Abstract
In aquaculture, the use of probiotics to improve growth, immunity, and stress resistance in crustaceans has gained increasing attention. This study examined the effects of incorporating different levels of Rhodotorula mucilaginosa (0.0, 0.1, 1.0, and 10.0 g/kg) into the diet on growth performance, antioxidant capacity (AOC), immune function, Toll/Imd, and JAK-STAT signaling pathways in red claw crayfish (Cherax quadricanatus). The investigation was conducted through a 56-day feeding trial. The main results are as follows: Compared with the control group (0.0 g/kg), different R. mucilaginosa levels significantly increased (p < 0.05) the specific growth rate (SGR) and weight gain rate (WGR) of red claw crayfish, significantly increased (p < 0.05) the activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione S-transferase (GST), total AOC (T-AOC), and acid phosphatase (ACP) in hepatopancreas of red claw crayfish, and significantly upregulated (p < 0.05) the relative expression levels of tumor necrosis factor receptor-associated protein 6, akirin, immunodeficiency homolog, interferon regulatory factor 4, Toll-like receptor (TLR) 6, TLR 2, Janus kinase, signal transducer activator of transcription, tumor necrosis factor α, interleukin-1β, transforming growth factor-β1 genes in the hepatopancreas of red claw crayfish. In conclusion, R. mucilaginosa significantly enhanced red claw crayfish's growth, AOC, and immune function, and activated the Toll/Imd and JAK-STAT signaling pathways. In this experimental context, the ideal addition level of R. mucilaginosa is 1.0 g/kg.
Keywords: crustaceans, feed additive, hepatopancreas, probiotics, signal pathway activation
1. Introduction
Red claw crayfish (Cherax quadricarinatus) is an aquaculture species with substantial economic importance. It demonstrates fast growth along with robust environmental adaptability, capable of withstanding low oxygen conditions, a broad spectrum of pH levels, variations in temperature, and even elevated ammonia concentrations [1, 2]. The red claw crayfish is widely farmed in various provinces stretching from Hainan to Jiangsu, Fujian, and Guangdong in China, and it is estimated that the market value will climb to $9.3 billion by 2024 [3]. With the expansion of farming scale, high-density aquaculture practices have become increasingly prevalent. However, under such intensive farming conditions, red claw crayfish experience significantly increased survival stress and become susceptible to various pathogens [4]. Particularly in high-density recirculating aquaculture systems, where water exchange is limited, pathogens can spread rapidly once introduced, often causing substantial economic losses [5]. This phenomenon has become a major bottleneck restricting the sustainable development of the red claw crayfish farming industry.
To address this challenge, antibiotics have traditionally been the primary approach for disease control [6]. Antibiotics can rapidly and effectively eliminate bacteria, thereby controlling disease outbreaks. However, prolonged antibiotic use may result in drug resistance and environmental contamination, negatively impacting both organisms and ecosystems [7]. The rising public concern over food safety and environmental protection has made the shortcomings of this control measure increasingly apparent. In response to these significant risks, researchers have begun exploring alternative approaches, with probiotics emerging as a focal point of investigation [8]. Probiotics, which serve as an alternative to antibiotics, improve water quality and enhance host immunity, have garnered considerable attention. They not only inhibit the growth and reproduction of pathogenic bacteria through competitive exclusion [9] but also promote nutrient absorption, thereby fostering the growth and development of aquatic organisms and improving feed efficiency [10]. These characteristics demonstrate the broad application prospects of probiotics in healthy aquaculture of red claw crayfish.
Rhodotorula mucilaginosa has drawn significant interest because of its exceptional adaptability and the wealth of bioactive substances it contains. It is capable of flourishing in a wide range of environments, from typical settings to extreme conditions like deep-sea hydrothermal vents and Arctic ice sheets [11]. This organism is abundant in natural carotenoids, lipids, and a variety of enzymes [12]. Research has shown that the carotenoids synthesized by R. mucilaginosa, such as β-carotene, torulene, and torularhodin, exhibit substantial antioxidant capabilities. These compounds efficiently eliminate free radicals and reduce the damage caused by oxidative stress to aquatic cells [13]. Additionally, the polyunsaturated fatty acids derived from R. mucilaginosa exhibit anti-inflammatory effects, contributing to the overall health of aquatic organisms [14]. In the past few years, significant advancements have been achieved in utilizing yeast within the aquaculture industry. Studies suggest that red yeast can function as a dietary supplement, improving both the growth and immune response of Nile tilapia (Oreochromis niloticus) [15] and koi carp (Cyprinus carpio var. koi) [16]. Furthermore, the marine red yeast demonstrates suppressive effects on Vibrio species, which enhances the survival probability of whiteleg shrimp (Litopenaeus vannamei) [17]. With ongoing advancements in research, the possible uses of red yeast in aquaculture are anticipated to increase considerably.
To date, there has been relatively little research examining the impacts of R. mucilaginosa supplementation on growth, antioxidant capability, immune response, and the related signaling pathways in red-clawed crayfish. As a result, the underlying mechanisms remain largely unknown. Therefore, this study selected an optimal strain of R. mucilaginosa as a feed additive for red claw crayfish to investigate its beneficial impacts on growth, antioxidant capacity (AOC), immune function, Toll/Imd, and JAK-STAT signaling pathways. This study seeks to explore how feed probiotics affect the innate immune response in red claw crayfish, thereby offering a scientific basis for the utilization and development of R. mucilaginosa in aquaculture feed.
2. Materials and Methods
2.1. Experimental Diets
The freeze-dried powder of R. mucilaginosa, supplied by Guangzhou Xinhaili Biotechnology Co., Ltd. in Guangzhou, China, contained over 1 × 1010 cells per gram. Based on wet weight, the nutritional profile of R. mucilaginosa was as follows: it comprised 81.17% moisture, 9.27% crude protein, 4.45% crude lipid, 4.2% total triglycerides, 1.3% β-glucan, 1.4 mg/kg of β-carotene, 1.0 mg/kg of astaxanthin, and 172 mg/kg of vitamin E. The R. mucilaginosa was preserved at −20°C in a refrigerator.
The safety assessment of R. mucilaginosa was conducted following the research method of Kang et al. [18]. Healthy juvenile crayfish (purchased from the South Propagation Base of Guangxi Academy of Fishery Sciences in Nanning, China; the initial body weight was 1.10 ± 0.09 g, and the initial body length was 3.08 ± 0.12 cm) were randomly divided into two groups (treatment group and control group), each comprising three replicates. A total of six aquaculture tanks (0.6 × 0.6 × 0.6 m) were utilized, with 10 crayfish housed in each tank. Water conditions were maintained as follows: water temperature at 26–28°C, dissolved oxygen at 4.7–6.2 mg/L, ammonia nitrogen at 0–0.25 ppm, nitrite at 0.01 ppm, nitrate at 0–10 ppm, pH at 7.6–7.8, and natural light (~10 h light: 14 h dark). In the treatment group, R. mucilaginosa was added to maintain a bacterial concentration of 1 × 108 CFU/mL in aquaculture water, while no bacteria were added to the control group. One-third of the water volume was renewed daily, bottom feces were removed, 1 × 108 CFU/mL R. mucilaginosa was supplemented (treatment group), and a basal diet was provided. The feeding behavior and survival of juvenile crayfish were observed for 7 days, with survival numbers recorded daily. By the conclusion of the cultivation period, all red claw crayfish demonstrated excellent growth and vitality, with no noticeable decline in feeding activity. Both the treatment and control groups achieved a survival rate of 100%.
All feed materials were of animal food grade and manufactured by Guangdong Hengxing Feed Industry Co., Ltd., located in Zhanjiang, China. Based on prior research findings [19] and safety assessments (1 × 108 CFU/mL), experimental diets were formulated with varying concentrations of R. mucilaginosa. To prepare the feed, varying quantities of freeze-dried R. mucilaginosa powder were incorporated into the basal feed and mixed uniformly to attain final concentrations of 0, 0.1, 1.0, and 10.0 g/kg. Initially, the basal feed was pulverized to a 60-mesh size using a hammer mill. Subsequently, the specified concentrations of R. mucilaginosa were added to the ground basal feed accordingly. All mixtures were blended uniformly for 15 min using a drum mixer. Sterile distilled water was then added to the mixtures at a ratio of 40% (w/w) to form dough-like feed. The moistened mixtures were extruded through a pellet mill to produce pellets with diameters ranging from 1.00 to 1.50 mm. The fabricated feed pellets were dried in the air at 30°C until their moisture level reached below 10 g per 100 g. Subsequently, the dried pellets were placed in airtight bags and kept at −20°C for later utilization. A new supply of feed was produced every week. The specific ingredients of the experimental diets are outlined in Table 1.
