Abstract
Chloroplasts are endosymbiotic organelles of cyanobacterial origin. It seems reasonable to assume that cell division and organelle division still share general principles, as shown for the FtsZ proteins. However, further components involved in this process are largely unknown. Here we describe ARTEMIS, a nuclear-encoded protein of chloroplast inner envelope membranes that is required for organelle division. ARTEMIS consists of three distinct modules: an N-terminal receptor-like region, a centrally positioned glycine-rich stretch containing a nucleoside triphosphate-binding site, and a C-terminal YidC/Oxa1p/Alb3 protein translocase-like domain. Analysis of Arabidopsis En-1 transposon mutants as well as ARTEMIS antisense plants revealed chloroplasts arrested in the late stages of division. Chloroplasts showed clearly separated and distinct multiple thylakoid systems, whereas the final organelle fission remained unaccomplished. Inactivation of a cyanobacterial gene with sequence similarity to the YidC/Oxa1p/Alb3-like domain of ARTEMIS resulted in aberrant cell division, which could be rescued by the Arabidopsis protein. ARTEMIS represents a so-far-unrecognized link between prokaryotic cell fission and chloroplast division.
Division of higher plant chloroplasts is a complex and still poorly understood process that combines mechanisms of organelle constriction with the assembly and expansion of envelope membranes and the thylakoid network. Four nuclear-encoded proteins have so far been implicated in chloroplast division. FtsZ is a tubulin-like GTPase that assembles into a ring structure at the bacterial cell midpoint and enables recruitment of other division proteins (1). Most of identified eukaryotic FtsZ genes are of cyanobacterial origin and are implicated in chloroplast division. In chromophyte and red algae, an additional α-proteobacterial-related FtsZ is involved in mitochondrial division (2). At least four FtsZ homologues encoded by two different gene families, FtsZ1 and FtsZ2, can be found in Arabidopsis (2, 3). Members of both families colocalize in the chloroplast stroma and form a contractile ring(s) at the plastid division site (3, 4). Apart from FtsZ ring(s), constriction of chloroplasts during division involves at least one additional ring structure located at the cytoplasmic surface of the outer envelope (5). This plastid-dividing (PD) ring is in red algae composed of so-far-unidentified component(s) that are not related to FtsZ proteins (6). It has been postulated that the FtsZ ring-based system evolved from cyanobacterial endosymbiont, whereas the PD ring probably originates from the eukaryotic host cell (7). Both systems appear to complement each other and are in dynamic transition during a division process (7). In plastids of moss Physcomitrella, FtsZ-GFP monomers polymerize to highly organized structures resembling cytoskeleton (8). Accordingly, the network has been designated plastoskeleton, although its existence in plastids of other species has yet to be established.
Akin to bacterial fission, placement of the plastid division initiation site on the stromal surface of the inner envelope is probably mediated by MinD and MinE proteins (9–11). In bacteria, MinD forms a complex with MinC and prevents Z-ring formation at all potential division sites, except the mid-cell (12). Plants with altered MinD expression form large plastids unable to carry out the fission reaction (9–10). MinE, on the other hand, prevents MinCD from inhibiting Z-ring formation at the proper mid-cell site (12). Arabidopsis plants overexpressing MinE accumulate large chloroplasts with similar morphology to MinD mutants (11). A unique feature of MinC and MinD is their ability to rapidly oscillate from one bacterial cell pole to another (12). In Bacillus subtilis, the MinCD complex appears to be stationary and equally distributed on both poles. The exact mechanisms of MinCD recruitment at polar zones are unclear and underline the general problem of how a cell can identify its midpoint (12). Almost equally elusive are the mechanisms of initial FtsZ tethering to the bacterial cytoplasmic membrane at very early stages of division. The cytoplasmic membrane protein ZipA has been proposed as an FtsZ receptor in bacteria (13). Except the already mentioned FtsZ homologues and the chloroplast MinC and MinE proteins, homologues of other bacterial cell division proteins have not been found in the Arabidopsis genome (3), suggesting that chloroplast division involves different or additional components. It remains to be clarified how chloroplast division proteins adhere to the inner envelope in a timely and a spatially coordinated manner. How are constrictions of FtsZ ring(s) and the plastid-dividing ring coordinated? Which components are involved in possible nuclear control of chloroplast division?
