Abstract
Neuroinflammation, α-synuclein pathology and dopaminergic cell loss are the hallmarks of Parkinson’s disease (PD), an incurable movement disorder. The presence of the blood-brain barrier (BBB) impedes the delivery of therapeutics and makes the design of drug-targeting delivery vehicles challenging. Nanomedicine is designed and has significantly impacted the scientific community. Over the last few decades, to address the shortcomings of synthetic nanoparticles, a new approach has emerged that mimic the physiological environment. Cell membrane-coated nanoparticles have been developed to interact with the physiological environment, enhance central nervous system drug delivery and mask toxic effects. Cell membranes are multifunctional, biocompatible platforms with the potential for surface modification and targeted delivery design. A synchronous design of cell membrane and nanoparticles is required for the cell membrane-based biomimetics, which can improve the BBB recognition and transport. This review summarizes the challenges in drug delivery and how cell membrane-coated nanoparticles can overcome them. Moreover, major cell membranes used in biomedical applications are discussed with a focus on PD.
Keywords: Parkinson’s disease, Cell membrane, Nanoparticles, Blood-brain barrier, Drug delivery
Graphical abstract
1. Introduction
Parkinson’s disease (PD) is an incurable movement disorder characterized by selective loss of dopaminergic neurons and abnormal aggregation of α-synuclein protein [1]. As the second most common neurodegenerative disorder worldwide [2], PD currently has treatments that are focused solely on symptom alleviation. Many investigations have been carried out to explore therapeutic interventions targeting multiple pathways; however, the major difficulty lies in the precise delivery of therapeutics to the brain. The central nervous system (CNS) and peripheral nervous system (PNS) are separated by the blood-brain barrier (BBB) serving a protective function by maintaining brain homeostasis and blocking harmful substances [[3], [4], [5], [6]]. However, the BBB's high selectivity also restricts therapeutic efficacy, resulting in nonspecific drug distribution and poor penetration. Disruption of the BBB may increase the risk of harmful substance accumulation in the CNS, potentially causing irreversible damage [7,8].
The emergence of nanoformulations and their various modifications has been observed as a feasible solution with suitable carriers [9,10]. But they also have certain drawbacks, including immune response, rapid systemic clearance and potential toxicity [11,12]. Even certain nanoparticles (NPs) have been reported to induce inflammatory responses and oxidative stress after reaching systemic circulation [13]. New interventions are required to overcome these effects and exert the full potency of targeted therapies. Therefore, recently scientific attention has shifted toward cell membrane-coated nanoparticles (CMNPs). These core-shell structures feature a NP core enveloped by a natural cell membrane layer, often further modified with targeting motifs to enhance biological specificity. CMNPs not only retain the benefits of NPs but also enhance the biocompatibility, prolonged drug circulation, reduce clearance, and enhance BBB penetration [14]. The major issue of drug delivery is addressed via the movement of cells by biological membranes via these biomimetics [15]. Moreover, the chemotactic abilities of leukocyte membranes are explored to target inflammation in CNS [16]. As they also retain membrane receptors and tightly adhere to the brain endothelial cells, they migrate toward the lesion site via chemokine receptor pathways [16]. These are drug-delivery cargoes with desired targeting abilities [17]. CMNPs have the advantage of combining the multifunctionality of biological membranes with synthetic NPs. This bestows their homologous targeting ability on the extraneous NPs to interact within the physiological environment.
In this review, we attempted to highlight the challenges in CNS targeting in the current scenario, and how different CNMPs can be unique with a special focus on PD. Numerous types of cell membranes derived from platelets, red blood cells (RBCs), cancer cells, neural cells, macrophages, white blood cells and numerous others have been discussed, along with their utilization in the fabrication of CMNPs. Moreover, the latest investigations carried out to address the clinical manifestations of PD and the effects of these biomimetics have been updated in this review.
2. Cell membrane-coated nanoplatforms
Drug delivery system (DDS) utilizes the principle of delivering therapeutic molecules to specific tissue or cells. Traditional systems were based on the administration of “naked drugs” via parenteral, topical, oral, inhalational and intravascular routes. However, each route has certain limitation. For instance, inhalational routes require specialized devices for administration and may cause respiratory tract irritation. This route is also limited to specific drugs only and results in variable absorption. Similarly, transdermal systems are limited to drugs with specific molecular size and lipophilicity, and may cause skin irritation or allergic reactions upon application. The rectal route is inconvenient, not suitable for all patient types, and can have variable absorption rates influenced by rectal contents. Similar issues frequently occur with the sublingual route, which limits dose capacity and affects compliance due to the taste [18,19]. Therefore, smart delivery systems became popular for delivering the maximum amount of drugs to the target site, and targeted drug delivery systems (TDDS) came into the limelight. When compared to other routes, TDDS offer significant advantages which mainly focus on enhancing therapeutic efficacy while minimizing the side effects. TDDS delivers drugs directly to the target site while sparing healthy tissue, leading to overall improved outcomes. By incorporating nanocarriers and other advanced delivery systems, TDDS has improved the solubility and stability of poorly water-soluble drugs, enhancing overall bioavailability. This is specifically beneficial for drugs that undergo significant first-pass metabolism. In addition to this, TDDS provides controlled and sustained release of therapeutic agents over extended periods. Moreover, the precision of TDDS enhances drug effectiveness by ensuring higher concentrations reach the intended site of action. A versatile range of therapeutics can be delivered by TDDS such as small molecules, nucleic acids, peptides and proteins [20,21].