Table 1.
The composition of the experimental diets for red claw crayfish (g/kg of dried diet).
| Ingredients | R. mucilaginosa levels (g/kg) | |||
|---|---|---|---|---|
| 0 | 0.1 | 1.0 | 10.0 | |
| R. mucilaginosa | 0 | 0.10 | 1.00 | 10.00 |
| Fish meal | 485.00 | 485.00 | 485.00 | 485.00 |
| Soybean meal | 119.30 | 119.20 | 118.30 | 109.30 |
| Sorghum flour | 106.50 | 106.50 | 106.50 | 106.50 |
| Wheat flour | 139.00 | 139.00 | 139.00 | 139.00 |
| Corn flour | 45.00 | 45.00 | 45.00 | 45.00 |
| Soy lecithin | 10.00 | 10.00 | 10.00 | 10.00 |
| Fish oil | 15.20 | 15.20 | 15.20 | 15.20 |
| Gelatin | 20.00 | 20.00 | 20.00 | 20.00 |
| Calcium carbonate | 10.00 | 10.00 | 10.00 | 10.00 |
| Choline chloride | 5.00 | 5.00 | 5.00 | 5.00 |
| Mineral premixesa | 20.00 | 20.00 | 20.00 | 20.00 |
| Vitamin premixesb | 20.00 | 20.00 | 20.00 | 20.00 |
| Vitamin C | 5.00 | 5.00 | 5.00 | 5.00 |
| Proximal composition (% dry matter) | ||||
| Dry material | 92.56 | 92.56 | 92.56 | 92.56 |
| Ash | 7.76 | 7.76 | 7.76 | 7.76 |
| Ethereal extract | 7.40 | 7.40 | 7.40 | 7.40 |
| Crude protein | 35.20 | 35.20 | 35.20 | 35.20 |
| Crude lipid | 7.84 | 7.84 | 7.84 | 7.84 |
| Fiber | 3.43 | 3.43 | 3.43 | 3.43 |
aMineral premixes (mg/kg): KCl, 0.5; MgSO4·7H2O, 0.5; ZnSO4·7H2O, 0.09; MnCl2·4H2O, 0.0234; CuSO4·5H2O, 0.005; KI, 0.005; CoCl2·2H2O, 0.0025; Na2HPO4, 2.37.
bVitamin premixes (mg/kg): vitamin B12, 0.02; vitamin A acetate, 5000 IU; vitamin D3, 4000 IU; α-tocopherol acetate, 100 IU; menadione, 5; thiamin HCl, 60; riboflavin, 25; pyridoxine HCl, 50; folic acid, 10; dl-capantothenic acid, 75; nicotinic acid, 5; biotin, 1; inositol, 5.
The viable count of R. mucilaginosa in the prepared feed was assessed by employing the plate counting technique [15]. A stock solution was prepared by vortexing 1 g of feed sample in 9 mL of sterilized normal saline. Serial dilutions ranging from 103 to 108 were prepared from the stock solution. For every dilution, 0.1 mL was plated onto sterile nutrient agar (NA) plates, with three replicates for each dilution level. The plates were then incubated anaerobically at 35°C for 48 h. After incubation, colonies were counted and randomly selected for identification to confirm their identity as R. mucilaginosa. The number of R. mucilaginosa colonies on each plate was calculated. Following colony counting, additional colonies were randomly selected for further identification and isolation of R. mucilaginosa strains. The calculation formula is as follows:
In the above formula, B is the total number of plate colonies on NA medium, C is the number of R. mucilaginosa colonies identified from 10 colonies, and f is the dilution multiple.
According to the NA plate count, no viable R. mucilaginosa was detected in the control group diet. In contrast, the viable counts of R. mucilaginosa in the experimental diets supplemented with 0.1, 1.0, and 10.0 g/kg were 0.89 × 106, 0.87 × 107, and 0.92 × 108 CFU/g, respectively.
2.2. Experimental Animals and Feeding Management
The red claw crayfish utilized in the experiment were obtained from the South Propagation Base of the Guangxi Academy of Fishery Sciences, located in Nanning, China. The initial body weight is 0.13 ± 0.06 g, and the initial body length is 0.58 ± 0.02 cm. All red claw crayfish experiments were conducted in accordance with the guidelines of Guangxi Minzu University, Nanning, China (Approval Number: GXUN 2023-018). Before the culture experiment, red claw crayfish were acclimated in the laboratory's water recirculation system for 7 days. After the acclimation period, young crayfish exhibiting consistent body morphology, absence of limb abnormalities, a healthy appearance, strong vitality, and currently in the intermolt phase were chosen for the subsequent cultivation experiment.
Based on varying concentrations of R. mucilaginosa diet, the experiment was designed with four groups: a control group (0 g/kg), a low-dose group (0.1 g/kg), a medium-dose group (1.0 g/kg), and a high-dose group (10.0 g/kg). Each group had three replicates, resulting in a total of 12 aquaculture tanks. Each tank contained 50 shrimp, for a total of 600 shrimp across all tanks. The experimental rearing tanks measured 1 × 2 × 1 m (L × W × H) with a water depth of 0.70 m. Each tank was equipped with 16 PVC pipes (0.75 m diameter, 0.40 m length) as shelters. Aeration stones provided continuous aeration and oxygenation, while aquatic plants were introduced to simulate a natural ecological environment, thereby mitigating stress responses and enhancing dissolved oxygen levels for the crayfish. The environmental conditions were carefully regulated, with the water temperature kept between 26–28°C and the dissolved oxygen levels ranging from 4.7 to 6.2 mg/L, ammonia nitrogen at 0–0.25 ppm, nitrite at 0.01 ppm, nitrate at 0–10 ppm, pH at 7.6–7.8, and natural light (~10 h light: 14 h dark).
Following the formal rearing period, the growth condition of each crayfish in every tank was monitored and documented before each feeding session, and any instances of mortality were recorded. According to the methods of Ren et al. [20], the daily feeding quantity was roughly equivalent to 5% of the crayfish's body weight, with modifications implemented according to actual feeding behaviors. Feedings took place twice a day, at 8:30 in the morning and 6:30 in the evening. Each feeding session consisted of two rounds, with a 30-min interval between them. Every morning after feeding, crayfish excrement was promptly removed, and one-third of the water volume in the rearing tanks was exchanged. The breeding trial lasted for 56 days.
2.3. Sampling
At the end of the culture experiment (56 days), the red claw crayfish was starved for 24 h, and then the red claw crayfish was randomly sampled. The 36 red-claw crayfish were randomly sampled in each group (12 red-claw crayfish were extracted from each aquaculture tank). After being rinsed with sterile saline, each crayfish was anesthetized in the ice-water mixture (0°C) for 10 min. According to the methods of Zhang et al. [21], then their body weight was measured individually to calculate the weight gain rate (WGR), specific growth rate (SGR), and feed conversion rate (FCR). The WGR, SGR, and FCR of red claw crayfish were calculated using the following formula:
After the growth performance indicators are calculated, transfer the crayfish to a clean, sterile dissection table. In a sterile environment, the red claw crayfish were carefully dissected, and the hepatopancreas was removed, immediately frozen in liquid nitrogen, and preserved at −80°C.
2.4. Determination of Biochemical Parameters and Antioxidant Enzyme Activities
According to the methods of Liu et al. [15], for the measurement of biochemical parameters and antioxidant enzyme activities in the hepatopancreas of red claw crayfish, the samples were first thawed, rinsed with sterile saline, and dried using filter paper. Precisely 1 g of hepatopancreas tissue was weighed and transferred into a 50 mL sterile, enzyme-free centrifuge tube, followed by the addition of nine volumes of physiological saline. The sample was mechanically homogenized on ice and subsequently centrifuged at 1000 × g for 10 min at 4°C. The resulting supernatant was collected and diluted with physiological saline to prepare a 1% homogenate for analysis.
The activities of alkaline phosphatase (AKP), acid phosphatase (ACP), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione S-transferase (GST), and total AOC (T-AOC) and the malondialdehyde (MDA) content in the hepatopancreas were measured using an ELISA analyzer (RT-6100, Rayto, Shenzhen, China) and assay kits. The measurements followed the instructions provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Detailed protocols can be accessed at http://www.njjcbio.com/ (accessed on 8 March 2025).
