Abstract
Background:
Multiple myeloma is still one of deadliest malignancies known. Although many attempts to prognosticate the disease have been done like the International Staging System (ISS), most of the proposed prognostic tools are based merely on laboratory tests and hence prone to analytical errors in large and high-volume centers. This study aims to evaluate the prognostic effectiveness of histopathologic components of bone marrow and compare it to the results of laboratory-based prognostic tools.
Methods:
This cross-sectional study, bone marrow specimens of 93 multiple myeloma patients underwent aspiration and biopsy evaluated. The primary outcome was overall survival (OS) based on plasma cell percentage. The secondary outcomes were also OS based on angiogenesis using IHC marker CD34, nuclear atypia level, BM involvement pattern and the presence of fibrosis in bone marrow specimens. All biopsy specimens assessed using light microscopy on Hematoxylin and Eosin and IHC staining. Giemsa staining assessed for aspirate specimens.
Results:
Of 93 patients, 63.4% were dead. Median survival was 34.0 months (95% CI [24.6; 43.3]) and the average age at diagnosis was 65 years (highest 84 and lowest 40). Patients with bone marrow plasma cell count of over 70%, had a hazard ratio (HR) of death of 4.7 times more than those with plasma cell count between 10-25%. Similarly, diffuse infiltration pattern (HR 4.67) and blastic morphology (HR 4.17) associated with a significant worse prognosis (p=0.03).
Conclusion:
Comparing to laboratory-based ISS, wider HR of death spectrum in this study proposes a potential more precise, robust and easy-to-use prognostication tool.
Key Words: Multiple myeloma, Histopathological prognosis, International staging system
Multiple myeloma (MM) is a neoplasm that arises as a result of the abnormal proliferation of plasma cells in the bone marrow (BM) and despite many advances in its treatment, it is still known as an incurable disease. Although rare, but with a prevalence of about 10% of all blood malignancies, it ranks second among these cancers (1). This disease belongs to plasma cell dyscrasia spectrum which ranges from monoclonal gammopathy of unknown significance (MGUS) to plasma cell leukemia (2-4). Multiple myeloma is mostly seen in the elderly with prevalence in the African American population twice more than those of the European American descendants. This disease itself constitutes a clinical and immunological phenotypic spectrum whose components differ considerable in terms of biological behavior and survival rate (5). Advances in the treatment of MM in the past few decades, beginning with the use of autologous stem cell transplantation and then with the use of new therapies such as immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs), have changed the natural course of the disease (5, 6).
and has prolonged the survival time; However, there is no definitive cure for this disease and its average survival is about 3 years, and 5-year survival in patients under standard treatment is only 24% (5, 6). The diagnosis of multiple myeloma is based on specific criteria that include the following: a high percentage of monoclonal plasma cells in the bone marrow, increased amounts of intact monoclonal immunoglobulin or its fragments (free light chain) in the serum or urine, along with evidence of damage to the end organs which include: hypercalcemia, renal failure, anemia, and bone lesions including osteoporosis or severe osteopenia, which are collectively known as CRAB criteria. (3, 7, 8). Several clinical, laboratory and histological variables help us in determining the prognosis of the disease (9), which include: serum beta-2 microglobulin, bone marrow plasma cell (BMPC) index, cytogenetics, plasmablastic morphology, serum lactate dehydrogenase (LDH), C-reactive protein (CRP) and Interleukin-6. a combination of different prognostic factors, provides more information than each factor alone (5). Unfortunately some of these criteria tests, including beta-2 microglobulin and interleukin-6 assays, are not readily available in many centers in developing countries (9).
