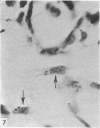
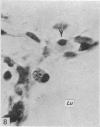
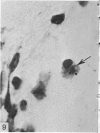
Abstract
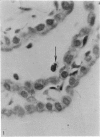
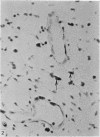
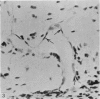
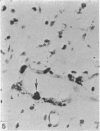
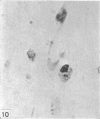
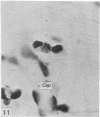
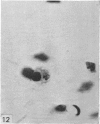
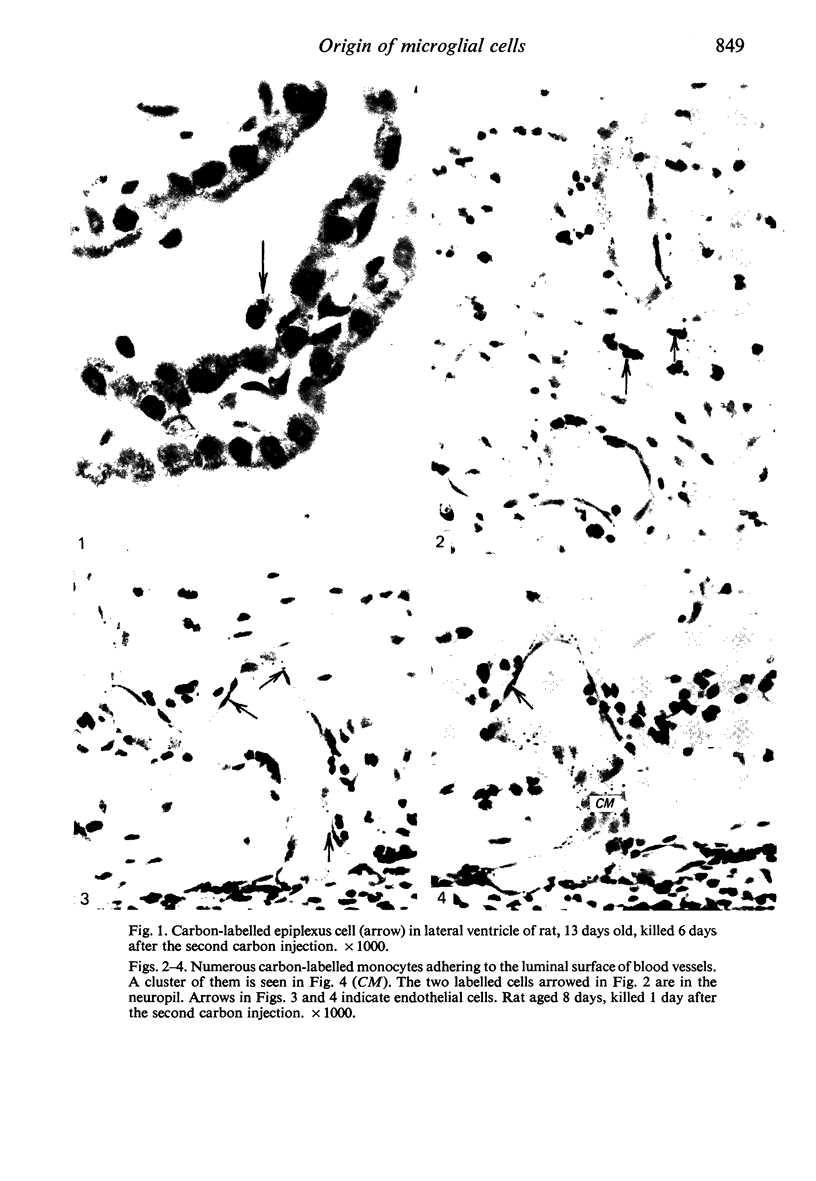
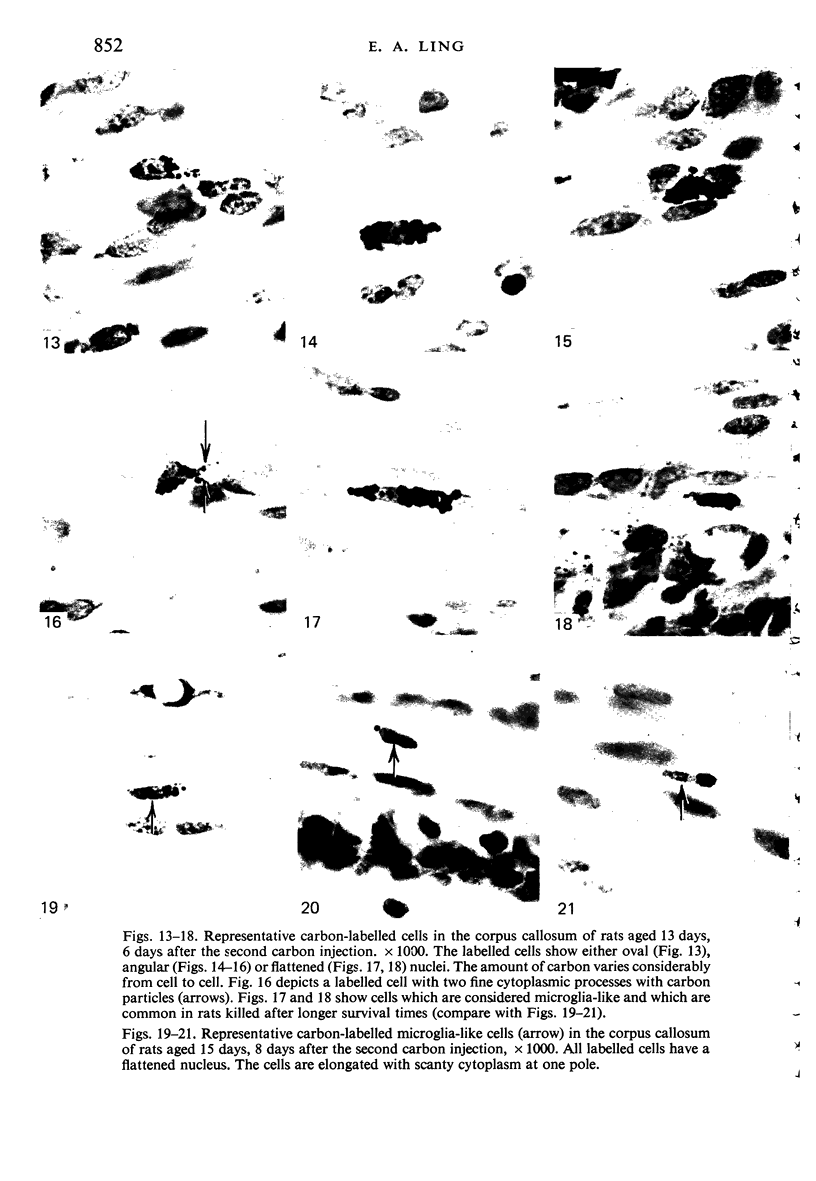
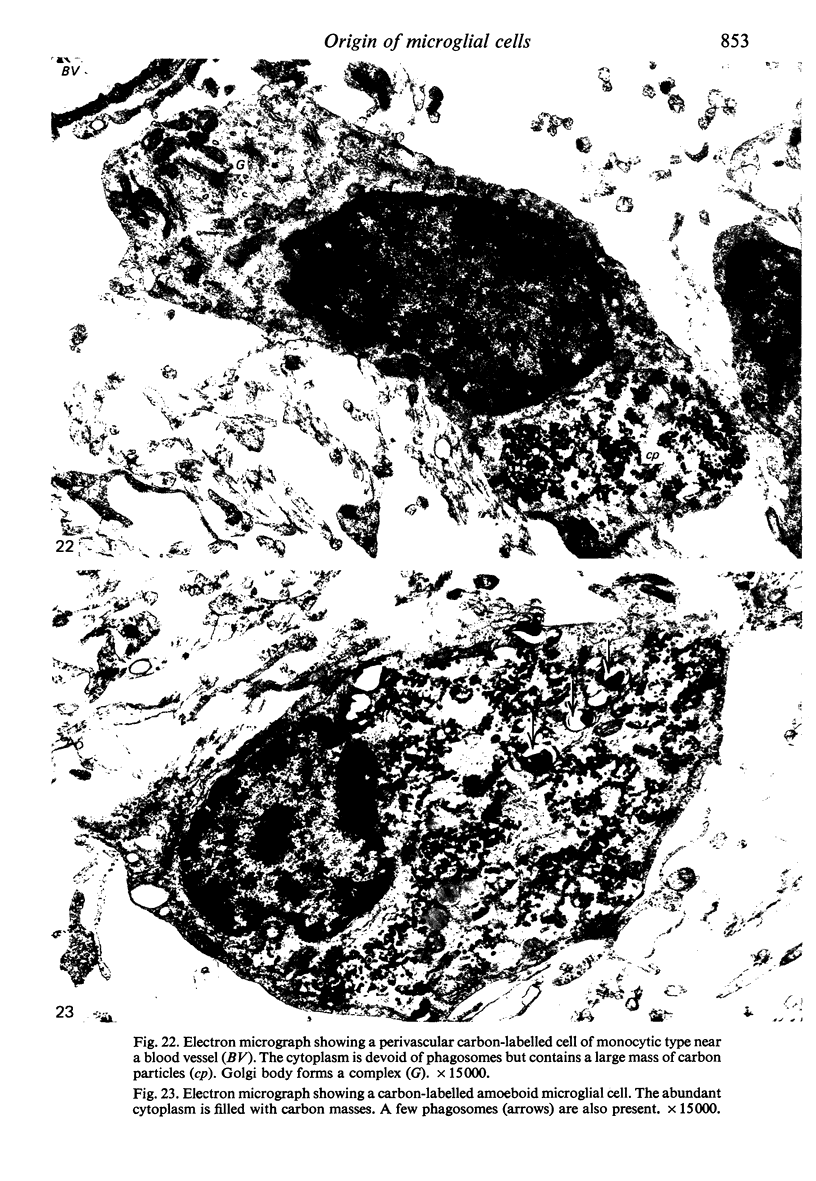
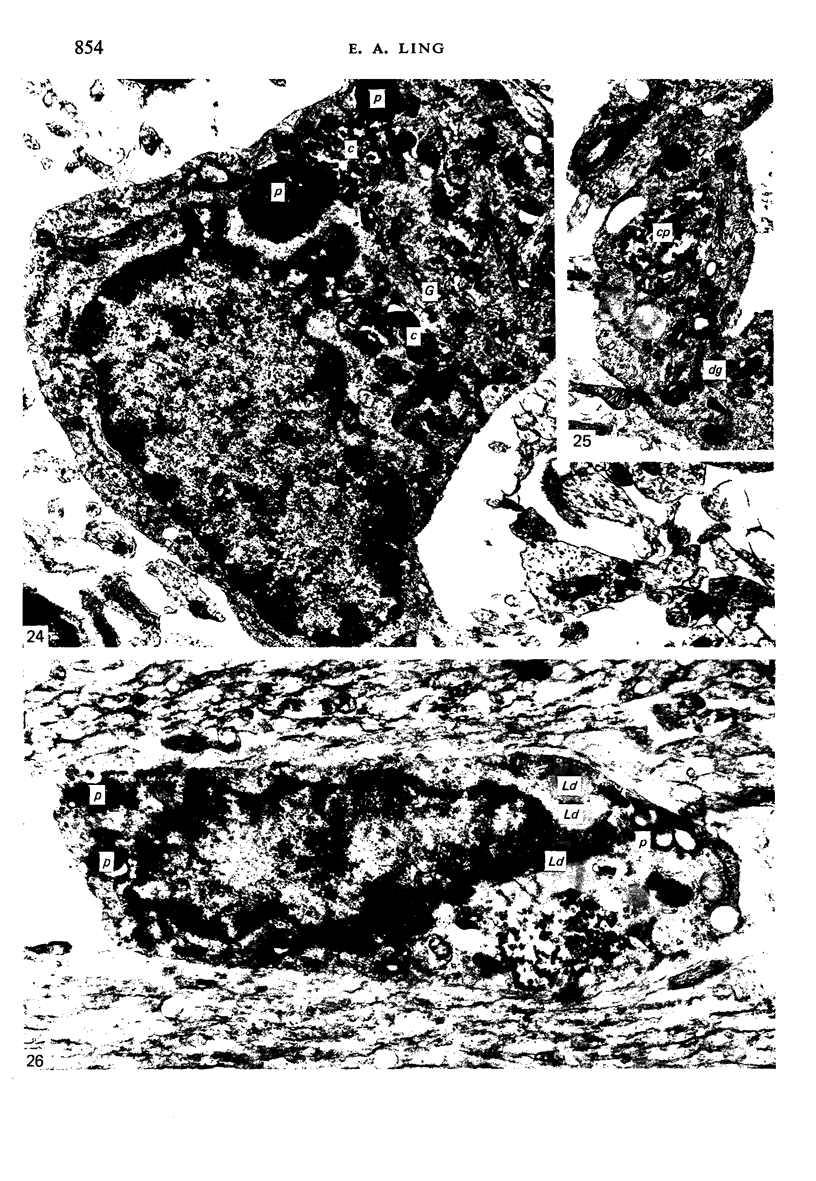
Two successive intravenous doses of carbon suspension were given at 24 hourly intervals into six days old rats. These animals were killed at intervals ranging from 1 to 9 days after the second injection. The corpus callosum and neighbouring structures were examined for cells containing ingested colloidal carbon particles in their cytoplasm. Twenty four hours after the second injection, a variable number of carbon-labelled monocytes were adherent to the luminal wall of blood vessels in the corpus callosum. Numerous carbon-labelled cells appeared to have left the lumen and entered the brain tissue surrounding the vessels. These perivascular carbon-labelled monocytes in the neuropil displayed a large pale nucleus with fine chromatin granules. The phagocytic amoeboid microglia in the corpus callosum were unlabelled at first, although a few cells of a similar nature in the cavum septi