The activities of AKP and ACP in the hepatopancreas were measured using a micro-enzyme-linked immunosorbent assay. The enzyme activities are expressed in King units per gram of protein (King unit/gprot). One King unit of AKP is defined as the amount of enzyme that produces 1 mg of phenol from the substrate in 15 min at 37°C, while one King unit of ACP is defined as the amount of enzyme that produces 1 mg of phenol from the substrate in 30 min at 37°C. The activity of SOD in the hepatopancreas was measured using the WST-1 method. One unit of SOD activity (U/mgprot) was defined as the amount of enzyme required to achieve 50% inhibition of superoxide radicals in a 1 mL reaction mixture containing 1 mg of tissue protein. The CAT activity in the hepatopancreas was determined by the ammonium molybdate method. One unit of CAT activity (U/mgprot) was defined as the amount of enzyme that decomposes 1 μmol of H2O2 per second per milligram of tissue protein. The activities of GPX and GST in the hepatopancreas were measured using a colorimetric method. The enzyme activity units were defined as follows: for GPX, one unit (U/mg protein) was defined as the amount of enzyme that caused a reduction of 1 μmol GSH per minute per milligram of tissue protein at 37°C. For GST, one unit (U/mg protein) was defined as the amount of enzyme that caused a reduction of 1 μmol GSH per minute per milligram of tissue protein at 37°C. The T-AOC (mmol/mg prot) in the hepatopancreas was assessed using the ABTS method. The MDA (nmol/mg prot) content in the hepatopancreas was measured using the thiobarbituric acid (TBA) assay.
2.5. Determination of Relative Expression Levels of Genes
According to the methods of Zhang et al. [2], the real-time quantitative polymerase chain reaction (RT-qPCR) was employed to determine the relative expression levels of tumor necrosis factor receptor-associated factor 6 (traf6), akirin, immune deficiency homolog (imd), interferon regulatory factor 4 (irf4), Toll-like receptor (TLR) 6 (tlr6), TLR 2 (tlr2), tumor necrosis factor α (tnf-α), interleukin-1β (il-1β), transforming growth factor-β1 (tgf-β1), Janus kinase (jak), signal transducer activator of transcription (stat), and β-actin in the hepatopancreas of red claw crayfish. β-actin was selected as the nonregulated internal reference gene. The forward and reverse primers for RT-qPCR were designed using Primer Premier 6.0 software, based on the mRNA sequences of red claw crayfish retrieved from the NCBI database. The synthesis of these primers was carried out by Shanghai Sangon Bioengineering Technology Co., Ltd. (Shanghai, China). For more detailed information regarding the primers, please refer to Table 2.
Table 2.
Primer sequences for RT-qPCR in red claw crayfish.
| Gene | Primer sequence (5′→3′) | Amplicon size (bp) | Tm (°C) | Gene bank |
|---|---|---|---|---|
| β-actin | F: CGCCTGTCCGCTGGAATAAT | 135 | 60 | XM_053800817.1 |
| R: ACGATGGAAGGGAAGACAGC | ||||
|
| ||||
| traf6 | F: GTGCCACAGTCCACCATTCT | 262 | 60 | XM_053772658.1 |
| R: TACCTCTGGCCGCATGAAAG | ||||
|
| ||||
| akirin | F: ACGCCGCAAGATATTACAGTGTGG | 112 | 60 | XM_053784413.1 |
| R: TGATGGTGAGGTAGGACAGACAGG | ||||
|
| ||||
| imd | F: CATACCTCCCCGTCTGTGTCA | [22] | 60 | [22] |
| R: CCATCTAACCCACCTGCTGTC | ||||
|
| ||||
| irf4 | F: CAGCGAAGTGTTCCGAGTTCCC | [23] | 60 | [23] |
| R: TATGCCTCCTCCCGTGTGTTCTC | ||||
|
| ||||
| tlr6 | F: CTACAGTGCCAATGATGCTACCTAC | 105 | 60 | XM_053797426.1 |
| R: TCGCTGAAGTCTCTGGAGTGAAG | ||||
|
| ||||
| tlr2 | F: CTCGGACAAGGAGCGGTTAGTTTC | 131 | 60 | XM_053771523.1 |
| R: TTCTGATTGATAACCTGCTGGAGTCTG | ||||
|
| ||||
| tnf-α | F: ACAGCATTAGTGAGAGCAGCAATC | 123 | 60 | XM_053772658.1 |
| R: CATTAGGACACATAACTGGTCTGAGG | ||||
|
| ||||
| il-1β | F: ACGGTCACAGCCTCTAATGGTAC | 78 | 60 | XM_053781109.1 |
| R: CTCTCGGTAGTTCGGATTGGTTTG | ||||
|
| ||||
| tgf-β1 | F: CTCCAACACCACCTGAAGATAGATTG | 98 | 60 | XM_053797306.1 |
| R: AGTAACAGTGACATAGCAGTAACCATC | ||||
|
| ||||
| jak | F: TGTGAGGCATAACAGTAACGAAGG | [24] | 60 | [24] |
| R: GCCCAAGGAACTCAATGGAATG | ||||
|
| ||||
| stat | F: CAGAAAATGTAGCCCACAGCCAG | [24] | 60 | [24] |
| R: TAAAGCAAGGGGATTATTATTCAGG | ||||
Note: F, forward primer; R, reverse primer. imd, immune deficiency homolog. β-actin, nonregulated reference gene.
Abbreviations: il-1β, interleukin-1β; irf4, interferon regulatory factor 4; jak, janus kinase; tgf-β1, transforming growth factor-β1; tlr2, Toll-like receptor 2; tlr6, Toll-like receptor 6; traf6, tumor necrosis factor receptor-associated factor 6; tnf-α, tumor necrosis factor α; stat, signal transducer activator of transcription.
The brief steps of the RT-qPCR method are as follows: The hepatopancreas tissue of red claw crayfish was homogenized with sterile physiological saline at a 1:10 (w/v) ratio and centrifuged at 1000 g for 10 min at 4°C to obtain the supernatant for RNA extraction. Total RNA was extracted using the Takara MiniBEST Universal RNA Extraction Kit (Takara Biomedical Technology, Beijing, China), with concentration and purity measured by a NanoDrop-2000 spectrophotometer. RNA samples meeting the quality criteria (30–1000 ng/μL concentration and A260/A280 ratio of 1.9–2.1) were subjected to integrity verification through 1% agarose TAE gel electrophoresis using GelRed nucleic acid stain (UVP, Upland, CA, USA), where intact RNA displayed three distinct bands (28, 18, and 5S) with the 28S band intensity being more than twice that of 18S. cDNA was synthesized from 1000 ng total RNA using PrimeScript RT Master Mix (Takara) in a 20 μL reaction system containing 10 μL 2× Taqman PCR mix, 1 μL each of forward and reverse primers, RNA template, and DEPC-treated ddH2O, under the following thermal conditions: 30°C for 10 min, 42°C for 15 min, 95°C for 5 min, and 5°C for 5 min. RT-qPCR was performed using the Q2000B Real-Time PCR System (LongGene) with TB Green Premix Ex Taq II (Tli RNaseH Plus, Takara) in a 20 μL reaction volume containing 2 μL cDNA, 10 μL 2× TB Green Premix, 6.4 μL DEPC water, and 0.8 μL each of forward and reverse primers. The RT-qPCR protocol consisted of initial denaturation at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 20 s. All procedures were carried out under RNase-free conditions to ensure RNA integrity throughout the experimental process.
2.6. Data Calculation and Statistics Analysis
In this study, all data were initially entered and sorted using Microsoft Excel 2016. Subsequently, one-way ANOVA was conducted using SPSS Statistics 25.0 to analyze the results data, followed by the creation of relevant charts. To assess significant differences between groups, the least significant difference (LSD) test was employed. GraphPad Prism 9 was utilized for generating the charts. The results of the significance tests are presented as “mean ± standard error” (mean ± SE). Different letters denote statistically significant differences (p < 0.05) between groups. Additionally, the relative expression levels of traf6, akirin, imd, irf4, tlr6, tlr2, tnf-α, il-1β, tgf-β1, jak, and stat genes from fluorescence quantification analysis were calculated using the 2−ΔΔCT method [25].
