Abstract
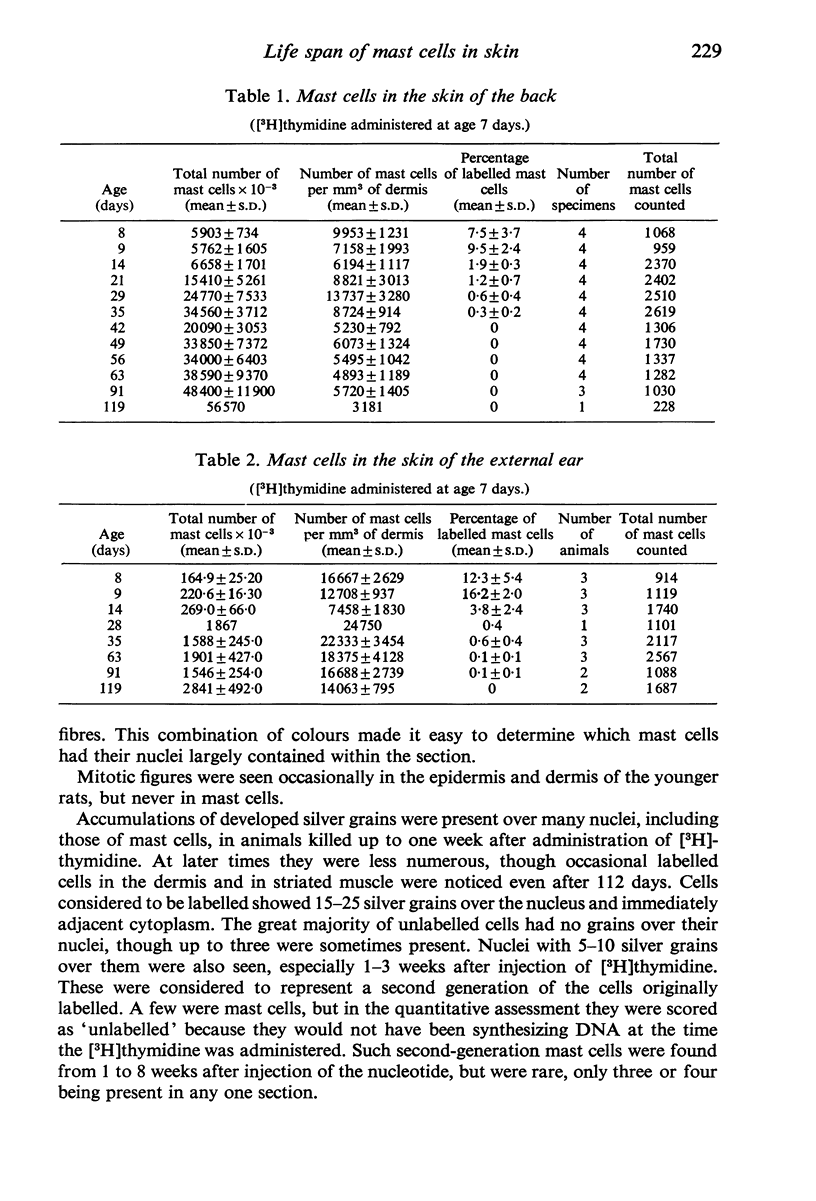
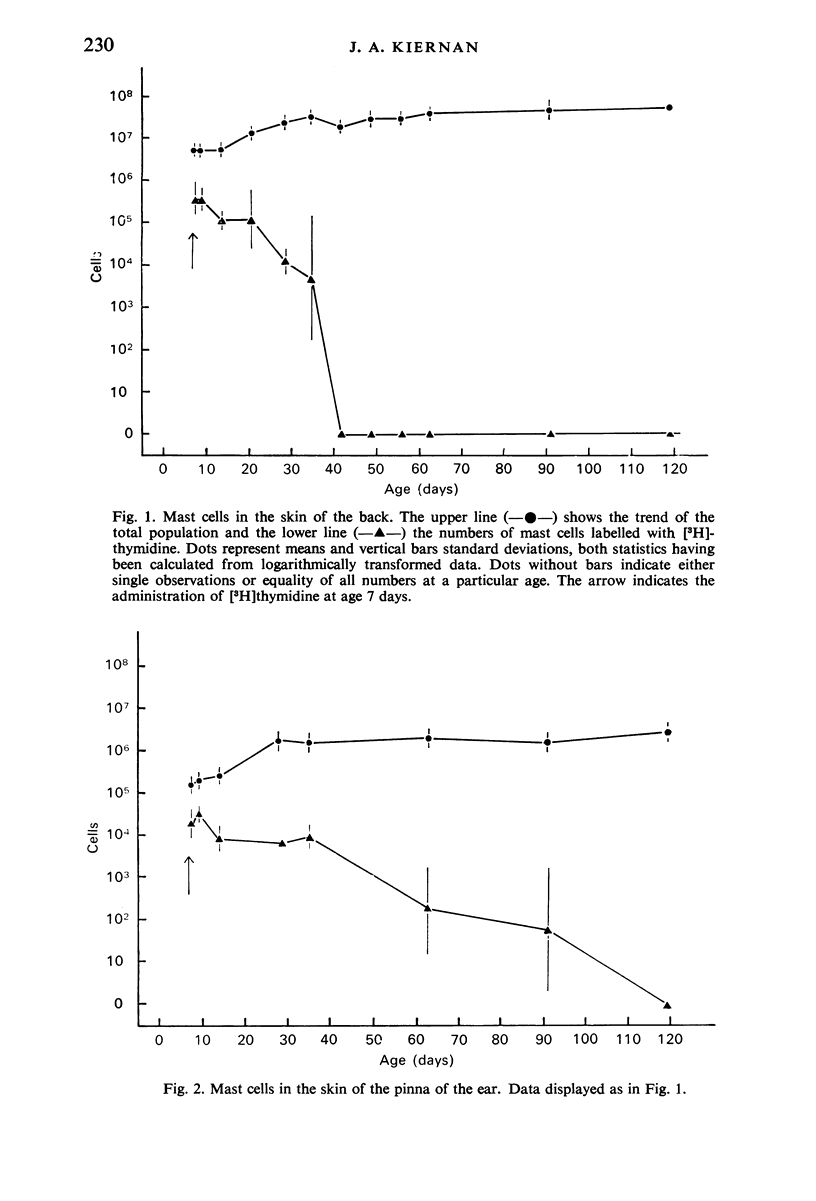
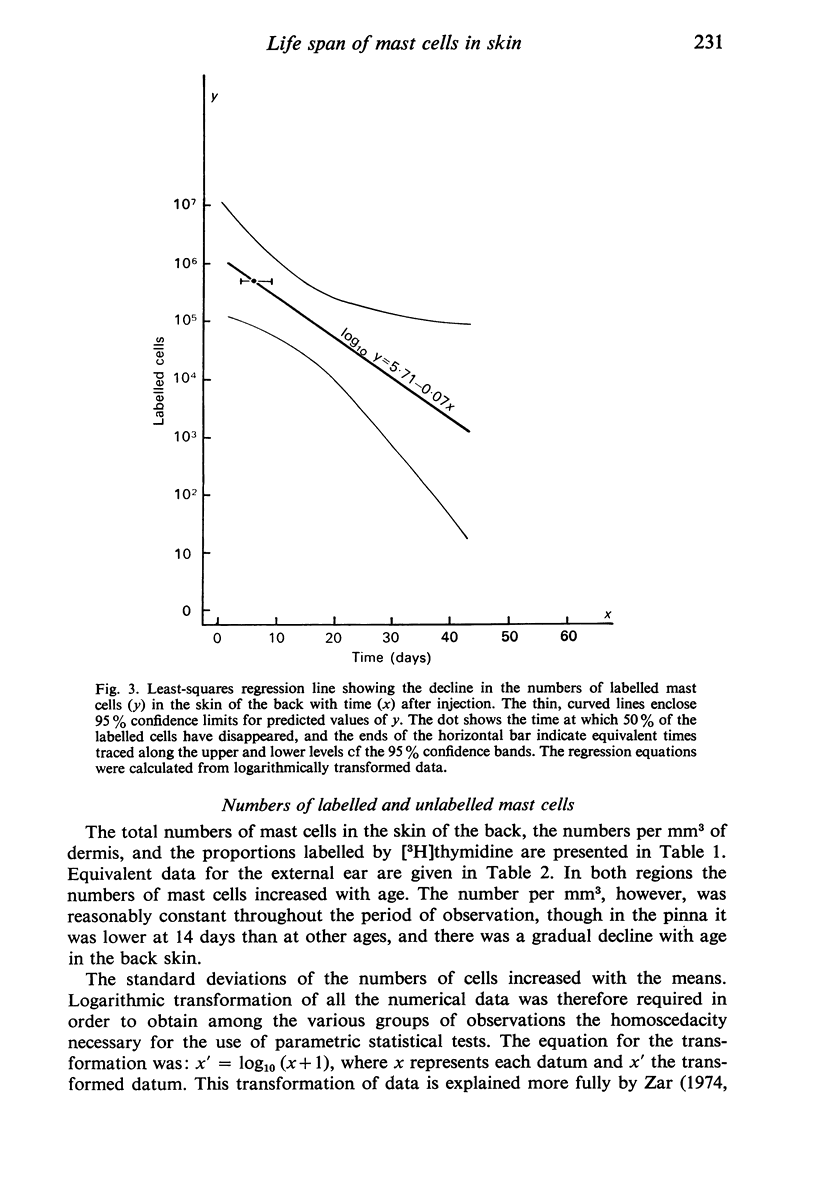
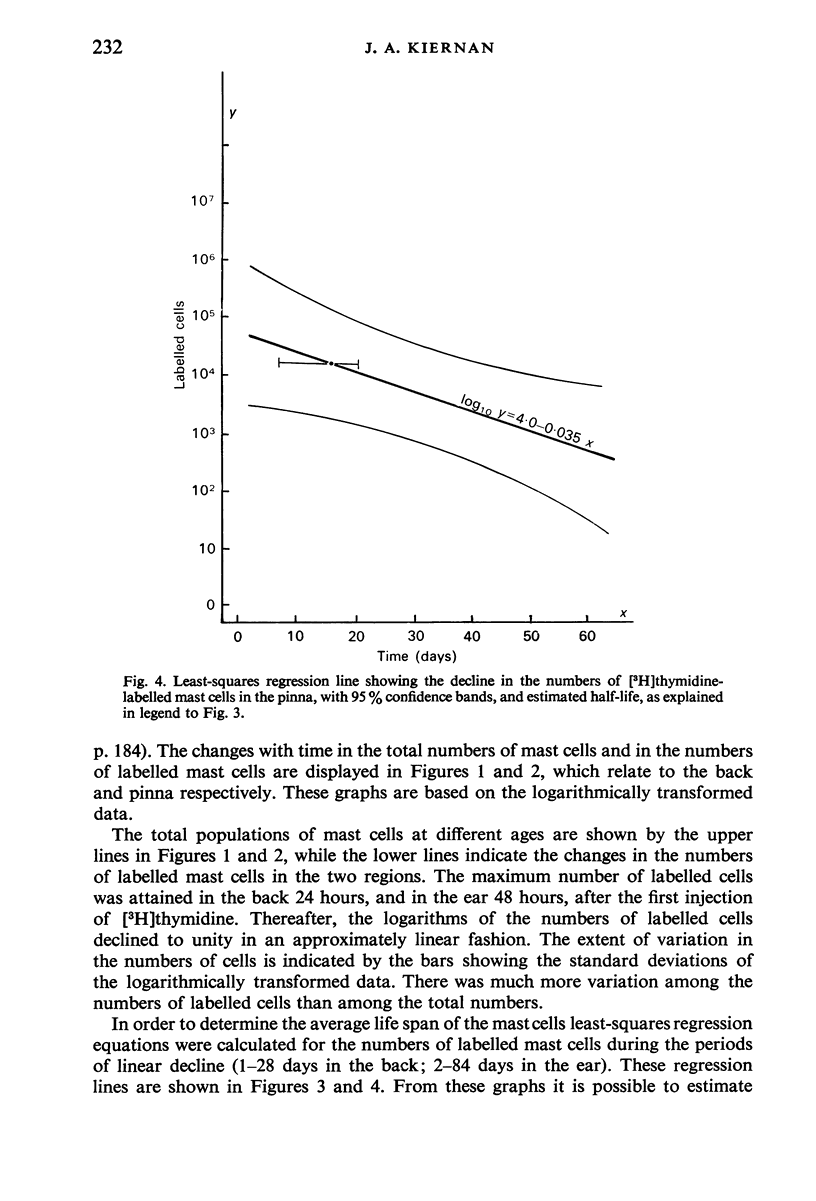
Tritiated thymidine was injected into young rats (age 7 days) in such a way as to be incorporated into the nuclei of all cells that were synthesizing DNA during a period of 22 hours. Specimens of the skin of the back and the pinna of the ear were taken at intervals from one to 112 days after injection of the [3H]thymidine. The mast cells were stained with Alcian blue, and autoradiographs were prepared. The nuclei were counterstained with alum-brazilin. Making due allowance for growth of the animals, and for shrinkage due to histological preparation, the total numbers of mast cells in the dorsal skin and in the pinna were determined. The numbers of mast cells containing [3H]thymidine were calculated from the proportions of those cells found to have radioactive nuclie. Using these data, the rates of appearance of labelled mast cells, and of decline in their numbers with time were determined for both regions of skin. No mitotic figures were seen in any mast cells. It is concluded that mast cells arise by the division of agranular precursor cells of unknown identity. The characteristic cytoplasmic granules appear to be produced by the daughter cells during the 24-48 hours following premitotic replication of DNA in the precursors. The differentiated cells have half-lives of 4-9 days in the skin of the back and 7-20 days in the external ear. All the labelled mast cells had disappeared after 28 days in the back and after 84 days in the ear. Comparison of these findings with the results of other investigators suggests that the mast cells produced early in life have much