The major focus has been on nanotechnological advancement that aim at sustained and controlled drug release. Commonly used nanoplatforms include biodegradable dendrimers, polymers, lipids and metal-based NPs, each with its own merits and demerits [22]. Encapsulation or conjugation of therapeutic drugs, miRNA, small molecule inhibitors and siRNA can improve solubility and bio-distribution properties. Thus, the development of therapeutic NPs has been encouraged primarily by their potential to advance treatment, prognosis and diagnosis [23]. Recently, cell membrane-coated nanoplatforms have gained much attention which are designed by wrapping cell-derived membranes around nanosized cores for therapeutically beneficial applications [24]. These natural systems have the benefit of effectively evading immune clearance and serving as longer-circulating drug carriers [25,26]. These membrane-based platforms can also detoxify harmful pathogens and molecules without prior danger signals and have better antigen-presenting properties [27]. These are highly encouraged for complex biological systems. To enhance efficiency and target specificity, the addition of function groups, improves the performance of these nanoplatforms [28]. Various methods are employed for the conjugation of functional ligands to the cell membrane, but damage to the cell membranes can occur due to aggregation of cell membrane proteins and disruption of immune integrity. Although method selection is important for minimizing membrane damage, it may limit the number of ligands and compatibility [29,30].
2.1. Basis for cell membrane coating nanosystems
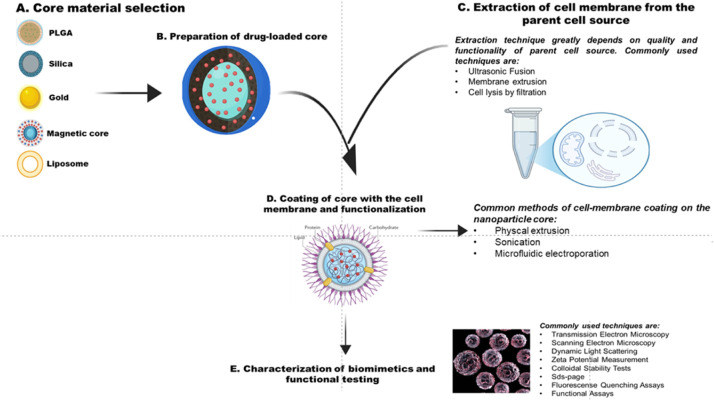
Cell membrane coating technology-based nanoplatforms came into existence in 2011 in an investigation based on leveraging the entire CMNPs. To develop the desirable properties of a cell, its outermost layer is transferred to encapsulate the NPs in a while complex setting preserving the bio-constituents of the membrane [31]. Cell membrane coating systems adopt advanced top-down approaches for intricate functionalities that have the potential to integrate with natural cell membranes [32]. The inherent feature of CMNPs to mimic the surface characteristics of target sites makes them unique by enhancing their biocompatibility, reducing macrophage cells-mediated uptake, improving tissue penetration, and extending circulation times [33,34]. A schematic representation of the process diagram is shown in Fig. 1.
Fig. 1.
Schematic illustration of the formation of core-shell and cell membrane coating on the NP core-shell.
Core-shell structures in cell membrane-based biomimetics are engineered to mimic natural cellular structures, enhancing their function for drug delivery and tissue engineering. Core fabrication, membrane extraction and membrane coating are the primary three steps of developing these biomimetics. The inner core is used to encapsulate drugs and other biological vectors and can be composed of various materials such as PLGA, inorganic substances and liposomes [21]. The core serves as a scaffold for the subsequent membrane coating, influencing the overall features of the final structure. Another crucial step is isolating the cell membrane from the parent cell, which needs to be performed delicately, as it is responsible for maintaining the integrity and functionality of the membrane. Common methods for membrane isolation include ultrasonic waves, hypotonic lysis, freeze-thaw cycles and homogenization. Different techniques are used to delicately monitor the yield and intactness of the membrane, and effectively replicate their natural functions when coated with the core. Further, when the membranes are effectively isolated, these are coated onto the inner core using methods such as extrusion, sonication or microfluidic electroporation [35,36]. In general, when fabricating these NPs, the surfaces are entirely and uniformly covered by the cell membranes, creating a structure like the integrated core-shell. However, to isolate the cell membranes, first the cell integrity must be disintegrated, and then, by applying external forces such as sonication or extrusion, the membranes fuse with the core of NPs [37]. The resulting core-shell structures exhibit characteristics such as improved drug delivery, reduced immune recognition and better cellular uptake. By utilizing various types of cell membranes- from RBCs to cancer cells-biomimetics can be tailored for specific therapeutic applications [38]. This was first demonstrated using the RBC membrane, as RBCs are responsible for oxygen delivery in the body and have a viability of 4 months. The specific markers of RBCs and their ability to circulate throughout the body make them desirable candidates for developing biomimetic systems [39]. Initially, membranes were lysed by incubating them in the hypotonic solution, and with the application of mechanical extrusion and sonication, vesicles were generated. These vesicles were then co-extruded with PLGA, resulting in the development of core-shell structured NPs surrounded by the membrane. Interestingly, these NPs were able to circulate in the mouse model for extended periods, having an effective half-life of 40 h, which is better than the PEGylated platforms [39]. When the cell membranes are lysed to develop cell-membrane-based biomimetics, nano- or micrometre-sized lipid vesicles are formed, retaining the membrane-bound components from the original cell. These vesicles retain the signaling networks and intrinsic functionalities of their parent cells. This specific property makes them more biocompatible and low in immunogenicity [40].