In recent years, different protein translocases have been identified in membranes of various subcellular compartments and organelles of eukaryotic cells and prokaryotic organisms (14). These auxiliary molecules not only transport proteins from one side of a membrane to another but also assist in protein insertion into the lipid bilayer. Formation of cellular membranes follows similar blueprints from prokaryotic organisms to eukaryotic organelles, and the conserved nature of certain protein translocases supports a bacterial origin of both mitochondria and chloroplasts (14). In mitochondria, Oxa1p protein was shown to be involved in the insertion of a subset of inner membrane proteins from the mitochondrial matrix (15–18). Alb3, a homologue of Oxa1p, is a chloroplast protein involved in the insertion of light-harvesting antenna proteins into the thylakoid membrane (19). The deletion of Alb3 leads to defective thylakoid assembly (20). Both Oxa1p and Alb3 seem to originate from the bacterial translocase YidC (21), which is associated with the SecYEG trimeric complex (22). YidC assists in sorting of proteins that were previously believed to insert into the membrane directly, without the aid of proteinaceous components. Most eubacteria possess only one YidC homologue, but species of Bacillus, Listeria, and Streptomyces contain an additional YidC-related protein (23). The function of this additional protein is poorly investigated; however, its disruption in B. subtilis cells leads to a cell cycle arrest in the intermediate stage of spore formation. The protein has accordingly been designated SpoIIIJ (stage III sporulation protein J) (24). Expression of spoIIIj is dispensable during vegetative growth; however, its sporulation-specific expression is crucial for efficient sporulation (25). During vegetative growth, SpoIIIJ localizes to the cell membrane, but in sporulating cells it accumulates at polar and engulfment septa (25). It remains unclear whether inactivation of spoIIIj leads to a block in spore formation because of impaired assembly of membrane proteins, either spore specific or unspecific.
Here we describe the identification of ARTEMIS, an additional chloroplast homologue of the YidC/Oxa1p/Alb3 translocase whose inactivation in the model plant Arabidopsis leads to a specific defect in chloroplast division. ARTEMIS is an integral inner envelope membrane protein that combines an N-terminal region similar to receptor protein kinases with a YidC/Oxa1p/Alb3-like C-terminal domain. Cyanobacterium Synechocystis contains an ARTEMIS-related protein whose inactivation leads to defects in cell division rather than general protein-targeting deficiencies. This defect can be complemented by ARTEMIS from Arabidopsis, suggesting an evolutionary and functional relationship between organellar and prokaryotic cell division.
Materials and Methods
Standard Methods.
The RNA isolation for reverse transcription–PCR was made by using RNeasy Plant Kit (Qiagen, Hilden, Germany). Hybridization of total RNA from Arabidopsis thaliana Col-0 was performed by using a 269-bp-long DNA probe corresponding to the region between nucleotides 1600 and 1869 of the art1 gene. Stringency of washing was 0.5 × SSC, 0.1% SDS at 65°C. DNA was isolated according to ref. 26. Total protein extracts were made by using extraction buffer (0.05 M Tris/HCl, pH 6.8/0.05 M EDTA/1% SDS/0.1% β-mercaptoethanol) and boiling at 95°C. Western transfer was made by using blotting buffer (25 mM Tris/192 mM glycine/10% Met-OH).
Photosynthesis and respiration rates were measured with a Clark-type electrode at 28°C, with a cell density of 5 μg of chlorophyll per milliliter. Respiration followed after a 5-min incubation in the dark; photosynthesis was measured at least three times for 10 min at 1,400 μmol m−2⋅s−1.
Total protein extracts were made as described in ref. 27. Synechocystis subfractionation was carried out according to ref. 28.
Protein Overexpression and Production of Antisera.
DNA fragments corresponding to amino acid stretches K537–K625 and G732–Q802 were cloned into BamHI/KpnI sites of pRSETA (Invitrogen) vector and overexpressed in Escherichia coli BL21 cells. Recombinant proteins were purified on Talon-Metal-Affinity Column (CLONTECH) and used as antigenes for immunization of two different rabbits. Antibody αArtA is raised against K537–K625 and αArtB against G732–Q802.
Identification of En-1 Transposon Lines.
Transposon lines were obtained from the ZIGIA collection (Max-Planck-Institut für Züchtungsforschung, Cologne, Germany) (29). The presence of the En-1 transposon was tested by genomic Southern hybridization. The position of the En-1 element in the art1 genomic sequence was determined with PCR by using oligonucleotide corresponding to the 18-bp stretch downstream of the art1 translation initiation codon and En-1-specific en205 (5′-AGAAGCACGACGGCTGTAGAATAGGA-3′). A 508-bp amplified fragment was sequenced. Placement was reconfirmed by PCR amplification from the opposite side using exon 3 reverse oligonucleotide and En-1-specific en8130 (5′-GAGCGTCGGTCCCCACACTTCTATAC-3′) and sequencing.
Generation of ARTEMIS Antisense Lines.