3. Results
3.1. Effects of Rhodotorula mucilaginosa on the Growth Performance of Red Claw Crayfish
After 56 days of feeding red claw crayfish with varying levels of R. mucilaginosa in their diets, the SGR and WGR in the 0.1, 1.0, and 10.0 g/kg groups were significantly higher than those in the control group (p < 0.05). Notably, the 1.0 g/kg group exhibited significantly higher SGR and WGR compared to the other treatment groups (p < 0.05), as shown in Figure 1.
Figure 1.

The effects of Rhodotorula mucilaginosa on the weight gain rate (WGR; A), specific growth rate (SGR; B), and feed conversion rate (FCR; C) of red claw crayfish. All the data are presented as mean ± SE (n = 3). Within the same figure, different superscript letters indicate significant differences between the values (p < 0.05).
After 56 days of feeding red claw crayfish with varying levels of R. mucilaginosa in their diets, the FCR in the 0.1, 1.0, and 10.0 g/kg groups was significantly lower than that in the control group (p < 0.05). Notably, the 1.0 g/kg group exhibited significantly lower FCR compared to the other treatment groups (p < 0.05), as shown in Figure 1.
3.2. Effects of Rhodotorula mucilaginosa on the Immune and Antioxidant Enzyme Activities in the Hepatopancreas of Red Claw Crayfish
After 56 days of feeding red claw crayfish with varying levels of R. mucilaginosa in their diets, the activities (contents) of SOD, CAT, GPX, GST, ACP, and T-AOC in the hepatopancreas in the 0.1, 1.0, and 10.0 g/kg groups were significantly higher than those in the control group (p < 0.05). The activity of AKP in the hepatopancreas in the 1.0 g/kg group was significantly higher than that in the control group (p < 0.05). However, no significant differences in AKP activity were observed between the 0.1 and 10.0 g/kg groups and the control group (p > 0.05). The MDA content in the hepatopancreas in the 0.1 and 1.0 g/kg groups was significantly lower than that in the control group (p < 0.05). However, there was no significant difference in MDA content between the 10.0 g/kg group and the control group (p > 0.05), as shown in Table 3.
Table 3.
The effects of Rhodotorula mucilaginosa on the immune and antioxidant enzyme activities in the hepatopancreas of red claw crayfish.
| Index | R. mucilaginosa levels (g/kg) | |||
|---|---|---|---|---|
| 0 | 0.1 | 1.0 | 10.0 | |
| SOD (U/mgprot) | 46.74 ± 2.06b | 54.77 ± 1.08a | 57.78 ± 2.36a | 57.73 ± 2.90a |
| CAT (U/mgprot) | 8.73 ± 0.33c | 12.05 ± 0.90b | 16.13 ± 0.42a | 14.25 ± 0.95ab |
| GPX (U/mgprot) | 574.01 ± 23.75c | 618.64 ± 24.11b | 683.07 ± 21.09a | 631.24 ± 20.32b |
| GST (U/mgprot) | 16.65 ± 3.17b | 19.27 ± 2.13a | 24.33 ± 4.01a | 27.16 ± 2.02a |
| T-AOC (mmol/mgprot) | 0.13 ± 0.01c | 0.18 ± 0.01b | 0.24 ± 0.01a | 0.20 ± 0.01b |
| ACP (King unit/gprot) | 278.99 ± 27.67c | 353.16 ± 43.55b | 424.36 ± 30.77a | 332.81 ± 34.23b |
| AKP (King unit/gprot) | 152.70 ± 16.52b | 151.34 ± 14.76b | 203.27 ± 16.42a | 164.01 ± 11.91b |
| MDA (nmol/mgprot) | 1.50 ± 0.05a | 1.30 ± 0.04b | 1.18 ± 0.04c | 1.45 ± 0.04a |
Note: All the data are presented as mean ± SE (n = 3). Within the same row, different superscript letters indicate significant differences between the values (p < 0.05).
Abbreviations: ACP, acid phosphatase; AKP, alkaline phosphatase; CAT, catalase; GPX, glutathione peroxidase; GST, glutathione s-transferase; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
3.3. Effects of Rhodotorula mucilaginosa on the Relative Expression Levels of Genes in the Hepatopancreas of Red Claw Crayfish
After 56 days of feeding red claw crayfish with varying levels of R. mucilaginosa in their diets, the relative expression levels of traf6, akirin, and imd genes in the hepatopancreas in the 0.1, 1.0, and 10.0 g/kg groups were significantly higher than those in the control group (p < 0.05). Notably, the 1.0 g/kg group exhibited significantly higher relative expression levels of traf6, akirin, and imd genes compared to the other treatment groups (p < 0.05), as shown in Figure 2.
Figure 2.
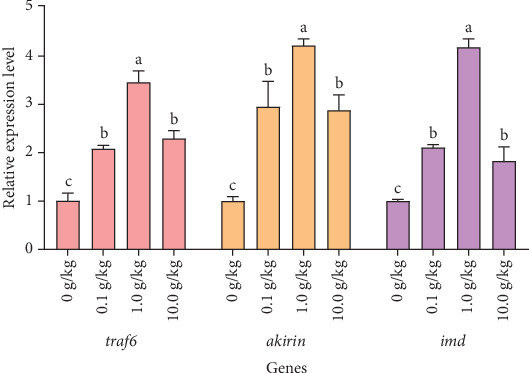
The effects of Rhodotorula mucilaginosa on the relative expression levels of tumor necrosis factor receptor-associated factor 6 (traf6), akirin, and immune deficiency homolog (imd) genes in the hepatopancreas of red claw crayfish. All the data are presented as mean ± SE (n = 3). Within the same figure, different superscript letters indicate significant differences between the values (p < 0.05).
After 56 days of feeding red claw crayfish with varying levels of R. mucilaginosa in their diets, the relative expression levels of irf4, tlr6, and tlr2 genes in the hepatopancreas in the 0.1, 1.0, and 10.0 g/kg groups were significantly higher than those in the control group (p < 0.05). Notably, the 1.0 g/kg group exhibited significantly higher relative expression levels of irf4, tlr6, and tlr2 genes compared to the other treatment groups (p < 0.05), as shown in Figure 3.
Figure 3.

The effects of Rhodotorula mucilaginosa on the relative expression levels of interferon regulatory factor 4 (irf4), Toll-like receptor 6 (tlr6), and Toll-like receptor 2 (tlr2) genes in the hepatopancreas of red claw crayfish. All the data are presented as mean ± SE (n = 3). Within the same figure, different superscript letters indicate significant differences between the values (p < 0.05).
After 56 days of feeding red claw crayfish with varying levels of R. mucilaginosa in their diets, the relative expression levels of tnf-α and il-1β genes in the hepatopancreas in the 1.0 g/kg group were significantly higher than those in the control group (p < 0.05). However, there was no significant difference in the relative expression levels of tnf-α and il-1β genes between the 0.1 and 10.0 g/kg groups and the control group (p > 0.05). After 56 days of feeding red claw crayfish with varying levels of R. mucilaginosa in their diets, the relative expression level of the tgf-β1 gene in the hepatopancreas in the 1.0 and 10.0 g/kg groups was significantly higher than that in the control group (p < 0.05). However, there was no significant difference in the relative expression level of the tgf-β1 gene between the 0.1 g/kg group and the control group (p > 0.05), as shown in Figure 4.
Figure 4.

The effects of Rhodotorula mucilaginosa on the relative expression levels of tumor necrosis factor α (tnf-α), interleukin-1β (il-1β), transforming growth factor-β1 (tgf-β1) genes in the hepatopancreas of red claw crayfish. All the data are presented as mean ± SE (n = 3). Within the same figure, different superscript letters indicate significant differences between the values (p < 0.05).
After 56 days of feeding red claw crayfish with varying levels of R. mucilaginosa in their diets, the relative expression levels of jak and stat genes in the hepatopancreas in the 0.1, 1.0, and 10.0 g/kg groups were significantly higher than those in the control group (p < 0.05). Notably, the 1.0 g/kg group exhibited significantly higher relative expression levels of jak and stat genes compared to the other treatment groups (p < 0.05), as shown in Figure 5.
Figure 5.

The effects of Rhodotorula mucilaginosa on the relative expression levels of Janus kinase (jak) and signal transducer activator of transcription (stat) genes in the hepatopancreas of red claw crayfish. All the data are presented as mean ± SE (n = 3). Within the same figure, different superscript letters indicate significant differences between the values (p < 0.05).