This biomimetic application of the vesicles can also be harnessed by using the outer membrane vesicles (OMVs) to deliver therapeutic proteins or vaccines directly to the target cells. Moreover, to improve stability, the surface properties of the vesicles can be modified, which can also help with the targeting capabilities and immune detection-related issues crucial for overall therapeutic efficacy [41]. Cell membranes thus are a more viable option than polymers as high targeting efficiency can be achieved due to their ability to recognize self-antigens [42]. Different cell membrane-based biomimetics specifically employed in neurodegenerative disorders have been schematically illustrated in Fig. 2. This platform has an outstanding ability to mimic intricate cellular functions for the development of newer therapeutic modalities. For instance, various CMNPs beneficially evade immune clearance by acquiring “markers of self” from the source cells, developing into better long-circulating drug carriers [31]. Various native affinity ligands on the parent cell have the exquisite capability of targeting the disease sites [43]. Others can work as cell decoys to detoxify pathogens and harmful molecules, imparting protection to the cells. Additionally, these biomimetics also have the ability for more relevant antigen presentation [44], while others can scavenge bacterial toxins and maintain their cell integrity. Given the need for multitasking and multifunctionality in complex biological systems, the development of CMNPs has become increasingly important. Having multifunctional ligands is beneficial to improve the performance of NPs. While cell membrane coatings offer better immune evasion and stealth, off-target side effects are limited due to target selectivity, ultimately enhancing therapeutic efficacy [45]. These NPs have better potential for immune uptake by presenting better antigenic information, and better control over immune activation for modulatory immunity [46]. Furthermore, responsiveness to the environment and a more dynamic bio-interface are characteristics of membrane-coated nanoformulations [47]. To introduce better functionalities, conjugation can be employed for groups such as biotin-, amine- and sulfhydryl-based reactions. These methods are employed for decorating functional ligands on the cell membrane for better targeting.
Fig. 2.
Schematic illustration of cell membrane based biomimetics in neurodegenerative disorders, which shows different organic and inorganic NPs encapsulated in bio membrane having surface proteins act on specific site in different neurogenerative diseases.
2.2. Types of cell membranes used for CMNPs
2.2.1. Neural cell membranes
Drug delivery application of cell membranes is a unique approach involving the transfer of the outermost layer of a cell onto the surface of drugs or drug-encapsulated nanosystems. However, this system remains unexplored for the nervous system. Specialized cell-to-cell contacts are often observed in the nervous system, such as those for oligodendrocytes, the myelinating cells that have close interactions with the neuronal cells [48]. In turn, neurons are in close contact with the surrounding glial and neuronal cells, which impacts neurite outgrowth [49]. Thus, cloaking drug delivery vehicles with suitable neural membranes can develop better neuronal biomimetics and positively enhance brain drug delivery while reducing the rapid clearance [50]. Exploiting this concept, in a recent study, rat cortex-derived four cell membranes—astrocytes, microglial, cortical neurons and oligodendrocyte progenitor cells (OPCs)—were used to coat di(thiophene-2-yl)-diketopyrrolopyrrole (DPP) conjugated poly(caprolactone) (PCL) NPs, which were fluorescently tagged [51]. When comparing CMNPs with non-coated ones, it was observed that the microglial activation was reduced in all three membrane-coated systems except for the cortical neuron cell membrane. This resulted in a more favorable system for neuronal cells to regenerate. In addition to this, efficient uptake and neural cell response to the membrane-coated nanosystem were observed. Overall, microglial cell coating was the most efficient among all [50]. This cell membrane has the potential for efficient targeted delivery as it biomimics the natural make-up of neuronal cells, can establish better communication signals with the target site, and can overcome the BBB limitations. However, the significant considerations before developing the neural cell membrane-based biomimetics include choosing the appropriate neural cell type, optimizing the size, surface charge and drug loading capacity, and extensive pre-clinical evaluation to assess the potential toxicity. Different sources of cell membranes used for the development of biomimetics and areas where improvement is needed is briefly discussed in Table 1.