The DNA fragment corresponding to the stretch between nucleotides 722 and 1329 was inserted in an antisense direction into SacI/XbaI sites of pGPTV phosphinotricin resistance binary vector (30). The construct was introduced to Arabidopsis plants by Agrobacterium-mediated transformation by using vacuum infiltration. Transformants were selected on 0.5 × Murashige and Skoog medium supplemented with 10 mg/ml of DL-phosphinotricin (Riedel-de Haën, Seelze, Germany). Seeds of resistant plants were grown on soil and reselected by spraying 2-wk-old seedlings with 250 mg⋅l−1 of phosphinotricin in 0.1% Tween 20.
Generation of Synechocystis Knockout and Complementation Lines.
Locus slr1471 was amplified by PCR from purified Synechocystis sp. PCC6803 genomic DNA by using oligonucleotides that allowed cloning into BamHI/KpnI sites of pBluescript to create pBSArt construct. The kanamycin-resistance cassette was inserted into the HindIII site of pBSArt located within the slr1471 sequence. pBSArt was transformed into Synechocystis sp. PCC6803 cells, and resistant colonies were selected on BG-11 plates supplemented with increasing concentrations of kanamycin (20 μg/ml end concentration). To ensure proper segregation, resistant colonies were screened through several rounds of replating on kanamycin-containing BG-11 medium. Efficiency of segregation was tested by PCR with slr1471-specific oligonucleotides and genomic Southern blotting. Δ1471 cells were grown autotrophically at 28°C in BG-11 under 80 μmol m−2⋅s−1 white light in flasks bubbled through with 2% CO2 in air. The promoter region of the sll0617 gene (31) was PCR amplified with oligonucleotides fitted with PstI/NdeI restriction sites and ligated with a nucleotide sequence fitted with NdeI/KpnI overhangs and corresponding to V538-R1013 stretch. The construct was inserted into PstI/KpnI sites of the pBluescript and transformed into Synechocystis Δ1471 cells. Transformants were selected on BG-11 plates containing 20 μg/ml of kanamycin and 30 μg/ml of ampicillin.
Results and Discussion
Characterization of ARTEMIS Protein.
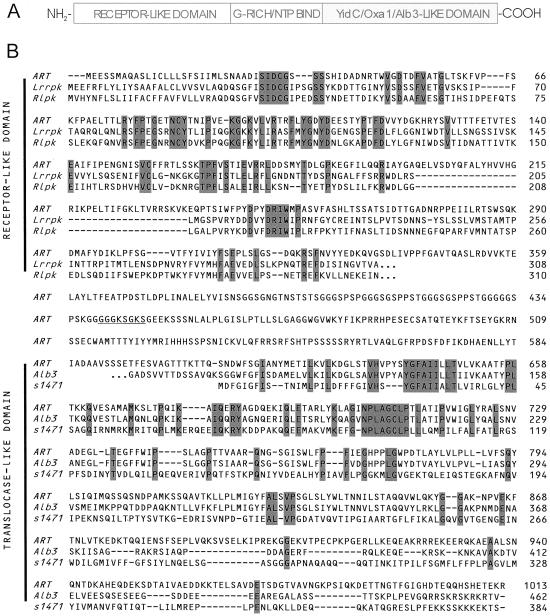
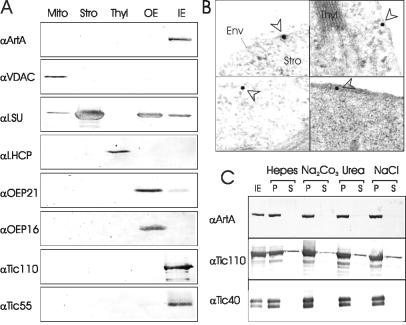
In a search of components involved in chloroplast biogenesis, we have identified an Arabidopsis protein (locus NP_173858 in GenBank) with a unique molecular structure. The 1,013-residue polypeptide (Fig. 1) encoded on chromosome 1 contains a COOH-terminal domain similar to the Alb3 protein (predicted polytopic region) with conserved YidC translocase elements. The middle portion contains a predicted ATP/GTP-binding domain, whereas the NH2-terminal region resembles receptor domains of receptor protein kinases. Northern blot analysis indicated a moderately abundant transcript of an estimated size of 3,550 bp in Arabidopsis plants (data not shown). We tested the hypothesis that this polypeptide is located in chloroplasts, which is indicated by a putative NH2-terminal chloroplast targeting signal (32). Antisera raised against different parts of the protein were used to analyze various cellular and chloroplast subfractions. A single immunoreactive band with an apparent molecular mass of 110 kDa was identified exclusively in inner envelope membranes of chloroplasts (Fig. 2A). The same 110-kDa protein band could be detected with an antibody raised against a conserved domain from E. coli Oxa1p (data not shown). Immunogold labeling of ultrathin sections (35) from leaves further corroborated the chloroplast localization (Fig. 2B). Consequently, the protein was designated ARTEMIS (Arabidopsis thaliana envelope membrane integrase). Inner envelope vesicles were extracted by using different salt and urea treatments. In every case, we observed ARTEMIS exclusively in the insoluble membrane fraction (Fig. 2C), strongly suggesting that ARTEMIS is an integral membrane protein. The membrane association properties of ARTEMIS are comparable to those of some translocon at the inner envelope of chloroplast (Tic) subunits (Fig. 2C).