shorter life spans than do most of the mast cells present in adult rats, The longer life span found in the pinna may account for the greater density of the cells there than in the back. This regional difference may reflect the greater need for mast cells in a region which is more susceptible to adverse environmental influences.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN A. M. Deoxyribonucleic acid synthesis and mitosis in mast cells of the rat. Lab Invest. 1962 Feb;11:188–191. [PubMed] [Google Scholar]
- ASBOE-HANSEN G., LEVI H., NIELSEN A., BENTZON M. W. PREMITOTIC UPTAKE OF TRITIATED THYMIDINE BY MAST CELLS. Acta Pathol Microbiol Scand. 1965;63:533–548. doi: 10.1111/apm.1965.63.4.533. [DOI] [PubMed] [Google Scholar]
- Arvier P. T., Chahl L. A., Ladd R. J. Modification by capsaicin and compound 48/80 of dye leakage induced by irritants in the rat. Br J Pharmacol. 1977 Jan;59(1):61–68. doi: 10.1111/j.1476-5381.1977.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTALANFFY F. D. TRITIATED THYMIDINE VERSUS COLCHICINE TECHNIQUE IN THE STUDY OF CELL POPULATION CYTODYNAMICS. Lab Invest. 1964 Aug;13:871–886. [PubMed] [Google Scholar]
- Blenkinsopp W. K. Mast cell proliferation in adult mice. Nature. 1967 May 27;214(5091):930–931. doi: 10.1038/214930a0. [DOI] [PubMed] [Google Scholar]
- Blenkinsopp W. K. Mast cell proliferation in adult rats. J Cell Sci. 1967 Mar;2(1):33–37. doi: 10.1242/jcs.2.1.33. [DOI] [PubMed] [Google Scholar]
- Burton A. L. Differentiation of mast cells in the subcutaneous connective tissue of rat embryos. Tex Rep Biol Med. 1967 Summer;25(2):240–250. [PubMed] [Google Scholar]
