Abstract
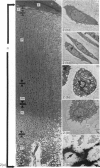
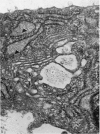
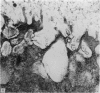
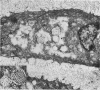
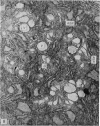
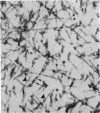
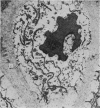
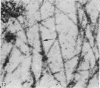
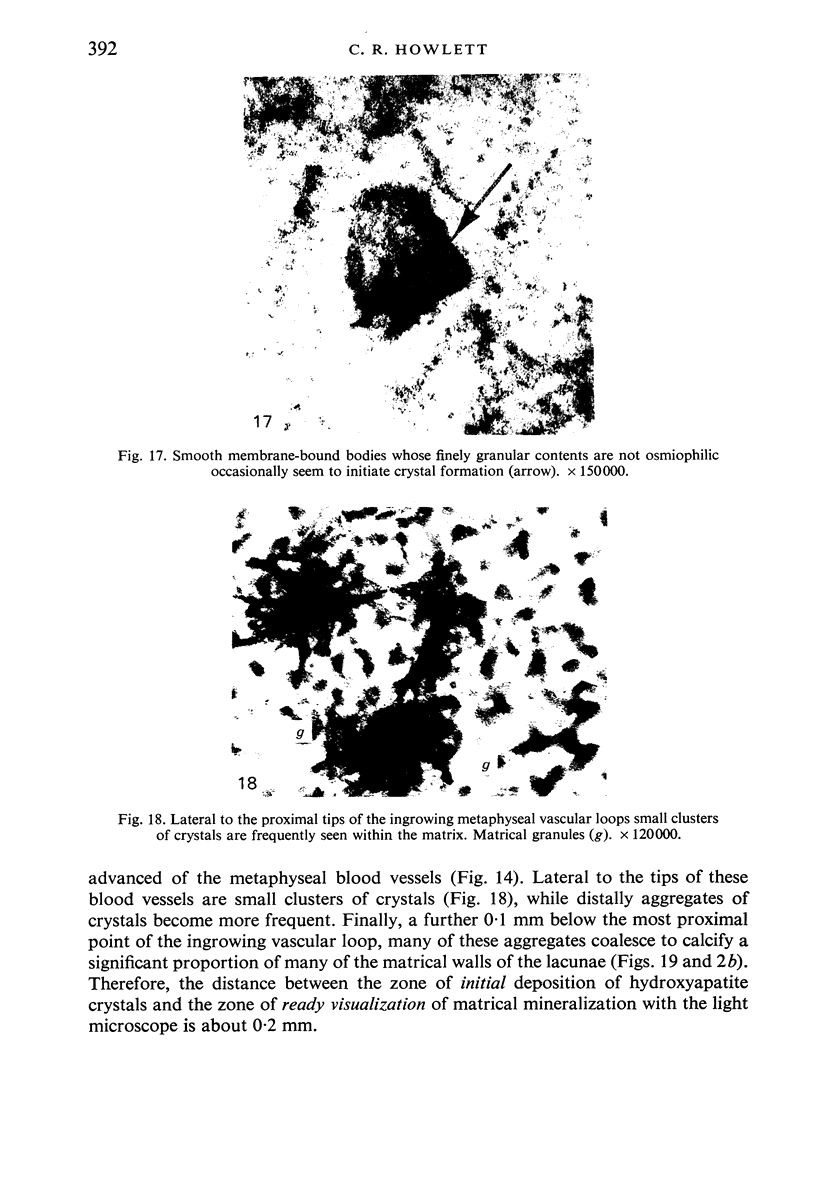
The ultrastructure of the proximal tibial growth plate of the 7 weeks old chicken has been described. Little ultrastructural difference could be ascertained between growth plates examined from normal white leghorn and commercial broiler chickens. The growth plate may be divided into five zones: interstitial, proliferating, prehypertrophic, hypertrophic, and degenerating hypertrophic. These zones reflect a maturation of chondrocytes, beginning with a stage of high mitotic and cytoplasmic activity passing through a stage of active secretion of matrical components (prehypertrophic and hypertrophic) and ending with degeneration of the cells and calcification of the matrix. Mineralization of the matrix appears to be initiated within matrical dense bodies, as in the mammal. Single hydroxyapatite crystals are first encountered about 0.1 mm proximal (i.e. towards the knee) to the limit of metaphyseal blood vessel ingrowth, while dense calcification is observed 0.1 mm distal to the tips of these metaphyseal vessels. The diameter of microfibrillary collagen in the growth plate matrix ranges from approximately 9 nm in the proximal zones to 19 nm in the distal zones. Many of the fibrils in the distal zones have a more or less distinct periodicity. Other major elements of the growth plate matrix are the ruthenium red-stained syncytial aggregates of mucopolysaccharides which are probably derived from the granules within the large intracellular Golgi vesicles. These findings have led the author to conclude that, while light microscopy indicates that avian and mammalian growth plates have very different structures, electron microscopy finds many similarities, suggesting that the physiological control mechanisms in these two vertebrate classes have much in common.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. E., Parker J. Electron microscopy of the epiphyseal cartilage plate. A critical review of electron microscopy observations on enchondral ossification. Clin Orthop Relat Res. 1968 May-Jun;58:225–241. [PubMed] [Google Scholar]
- Anderson H. C., Matsuzawa T., Sajdera S. W., Ali S. Y. Membranous particles in calcifying cartilage matrix. Trans N Y Acad Sci. 1970 May;32(5):619–630. doi: 10.1111/j.2164-0947.1970.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Anderson H. C., Sajdera S. W. The fine structure of bovine nasal cartilage. Extraction as a technique to study proteoglycans and collagen in cartilage matrix. J Cell Biol. 1971 Jun;49(3):650–663. doi: 10.1083/jcb.49.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H. C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969 Apr;41(1):59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonucci E. Fine structure and histochemistry of "calcifying globules" in epiphyseal cartilage. Z Zellforsch Mikrosk Anat. 1970;103(2):192–217. doi: 10.1007/BF00337312. [DOI] [PubMed] [Google Scholar]