Fig 1.
Deduced ARTEMIS protein sequence and comparison with related proteins. (A) Schematic representation of ARTEMIS three-domain structure. (B) Alignment of ARTEMIS deduced amino acid sequence (ART) with Lrrpk (A. thaliana, light repressible receptor protein kinase, locus. NP_172061), Rlpk (A. thaliana, receptor-like protein kinase, locus AC003058), Alb3 (A. thaliana, albino3, locus AAB61458), and s1471 (Synechocystis sp. PCC6803, putative inner membrane protein slr1471, locus S75683). Residues conserved in all three sequences are shaded. A predicted nucleotide binding site is underlined. Continuation of amino acid sequence beyond the region of homology is indicated by three dots. Sequence alignment was performed by using the CLUSTAL method.
Fig 2.
Subcellular localization and membrane association properties of ARTEMIS. (A) Immunoblot using αArtB serum to probe mitochondrial (Mito), stromal (Stro), tylakoid membrane (Thyl), chloroplast outer envelope (OE), and chloroplast inner envelope (IE) fractions resolved on SDS/PAGE. Mitochondria and chloroplast subfractions were isolated from pea (Pisum sativum) as described (33, 34). The identity of various subfractions was tested by using immunoblotting with antisera against: voltage-dependent anion-selective channel (VDAC), large subunit of Rubisco (LSU), light harvesting complex protein (LHCP), outer envelope protein of 21 kDa (OEP21), outer envelope protein of 16 kDa (OEP16), translocon at the inner envelope component of 110 kDa (Tic110), and translocon at the inner envelope component of 55 kDa (Tic55). (B) Immunogold labeling of leaf ultrathin sections with αArtA. Gold particles are highlighted with arrowheads. Env, envelope membranes; Stro, stroma; Thyl, thylakoid membranes. (C) ARTEMIS is an integral inner envelope protein. Purified inner envelopes were treated with 5 mM Hepes/KOH, pH 7.6/1 M NaCl.0.5 M Na2CO3 or 4 M urea, as indicated and separated into soluble (S) and insoluble (P) protein fractions. ARTEMIS, Tic 110, and Tic 40 were determined by immunoblotting.
In ARTEMIS, the receptor part and the YidC/Oxa1p/Alb3-like domain are connected by a glycine-rich region harboring a predicted ATP/GTP-binding motif (P-loop) (Fig. 1B). To test whether ARTEMIS can bind nucleoside triphosphates, detergent-solubilized inner envelope vesicles were incubated with GTP-agarose matrix. Bound proteins were eluted with an excess amount of either GTP or ATP, and ARTEMIS was detected by immunoblot analysis. ARTEMIS could bind to the GTP matrix and was efficiently eluted only with GTP but barely with ATP, indicating a specificity for this nucleoside triphosphate (Fig. 3A). It was also possible to directly label ARTEMIS with [α32-P] GTP (36) in isolated inner membrane vesicles, providing further evidence of nucleoside triphosphate binding and possibly GTP-dependent regulation of ARTEMIS function (Fig. 3B). Although the predicted P-loop most likely participates in nucleoside triphosphate binding, other unidentified structural elements of ARTEMIS might be necessary for determination of specificity and strength of interaction. GTP could also assist in a possible homo- or heterooligomerization of ARTEMIS via the receptor domain.
Fig 3.

Nucleoside triphosphate binding of ARTEMIS. (A) Fifty micrograms of inner envelope vesicles was solubilized with 1% TritonX-100, diluted 10 times with binding buffer (20 mM Tris, pH 7.5/100 mM NaCl/5 mM MgCl2) and incubated for 20 min at 4°C with GTP-agarose resin. After washing with 40 resin volumes of binding buffer, proteins were released with 10 mM ATP or GTP. ARTEMIS was detected by immunoblotting. (B) Interaction of ARTEMIS with [α-32P]GTP. Chemical crosslinking was performed according to ref. 36. Protein–GTP complexes were immunoprecipitated with αArtA antibody, resolved on SDS/PAGE and detected by autoradiography. (C) Expression profiling of ARTEMIS in different organs. Ten micrograms of total cellular proteins from various tissues (as indicated) was immunostained with αArtA antiserum. (Left) Positions of molecular weight standards.
Arabidopsis ARTEMIS Mutants Have Defective Chloroplast Division.