Table 1.
Different sources of cell membranes, their emerging role in developing biomimetics, development status, and areas requiring focus.
| Cell membrane source | Emerging role in biomimetic system | Improvement areas | Development status | Ref. |
|---|---|---|---|---|
| Erythrocytes (RBCs) | Long circulation time; immune evasion due to self-markers | Enhance targeting capabilities for certain tissues | Widely used; ongoing research for hybridization with other membranes | [52,53] |
| Cancer cells | Targeting efficiency due to unique surface proteins | Optimization of extraction methods to maintain membrane integrity | Successful applications with targeted therapy | [52] |
| Platelets | Targeting efficiency due to adhesion properties | Stability and circulation time | Actively researched, hybrid membranes with RBCs show promising results | [53] |
| Macrophages | Immune modulation and targeting of inflammatory sites | Enhance functionalization for better targeting and uptake | Under development for macrophage-based NP efficacy | [53,54] |
| Leukocytes (WBCs) |
Modulates immune responses | Need improvement in extraction technique to preserve cell functionality | Emerging for its therapeutic applications | [53,54] |
| Hybrid membrane | Combine features of different cell types for enhanced functionality | Needs standardization for fusion techniques and assessment in long-term stability. | Innovative approaches are being explored with promising results in biocompatibility and targeting efficiency | [52,53] |
| Neural cells | Biomimics the CNS components; potential for neuroprotection | Needs improvement for penetration through BBB | Developing rapidly specifically for neurodegenerative therapies | [54] |
| Mesenchymal stem cells (MSCs) | Tissue regeneration and repair; immunomodulation properties | Needs enhancement in homing capabilities to target specific tissues effectively | Actively researched for regenerative medicine applications | [53] |
| Bacterial cell | Mimics pathogen interactions | Needs improvement in stability and functionality in diverse environments | Emerging interests for DDSs | [52,53] |
| Endothelial cells | Targets vascular systems; promotes tissue repair | Needs enhancement for membrane integrity and functionalization to target | On-going research focusing on DDSs | [53,54] |
2.2.2. Cancer cell membranes
Due to high target specificity and similar mechanism for adhesion to other cancer cells, membranes isolated from cancer cells are considered excellent sources to encapsulate nanomedicines [44,55]. Their unique properties, such as self-targeting, make them suitable for replicative immortality, angiogenesis, drug delivery, and activating metastasis and invasion [56]. These biomimetics are stable and can efficiently transfer antigens to the target sites from tumor membranes, overcoming the inefficient ability of therapeutic drugs to target tumors [44]. Beyond oncology, cancer cell membrane-based nanoplatforms have opened new avenues for neurodegenerative disorders such as PD. The transfer of the cell membrane to the NP surface has various advantages, as the membrane is the most basic component of the cell that participates in cell-environment interactions and multiple cellular functions, including signal transduction and self-recognition. Direct transfer of the membrane to the cell surface retains all the biological components and functions of the membrane. In comparison, cancer membranes are more notorious than other membranes. The foremost role of cancer membranes is the expression of CD47 protein, a ligand for signal-regulated protein alpha (SIRPα) that assists in sending the “don’t eat me” signals to macrophages and bypasses the engulfment process, hence achieving immune escape [57]. Immune escape is significant as it can reduce the inflammatory responses that can exacerbate neuronal damage in PD. For NP-based DDS, it can enhance the their efficacy by prolonging the circulation time without being recognized and cleared by the immune system. The increased half-life assists in the effective delivery of therapeutic agents to the brain, which is crucial for managing PD symptoms and halting disease progression. Particularly, this NP design is significant to cross the BBB. Furthermore, cancer cell membranes are rich in adhesion molecules, including N-cadherin which also plays a crucial role in the pathology of PD. N-cadherin is essential for the differentiation and maturation of dopaminergic neurons [58]. Various reports suggest that particularly N-cadherin expression could promote the apoptotic signals in the neuronal cells, thereby protecting the dopaminergic neuronal population [59]. Thus, cancer CMNPs can be utilized to effectively deliver the neuroprotective agents that may target the PD pathology at the molecular level. Cancer cell membrane-camouflaged NPs represent a promising strategy for addressing some of the challenges associated with delivering therapies for PD. Recent investigations have demonstrated that these nanoplatforms improved outcomes in various biomedical applications. For instance, paclitaxel-loaded PCL NPs for targeting 4T1 tumor [60] and doxorubicin loaded Fe2O3 NPs for improved internalization and retained membrane markers [61]. Another important application of this nanoplatforms is vaccines. In a recent investigation, researchers developed cancer CMNPs with an adjuvant layer of monophosphorylipid A (MLPA), a TLR4agonist, used as a vaccine. These NPs were used to investigate the release of antigens specific for tumors and dendritic cell maturation. Furthermore, dendritic cells were combined with the tumor antigen-specific pmel-1 transgenic murine splenocytes, which assisted in confirming the accumulation of T cells and secretion of interferon-gamma around the dendritic cells and cancer cell membrane having MLPA-coated NPs [62].