4. Discussion
4.1. Effects of Rhodotorula mucilaginosa on the Growth Performance of Red Claw Crayfish
SGR, WGR, and FCR are critical metrics for evaluating the growth performance of aquatic animals, directly reflecting their growth efficiency and health status [26]. This research showed that adding R. mucilaginosa to the diet greatly improved the WGR and SGR of red claw crayfish and decreased the FCR. Notably, the most pronounced growth promotion was observed when the R. mucilaginosa concentration in the diet reached 1.0 g/kg. This phenomenon can be ascribed to the comprehensive nutrient composition of yeast, which comprises amino acids, fatty acids, vitamins, minerals, and substances that regulate immune responses [27, 28]. For instance, lysine, leucine, and arginine in yeast have been shown to attract aquatic animals and promote their growth and development [12]. Furthermore, the β-glucan and mannan oligosaccharides present in the yeast cell wall improve nutrient absorption, which in turn supports increased growth [29]. Consistent with these findings, Yang et al. [19] observed significant enhancements in WGR, SGR, and survival rate of whiteleg shrimp when fed diets supplemented with 108 CFU/g of marine red yeast. Furthermore, this study revealed a dose-dependent response in red claw crayfish, where WGR and SGR initially increased but subsequently declined with increasing dietary levels of R. mucilaginosa. This parabolic growth response suggests that excessive yeast supplementation may exert negative effects on growth performance, ultimately leading to reduced WGR and SGR. When the addition of R. mucilaginosa reached 10.0 g/kg, it significantly exceeded the optimal dosage, leading to excessive and sustained immune activation. This process consumed a substantial amount of energy and protein resources that should have been allocated for growth. Furthermore, over-supplementation with probiotics can disrupt the normal intestinal microbiota, potentially causing dysbiosis and a decline in digestive and absorptive functions. Additionally, the accumulation of trace metabolic byproducts may exert adverse effects [30, 31]. A parallel phenomenon was observed in juvenile tilapia, where dietary supplementation with R. mucilaginosa enhanced WGR, SGR, and FCR, with optimal inclusion levels ranging between 0.53% and 0.60%. Beyond this threshold, excessive yeast supplementation resulted in diminished growth performance in tilapia juveniles [15].
4.2. Effects of Rhodotorula mucilaginosa on the Immune and Antioxidant Enzyme Activities of Red Claw Crayfish
SOD, CAT, GPX, GST, T-AOC, and MDA play essential roles as key markers for evaluating the AOC and oxidative damage in living organisms [32]. This study demonstrated that supplementation with R. mucilaginosa significantly enhanced the activities of SOD, CAT, GSH-PX, GST, and T-AOC in the hepatopancreas of red claw crayfish while markedly reducing MDA levels. These results indicate that R. mucilaginosa can enhance the antioxidant and immune functions of red claw crayfish. The mechanisms may be attributed to the following factors: First, carotenoids in yeast hydrolysate effectively scavenge oxygen free radicals [33], thereby mitigating cellular oxidative damage and modulating the activities of SOD and CAT [34]. Second, astaxanthin in R. mucilaginosa helps maintain cellular redox balance and reduces ROS levels, indirectly influencing the activities of GPX and GST [35]. Third, yeast cell wall polysaccharides, including β-glucan and mannan, can form complexes with Fe2+. This interaction suppresses the generation of ·OH radicals and interrupts lipid peroxidation chain reactions, leading to a reduction in MDA levels [36]. Comparable research has indicated that Rhodotorula paludigena can boost the antioxidant and immune capabilities of whiteleg shrimp [37].
ACP is implicated in apoptosis and immune responses, whereas AKP is involved in digestion and immune functions in aquatic animals [38]. In the present research, a notable rise in the activities of both ACP and AKP was detected. This increase could potentially be ascribed to the β-glucan derived from the cell wall of R. mucilaginosa. The β-glucan interacts with β-glucan-binding protein-high-density lipoprotein, thereby triggering the prophenoloxidase system. Consequently, this activation enhances the activities of immune-related enzymes [39], thereby promoting increased ACP and AKP enzyme activities. Similar findings were reported in shrimp (Penaeus vannamei), where R. mucilaginosa was shown to enhance immune enzyme activities and thereby improve host immune responses [40].
4.3. Effects of Rhodotorula mucilaginosa on the Relative Expression Levels of Genes of Red Claw Crayfish
Crustaceans possess a relatively straightforward immune system that mainly relies on innate immunity to defend against pathogen intrusion. Among the key mechanisms, the Toll and IMD pathways play essential roles in triggering immune genes when faced with pathogen threats [41]. TLR2 and TLR6 are key members of the TLR family, primarily responsible for recognizing pathogen-associated molecular patterns (PAMPs), such as bacterial lipopolysaccharides and fungal β-glucans [42, 43]. In the Toll signaling pathway, TLR2/6 recognizes pathogens by forming heterodimers [44] and recruits the adaptor protein MyD88, subsequently activating TRAF6. TRAF6 then activates TAK1 through K63-linked ubiquitination, ultimately leading to nuclear translocation of NF-κB and expression of antimicrobial peptides (e.g., crustin and lysozyme) [45]. This research discovered that supplementing the diet with R. mucilaginosa considerably increased the expression levels of the tlr2 and tlr6 genes in the hepatopancreas of red claw crayfish. This upregulation suggests that β-glucans and other components in the red yeast can be recognized by TLR2/6, activating downstream signaling pathways and inducing antimicrobial peptide expression, thereby enhancing immune defense [46]. The increased expression of TLR2/6 not only improves pathogen recognition in crayfish but also systematically clears pathogens through Toll pathway activation, generating immune responses [47]. Similar findings were reported in kuruma shrimp (Marsupenaeus japonicus), where upregulated tlr1/2 expression was closely associated with antiviral responses [48].
The protein encoded by IMD acts as the central element in the IMD signaling pathway, triggering innate immune responses by identifying PAMPs [49]. In the IMD signaling pathway, the protein IMD triggers TAK1 and the IKK complex through adaptor proteins FADD and DREDD. This process subsequently results in the activation of the NF-κB-like transcription factor known as Relish. After relocating to the nucleus, Relish promotes the expression of antimicrobial peptides, which strengthens the host's ability to combat bacterial infections [50]. Our research showed that adding R. mucilaginosa to the diet led to a significant increase in imd gene expression within the hepatopancreas of red claw crayfish. This pattern of enhancement suggests that R. mucilaginosa is capable of strengthening the innate immune response in crayfish by activating the IMD pathway [51]. Activation of the IMD pathway not only facilitates pathogen clearance but also strengthens host immune defense mechanisms by inducing antimicrobial peptide production [52]. These findings are corroborated by parallel research in oriental river prawn (Macrobrachium nipponense), where dietary supplementation with Bacillus coagulans was shown to significantly upregulate imd expression [53].
The protein encoded by traf6, serving as a crucial adaptor molecule in both Toll and IMD signaling pathways, exerts its immunomodulatory function by initiating ubiquitination cascades that activate TAK1 and IKK complexes, thereby stimulating NF-κB and MAPK pathways which ultimately induce NF-κB nuclear translocation and antimicrobial peptide expression to regulate immune responses and inflammatory cytokine production [45], while our study demonstrates that R. mucilaginosa significantly upregulates traf6 expression levels, indicating its capacity to enhance innate immunity in red claw crayfish through dual activation of Toll and IMD pathways while maintaining immune homeostasis via inflammatory cytokine modulation [54], a regulatory mechanism corroborated by similar traf6 upregulation observed in Chinese mitten crab (Eriocheir sinensis) [55].
The transcription factors irf4 and akirin, known as key regulatory components in both Toll and IMD signaling pathways [56, 57], exhibit distinct but complementary immunomodulatory functions, where irf4 mediates TLR signal-dependent regulation of NF-κB-driven gene expression to orchestrate inflammatory responses and immune cell differentiation [58], while akirin interacts with the Bap60 subunit and 14-3-3 proteins to modulate NF-κB activity while also forming complexes with Relish to negatively regulate the IMD pathway and suppress target antimicrobial peptide expression [59], with our study demonstrating significant upregulation of both irf4 and akirin expression following R. mucilaginosa supplementation, indicating this yeast enhances red claw crayfish immunity through coordinated NF-κB pathway modulation, a conclusion consistent with Chen and Wang's [22] findings during Vibrio parahaemolyticus challenge experiments in the same species.