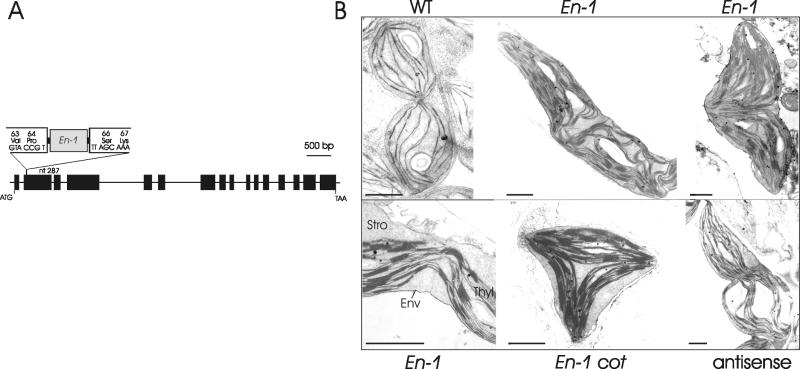
We subsequently performed a screen in a population of maize En-1 transposon mutagenized Arabidopsis plants. The 7AR137 mutant contained a single En-1 element inserted into the second exon of the art1 gene (Fig. 4A). The location of the En-1 insertion, combined with drastically reduced levels of ARTEMIS protein in mutant plants, indicated that 7AR137 is impaired in ARTEMIS synthesis. The phenotype of 7AR137 did not significantly differ from the wild type, although 7AR137 plants began flowering about 4 days earlier on average. The ultrastructure of mutant plants revealed extended, seemingly duplicated or triplicated, yet undivided chloroplasts of irregular shapes (Fig. 4B). Each half of a mutant chloroplast contained a wild-type-like thylakoid network with normally stacked membranes, indicating that thylakoid biogenesis is undisturbed, and that unlike the alb3 mutation (20), ARTEMIS does not influence light-harvesting complex protein insertion and photosystem II assembly. Other chloroplast division mutants are also able to carry out normal photosynthesis, providing additional evidence that thylakoid assembly and function are not affected by the inability of chloroplasts to carry out the division (9, 10). Only at the midpoint of the two undivided organelles were fewer membranes observed. They appeared deformed and pulled toward one side of the envelope membranes. In every case, envelope membranes were continuous and seemed unconstricted. Sometimes, chloroplasts formed long filament-like structures (not shown). Apparently, constriction and formation of the envelope membrane furrow were not initiated in mutant plastids and were uncoupled from thylakoid partitioning and elongation (Fig. 4B Lower Left). Even more, developing cotyledons contained tripolar chloroplasts, with three individual thylakoid systems (Fig. 4B Lower Center). We have identified an F2-generation plant in which the transposon element was no longer detectable in the art1 sequence. This plant contained only wild-type-like chloroplasts but still contained the En-1 element, however, in a different region of the genome as tested by PCR and Southern hybridization. To further substantiate these findings, we created transgenic Arabidopsis plants harboring an antisense construct of art1. Electron microscopy of leaf ultrathin sections revealed plastids with comparable phenotypic characteristics as observed in the 7AR137 line (Fig. 4B Lower Right). We conclude that mutation in the art1 gene has a specific effect on late stages of plastokinesis, probably the positioning of constriction ring(s) and recruitment of the envelope-located division apparatus.
Fig 4.
(A) Schematic representation of art1 gene in Arabidopsis mutant 7AR137. Position of En-1 element in the second exon is depicted. Exons are represented by black boxes; introns are indicated as lines. ATG, translation initiation codon; TAA, translation termination codon. (B) Ultrastructure of ARTEMIS mutant chloroplasts. Young dividing wild-type Arabidopsis chloroplast (WT), undivided chloroplasts of 7AR137 line (En-1), enlarged region of plastidal midpoint lacking envelope isthmus (Lower Left), triangular chloroplast of 5-day-old cotyledons (cot), and undivided chloroplast of antisense plants (Lower Right). Env, envelope membranes; Stro, stroma; Thyl, thylakoid membranes. (Bar = 1 μm.)