- CAMERON I. L., GREULICH R. C. Evidence for an essentially constant duration of DNA synthesis in renewing epithelia of the adult mouse. J Cell Biol. 1963 Jul;18:31–40. doi: 10.1083/jcb.18.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRONKITE E. P., BOND V. P., FLIEDNER T. M., RUBINI J. R. The use of tritiated thymidine in the study of DNS synthesis and cell turnover in hemopoietic tissues. Lab Invest. 1959 Jan-Feb;8(1):263–277. [PubMed] [Google Scholar]
- Chahl L. A., Ladd R. J. Local oedema and general excitation of cutaneous sensory receptors produced by electrical stimulation of the saphenous nerve in the rat. Pain. 1976 Mar;2(1):25–34. doi: 10.1016/0304-3959(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Chiu H., Lagunoff D. Histochemical comparison of frog and rat mast cells. J Histochem Cytochem. 1971 Jun;19(6):369–375. doi: 10.1177/19.6.369. [DOI] [PubMed] [Google Scholar]
- Combs J. W., Lagunoff D., Benditt E. P. Differentiation and proliferation of embryonic mast cells of the rat. J Cell Biol. 1965 Jun;25(3):577–592. doi: 10.1083/jcb.25.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs J. W. Maturation of rat mast cells. An electron microscope study. J Cell Biol. 1966 Dec;31(3):563–575. doi: 10.1083/jcb.31.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaba G., Forgács A. The ontogenesis of mast cells. Acta Biol Acad Sci Hung. 1971;22(4):423–430. [PubMed] [Google Scholar]
- Csaba G., Hodinka L. The thymus as the source of mast cells in the blood. Acta Biol Acad Sci Hung. 1970;21(3):333–334. [PubMed] [Google Scholar]
- Diamant B., Krüger P. G. Structural changes of isolated rat peritoneal mast cells induced by adenosine triphosphate and adenosine diphosphate. J Histochem Cytochem. 1968 Nov;16(11):707–716. doi: 10.1177/16.11.707. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P., De Oca H. M. Production of heparin related mucopolysaccharides by mammalian cells in culture. Proc Soc Exp Biol Med. 1970 Sep;134(4):955–962. doi: 10.3181/00379727-134-34920. [DOI] [PubMed] [Google Scholar]
