–border membranes (43). The genes responsible for human X-linked hypophosphatemia and autosomal dominant hypophosphatemic rickets were identified as PHEX, encoding an endopeptidase, and FGF23, a growth factor, respectively. The mechanism by which these genetic defects modified Npt2 function in the brush–border membranes is not understood. Although the Npt2 (−/−) mice recapitulate some of the phenotype of human hereditary hypophosphatemic rickets with hypercalciuria, they also did not provide direct access to mechanisms that dictate Npt2 trafficking. In this regard, disruption of the mouse gene encoding NHERF-1, a protein that directly associated with Npt2 and regulated its trafficking and membrane insertion, provided an important tool for understanding the regulatory defects associated with human disease.
Acknowledgments
We thank Cheryl Bock of Duke University Comprehensive Cancer Center Transgenic Mouse Facility for generating the NHERF-1 mutant mice. We acknowledge the excellent technical support provided by Feng Ying Wang, Deborah Steplock, Jie Liu, and Kausik Roy. Dr. M. A. Knepper (National Heart, Lung, and Blood Institute, Bethesda) provided the rabbit polyclonal antibodies directed against Npt2. These studies were supported by National Institutes of Health Grants DK55881 (to E.J.W. and S.S.) and DK32839 (to J.B.W.), and by a grant from Research Service, Department of Veterans Affairs (to E.J.W.). J.W.V. is supported by a Department of Defense Breast Cancer Research Program Predoctoral Training Fellowship (DAMD17-98-1-8070).
Abbreviations
NHERF, Na+/H+ exchanger regulatory factor
WT, wild type
PDZ, PSD-95/Discs large/ZO-1
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Weinman E. J., Minkoff, C. & Shenolikar, S. (2000) Am. J. Physiol. Renal Physiol. 279, F393-F399. [DOI] [PubMed] [Google Scholar]
- 2.Yun C. H., Oh, S., Zizak, M., Steplock, D., Tsao, S., Tse, C. M., Weinman, E. J. & Donowitz, M. (1997) Proc. Natl. Acad. Sci. USA 94, 3010-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Norian, J. M., Magyar, C. E., Holstein-Rathlou, N. H., Mircheff, A. K. & McDonough, A. A. (1999) Am. J. Physiol. 276, F711-F719. [DOI] [PubMed] [Google Scholar]
- 4.Reczek D., Berryman, M. & Bretscher, A. (1997) J. Cell Biol. 139, 169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinman E. J., Steplock, D. & Shenolikar, S. (1993) J. Clin. Invest. 92, 1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voltz J. W., Weinman, E. J. & Shenolikar, S. (2001) Oncogene 20, 6309-6314. [DOI] [PubMed] [Google Scholar]
- 7.Gisler S. M., Stagljar, I., Traebert, M., Bacic, D., Biber, J. & Murer, H. (2001) J. Biol. Chem. 276, 9206-9213. [DOI] [PubMed] [Google Scholar]
- 8.Kanai F., Marignani, P. A., Sarbassova, D., Yagi, R., Hall, R. A., Donowitz, M., Hisaminato, A., Fujiwara, T., Ito, Y., Cantley, L. C. & Yaffe, M. B. (2000) EMBO J. 19, 6778-6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeMarco S. J., Chicka, M. C. & Strehler, E. E. (2002) J. Biol. Chem. 277, 10506-10511. [DOI] [PubMed] [Google Scholar]
- 10.Hall R. A., Spurney, R. F., Premont, R. T., Rahman, N., Blitzer, J. T., Pitcher, J. A. & Lefkowitz, R. J. (1999) J. Biol. Chem. 274, 24328-24334. [DOI] [PubMed] [Google Scholar]
- 11.Ediger T. R., Kraus, W. L., Weinman, E. J. & Katzenellenbogen, B. S. (1999) Endocrinology 140, 2976-2982. [DOI] [PubMed] [Google Scholar]
- 12.Stemmer-Rachamimov A. O., Wiederhold, T., Nielsen, G. P., James, M., Pinney-Michalowski, D., Roy, J. E., Cohen, W. A., Ramesh, V. & Louis, D. N. (2001) Am. J. Pathol. 158, 57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wade J. B., Welling, P. A., Donowitz, M., Shenolikar, S. & Weinman, E. J. (2001) Am. J. Physiol. Cell Physiol. 280, C192-C198. [DOI] [PubMed] [Google Scholar]
- 14.Ingraffea J., Reczek, D. & Bretscher, A. (2002) Eur. J. Cell Biol. 81, 61-68. [DOI] [PubMed] [Google Scholar]
- 15.Weinman E. J., Steplock, D., Zhang, X., Akhter, S. & Shenolikar, S. (1999) Biochim. Biophys. Acta 1447, 71-76. [DOI] [PubMed] [Google Scholar]