Although some basic elements of the division machinery are conserved, e.g., FtsZ GTPase, from prokaryotes to chloroplasts (37), plastokinesis in algae and higher plants is likely more complex than bacterial binary fission. Together with division of the envelope membranes, the photosynthetic membrane network has to be separated. The genome of Arabidopsis contains several FtsZ genes (38), and the assembly of at least two distinct division rings has been documented in chloroplasts (39). Division site determining factors MinD and MinE have been shown to participate in positioning of the FtsZ ring in chloroplasts (9–11), but other factors involved in division have largely remained elusive. Our findings suggest that constriction and division of thylakoid membranes can be accomplished independently of envelope invagination. ARTEMIS seems to orchestrate these apparently independent division processes. Formation of tripolar chloroplasts and thylakoids curved to specific regions of the inner membrane may be indicative of an improper and redundant placement of envelope division initiation sites or a failure to insert essential components of envelope biogenesis or of chloroplast division into the envelope membrane via the YidC/Oxa1p/Alb3-like module of ARTEMIS. The apparently normal phenotype of thylakoid network and of envelope membranes suggests that ARTEMIS is not involved, at least not directly, in general protein translocation into chloroplasts. It is, however, possible that ARTEMIS influences translocation of a specific subset of proteins that are necessary for chloroplast division or positioning of the organellar midpoint.
Consistent with its role in plastid division, ARTEMIS accumulates in different plant organs (Fig. 3C). The highest concentration is found in green tissues, although low but detectable levels can be found in etiolated seedlings. Fully differentiated roots and flowers, containing mostly leucoplasts and amyloplasts, accumulate barely detectable levels of ARTEMIS protein (slightly higher accumulation in flowers could be a result of chloroplast contamination from the green tissue of the floral base). This indicates that ARTEMIS is relatively abundantly expressed in organs containing actively dividing plastids, whereas those organs with only few meristematic or dividing cells accumulate only low amounts of ARTEMIS.
Synechocystis ARTEMIS-Related Protein Is Involved in Cell Division.
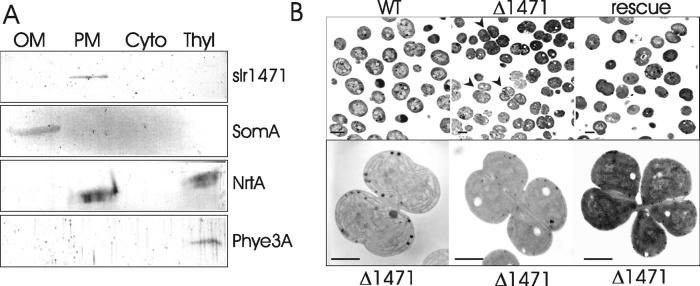
Chloroplasts have evolved from cyanobacterial ancestors, and it is reasonable to assume that certain mechanisms of cell division have been retained throughout evolution. However, only two (FtsZ and FtsI) of the nine E. coli cell division proteins identified so far have been detected in the Synechocystis PCC6803 genome (7). We have performed a search of the Synechocystis genome using the YidC/Oxa1p/Alb3-like domain of ARTEMIS as a query and have identified a locus, slr1471 (Fig. 1B), which encodes a putative plasma membrane protein of 384 residues. To verify the proposed localization, we have performed immunoblot analysis of various Synechocystis subfractions using the E. coli antibody. A single immunoreactive band with apparent molecular mass of 43 kDa could be detected in the plasma membrane fraction (Fig. 5A), strongly indicating that slr1471 is indeed located on the plasma membrane. To assess the function of the slr1471 gene product, we created a Δ1471 deletion mutant cell line. Synechocysis Δ1471 cells have a changed morphology and frequently form tetrameric or even hexameric clusters evidently arrested in late stages of division (Fig. 5B Lower). Cells also seem to initiate their fission unevenly, creating cells of irregular shapes. As in chloroplasts, thylakoid membrane biogenesis was not affected. Measurements of photosynthetic activity indicated no significant differences between wild-type cells and Δ1471 mutants (data not shown). In wild-type Synechocystis, only monomeric or dimeric cells undergoing normal division are detectable. The morphological phenotype of cyanobacterial Δ1471 cells resembles those of undivided Arabidopsis chloroplasts. We conclude that altered morphology of Δ1471 cells might be a result of an aberrant assembly of the cell division machinery and not a general phenomenon caused by an improper membrane biogenesis.
Fig 5.
Subcellular localization of slr1471 and the ultrastructure of Synechocystis Δ1471 cells. (A) Immunoblot analysis of outer membrane (OM), plasma membrane (PM), cytoplasm (Cyto), and thylakoid membrane (Thyl) fractions using antisera against the conserved domain of E. coli YidC (slr1471), Synechocystis porin protein SomA, a component of nitrate transporter NrtA, and a rod-linker protein of phycobilisomes Phye3A. (B Upper) overview image of wild-type Synechocystis cells (WT), mutant cells forming cell clusters (Δ1471, indicated by arrowheads), and restored cell division of Δ1471 cells rescued by pVipp1art1 (Right). (Lower) Enlarged clusters of undivided Δ1471 cells. (Bar = 1 μm.)
ARTEMIS Can Rescue Δ1471 Synechocystis Mutant.