- Escobar A., Sampedro E. D., Dow R. S. Quantitative data on the inferior olivary nucleus in man, cat and vampire bat. J Comp Neurol. 1968 Mar;132(3):397–403. doi: 10.1002/cne.901320303. [DOI] [PubMed] [Google Scholar]
- FAWCETT D. W. An experimental study of mast cell degranulation and regeneration. Anat Rec. 1955 Jan;121(1):29–51. doi: 10.1002/ar.1091210104. [DOI] [PubMed] [Google Scholar]
- GINSBURG H., SACHS L. FORMATION OF PURE SUSPENSIONS OF MAST CELLS IN TISSUE CULTURE BY DIFFERENTIATION OF LYMPHOID CELLS FROM THE MOUSE THYMUS. J Natl Cancer Inst. 1963 Jul;31:1–39. [PubMed] [Google Scholar]
- GINSBURG H. The in vitro differentiation and culture of normal mast cells from the mouse thymus. Ann N Y Acad Sci. 1963 Feb 26;103:20–39. doi: 10.1111/j.1749-6632.1963.tb53690.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg H., Lagunoff D. The in vitro differentiation of mast cells. Cultures of cells from immunized mouse lymph nodes and thoracic duct lymph on fibroblast monolayers. J Cell Biol. 1967 Dec;35(3):685–697. doi: 10.1083/jcb.35.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEROUX O. Mast cells in the skin of the ear of the rat exposed to cold. Can J Biochem Physiol. 1961 Dec;39:1871–1878. [PubMed] [Google Scholar]
- JAQUES L. B. Heparin and the mast cell. Can J Biochem Physiol. 1961 Mar;39:643–651. doi: 10.1139/o61-064. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. A pharmacological and histological investigation of the involvement of mast cells in cutaneous axon reflex vasodilatation. Q J Exp Physiol Cogn Med Sci. 1975 Apr;60(2):123–130. doi: 10.1113/expphysiol.1975.sp002298. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. A study of chemically induced acute inflammation in the skin of the rat. Q J Exp Physiol Cogn Med Sci. 1977 Apr;62(2):151–161. doi: 10.1113/expphysiol.1977.sp002385. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. Degranulation of mast cells following antidromic stimulation of cutaneous nerves. J Anat. 1972 Feb;111(Pt 2):349–350. [PubMed] [Google Scholar]
- Kiernan J. A. The development of mast cells in vitro. J Anat. 1974 Dec;118(Pt 3):517–529. [PMC free article] [PubMed] [Google Scholar]
- Kiernan J. A. The involvement of mast cells in vasodilatation due to axon reflexes in injured skin. Q J Exp Physiol Cogn Med Sci. 1972 Jul;57(3):311–317. doi: 10.1113/expphysiol.1972.sp002164. [DOI] [PubMed] [Google Scholar]
- Lampe M., Kiernan J. A. Cytochemical variation of mast cells in the skin of the rat. Arch Dermatol Res. 1977 Mar 25;258(1):69–80. doi: 10.1007/BF00582869. [DOI] [PubMed] [Google Scholar]
- Lampe M., Kiernan J. A. Degranulation of dermal mast cells: effects of fixation and of antidromic nervous impulses on two histochemically identified cell-types. Arch Dermatol Res. 1976 Dec 15;257(2):125–129. doi: 10.1007/BF00558085. [DOI] [PubMed] [Google Scholar]
- MESSIER B., LEBLOND C. P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960 May;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- NYGAARD O. F., POTTER R. L. Effect of x-radiation on DNA metabolism in various tissues of the rat. I. Incorporation of C14-thymidine into DNA during the first 24 hours postirradiation. Radiat Res. 1959 Apr;10(4):462–476. [PubMed] [Google Scholar]
- Padawer J. Mast cell degranulation in fixed preparations. Proc Soc Exp Biol Med. 1965 Nov;120(2):318–321. doi: 10.3181/00379727-120-30523. [DOI] [PubMed] [Google Scholar]