IL-1β and TNF-α belong to the proinflammatory cytokine group. These molecules can stimulate various cells to generate inflammatory mediators and are essential in regulating the inflammatory response [60]. TGF-β1 is a multifunctional cytokine that not only inhibits inflammation but also promotes the apoptosis of inflammatory cells [60]. This research found that R. mucilaginosa enhanced the expression of tnf-α, il-1β, and tgf-β1 genes in the hepatopancreas of red claw crayfish. The upregulation of tnf-α and il-1β expressions may be attributed to R. mucilaginosa activating TLR2 to recognize foreign antigens, thereby promoting inflammatory signal transduction and enhancing phagocyte function [61, 62]. The increased expression of anti-inflammatory factors such as tgf-β1 could be a result of immune regulation within the body to prevent excessive activation of proinflammatory factors [60]. Comparable immunomodulatory effects were also noted in marron (Cherax cainii). The addition of Lactobacillus acidophilus and L. plantarum to the diet led to a notable increase in the expression of the proinflammatory cytokines tnf-α and il-1β. This indicates that probiotic-driven immune activation mechanisms are preserved across different species within the Cherax genus [63].
The JAK/STAT signaling pathway, which is also referred to as the cytokine-induced signaling pathway, facilitates the activation of neutrophils and macrophages. It additionally modulates inflammatory reactions and tissue repair, and it is essential in the immune responses of crustaceans [64]. Its core components include cytokine receptors, JAK tyrosine kinases, and STAT transcription factors [41]. When a cytokine signal is detected by its receptor, the JAK tyrosine kinase undergoes phosphorylation. This process triggers the activation and subsequent movement of STAT proteins into the nucleus, where they control the expression of antibacterial peptides [65]. In this research, it was noted that incorporating R. mucilaginosa into the diet of red claw crayfish markedly increased the expression levels of jak and stat genes within the hepatopancreas. This upregulation may represent an adaptive response of the crayfish's immune system to external stimuli. The JAK-STAT signaling pathway is central to immune regulation, promoting the activation, proliferation, and differentiation of immune cells and modulating cytokine production [66]. Consistent with these findings, Sriphuttha et al. [37] demonstrated that Rhodotorula paludigena CM33 could activate the JAK-STAT signaling pathways in Litopenaeus vannamei, thereby enhancing shrimp immunity.
5. Conclusion
In summary, R. mucilaginosa markedly promoted growth, boosted the antioxidant capabilities, and strengthened the immune responses of red claw crayfish while activating the Toll/Imd and JAK-STAT signaling pathways. In this experimental context, the ideal addition level of R. mucilaginosa was determined to be 1.0 g/kg. Future research will refine this dosage by incorporating a narrower gradient (e.g., 0.8 and 1.2 g/kg) to determine the most effective addition amount for practical application. These findings will provide a solid scientific foundation for developing efficient compound probiotic feed specifically tailored for red claw crayfish.
Contributor Information
Yongqiang Liu, Email: liuyongqiang@gxmzu.edu.cn.
Huizan Yang, Email: yhzyang@163.com.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
All red claw crayfish experiments were conducted in accordance with the guidelines of Guangxi Minzu University, Nanning, China, and this research does not contain any studies with human participants (Approval Number: GXUN 2023-018).
Disclosure
All authors have thoroughly reviewed and provided their consent to the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Qin Zhang: conceptualization, methodology, investigation, data curation, writing – review and editing, funding acquisition, validation, visualization, formal analysis. Liuqing Meng: methodology, investigation, data curation, writing – original draft preparation, writing – review and editing, supervision, validation, visualization, formal analysis, software. Jiqing Li, Luoqing Li, Qinghui Zeng, Rui Wang, Dapeng Wang, and Tong Tong: methodology, investigation, validation, visualization. Yongqiang Liu and Huizan Yang: conceptualization, methodology, investigation, data curation, funding acquisition, validation, visualization, formal analysis, project administration. Qin Zhang and Liuqing Meng contributed equally to this work.
Funding
This study was supported by the Guangxi Science and Technology Base and Talent Special Project (Grant GuikeAD24010034) and the Scientific Research Fund for the Introduction of Excellent Talents in Guangxi University for Nationalities (Grant 2018KJQD14).
References
- 1.Haubrock P. J., Oficialdegui F. J., Zeng Y., Patoka J., Yeo D. C. J., Kouba A. The Redclaw Crayfish: A Prominent Aquaculture Species With Invasive Potential in Tropical and Subtropical Biodiversity Hotspots. Reviews in Aquaculture . 2021;13(3):1488–1530. doi: 10.1111/raq.12531. [DOI] [Google Scholar]
- 2.Zhang Q., Xie Y., Tang J., et al. Effects of Dietary Chitosan on Growth Performance, Serum Biochemical Indices, Antioxidant Capacity, and Immune Response of Juvenile Tilapia (Oreochromis niloticus) Under Cadmium Stress. Animals . 2024;14(15) doi: 10.3390/ani14152259.2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei J., Huang C., Nie X., et al. Analysis of Seven Populations of Cultured Redclaw Crayfish, Cherax quadricarinatus, Using Newly Developed Microsatellite Markers. Aquaculture Reports . 2024;35 doi: 10.1016/j.aqrep.2024.102024.102024 [DOI] [Google Scholar]
- 4.El-Saadony T. M., Swelum A. A., Abo Ghanima M. M., et al. Shrimp Production, the Most Important Diseases That Threaten It, and the Role of Probiotics in Confronting These Diseases: A Review. Research in Veterinary Science . 2022;144:126–140. doi: 10.1016/j.rvsc.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y., Yu M., Liu J., et al. Bacterial Community Associated With Healthy and Diseased Pacific White Shrimp (Litopenaeus vannamei) Larvae and Rearing Water Across Different Growth Stages. Frontiers in Microbiology . 2017;8 doi: 10.3389/fmicb.2017.01362.1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo W. Y., Chen Z., Leung H. M., Leung A. O. W. Application of Veterinary Antibiotics in China’s Aquaculture Industry and Their Potential Human Health Risks. Environmental Science and Pollution Research . 2017;24(10):8978–8989. doi: 10.1007/s11356-015-5607-z. [DOI] [PubMed] [Google Scholar]
- 7.Licona-Jain A., Campa-Córdova Á., Luna-González A., Racotta I. S., Tello M., Angulo C. Dietary Supplementation of Marine Yeast Yarrowia lipolytica Modulates Immune Response in Litopenaeus Vannamei. Fish & Shellfish Immunology . 2020;105:469–476. doi: 10.1016/j.fsi.2020.07.043. [DOI] [PubMed] [Google Scholar]
- 8.Hasan K. N., Banerjee G. Recent Studies on Probiotics as Beneficial Mediator in Aquaculture: A Review. The Journal of Basic and Applied Zoology . 2020;81(1) doi: 10.1186/s41936-020-00190-y.53 [DOI] [Google Scholar]
- 9.Knipe H., Temperton B., Lange A., Bass D., Tyler C. R. Probiotics and Competitive Exclusion of Pathogens in Shrimp Aquaculture. Reviews in Aquaculture . 2021;13(1):324–352. doi: 10.1111/raq.12477. [DOI] [Google Scholar]
- 10.Rohani M. F., Islam S. M. M., Hossain M. K., et al. Probiotics, Prebiotics and Synbiotics Improved the Functionality of Aquafeed: Upgrading Growth, Reproduction, Immunity and Disease Resistance in Fish. Fish & Shellfish Immunology . 2022;120:569–589. doi: 10.1016/j.fsi.2021.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Castañeda-Tamez P., Chiquete-Félix N., Uribe-Carvajal S., Cabrera-Orefice A. The Mitochondrial Respiratory Chain From Rhodotorula mucilaginosa, an Extremophile Yeast. Biochimica et Biophysica Acta (BBA) - Bioenergetics . 2024;1865(2) doi: 10.1016/j.bbabio.2024.149035.149035 [DOI] [PubMed] [Google Scholar]