To determine whether defects in the Synechocystis Δ1471 line could be complemented by the YidC/Oxa1p/Alb3-like domain of Arabidopsis ARTEMIS, the C-terminal domain was fused to a 450-bp-long promoter region of the sll0617 gene (31) to form a pVipp1art1 construct. We performed a rescue transformation of Synechocystis Δ1471 by using pVipp1art1 plasmidal expression and found that cell division was restored, i.e., only monomeric and dimeric cells as in wild type were detected (Fig. 5B Upper Right). The YidC/Oxa1p/Alb3 module of ARTEMIS can thus complement slr1471 deletion, suggesting that both proteins are evolutionarily and functionally related.
The large NH2-terminal receptor-like domain of ARTEMIS, including the nucleoside triphosphate-binding site, has no significant protein homologue in Synechocystis and has most likely been fused with the YidC/Oxa1p/Alb3 domain on evolution of chloroplasts as eukaryotic organelles. Interestingly, homologous sequences can be found in a number of plant transmembrane receptor protein kinases (Fig. 1B). These proteins are involved in an array of cellular signaling events and can form homo- and heterooligomeric complexes with themselves or other receptors (40). It is intriguing to speculate that the receptor domain of ARTEMIS might be a protein module of eukaryotic origin to establish the nuclear control over organelle division, whereas the YidC/Oxa1p/Art translocase domain assists in the integration and positioning of the division machinery into the inner envelope. Several bacterial division proteins are transmembrane proteins and at least one of them, FtsQ, has been shown to interact with the YidC translocase (41). As in the case of receptor kinases involved in meristem development, the ligand(s) perceived by the receptor domain could be peptide(s) (40). It is also tempting to speculate that common ligands may orchestrate plastid division with cell expansion and development. GTP most probably plays a regulatory role, as in the case of outer envelope protein import receptor Toc34, or may provide a switch for ligand binding or homo-/heterooligomerization. Addition of such a large regulatory domain onto a conserved YidC/Oxa1p/Art core domain could have been evolutionarily necessary to establish coordination between the host cell and the cyanobacterial endosymbiont.
Conclusion
We propose that ARTEMIS evolved during endosymbiosis from a single-domain single-function polypeptide involved in prokaryotic cell fission into a multidomain multifunctional polypeptide. Today, ARTEMIS most likely combines regulatory circuits engaged between organelle and nucleus to control chloroplast division with a protein translocase function for the integration of membrane components into the inner envelope to enable chloroplast division.
Acknowledgments
We thank B. Zeppenfeld and A. Daniel for excellent technical assistance. Antibody against Oxa1p was a kind gift of J. W. de Gier, Department of Biochemistry and Biophysics, the Arrhenius Laboratory for Natural Sciences, Stockholm University, Stockholm, Sweden. The research was supported by grants from the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich TR-1, and Fonds der Chemischen Industrie.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Rothfield L, Justice, S. & Garcia-Lara, J. (1999) Annu. Rev. Genet. 33 423-448. [DOI] [PubMed] [Google Scholar]
- 2.Reski R. (2002) Trends Plant Sci. 7 103-105. [DOI] [PubMed] [Google Scholar]
- 3.Osteryoung K. W. & McAndrew, R. S. (2001) Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 315-333. [DOI] [PubMed] [Google Scholar]
- 4.McAndrew R. S., Froehlich, J. E., Vitha, S., Stokes, K. D. & Ostryoung, K. W. (2001) Plant Physiol. 127 1656-1666. [PMC free article] [PubMed] [Google Scholar]
- 5.Pyke K. A. (1999) Plant Cell 11 549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyagishima S., Takahara, M. & Kuroiwa, T. (2001) Plant Cell 13 707-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyagishima S., Takahara, M., Mori, T., Kuroiwa, H., Higashiyama, T. & Kuroiwa, T. (2001) Plant Cell 13 2257-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiessling J., Kruse, S., Rensing, S. A., Harter, K., Decker, E. L. & Reski, R. (2000) J. Cell Biol. 151 945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colletti K. S., Tattersall, E. A., Pyke, K. A., Froelich, J. E., Stokes, K. D. & Osteryoung, K. W. (2000) Curr. Biol. 10 507-516. [DOI] [PubMed] [Google Scholar]