The international staging system (ISS) for MM is a powerful prognostic tool which divides patients into three stages based on serum beta-2 microglobulin and albumin level (10) which is depicted in table 1. Some studies propose that different histological parameters can be effective on prognosis: the percentage of plasma cells in the bone marrow (mostly over 10%), the infiltration pattern of plasma cells, cell maturity, bone marrow fibrosis and mitotic index (4, 9, 11). Survival is estimated to be shorter in patients with more than 25% myeloma cells in the bone marrow along with a higher rate of tumor cell infiltration (12). Myeloma cells are located in the bone marrow microenvironment, which includes cells (tumor cells, stromal cells), extracellular matrix and various molecules, all of which contribute to the survival and proliferation of cancer (13). The interaction between myeloma cells and bone marrow stroma is not solely based on intercellular interactions, but also on autocrine and paracrine circuits mediated by soluble or insoluble components (e.g. : cytokines, angiogenesis factors) (2). Angiogenesis is necessary for the growth of plasma cells and hence affects the prognosis (14) and is probably associated with other prognostic factors such as beta-2 microglobulin and CRP, as well as lower treatment response (15, 16). As a result, targeting angiogenesis can theoretically halt disease progression, reduce drug resistance, and prolong the patient's survival (17, 18).
Table 1.
International staging system (ISS) for multiple myeloma
| Stage | Criteria | Median survival (months) |
|---|---|---|
| I | serum albumin ≥3.5 g/dl and a serum beta-2 microglobulin <3.5 mg/l | 62 |
| II | patients who did not fulfill the stage 1 or 3 criteria | 45 |
| III | serum beta-2 microglobulin ≥5.5 mg/l | 29 |
Inhibition of proangiogenic cytokines has been widely used in the treatment of MM. For example, in addition to proteasome inhibitors and immunomodulators that have indirect anti-angiogenesis activity, bisphosphonates are other compounds that, although initially used to reduce bone loss in MM, have shown direct anti-angiogenic activity. Zoledronic acid shows direct cytotoxic activity, suppressing angiogenesis and inhibiting proliferation of endothelial cells dependent on fibroblast growth factor (FGF-2) and vascular endothelial growth factor (VEGF) (19, 20). Collectively, the mentioned histological parameters in the bone marrow can potentially predict the survival of the patients based on the bone marrow biopsy sample which can help to determine the most beneficent therapy and avoid unnecessary overtreatment. In this study, we examined these parameters together. The primary end point was to assess the effect of BMPC percentage on overall survival (OS) in multiple myeloma patients. The same survival impact was also investigated for angiogenesis (using CD34), cell maturity, BM involvement pattern, and presence of fibrosis in bone marrow aspiration and biopsy specimens as secondary outcomes.
Methods
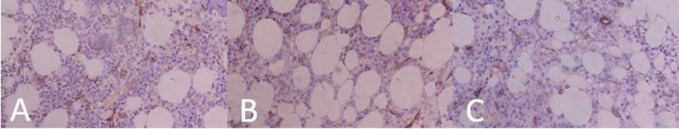
This was a cross-sectional study of multiple myeloma patients referred to Ayatollah Rouhani Hospital in Babol city between April 2012 and March 2016 who underwent aspiration and bone marrow biopsy for confirmation. The research was approved by the Ethics Committee of Babol University of Medical Sciences under the code: IR.MUBABOL.HRI.REC.1400.192. Informed consent was obtained from all participants involved in the study. All pathology blocks were safely conserved in the pathology ward. Patients whose pathologic data were missed, did not contain enough tissue for a comprehensive IHC, those who did not answer the follow-up calls or those who were reluctant to participate in the study were excluded. The primary outcome was overall survival (OS) based on plasma cell percentage. The secondary outcomes were also OS based on angiogenesis via IHC marker CD34, nuclear atypia level, BM involvement pattern, and the presence of fibrosis in bone marrow specimens. Age of the patients at diagnosis was regarded as a potential confounder by the investigators which was determined to be addressed at final analysis by multivariate cox regression. No other variable was hypothesized to be a major confounder. Gender of the patients was presumed to be a potential effect modifier, although data analysis did not show any significant difference between survivals in either gender and overall survival. All biopsy specimens were assessed using light microscopy on hematoxylin and eosin (H&E) and