pellucidi did show carbon particles in their cytoplasm. Four or five days after the second carbon injection perivascular carbon-labelled monocytes were rare, but carbon particles were now present in the amoeboid microglia. At 8 days amoeboid microglia were virtually absent from the corpus callosum but carbon particles now appeared in cells which closely resembled microglia (flattened nucleus, coarse chromatin, scanty cytoplasm at one pole). The sequential appearance of carbon particles in monocytes, amoeboid microglia, and microglia, suggests that monocytes transform into microglia by way of an amoeboid microglial stage.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barón M., Gallego A. The relation of the microglia with the pericytes in the cat cerebral cortex. Z Zellforsch Mikrosk Anat. 1972;128(1):42–57. doi: 10.1007/BF00306887. [DOI] [PubMed] [Google Scholar]
- Booz K. H., Felsing T. Uber ein transitorisches, perinatales subependymales Zellsystem der weissen Ratte. Z Anat Entwicklungsgesch. 1973;141(3):275–288. [PubMed] [Google Scholar]
- Cammermeyer J. The life history of the microglial cell: a light microscopic study. Neurosci Res (N Y) 1970;3:43–129. doi: 10.1016/b978-0-12-512503-1.50008-6. [DOI] [PubMed] [Google Scholar]
- Carpenter S. J., McCarthy L. E., Borison H. L. Electron microscopic study of the epiplexus (Kolmer) cells of the cat choroid plexus. Z Zellforsch Mikrosk Anat. 1970;110(4):471–486. doi: 10.1007/BF00330099. [DOI] [PubMed] [Google Scholar]