Altogether, these studies confirm the induced tumor-specific immune responses by using cancer-cell-derived membranes that stimulate the tumor-associated antigens and adjuvant coating that can be effective for cancer immunotherapies.
2.2.3. Mesenchymal stem cell (MSC) membranes
MSC membrane camouflages NPs can potentially help in PD by allowing targeted delivery of therapeutic moieties directly to brain regions, evading the immune system due to the cloaking effect of the cell membrane, and modulating inflammatory responses within the brain, all while promoting better drug penetration across the BBB due to the cell membrane’s natural ability to interact with brain tissue. Certain benefits of MSCs include multi-lineage differentiation, immunomodulation and homing, and high-speed proliferation [63], which are crucial for PD. A very recent investigation clarified how MSCs-based cell membrane-derived nanocarriers can help in PD. The main benefits include the MSCs and MSCs-derived neuron-like cell membrane-coated therapeutics that can specifically target the disease site and are biocompatible. In this study, microfluidic electroporation chips prepared MSC-derived neuron-like cell membrane-coated curcumin PLGA NPs (MM-Cur-NPs), which were investigated in cellular and animal PD models. As per the results, cellular mitochondrial membrane potential and oxidative stress were restored to normal, and improved accumulation and distribution were observed in PD mice, restoring damaged dopaminergic neurons. Moreover, fluctuated neurotransmitter metabolites were restored to normal levels; genes expressing anti-inflammatory factors increased, and genes expressing pro-inflammatory factors were reduced significantly. In addition, microglial expression of IBA-1, and neuronal apoptosis were inhibited. This study in conclusion demonstrated the synergistic effect of cell membrane-derived nanoplatforms for neuronal protection and alleviation of movement disorder [64].
Stromal stem cells, better known as MSCs, have the potential for self-renewal and are also considered as multipotent progenitor cells. They greatly influence the field of regenerative medicine, since they can decrease the side effects induced by chemotherapeutic drugs and are utilized to treat autoimmune disorders. Membranes extracted from these cells can be used to develop nanomedicines by coating NPs, as these cell membranes have the advantage of easy isolation and store abundant information for large-scale synthesis [65,66]. In a DDS using PLGA NPs loaded with doxorubicin exhibited effective tumor localization and antitumor effect [67]. MSC membranes have also been utilized to mask NPs for enhancing deep tissue tumor treatment by photodynamic therapy [90]. Interestingly, using these cell membranes in regenerative medicine does not have any impact on the interactions between diseased cells and stem cells [68]. Similarly, magnetic resonance imaging (MRI) has also been carried out by coating iron oxide NPs with adipose-derived MSC membranes. Doxorubicin co-encapsulated with superparamagnetic iron oxide NPs and coated with MSC membranes showed effective therapy of colon cancer. Efficiently, these NPs improved the anti-tumor effect, and cellular uptake, attenuated the immune response and better targeting efficiency was confirmed [69]. Another research team developed stem cell-coated nanocarriers with C-X-C chemokine receptor type 4 (CXCR4) expression that reduced uptake by murine and human macrophages while enhancing the endothelial barrier penetration [70]. Thus, this coating system has proven efficient for several therapeutic applications. In a recent investigation, glyburide (a protective agent) has been used to improve ischemic stroke outcomes such as hemorrhagic transformation, edema and infarct volume. To overcome glyburide's limited penetration, neural stem cells have been employed for targeted delivery, with CXCR4 overexpression used to specifically target stroke area. The results demonstrated a better accumulation of drugs in the infarct zone [71,72].
Overall, this coating system thus has proven efficient for several therapeutic applications. Current literatures suggest that the use of MSC membrane-camouflaged NPs holds promise as a strategy to enhance targeted drug delivery by leveraging the unique homing and immunomodulatory features of MSCs. However, applying the membrane of stem cells only means utilizing its functionality and not its distinct differentiation capacity. Future researchers should focus on optimizing membrane extraction methods, improving NP coating techniques, and evaluating potential toxicity concerns.
2.2.4. Bacterial cell membranes
Lack of cell organelles and nuclei is a specific trait of bacterial cells [73]. These pathogens have also been exploited for their cell membrane isolation to develop biomimetics for treating and curing diseases. For instance, E. Coli membrane was extracted and used to coat drugs delivered to neutrophils, which are the predominant immune cells near inflamed areas. This coating efficiently maintained biocompatibility by facilitating neutrophil-mediated endocytosis in contrast to non-functionalized NPs [74]. Similarly, PLGA-encapsulated NPs were coated by the extracellular vesicles obtained from S. aureus strains. These NPs demonstrated improved targeting of infection sites and infected macrophages, showing higher accumulation at the sites with greater bacterial load compared to non-infected regions. These were also explored by loading the antibiotics in the NPs further decreasing the bacterial loads [75]. So far, no study has demonstrated the effect of bacterial cell membrane-covered NPs in PD. However, their potential in other disorders suggests promising applications Given the ability of these membranes to cross the BBB, they could be exploited for drug delivery in PD. NPs coated with the bacterial membrane can evade the immune system and allow targeted delivery of therapeutic agents directly to the affected brain regions.