IHC staining by a single certified hematopathologist and was reviewed by a senior hematopathologist. The aspirate specimens were assessed using Giemsa staining. In case of a diagnosis conflict, a third senior hematopathologist was consulted. Feature assessed include: angiogenesis rate by counting CD34+ cells, plasma cell percentage in BM, cell maturity (i.e.: Plasmacytic or mature, pleomorphic, or plasmablastic), infiltration pattern of plasma cells (i.e.: focal, interstitial, multifocal, or diffuse) and existence of any degree of fibrosis. Biopsy and aspiration samples were considered sufficient if they contained at least 3 subcortical spaces and a suitable number of cell particles respectively. The percentage of plasma cells in the biopsy sample was estimated by counting 500 cells using H&E staining and IHC for CD138 membrane staining with X100 and X400 magnification in at least 5 areas. To determine the presence of fibrosis, biopsy samples were examined with Masson's Trichrome staining and checked in at least 5 areas; those with any level of fibrosis bundles were identified as positive. To check for the presence of angiogenesis, after H&E and CD34 membrane staining, the samples were first identified with 100x magnification and then we counted microvessel density (MVD) – the mean number of vessels per area in 400x in at least 5 areas using two controls (figure 1). Based on previous studies, we knew that normal BM has a mean MVD of 6.80±1.59 vessels/mm2 and MM-inflicted bone marrow has a median MVD of 48 vessels/mm2 (16, 21). So we used a cut-off of ≥10 vessels/mm2 (over two standard deviation from normal BM mean) to mark a specimen as angiogenesis positive. Finally for assessment of plasma cell morphology, aspiration specimens were stained with Wright-Giemsa stain and seen under 400x in at least 5 areas. In case of conflicting results between biopsy and aspirate specimens, biopsy was given priority.
Figure 1.
CD34 IHC stained Bone marrow specimens showing different staining patterns. A: BM infiltrated with MM cells. A high and crazy pattern of angiogenesis is seen. B: control 1 containing Normal BM and normal vasculature. C: control 2. IHC, immunohistochemistry; BM, bone marrow; MM, multiple myeloma.
Overall survival was defined as the time from pathological diagnosis to death which was asked from patient or his/her family by phone call and approved by death certificates and right censoring in cases of missing or conflicting data (8% of cases). Potential sources of bias were hypothesized to be either a recall bias or measurement bias. Recall bias could potentially affect the reliability of the survival outcome, as in some cases, survival was assessed by phone calls after 5 years. This was completely eliminated using mandatory death certificate verification with the help of national authorities. Another bias that was at least partially addressed was measurement bias. All of the outcomes were subjective variables that could be reported differently by different people or by the same physician at different times. To deals with this, all samples were obligatory studied by a single certified hematopathologist and was reviewed by a senior hematopathologist for possible errors.
Statistics:
Based on a recent study that showed an approximately twice 4-year survival for patients with less than 50% BMPC compared to those with more than 50% (22), a survival odd ratio (OR) of 2 was regarded as the effect size. The sample size was calculated to be 93 cases using PASS v21.0 software for two-tails logistic regression to find an OR of survival of 2, assuming a 15% prevalence of plasma cells in the bone marrow (with an alpha of 5% and a power of 80%). Census was the method of sampling. Data were collected using checklists and then registered into computer on a weekly basis with regular back-ups. At least two different histopathology images were collected and submitted to each patient's portfolio for necessary possible reviews and random audition by the third pathologist. Plasma cell percentage was categorized into four groups similar to previous studies: Group 1(10-25%), group 2 (25-50%), group 3 (50-70%), and group 4 (>70%). Using SPSS v.26, demographic data was checked for normality and central tendency parameters and dispersion was drawn. Descriptive data were analyzed for histopathological relation using chi-square test. Survival for each histopathological component was drawn with Kaplan-Meier curve and the amount of difference between them was analyzed using multivariate logistic regression model.