- 12.Li Z., Li C., Cheng P., Yu G. Rhodotorula mucilaginosa—Alternative Sources of Natural Carotenoids, Lipids, and Enzymes for Industrial Use. Heliyon . 2022;8(11) doi: 10.1016/j.heliyon.2022.e11505.e11505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosqueda-Martínez E., Chiquete-Félix N., Castañeda-Tamez P., et al. In Rhodotorula mucilaginosa, Active Oxidative Metabolism Increases Carotenoids to Inactivate Excess Reactive Oxygen Species. Frontiers in Fungal Biology . 2024;5 doi: 10.3389/ffunb.2024.1378590.1378590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogusławska-Wąs E., Dłubała A., Laskowska M. The Role of Rhodotorula mucilaginosa in Selected Biological Process of Wild Fish. Fish Physiology and Biochemistry . 2019;45(2):511–521. doi: 10.1007/s10695-018-0591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Huang E., Li X., et al. Effects of Dietary Marine Red Yeast Supplementation on Growth Performance, Antioxidant, Immunity, Lipid Metabolism and mTOR Signaling Pathway in Juvenile Tilapia (Oreochromis niloticus) Aquaculture Reports . 2024;37 doi: 10.1016/j.aqrep.2024.102196.102196 [DOI] [Google Scholar]
- 16.Linh N. V., Wannavijit S., Sumon M. A. A., et al. Immunomodulatory and Growth-Promoting Effects of Supplementing Red Yeast (Sporidiobolus pararoseus) in Fish Meal-Based Diets for Koi Carp (Cyprinus carpio Var. koi) Cultured in a Biofloc System. Aquaculture International . 2025;33(1) doi: 10.1007/s10499-024-01738-3.17 [DOI] [Google Scholar]
- 17.Yun L., Wang W., Li Y., et al. PLoS ONE. Potential Application Values of a Marine Red Yeast, Rhodosporidiums Sphaerocarpum, YLY01, in Aquaculture and Tail Water Treatment Assessed by the Removal of Ammonia Nitrogen, the Inhibition to Vibrio Spp., and Nutrient Composition . 2021;16(2) doi: 10.1371/journal.pone.0246841.e0246841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang M., Su X., Yun L., et al. Evaluation of Probiotic Characteristics and Whole Genome Analysis of Bacillus Velezensis R-71003 Isolated From the Intestine of Common Carp (Cyprinus carpio L.) for its Use as a Probiotic in Aquaculture. Aquaculture Reports . 2022;25 doi: 10.1016/j.aqrep.2022.101254.101254 [DOI] [Google Scholar]
- 19.Yang S.-P., Wu Z.-H., Jian J.-C., Zhang X.-Z. Effect of Marine Red Yeast Rhodosporidium paludigenum on Growth and Antioxidant Competence of Litopenaeus vannamei. Aquaculture . 2010;309(1–4):62–65. doi: 10.1016/j.aquaculture.2010.09.032. [DOI] [Google Scholar]
- 20.Ren X., Liu X., Zhu X., Xiong L., Bai X. Trained Immunity Can Improve the Disease Resistance of Red Swamp Crayfish (Procambarus clarkii) Fish & Shellfish Immunology . 2023;132 doi: 10.1016/j.fsi.2022.108468.108468 [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q., Guo M., Li F., et al. Evaluation of Fermented Soybean Meal to Replace a Portion Fish Meal on Growth Performance, Antioxidant Capacity, Immunity, and mTOR Signaling Pathway of Coho Salmon (Oncorhynchus kisutch) Aquaculture Nutrition . 2023;8 doi: 10.1155/2023/2558173.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D., Wang H. Redclaw Crayfish (Cherax quadricarinatus) Responds to Vibrio parahaemolyticus Infection by Activating Toll and Immune Deficiency Signaling Pathways and Transcription of Associated Immune Response Genes. Fish & Shellfish Immunology . 2022;127:611–622. doi: 10.1016/j.fsi.2022.06.069. [DOI] [PubMed] [Google Scholar]
- 23.Chen D. Effects of Vibrio Parahaemolyticus Infection on the Transcriptome and Metabolome of the Hepatopancreas and Functional Analysis of Relish and TRAF6 Genes in Redclaw Crayfish . Shandong Agricultural University; 2022. [Google Scholar]
- 24.Chen X. Molecular Characterization of Cytokine Receptor Domeless and Its Functional Research on WSSV Infection of Cherax quadricarinatus . East China University of Science and Technology; 2018. [Google Scholar]
- 25.Schmittgen T. D., Livak K. J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nature Protocols . 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Ke Q., Liu J., Zhao J., et al. Genomic Selection of Large Yellow Croaker (Larimichthys crocea) With a High Plant Protein Diet Enhances the Growth Performance of Offspring. Marine Biotechnology . 2024;26(4):732–740. doi: 10.1007/s10126-024-10341-9. [DOI] [PubMed] [Google Scholar]
- 27.Gainza O., Romero J. Effect of Mannan Oligosaccharides on the Microbiota and Productivity Parameters of Litopenaeus vannamei Shrimp Under Intensive Cultivation in Ecuador. Scientific Reports . 2020;10(1) doi: 10.1038/s41598-020-59587-y.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torrecillas S., Montero D., Izquierdo M. Improved Health and Growth of Fish Fed Mannan Oligosaccharides: Potential Mode of Action. Fish & Shellfish Immunology . 2014;36(2):525–544. doi: 10.1016/j.fsi.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Ettefaghdoost M., Haghighi H. Impact of Different Dietary Lutein Levels on Growth Performance, Biochemical and Immuno-Physiological Parameters of Oriental River Prawn (Macrobrachium nipponense) Fish & Shellfish Immunology . 2021;115:86–94. doi: 10.1016/j.fsi.2021.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Chen X.-Q., Zhao W., Xie S.-W., et al. Effects of Dietary Hydrolyzed Yeast (Rhodotorula mucilaginosa) on Growth Performance, Immune Response, Antioxidant Capacity and Histomorphology of Juvenile Nile Tilapia (Oreochromis niloticus) Fish & Shellfish Immunology . 2019;90:30–39. doi: 10.1016/j.fsi.2019.03.068. [DOI] [PubMed] [Google Scholar]
- 31.Haridevamuthu B., Chandran A., Raj D., et al. Growth Performance and Immunomodulatory Effect of, Terminalia catappa, L. Diet on Litopenaeus vannamei Against Vibrio parahaemolyticus Challenge. Aquaculture International . 2024;32(3):2549–2570. doi: 10.1007/s10499-023-01284-4. [DOI] [Google Scholar]
- 32.Hoseinifar S. H., Yousefi S., Van Doan H., et al. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Reviews in Fisheries Science & Aquaculture . 2021;29(2):198–217. doi: 10.1080/23308249.2020.1795616. [DOI] [Google Scholar]
- 33.Wade N. M., Gabaudan J., Glencross B. D. A Review of Carotenoid Utilisation and Function in Crustacean Aquaculture. Reviews in Aquaculture . 2017;9(2):141–156. doi: 10.1111/raq.12109. [DOI] [Google Scholar]
- 34.Kieliszek M., Kot A. M., Kolotylo V. Bioaccumulation of Selenium and Production of Carotenoids by the Yeast Rhodotorula mucilaginosa. Biocatalysis and Agricultural Biotechnology . 2023;53 doi: 10.1016/j.bcab.2023.102903.102903 [DOI] [Google Scholar]
- 35.Jiang G., Yang Z., Wang Y., et al. Enhanced Astaxanthin Production in Yeast Via Combined Mutagenesis and Evolution. Biochemical Engineering Journal . 2020;156 doi: 10.1016/j.bej.2020.107519.107519 [DOI] [Google Scholar]
- 36.Tang Q., Huang G., Zhao F., Zhou L., Huang S., Li H. The Antioxidant Activities of Six (1 → 3)-β- D—Glucan Derivatives Prepared From Yeast Cell Wall. International Journal of Biological Macromolecules . 2017;98:216–221. doi: 10.1016/j.ijbiomac.2017.01.132. [DOI] [PubMed] [Google Scholar]
- 37.Sriphuttha C., Limkul S., Pongsetkul J., et al. Effect of Fed Dietary Yeast (Rhodotorula paludigena CM33) on Shrimp Growth, Gene Expression, Intestinal Microbial, Disease Resistance, and Meat Composition of Litopenaeus vannamei. Developmental & Comparative Immunology . 2023;147 doi: 10.1016/j.dci.2023.104896.104896 [DOI] [PubMed] [Google Scholar]