2.2.5. Red blood cells membranes (RBCMs)
RBCMs are the most promising cell type among all for developing and treating numerous disorders as they are efficient transporters for drugs, nucleic acids, proteins and active therapeutic molecules. They are abundant in blood and have a life span of ∼120 d in humans [76]. Their lack of organelles simplifies membrane extraction and purification, making them a pioneer choice for biomimetic applications. RBCM-based systems are widely used due to their rapid manufacturability and biocompatibility. The highly hydrophilic polysaccharides on the surface of RBCs enhance the stability of coated NPs. RBCMs are advantageous for brain targeting because the brain has a rich blood supply, facilitating unobstructed NP delivery [77]. However, there are certain drawbacks that limits its use for brain delivery including limited targeting ability and potential damage to integrity, stability and functional proteins during chemical modification [77]. Despite these limitations, its widespread use for various biomedical applications has been observed in recent times.
These membranes were initially isolated by Hu et al., through hypotonic treatment of RBCs [31]. Subsequent studies led to the development of RBCs with prolonged circulation time and enhanced oxygen-carrying capacity [78]. With the TEM demonstrations, it was found that RBCMs can efficiently coat NPs (65–340 nm in diameter) while preserving surface proteins, glucans and lipid membrane stability [79]. Recently, reactive oxygen species (ROS) responsive NPs modified by stroke homing peptide (SHp) encapsulated with RBCM designated as SHP-RBC—NP/NR2B9C were designed for ischemic stroke where NR2B9C (a therapeutic peptide) prevents the neurotoxicity produced by the N-methyl-d-aspartate receptor (NMDAR) without interfering in its primary activity. However, its high molecular weight and hydrophilic nature limits its BBB penetration. To overcome this, the scaffold was coated with RBCM, significantly improving BBB crossing efficiency—1.71% for RBC-coated drugs, 1.44% for drug-loaded NPs, and only 0.53% for the free drug [80]. Furthermore, the researchers inserted SHp in the RBCM for better targeted delivery to the ischemic region [81,82].
Conversely, studies have reported electrostatic repulsions between negatively charged NP core and membrane extracellular regions, which confirms that the surface proteins reside on the outer region of the membrane when RBCMs coat the NPs. And conventionally this NP offers a better half-life than uncoated NPs [31,79]. These coatings have been widely exploited for MRI applications through the incorporation of magnetic iron oxide groups with the NPs. They serve as better contrasting agents while demonstrating enhanced capacities for heat conversion [83,84]. Essentially, hypotonic method of isolation can better preserve the biophysical and immune properties of the cell [85,86]. In cancer biology also, doxorubicin-loaded NP cores coated with RBCMs have demonstrated efficient drug loading capacity and sustained release kinetics [87]. In another investigation, inhibition of phagocytosis by macrophages was achieved by specific RBCM marker CD47, which protected gold particles from various interactions and resulted in better half-life of particles in the biological system [88,89]. Similarly, various studies have been carried out to address inflammatory diseases, atherosclerosis, bacterial infections and leukemia [43,[90], [91], [92]].
Current literature suggests the beneficial effects of RBCM-based platforms for neurological disorders, particularly PD, due to their unique properties. RBCMs significantly enhance the circulation time of NPs in the bloodstream and minimize the immunogenicity, ensuring therapeutic agents reach their target site in brain without being rapidly cleared from circulation. Another important factor that makes the RBCMs suitable as drug delivery vehicles for PD is their capability to inhibit inflammation and apoptosis, which are significant contributors to neuronal damage in the PD. In addition, these nanoplatforms have natural biomimic characteristics. Hence, they can more effectively navigate biological barriers and are particularly suitable for enhancing BBB penetration, making them more suitable for PD DDSs. This has been demonstrated in a recent investigation where a RBCM/UCMG DDS was designed, which wrapping lanthanide ion (Ln3+)-doped upconverting NPs (UCNPs) with RBCMs. These NPs can excite nitric oxide (NO) donors after converting near-infrared (NIR) light to higher energy green light. While this scaffold was initially employed for cancer therapy, the investigators evaluated it for PD-related neuroinflammation. The scaffold effectively crossed the BBB and post-irradiation with an NIR beam of 980 nm, NO was released in the brain. This significantly decreased the pro-inflammatory mediators, protected dopaminergic neurons, and ultimately improved PD outcomes [2].