Results
Of the 93 patients, 59 (63.4 %) were dead. Median survival was 34.0 months (95% CI [24.6; 43.3]) and the average age at diagnosis was 65 years (highest 84 and lowest 40). Table 2 displayed the demographic and clinical characteristics of the patients in this study. Figure 2 shows survival of patients with regard to BMPC percentage. In Cox regression analysis, age-adjusted hazard ratio (HR) of death was significantly higher as BMPCs increased. Compared to G1 patients (in which median survival was not reached at 5 years), median OS was 45 months in G2 (HR 1.78 (95% CI [0.7; 4.3])), 13 months (95% CI [8.8; 17.2]) in G3 (HR 3.92 (95% CI [1.7; 9.0])), and 23 months (95% CI [19.3; 26.7]) in G4 patients (HR 4.7 (95% CI [2.2; 10])). CD34 positive (cut-off of ≥10 vessels/mm2) patients faced significantly increased mortality compared to CD34 negative patients (age-adjusted median survival 23 months (95% CI [10.6; 35.4]) vs. 36 months (95% CI [25.8; 46.2]); HR 2.38, (95% CI [1.3; 4.4]), P= 0.01)).
Table 2.
Demographic and clinical features of patients
| Variable | Number (%) |
|---|---|
| Gender | |
| Male Female |
56 (60.2%) 37 (39.8%) |
| Survival status | |
| Dead Alive |
59 (63.4%) 34 (36.6%) |
| BM plasma cell (%) | |
| G1: 10-25% G2: >25-50% G3: >50-70% G4: >70% |
27 (29%) 14 (15%) 14 (15%) 38 (40%) |
| CD34 status | |
| Positive Negative |
15 (16.1%) 78 (83.9%) |
| Cell maturity | |
| Mature Pleomorphic Blastic |
52 (55.9%) 22 (23.7%) 19 (20.4%) |
| BM involvement pattern | |
| Focal Multifocal Interstitial Diffuse |
27 (29%) 11 (11.8%) 16 (17.2%) 39 (41.9%) |
| Fibrosis | |
| Present Absent |
78 (84%) 15 (16%) |
Figure 2.
Kaplan-Meier survival curve of patients according to their bone marrow plasma cell percent. Blue line represents group 1 patients (10-25% plasma cells) with the best median survival and red line represents group 4 patients (>70% plasma cells) with worst (not reached vs. 23 months (95% CI [19.3;26.7]); log-rank, p= 0.01)).
Mature cells had shown the best median survival (not reached), followed by pleomorphic (23 months (95% CI [19.6; 26.4])) and blastic cells (13 months (95% CI [10.6; 35.4])) respectively (log-rank, P= 0. 00). Patients with pleomorphic cell phenotype experienced over twice mortality compared to those with mature cell phenotype (HR 2.44, (95% CI [1.3; 4.5]), P= 0. 01), while those with the blastic phenotype had over 4 times increased mortality (HR 4.17, (95% CI [2.2; 7.9]), P= 0. 00). Bone marrow infiltration pattern also had a statistically significant effect on mortality. Focal pattern was associated with best survival (median OS not reached), and median OS for interstitial, multifocal and diffuse patterns were 46 months, 23 months (95% CI [6.8; 39.2]), and 20 months (95% CI [6.5; 33.5]) respectively (log-rank, P= 0.00).
The diffuse pattern was associated with the most lethal behavior (HR 4.67, (95% CI [2.2; 10]), P= 0. 00) compared to focal pattern. Similarly multifocal pattern had near 4 times more mortality than focal pattern (HR 3.85, (95% CI [1.5; 9.8]), P= 0.01), while no statistically significant difference in death was found for interstitial pattern despite having more patients than multifocal group (HR 1.43, (95% CI [0.6; 3.7]), P= 0.46). Regarding fibrosis, there was a significant increase in mortality in patients with BM fibrosis compared to those without fibrosis (median OS 22.8 months (95% CI [15.1; 30.5]) vs. 37.8 months (95% CI [33.1; 42.4]; HR 2.68, (95% CI [1.4; 4.1]), P= 0.00).