- Fujita S., Kitamura T. Origin of brain macrophages and the nature of the so-called microglia. Acta Neuropathol Suppl. 1975;Suppl 6:291–296. doi: 10.1007/978-3-662-08456-4_51. [DOI] [PubMed] [Google Scholar]
- Imamoto K., Leblond C. P. Presence of labeled monocytes, macrophages and microglia in a stab wound of the brain following an injection of bone marrow cells labeled with 3H-uridine into rats. J Comp Neurol. 1977 Jul 15;174(2):255–279. doi: 10.1002/cne.901740205. [DOI] [PubMed] [Google Scholar]
- Imamoto K., Leblond C. P. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol. 1978 Jul 1;180(1):139–163. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- Lewis P. D. The fate of the subependymal cell in the adult rat brain, with a note on the origin of microglia. Brain. 1968;91(4):721–736. doi: 10.1093/brain/91.4.721. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Brain macrophages in rats following intravenous labelling of mononuclear leucocytes with colloidal carbon. J Anat. 1978 Jan;125(Pt 1):101–106. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Leblond C. P. Investigation of glial cells in semithin sections. II. Variation with age in the numbers of the various glial cell types in rat cortex and corpus callosum. J Comp Neurol. 1973 May 1;149(1):73–81. doi: 10.1002/cne.901490105. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Light and electron microscopic demonstration of some lysosomal enzymes in the amoeboid microglia in neonatal rat brain. J Anat. 1977 Jul;123(Pt 3):637–648. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A. Some aspects of amoeboid microglia in the corpus callosum and neighbouring regions of neonatal rats. J Anat. 1976 Feb;121(Pt 1):29–45. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Tan C. K. Amoeboid microglial cells in the corpus callosum of neonatal rats. Arch Histol Jpn. 1974 Mar;36(4):265–280. doi: 10.1679/aohc1950.36.265. [DOI] [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Identification of microglia in light and electron microscopy. J Comp Neurol. 1969 Jan;135(1):57–80. doi: 10.1002/cne.901350104. [DOI] [PubMed] [Google Scholar]
- Penfield W. Microglia and the Process of Phagocytosis in Gliomas. Am J Pathol. 1925 Jan;1(1):77–90.15. [PMC free article] [PubMed] [Google Scholar]
- Stensaas L. J., Reichert W. H. Round and amoeboid microglial cells in the neonatal rabbit brain. Z Zellforsch Mikrosk Anat. 1971;119(2):147–163. doi: 10.1007/BF00324517. [DOI] [PubMed] [Google Scholar]
- Vaughn J. E., Hinds P. L., Skoff R. P. Electron microscopic studies of Wallerian degeneration in rat optic nerves. I. The multipotential glia. J Comp Neurol. 1970 Oct;140(2):175–206. doi: 10.1002/cne.901400204. [DOI] [PubMed] [Google Scholar]
- Vaughn J. E., Peters A. A third neuroglial cell type. An electron microscopic study. J Comp Neurol. 1968 Jun;133(2):269–288. doi: 10.1002/cne.901330207. [DOI] [PubMed] [Google Scholar]