2.2.6. White blood cells (WBCs) membranes
Based on their functionality, WBCs (or leukocytes) are divided into different subtypes including eosinophils, mast cells, lymphocytes, basophils, macrophages, granulocytes, monocytes and neutrophils. These cells mainly carry out immune functions and are abundantly recruited to different tissues in response to stress. Since they contain intricate intracellular components and nuclei, the membrane isolation process is very difficult. Their unique infection-fighting and chemotactic properties make them suitable for coating purposes [93]. WBCs based membrane coatings are mainly used for the delivery of chemotherapeutic drugs, as these cells can effectively locate inflammatory sites, which are useful targets for tumor vasculature remodeling [94]. In a study, WBC membranes were coated onto nanoporous silicon using high-density sucrose for purification. To enhance stability, positive (3-aminopropyl) triethoxysilane (APTES) was subsequently applied [95].
Monocytes, derived from hematopoietic stem cells, are the precursors of macrophages that tend to accumulate at the tumor site, hence playing an essential role in tumor progression and metastasis [96]. To exploit this property, researchers coated doxorubicin-loaded PLGA-NPs with monocyte membranes. As a result, it was found that these NPs target the tumor cells and maintain the integrity of membrane-based heteroproteins much more effectively than uncoated NPs. Another investigation established the advantages of the monocyte coating system, showing enhanced stability against the invasion of mononuclear phagocytes due to chemokine receptor-mediated tumor tropism [97]. Another crucial membrane of this class is macrophages derived from monocytes [98]. They play a key role in cancer immunology by recognizing and engulfing foreign substances or tumor cells lacking surface biomarkers [99]. In a report, WBC-like vectors were designed by coating the nanoporous silicon NPs, which influenced the circulation time and accumulation at the tumor site in contrast to uncoated nanoparticles, along with better endothelial penetration and transport capabilities [100]. A recent study developed neutrophil-like cell-membrane-coated mesoporous Prussian blue nanozyme (MPBzyme@NCM) for targeted therapy in ischemic stroke. This scaffold effectively crosses the BBB by actively binding to the inflamed microvascular endothelial cells of the brain. Effective accumulation of nano-scaffold was observed in the damaged brain and was efficiently phagocytized by the brain microglial cells to produce long-term benefits. In addition to this, the developed nanozyme also induced microglial polarization, neutrophil recruitment, decreased neuronal apoptosis, and neural stem cell, precursor cells and neuronal cells proliferation [101]. This application of neutrophil cell membrane to cross the BBB also opens avenues for PD. These cells can migrate to injured and inflammatory sites, making them desirable for targeting the neuroinflammatory processes associated with PD. By utilizing the WBC membranes, their homing abilities can be inherently exploited to deliver the therapeutic agents directly to the brain regions. These membranes can also evade the immune system and decrease the likelihood of rapid clearance from the system. In addition to this, WBCs also secrete various neuroprotective factors which mitigates oxidative stress and inflammation. As such, these nanoplatforms represent a promising strategy for enhancing drug delivery and providing neuroprotective effects.
One of the WBC membranes includes bone-marrow-derived macrophage (BMM) membrane, which was explored in PD about a decade ago and has shown versatile results. These cellular membranes are innovative in drug delivery research particularly due to their affinity towards the endothelial cells and biological properties. In an earlier investigation, a group of researchers found that BMM were used to load a significant amount of catalase (an antioxidant enzyme) for sustained release over 5–7 d in the MPTP-intoxicated murine model of PD. To effectively eliminate the issue of early degradation, the nanoscale block ionomer complex was used to package the catalase enzyme which further decomposed microglial hydrogen peroxide upon activation by tumor necrosis factor-alpha (TNF-α) or nitrated alpha-synuclein (N-α-syn). Interestingly, substantial amounts of catalase were released and found to accumulate in the mice's brains after the implantation of the nanozyme-laden BMMs [16]. This study provided essential insights into the loading capacity of the carrier, the neuroprotective effects of the nano-scaffold, and the safety cellular study. This laid the groundwork for a cell-mediated delivery system for PD therapeutics. Further, to improve the given delivery system, researchers developed polyion complexes obtained through the coupling of different block copolymers. This resulted in the formation of an insoluble polyion core, which positively modulated the uptake, viability, kinetics and release studies, contributing to development of optimal carrier for PD [102]. All these reports suggest the biomedical advantages of using WBCs derived membranes presents a wide variety of applications with effective abilities of immune evasion, inflammation resolution, elimination of toxins and tumor targeting.
2.2.7. Platelets cell membranes
Thrombocytes or platelets originate in the bone marrow and lack a nucleus. They help in maintaining homeostasis and accumulate at the site of injury, initiating the healing process by forming clots. The membranes isolated from these cells are novel carriers for drugs, treat immune thrombocytopenia and various types of cancers [100,103,104]. In an initial study, platelet-derived membranes were used to coat the PLGA-NPs, which maintained the surface marker integrity and functionality of immunomodulatory and binding proteins. With the assistance of membrane glycoproteins, these NPs were found to bind with human-type IV collagen. In contrast to uncoated NPs, macrophage uptake was decreased, forming stable and biocompatible material. These NPs were loaded with docetaxel for the intervention of restenosis following angioplasty in vivo settings. They were specifically guided to the damaged vasculature and remained stable for 5 d [105]. In another study, verteporfin loaded into PLGA-NPs and encapsulated by platelet-derived membrane was used to establish a link between ROS and tumor cells, fighting the melanoma without damaging the skin [104]. In a different study, doxorubicin and melanin-loaded NPs coated with platelet membrane were developed and functionalized with arginyl-glycyl-aspartic-peptide for better immunological evasion property and recruitment of the peptide towards the αvβ3 integrin, thereby improving the targeting of tumor vasculature [106].