Discussion
The results of our study showed that in patients with newly diagnosed multiple myeloma, the presence of angiogenesis, higher percentages of BMPCs, less mature neoplasm, more widespread involvement of the BM, and the presence of fibrosis have a significant relationship with poor prognosis and reduced survival. Although a multivariate regression test did not show any independent relationship between any of the determinants and survival, the covariates in this study have not been primarily regarded to be a possible confounder or effect modifier (except for age).
The fact that higher amount of bone marrow plasma cells is a prognostic factor has been elucidated in multiple previous studies (7, 22-24), yet they introduced different thresholds for prognostic groups. Higher plasma cells in BM are a representative of higher burden of the disease throughout the body and higher complications. In our study there was a near four times more probability of death at 5 years if BMPCs were over 50% than less (compared to near two times seen in the Greek study (22) this discrepancy can partially be attributed to smaller sample size and a robust smear reviewing strategy which prevents group migration. 50% is an easy threshold to assess and could provide future prognostic tools like nomograms with a simple dichotomous outcome. Survival greatly improves as the number of plasma cells reach below 50%. This is implied in a 45-months median survival for those patients with BMPC between 25-50%, exactly what has been seen in ISS stage 2 patients. Cancer angiogenesis have proven to be a strong predictor of survival (HR=2.38) in MM, just as been previously postulated by some papers (21, 25). None of the cases with G1 (10-25%) BM plasma cell had evidence of angiogenesis. As the BM plasma cell percent increased, the level of angiogenesis also increased (5.2% for G2, 22.2% for G3, and 35.7% for G4, Chi-square P= 0.00). This confirms that as the bulk of tumor grows in the BM, the need for angiogenesis increases and by measuring MVD, one can estimate the survival of patient. Blastic morphology of MM cells had a powerful impact on survival (HR 4.17) compared to mature cancer cells. There was a surprising high median survival for these patients (13 months), slightly better than those reported long ago (26).
Better survival in this study is not surprising as treatment strategies of MM have exploded recently. There was no significant age difference between patients with blastic and mature morphology (65 vs. 61 years). Pleomorphic cells stand between mature and blastic cell in prognosis which is near ISS group II (23 vs. 29 months). There is a little caveat in our work here. Based on some previous studies, it is postulated that some CD138 negative MM cells are undifferentiated or dedifferentiated in nature and typically present with aggressive blastic morphology. Although these cells may represent only a small fraction of malignant cells (2-5%), their contribution to survival cannot be ignored (27, 28). It is well known that the higher level of plasma cell infiltration in the bone marrow is associated with a higher prevalence of anemia, reduction of red blood cell progenitor cells, and as a result, a poor prognosis and lower survival of patients (29).
This has also been exploited as an index for prognostication based on MRI (30). In our study, diffuse pattern was associated with the worst median survival compared to focal pattern (HR 4.67) followed by multifocal pattern (HR 3.85). Also fibrosis in bone marrow is associated with worse prognosis which is in concordance with previous literature (HR 2.68) (31, 32). Among all histopathologic components of BM, Blastic morphology was associated with worst median survival (13 months), followed by diffuse pattern and >50% BM plasma cell infiltration. The results of this confirms the powerful impact of BM histopathology components on MM patients' survival. Comparing to laboratory-based ISS, wider HR of death spectrum in this study, proposes a potential more precise, robust and easy-to-use prognostication tool.
Acknowledgments
The authors thankfully acknowledge the scientific support provided by the Research and Technology Empowerment Committee of Babol University of Medical Sciences, and Student Research Committee of Babol University of Medical Science. They also express their gratitude to the Clinical Research Development Unit of Rouhani Hospital.
Funding:
The entire budget of this study was provided by Research and Technology Vice-Chancellery of Babol University of Medical Sciences.
Ethics approval:
This study was approved by the Ethics Committee of Babol University of Medical Sciences under the code: IR.MUBABOL.HRI.REC.1400.192.
Conflict of interests:
The authors disclose that they have no conflicts of interest.