- Padawer J. Mast cells: extended lifespan and lack of granule turnover under normal in vivo conditions. Exp Mol Pathol. 1974 Apr;20(2):269–280. doi: 10.1016/0014-4800(74)90059-8. [DOI] [PubMed] [Google Scholar]
- STANNERS C. P., TILL J. E. DNA synthesis in individual L-strain mouse cells. Biochim Biophys Acta. 1960 Jan 29;37:406–419. doi: 10.1016/0006-3002(60)90496-0. [DOI] [PubMed] [Google Scholar]
- Tas J. The Alcian blue and combined Alcian blue--Safranin O staining of glycosaminoglycans studied in a model system and in mast cells. Histochem J. 1977 Mar;9(2):205–230. doi: 10.1007/BF01003632. [DOI] [PubMed] [Google Scholar]
- Thiede A., Müller-Hermelink H. K., Sonntag H. G., Müller-Ruchholtz W., Leder L. D. Preliminary report on the origin of rat mast cells. Klin Wochenschr. 1971 Apr 1;49(7):435–436. doi: 10.1007/BF01485001. [DOI] [PubMed] [Google Scholar]
- Uvnäs B. Histamine storage and release. Fed Proc. 1974 Oct;33(10):2172–2176. [PubMed] [Google Scholar]
- WALKER B. E. Mast cell turn-over in adult mice. Nature. 1961 Dec 9;192:980–981. doi: 10.1038/192980a0. [DOI] [PubMed] [Google Scholar]
- WATSON W. C., KENNEDY J. S. Regeneration of the tissue mast cell: an autoradiographic study. Br J Exp Pathol. 1960 Aug;41:385–388. [PMC free article] [PubMed] [Google Scholar]
- WICHMANN B. E. The mast cell count during the process of wound healing; an experimental investigation on rats. Acta Pathol Microbiol Scand Suppl. 1955;(Suppl 108):1–35. [PubMed] [Google Scholar]
- Yong L. C., Watkins S., Wilhelm D. L. The mast cell: distribution and maturation in the peritoneal cavity of the adult rat. Pathology. 1975 Oct;7(4):307–318. doi: 10.3109/00313027509081687. [DOI] [PubMed] [Google Scholar]