- Bonucci E. The locus of initial calcification in cartilage and bone. Clin Orthop Relat Res. 1971;78:108–139. doi: 10.1097/00003086-197107000-00010. [DOI] [PubMed] [Google Scholar]
- CAMERON D. A. The fine structure of bone and calcified cartilage. A critical review of the contribution of electron microscopy to the understading of osteogenesis. Clin Orthop Relat Res. 1963;26:199–228. [PubMed] [Google Scholar]
- Crissman R. S., Low F. N. A study of fine structural changes in the cartilage-to-bone transition within the developing chick vertebra. Am J Anat. 1974 Aug;140(4):451–469. doi: 10.1002/aja.1001400402. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Lutfi A. M. The ultrastructure of cartilage cells in the epiphyses of long bones in the domestic fowl. Acta Anat (Basel) 1974;87(1):12–21. doi: 10.1159/000144152. [DOI] [PubMed] [Google Scholar]
- Matukas V. J., Krikos G. A. Evidence for changes in protein polysaccharide associated with the onset of calcification in cartilage. J Cell Biol. 1968 Oct;39(1):43–48. doi: 10.1083/jcb.39.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matukas V. J., Panner B. J., Orbison J. L. Studies on ultrastructural identification and distribution of protein-polysaccharide in cartilage matrix. J Cell Biol. 1967 Feb;32(2):365–377. doi: 10.1083/jcb.32.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Matukas V. J. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. D., Low F. N. The fine structure of developing cartilage in the chick embryo. Am J Anat. 1971 Jun;131(2):197–215. doi: 10.1002/aja.1001310205. [DOI] [PubMed] [Google Scholar]
- REVEL J. P., HAY E. D. AN AUTORADIOGRAPHIC AND ELECTRON MICROSCOPIC STUDY OF COLLAGEN SYNTHESIS IN DIFFERENTIATING CARTILAGE. Z Zellforsch Mikrosk Anat. 1963 Oct 8;61:110–144. doi: 10.1007/BF00341524. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- TAKUMA S. Electron microscopy of the developing cartilagenous epiphysis. Arch Oral Biol. 1960 Jul;2:111–119. doi: 10.1016/0003-9969(60)90059-5. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Friberg U. Ultrastructure and acid phosphatase activity of matrix vesicles and cytoplasmic dense bodies in the epiphyseal plate. J Ultrastruct Res. 1970 Dec;33(5):554–573. doi: 10.1016/s0022-5320(70)90181-4. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Friberg U. Ultrastructure of the epiphyseal plate of the normal guinea pig. Z Zellforsch Mikrosk Anat. 1971;122(2):254–272. doi: 10.1007/BF00337633. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Lohmander S., Friberg U. Electron microscopic demonstration of proteoglycans in guinea pig epiphyseal cartilage. J Ultrastruct Res. 1973 Dec;45(5):407–427. doi: 10.1016/s0022-5320(73)80070-x. [DOI] [PubMed] [Google Scholar]
- WOLBACH S. B., HEGSTED D. M. Endochondral bone growth in the chick. AMA Arch Pathol. 1952 Jul;54(1):1–12. [PubMed] [Google Scholar]
- Wise D. R., Jennings A. R. The development and morphology of the growth plates of two long bones of the turkey. Res Vet Sci. 1973 Mar;14(2):161–166. [PubMed] [Google Scholar]