Authors’ contribution:
Author 1: Project development, Data collection; Author 2: Manuscript writing/editing; Author 3: Manuscript editing; Author 4: Manuscript writing; Author 5: Data analysis; Author 6: Designed the manuscript, Approved the final manuscript.
References
- 1.Ria R, Reale A, Mangialardi G, et al. The bone marrow microenvironment in multiple myeloma: Cellular and molecular basis of disease progression. Cell Respiration and Cell Survival: Processes, Types and Effects. Nova Science Publishers, Inc; 2011 . pp. 94–124. [Google Scholar]
- 2.Melaccio A, Reale A, Saltarella I, et al. Pathways of angiogenic and inflammatory cytokines in multiple myeloma: Role in plasma cell clonal expansion and drug resistance. J Clin Med. 2022;11:6491. doi: 10.3390/jcm11216491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee N, Moon S, Lee J, et al. Discrepancies between the percentage of plasma cells in bone marrow aspiration and BM biopsy: Impact on the revised IMWG diagnostic criteria of multiple myeloma. Blood Cancer J. 2017;7:e530. doi: 10.1038/bcj.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartl R, Frisch B, Fateh-Moghadam A, et al. Histologic classification and staging of multiple myeloma: a retrospective and prospective study of 674 cases. Am J Clin Pathol. 1987;87:342–55. doi: 10.1093/ajcp/87.3.342. [DOI] [PubMed] [Google Scholar]
- 5.Alexanian R, Balcerzak S, Bonnet JD, et al. Prognostic factors in multiple myeloma. Cancer. 1975;36:1192–1201. doi: 10.1002/1097-0142(197510)36:4<1192::aid-cncr2820360403>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol. 2016;43:676–81. doi: 10.1053/j.seminoncol.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buon E, Guang B, Bianchi G. Plasma cell neoplasms: an overview. Atlas Genet Cytogenet Oncol Haematol. 2018;12:497–506. [Google Scholar]
- 8.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–57. [PubMed] [Google Scholar]
- 9.Wei A, Westerman D, Feleppa F, Trivett M, Juneja S. Bone marrow plasma cell microaggregates detected by immunohistology predict earlier relapse in patients with minimal disease after high-dose therapy for myeloma. Haematologica. 2005;90:1147–9. [PubMed] [Google Scholar]
- 10.Smith A, Wisloff F, Samson D. Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol. 2006;132:410–51. doi: 10.1111/j.1365-2141.2005.05867.x. [DOI] [PubMed] [Google Scholar]
- 11.Ribourtout B, Zandecki M. Plasma cell morphology in multiple myeloma and related disorders. Morphologie. 2015;99:38–62. doi: 10.1016/j.morpho.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Štifter S, Babarović E, Valković T, et al. Combined evaluation of bone marrow aspirate and biopsy is superior in the prognosis of multiple myeloma. Diagn Pathol. 2010;5:30. doi: 10.1186/1746-1596-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akhtar S, Ali TA, Faiyaz A, et al. Cytokine-mediated dysregulation of signaling pathways in the pathogenesis of multiple myeloma. Int J Mol Sci. 2020;21:5002. doi: 10.3390/ijms21145002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajkumar SV, Leong T, Roche PC, et al. Prognostic Value of Bone Marrow Angiogenesis in Multiple Myeloma. Clin Cancer Res. 2000;6:3111–6. [PubMed] [Google Scholar]
- 15.Rana C, Sharma S, Agrawal V, Singh U. Bone marrow angiogenesis in multiple myeloma and its correlation with clinicopathological factors. Ann Hematol. 2010;89:789–94. doi: 10.1007/s00277-010-0919-z. [DOI] [PubMed] [Google Scholar]
- 16.Sezer O, Niemöller K, Eucker J, et al. Bone marrow microvessel density is a prognostic factor for survival in patients with multiple myeloma. Ann Hematol. 2000;79:574–7. doi: 10.1007/s002770000236. [DOI] [PubMed] [Google Scholar]