- 38.Yohana M. A., Ray G. W., Yang Q., et al. Comprehensive Analysis of Butyric Acid Impact on Immunology, Histopathology, Gene Expression, and Metabolomic Responses in Pacific Shrimp Experiencing Cold Stress. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics . 2024;52 doi: 10.1016/j.cbd.2024.101293.101293 [DOI] [PubMed] [Google Scholar]
- 39.Thitamadee S., Srisala J., Taengchaiyaphum S., Sritunyalucksana K. Double-Dose β-Glucan Treatment in WSSV-Challenged Shrimp Reduces Viral Replication But Causes Mortality Possibly Due to Excessive ROS Production. Fish & Shellfish Immunology . 2014;40(2):478–484. doi: 10.1016/j.fsi.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 40.Wang B., Liu Y., Luo K., et al. ‘Biotic’ Potential of the Red Yeast Rhodotorula mucilaginosa strain JM-01 on the Growth, Shell Pigmentation, and Immune Defense Attributes of the Shrimp, Penaeus vannamei. Aquaculture . 2023;572 doi: 10.1016/j.aquaculture.2023.739543.739543 [DOI] [Google Scholar]
- 41.Li F., Xiang J. Signaling Pathways Regulating Innate Immune Responses in Shrimp. Fish & Shellfish Immunology . 2013;34(4):973–980. doi: 10.1016/j.fsi.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 42.Akira S., Uematsu S., Takeuchi O. Pathogen Recognition and Innate Immunity. Cell . 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Travassos L. H., Girardin S. E., Philpott D. J., et al. Toll-Like Receptor 2-Dependent Bacterial Sensing Does Not Occur Via Peptidoglycan Recognition. EMBO reports . 2004;5(10):1000–1006. doi: 10.1038/sj.embor.7400248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Neill L. A. J., Golenbock D., Bowie A. G. The History of Toll-Like Receptors—Redefining Innate Immunity. Nature Reviews Immunology . 2013;13(6):453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J., Kong X., Zhou C., Li L., Nie G., Li X. Toll-Like Receptor Recognition of Bacteria in Fish: Ligand Specificity and Signal Pathways. Fish & Shellfish Immunology . 2014;41(2):380–388. doi: 10.1016/j.fsi.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 46.Kawai T., Akira S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-Like Receptors. Nature Immunology . 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 47.Wan H., Mu S., Baohua D., Guo S., Kang X. Genome-Wide Investigation of Toll-Like Receptor Genes (TLRs) in Procambarus clarkia and Their Expression Pattern in Response to Black May Disease. Fish & Shellfish Immunology . 2022;131:775–784. doi: 10.1016/j.fsi.2022.10.066. [DOI] [PubMed] [Google Scholar]
- 48.Maeda M., Shibata A., Biswas G., et al. Isolation of Lactic Acid Bacteria From Kuruma Shrimp (Marsupenaeus japonicus) Intestine and Assessment of Immunomodulatory Role of a Selected Strain as Probiotic. Marine Biotechnology . 2014;16(2):181–192. doi: 10.1007/s10126-013-9532-1. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y., Ren Q. Research Progress in Innate Immunity of Freshwater Crustaceans. Developmental & Comparative Immunology . 2020;104 doi: 10.1016/j.dci.2019.103569.103569 [DOI] [PubMed] [Google Scholar]
- 50.Bai L., Zhou K., Li H., Qin Y., Wang Q., Li W. Bacteria-Induced IMD-Relish-AMPs Pathway Activation in Chinese Mitten Crab. Fish & Shellfish Immunology . 2020;106:866–875. doi: 10.1016/j.fsi.2020.08.046. [DOI] [PubMed] [Google Scholar]
- 51.Lan J.-F., Zhou J., Zhang X.-W., et al. Characterization of an Immune Deficiency Homolog (IMD) in Shrimp (Fenneropenaeus chinensis) and Crayfish (Procambarus clarkii) Developmental and Comparative Immunology . 2013;41(4):608–617. doi: 10.1016/j.dci.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Rajendran K. V., Sreedharan K., Deepika A., Kulkarni A. Fish Immune System and Vaccines . Singapore: Springer; 2022. Shrimp Immune System and Immune Responses; pp. 17–43. [DOI] [Google Scholar]
- 53.Wang Y., Liang Y., Yu J., et al. Effects of Polysaccharide Fermentation With Bacillus coagulans on Growth, Antioxidant and Immunity of Macrobrachium nipponense (Riental River Prawn) Frontiers in Marine Science . 2024;11 doi: 10.3389/fmars.2024.1514651.1514651 [DOI] [Google Scholar]
- 54.Bhoj V. G., Chen Z. J. Ubiquitylation in Innate and Adaptive Immunity. Nature . 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 55.Li J., Ma Y., Wu Z., et al. The Involvement of Tumor Necrosis Factor Receptor-Associated Factor 6 in Regulating Immune Response by NF-κB at Pre-Molt Stage of Chinese Mitten Crab (Eriocheir sinensis) Fish & Shellfish Immunology . 2024;153 doi: 10.1016/j.fsi.2024.109842.109842 [DOI] [PubMed] [Google Scholar]
- 56.Ai K., Luo K., Li Y., et al. Expression Pattern Analysis of IRF4 and Its Related Genes Revealed the Functional Differentiation of IRF4 Paralogues in Teleost. Fish & Shellfish Immunology . 2017;60:59–64. doi: 10.1016/j.fsi.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 57.Xiong H., Jiang Y., Ji T., Zhang Y., Wei W., Yang H. The Identification of a Nuclear Factor Akirin With Regulating the Expression of Antimicrobial Peptides in Red Swamp Crayfish (Procambarus clarkii) International Journal of Biological Macromolecules . 2021;183:707–717. doi: 10.1016/j.ijbiomac.2021.04.153. [DOI] [PubMed] [Google Scholar]
- 58.Chen D., Wang Z., Cao S., et al. Molecular Characterization and Functional Analysis of Interferon Regulatory Factor-4 in the Red Claw Crayfish (Cherax quadricarinatus) Aquaculture Reports . 2023;28 doi: 10.1016/j.aqrep.2022.101456.101456 [DOI] [Google Scholar]
- 59.Liu N., Wang X.-W., Sun J.-J., et al. Akirin Interacts With Bap60 and 14-3-3 Proteins to Regulate the Expression of Antimicrobial Peptides in the Kuruma Shrimp (Marsupenaeus japonicus) Developmental & Comparative Immunology . 2016;55:80–89. doi: 10.1016/j.dci.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Hoseinifar S. H., Zou H. K., Van Doan H., Kolangi Miandare H., Hoseini S. M. Evaluation of Some Intestinal Cytokines Genes Expression and Serum Innate Immune Parameters in Common Carp (Cyprinus carpio) Fed Dietary Loquat (Eriobotrya japonica) Leaf Extract. Aquaculture Research . 2018;49(1):120–127. doi: 10.1111/are.13440. [DOI] [Google Scholar]
- 61.Corripio-Miyar Y., Bird S., Tsamopoulos K., Secombes C. J. Cloning and Expression Analysis of Two Pro-Inflammatory Cytokines, IL-1β and IL-8, in Haddock (Melanogrammus aeglefinus) Molecular Immunology . 2007;44(6):1361–1373. doi: 10.1016/j.molimm.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Yuan C., Pan X., Gong Y., et al. Effects of Astragalus Polysaccharides (APS) on the Expression of Immune Response Genes in Head Kidney, Gill and Spleen of the Common Carp, Cyprinus carpio, L. International Immunopharmacology . 2008;8(1):51–58. doi: 10.1016/j.intimp.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Foysal M. J., Fotedar R., Siddik M. A. B., Tay A. Lactobacillus acidophilus and L. plantarum Improve Health Status, Modulate Gut Microbiota and Innate Immune Response of Marron (Cherax cainii) Scientific Reports . 2020;10(1) doi: 10.1038/s41598-020-62655-y.5916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deng H., Xu X., Hu L., et al. A Janus Kinase From Scylla paramamosain Activates JAK/STAT Signaling Pathway to Restrain Mud Crab Reovirus. Fish & Shellfish Immunology . 2019;90:275–287. doi: 10.1016/j.fsi.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 65.Li C., Li H., Chen Y., et al. Activation of Vago by Interferon Regulatory Factor (IRF) Suggests an Interferon System-Like Antiviral Mechanism in Shrimp. Scientific Reports . 2015;5(1):1–13. doi: 10.1038/srep15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo L., Zhou M., Chen D., et al. A New Insight to Characterize Immunomodulation Based on Hepatopancreatic Transcriptome and Humoral Immune Factor Analysis of the Cherax quadricarinatus Infected With Aeromonas veronii. Ecotoxicology and Environmental Safety . 2021;219 doi: 10.1016/j.ecoenv.2021.112347.112347 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