- 16.Weinman E. J., Steplock, D., Wang, Y. & Shenolikar, S. (1995) J. Clin. Invest. 95, 2143-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinman E. J., Steplock, D., Tate, K., Hall, R. A., Spurney, R. F. & Shenolikar, S. (1998) J. Clin. Invest. 101, 2199-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim G.-H., Martin, S. W., Fernandez-Llama, P., Masilamani, S., Packer, R. K. & Knepper, M. A. (2000) Am. J. Physiol. Renal Physiol. 279, F459-F467. [DOI] [PubMed] [Google Scholar]
- 19.Lowry O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. (1951) J. Biol. Chem. 193, 265-275. [PubMed] [Google Scholar]
- 20.Shenolikar S., Minkoff, C. M., Steplock, D. A., Evangelista, C., Liu, M. & Weinman, E. J. (2001) FEBS Lett. 489, 233-236. [DOI] [PubMed] [Google Scholar]
- 21.Beck L., Karaplis, A. C., Amizuka, N., Hewson, A. S., Ozawa, H. & Tenenhouse, H. S. (1998) Proc. Natl. Acad. Sci. USA 95, 5372-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murer H., Hernando, N., Forster, I. & Biber, J. (2000) Physiol. Rev. 80, 1373-1409. [DOI] [PubMed] [Google Scholar]
- 23.Short D. B., Trotter, K. W., Reczek, D., Kreda, S. M., Bretscher, A., Boucher, R. C., Stutts, M. J. & Milgram, S. L. (1998) J. Biol. Chem. 273, 19797-19801. [DOI] [PubMed] [Google Scholar]
- 24.Sun F., Hug, M. J., Bradbury, N. A. & Frizzell, R. A. (2000) J. Biol. Chem. 275, 14360-14366. [DOI] [PubMed] [Google Scholar]
- 25.Moyer B. D., Duhaime, M., Shaw, C., Denton, J., Reynolds, D., Karlson, K. H., Pfeiffer, J., Wang, S., Mickle, J. E., Milewski, M., et al. (2000) J. Biol. Chem. 275, 27069-27074. [DOI] [PubMed] [Google Scholar]
- 26.Bagorda A., Guerra, L., Di Sole, F., Hemle-Kolb, C., Cardone, R. A., Fanelli, T., Reshkin, S. J., Gisler, S. M., Murer, H. & Casavola, V. (2002) J. Biol. Chem. 277, 21480-21488. [DOI] [PubMed] [Google Scholar]
- 27.Kleizen B., Braakman, I. & de Jonge, H. R. (2000) Eur. J. Cell Biol. 79, 544-556. [DOI] [PubMed] [Google Scholar]
- 28.Ahn W., Kim, K. H., Lee, J. A., Kim, J. Y., Choi, J. Y., Moe, O. W., Milgram, S. L., Muallem, S. & Lee, M. G. (2001) J. Biol. Chem. 276, 17236-17243. [DOI] [PubMed] [Google Scholar]
- 29.Weinman E. J., Steplock, D., Donowitz, M. & Shenolikar, S. (2000) Biochemistry 39, 6123-6129. [DOI] [PubMed] [Google Scholar]
- 30.Hall R. A., Premont, R. T., Chow, C. W., Blitzer, J. T., Pitcher, J. A., Claing, A., Stoffel, R. H., Barak, L. S., Shenolikar, S., Weinman, E. J., et al. (1998) Nature (London) 392, 626-630. [DOI] [PubMed] [Google Scholar]
- 31.Cao T. T., Deacon, H. W., Reczek, D., Bretscher, A. & von Zastrow, M. (1999) Nature (London) 401, 286-290. [DOI] [PubMed] [Google Scholar]
- 32.Brdickova N., Brdicka, T., Andera, L., Spicka, J., Angelisova, P., Milgram, S. L. & Horejsi, V. (2001) FEBS Lett. 507, 133-136. [DOI] [PubMed] [Google Scholar]
- 33.Li X., Galli, T., Leu, S., Wade, J. B., Weinman, E. J., Leung, G., Cheong, A., Louvard, D. & Donowitz, M. (2001) J. Physiol. 537, 537-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y., Tang, J., Chen, Z., Trost, C., Flockerzi, V., Li, M., Ramesh, V. & Zhu, M. X. (2000) J. Biol. Chem. 275, 37559-37564. [DOI] [PubMed] [Google Scholar]
- 35.Breton S., Wiederhold, T., Marshansky, V., Nsumu, N. N., Ramesh, V. & Brown, D. (2000) J. Biol. Chem. 275, 18219-18224. [DOI] [PubMed] [Google Scholar]
- 36.Maudsley S., Zamah, A. M., Rahman, N., Blitzer, J. T., Luttrell, L. M., Lefkowitz, R. J. & Hall, R. A. (2000) Mol. Cell. Biol. 20, 8352-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang J. I., Heo, K., Shin, K. J., Kim, E., Yun, C., Ryu, S. H., Shin, H. S. & Suh, P. G. (2000) J. Biol. Chem. 275, 16632-16637. [DOI] [PubMed] [Google Scholar]
- 38.Mohler P. J., Kreda, S. M., Boucher, R. C., Sudol, M., Stutts, M. J. & Milgram, S. L. (1999) J. Cell. Biol. 147, 879-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shenolikar S. & Weinman, E. J. (2001) Am. J. Physiol. Renal Physiol. 280, F389-F395. [DOI] [PubMed] [Google Scholar]
- 40.Lau A. G. & Hall, R. A. (2001) Biochemistry 40, 8572-8580. [DOI] [PubMed] [Google Scholar]
- 41.Hu M. C., Fan, L., Crowder, L. A., Karim-Jimenez, Z., Murer, H. & Moe, O. W. (2001) J. Biol. Chem. 276, 26906-26915. [DOI] [PubMed] [Google Scholar]
- 42.Pfister M. F., Ruf, I., Stange, G., Ziegler, U., Lederer, E., Biber, J. & Murer, H. (1998) Proc. Natl. Acad. Sci. USA 95, 1909-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller Y. L., Collins, J. F. & Ghishan, F. K. (1998) Pediatr. Res. 44, 633-638. [DOI] [PubMed] [Google Scholar]