- 17.Ria R, Melaccio A, Racanelli V, Vacca A. Anti-VEGF drugs in the treatment of Multiple Myeloma Patients. J Clin Med. 2020;9:1765. doi: 10.3390/jcm9061765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cury PCdC, Higashi F, Zacchi FFS, et al. Effect of thalidomide on bone marrow angiogenesis in multiple myeloma patients. Hematol Transfus Cell Ther. 2020;42:159–63. doi: 10.1016/j.htct.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ria R, Reale A, Moschetta M, et al. A retrospective study of skeletal and disease-free survival benefits of zoledronic acid therapy in patients with multiple myeloma treated with novel agents. Int J Clin Exp Med. 2013;6:30–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Ribatti D, Nico B, Mangieri D, et al. Neridronate inhibits angiogenesis in vitro and in vivo. Clin Rheumatol. 2007;26:1094–8. doi: 10.1007/s10067-006-0455-3. [DOI] [PubMed] [Google Scholar]
- 21.Somasundaram V, Tevatia MS, Purohit A, et al. Evaluation of bone marrow microvessel density in patients with aplastic anemia. Indian J Hematol Blood Transfus. 2017;33:169–74. doi: 10.1007/s12288-016-0707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalayanni C, Iskas M, Vadikoliou C, et al. Prognostic value of diagnostic bone marrow plasma cell percentage in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2017;17:e43. [Google Scholar]
- 23.Fernández de Larrea C, Kyle R, Rosiñol L, et al. Primary plasma cell leukemia: consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. 2021;11:1–5. doi: 10.1038/s41408-021-00587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muchtar E, Gertz MA, Kourelis TV, et al. Bone marrow plasma cells 20% or greater discriminate presentation, response, and survival in AL amyloidosis. Leukemia. 2020;34:1135–43. doi: 10.1038/s41375-019-0655-x. [DOI] [PubMed] [Google Scholar]
- 25.Palta A, Kaur M, Tahlan A, Dimri K. Evaluation of angiogenesis in multiple myeloma by VEGF immunoexpression and microvessel density. J Lab Physicians. 2020;12:38–43. doi: 10.1055/s-0040-1714933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greipp PR, Raymond NM, Kyle RA, O'Fallon WM. Multiple myeloma: significance of plasmablastic subtype in morphological classification. 1985;65:305–10. [PubMed] [Google Scholar]
- 27.Wu D, Zhang P, Li F, et al. CD138-multiple myeloma cells express high level of CHK1 which correlated to overall survival in MM patient. Aging (Albany NY) 2020;12:23067. doi: 10.18632/aging.104066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuranda K, Berthon C, Dupont C, et al. A subpopulation of malignant CD34+ CD138+ B7-H1+ plasma cells is present in multiple myeloma patients. Exp Hematol. 2010;38:124–31. doi: 10.1016/j.exphem.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian R, Basu D, Dutta T. Prognostic significance of bone marrow histology in multiple myeloma. Indian J Cancer. 2009;46:40–5. doi: 10.4103/0019-509x.48594. [DOI] [PubMed] [Google Scholar]
- 30.Lee SY, Kim HJ, Shin YR, et al. Prognostic significance of focal lesions and diffuse infiltration on MRI for multiple myeloma: a meta-analysis. Eur Radiol . 2017;27:2333–47. doi: 10.1007/s00330-016-4543-8. [DOI] [PubMed] [Google Scholar]
- 31.Paul B, Zhao Y, Loitsch G, et al. The impact of bone marrow fibrosis and JAK2 expression on clinical outcomes in patients with newly diagnosed multiple myeloma treated with immunomodulatory agents and/or proteasome inhibitors. Cancer Med. 2020;9:5869–80. doi: 10.1002/cam4.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao J, Ma L, Guan JH. Pathological characteristics of bone marrow in multiple myeloma patients with secondary myelofibrosis and their relationship with prognosis. Zhongguo shi yan xue ye xue za zhi. 2017;25:1080–5. doi: 10.7534/j.issn.1009-2137.2017.04.021. [DOI] [PubMed] [Google Scholar]