- 10.Dinkins R., Reddy, M. S. S., Leng, M. & Collins, G. B. (2001) Planta 214 180-188. [DOI] [PubMed] [Google Scholar]
- 11.Itoh R., Fujiwara, M., Nagata, N. & Yoshida, S. (2001) Plant Physiol. 127 1644-1655. [PMC free article] [PubMed] [Google Scholar]
- 12.Rothfield L. I., Shih, Y.-L. & King, G. (2001) Cell 106 13-16. [DOI] [PubMed] [Google Scholar]
- 13.Hale C. & de Boer, P. (1997) Cell 88 175-185. [DOI] [PubMed] [Google Scholar]
- 14.Stuart R. A. & Neupert, W. (2000) Nature (London) 406 575. [DOI] [PubMed] [Google Scholar]
- 15.Bonnefoy N., Chalvet, F., Hamel, P., Slonimski, P. P. & Djuradin, G. (1994) J. Mol. Biol. 239 201-212. [DOI] [PubMed] [Google Scholar]
- 16.He S. & Fox, T. D. (1997) Mol. Biol. Cell 8 1449-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann J. M., Neupert, W. & Stuart, R. A. (1997) EMBO J. 16 2217-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hell K., Neupert, W. & Stuart, R. A. (2001) EMBO J. 20 1281-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore M., Harrison, M. S., Peterson, E. C. & Henry, R. (2000) J. Biol. Chem. 275 1529-1532. [DOI] [PubMed] [Google Scholar]
- 20.Sundberg E., Slagter, G. S., Fridborg, I., Cleary, S. P., Robinson, C. & Coupland, G. (1997) Plant Cell 9 717-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samuelson J. C., Chen, M., Jiang, F., Moller, I., Widmann, M., Kuhn, A., Phillips, G. J. & Dalbey, R. E. (2000) Nature (London) 406 637-641. [DOI] [PubMed] [Google Scholar]
- 22.Scotti P. A., Urbanas, M. L., Brunner, J., de Gier, J. W., von Heijne, G., van der Does, C., Driessen, A. J. M., Oudega, B. & Luirink, J. (2000) EMBO J. 19 542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lurink J., Samuelsson, T. & de Gier, J.-W. (2001) FEBS Lett. 501 1-5. [DOI] [PubMed] [Google Scholar]
- 24.Errington J., Appleby, L., Daniel, R. A., Goodfelow, H., Partridge, S. R & Yudkin, M. D. (1992) J. Gen. Microbiol. 138 2609-2618. [DOI] [PubMed] [Google Scholar]
- 25.Murakami T., Haga, K., Takeuchi, M. & Sato, T. (2002) J. Bacteriol. 184 1998-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dellaporta S. L., Wood, J. & Hicks, J. B. (1983) Plant Mol. Biol. Rep. 1 19-21. [Google Scholar]
- 27.Fulgosi H., Vener, A. V., Altschmied, L., Herrmann, R. G. & Andersson, B. (1998) EMBO J. 17 1577-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolter B., Soll, J., Schulz, A., Hinnah, S. & Wagner, R. (1998) Proc. Natl. Acad. Sci. USA 95 15831-15836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiner-Lange S., Gremse, M., Kuckenberg, M., Nissing, E., Schaechtel, D., Spenrath, N., Wolff, M., Saedler, H. & Dekker, K. (2001) Plant Biol. 3 391-397. [Google Scholar]
- 30.Becker D., Kemper, E., Schell, J. & Masterson, R. (1992) Plant Mol. Biol. 20 1195-1197. [DOI] [PubMed] [Google Scholar]
- 31.Westphal S., Heins, L., Soll, J. & Vothknecht, U. C. (2001) Proc. Natl. Acad. Sci. USA 98 4243-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Heijne G., Steppuhn, J. & Herrmann, R. G. (1989) Eur. J. Biochem. 180 535-545. [DOI] [PubMed] [Google Scholar]
- 33.Braun H. P. & Schmitz, U. K. (1995) Biochim. Biophys. Acta 1229 181-186. [DOI] [PubMed] [Google Scholar]
- 34.Lübeck J., Soll, J., Akita, M., Nielsen, E. & Keegstra, K. (1996) EMBO J. 15 4230-4238. [PMC free article] [PubMed] [Google Scholar]
- 35.Stahl T., Glockmann, C., Soll, J. & Heins, L. (1999) J. Biol. Chem. 274 37467-37472. [DOI] [PubMed] [Google Scholar]
- 36.Peter M. E, She, J., Huber, L. A. & Terhorst, C. (1993) Anal. Biochem. 210 77-85. [DOI] [PubMed] [Google Scholar]
- 37.Margolin W. (2000) Curr. Biol. 10 328-330. [DOI] [PubMed] [Google Scholar]
- 38.Vitha S., McAndrew, R. S. & Osteryoung, K. W. (2001) J. Cell Biol. 153 111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osteryoung K. W., Stokes, K. D., Rutheford, S. M., Percival, A. L. & Lee, W. Y. (1998) Plant Cell 10 1991-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torii K. U. (2000) Curr. Opin. Plant Biol. 3 361-367. [DOI] [PubMed] [Google Scholar]
- 41.van der Laan M., Houben, E. N. G., Nouwen, N., Lurink, J. & Driessen, A. J. M. (2001) EMBO Rep. 2 519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]