Platelet-based nanoplatforms have not been extensively explored but represent an emerging and promising strategy for targeted therapy in various disorders. They could potentially be applied in PD due to their multifunctional features, including natural ability to target sites of injury and inflammation. Since neuroinflammation plays a key role in the progression of PD, their ability to cross the BBB under stress conditions also makes them a suitable candidate to be explored in PD. In a recent investigation on intracerebral hemorrhage (ICH), platelet-membrane-modified-polydopamine NPs (Menp@PLT) were developed, demonstrating high targeting efficiency toward the hemorrhage site. These nano-scaffolds have ROS-scavenging properties, alleviate the neuroinflammation microenvironment of ICH, reduce the hemorrhage volume, and repair the blood vessel injury [107]. This demonstrates that the platelet-based nanoplatforms have a high tendency to reach the different brain regions, evade the immune responses, and potentially modulate the neuroinflammation, opening broader possibilities for effective drug delivery designs in PD treatment.
2.2.9. Endothelial cell membranes
These are the major cells for maintaining the integrity of healthy vasculature. For instance, Fe3O4-NPs have been employed, encapsulated with HUVECs derived cell membrane, and were well internalized and directed with the help of magnetic field [108]. As a result, a variety of drug delivery nanosystem designs can be developed using this method. BBB is a complex, dynamic, multicellular barrier mostly composed of endothelial cells [109]. Vascular endothelial cells are the component of BBB, making them an excellent source for the production of CMNPs, which is also attributed to their self-targeting property [110]. Few investigations have been carried out for CNS targeted delivery using vascular endothelial cells membranes to fabricate NPs. For instance, dihydroartemisinin (DHA) was formulated as DMSN-DHA@BMECM, encapsulated with vascular endothelial cells membranes, against the experimental cerebral malaria (CM), and for specific targeting, plasmodium falciparum RBCM protein 1 (PfEMP1) was used which is highly expressed on the infected RBCs [111,112]. As a suitable candidate capable of crossing the BBB, endothelial cells can be further explored for the application in PD, as these membranes can mimic the natural cellular interactions and directly deliver therapeutic agents to neuronal cells potentially enhancing the therapeutic efficacy.
2.2.10. Hybrid cell membranes
In recent times, multifunctional hybrid cell membranes have been developed to fabricate NPs that utilize multiple cell membranes, and their hybridization is carried out for unique biological functions. Various hybrid cell membranes have been used such as dendritic and cancer cell [113], platelets, and cancer cells [114], RBCs and cancer cells [115], and neutrophils and macrophages hybridized membranes. These scaffolds inherit the advantages of multiple membranes and overcome the disadvantages of individual cell membranes. For instance, platelet cell membranes having a better immune evasion activity when combined with cancer cell membranes improves the homologous targeting and reduces the drug clearance by the immune system [114]. More importantly, these cell membrane-based nanoplatforms mimic the surface functions and features of the source cells, having a major impact on the therapeutic efficacy. In a very recent study, a novel cell membrane-based design was developed by hybridizing the chemokine (C—C motif) receptor 2 (CCR2) and platelet membranes to overcome the BBB and target neuroinflammatory lesions. This bio-engineered scaffold was explored in the 5xFAD mice. This bio-engineered scaffold was loaded with rapamycin and 1‐trifluoromethoxyphenyl‐3‐(1‐propionylpiperidin‐4‐yl) urea (TPPU). Administration of these drug-loaded bioengineered scaffolds has demonstrated a significant reduction in cognitive impairment, neuroinflammation, and amyloid plaque deposition [116]. These membranes can be versatile platforms for PD. They can effectively target the specific brain regions where dopamine neurons are degenerating and enhance the overall drug delivery efficiency. The hybrid cell membranes can be developed in a way that they may mimic the natural properties of the brain cells and neurons, which can selectively cross the BBB and these membranes can be developed in a way that can help mask the NPs from the immune system maintaining the sustained release of the nano-formulation. By combining the membranes from different cell sources, researchers can also develop the formulation with multiple functionalities, such as delivering neuroprotective agents alongside dopamine precursors to address various aspects of PD pathology. The only things to be considered are the targeted delivery, high loading capacity, and thorough safety testing.
2.3. Basic layout of synthesis and characterization of membrane-coated nanoplatforms
The main process for well-synthesized and characterized membrane-coated NPs includes extraction of membrane fragments or vesicles followed by fusion of these membranes to the NPs and finally characterization of the resulting scaffold. For instance, RBCs are devoid of nuclei, making their isolation process straightforward. During processing, RBCs are cleared out of PBS, serum, buffy coat and hemoglobin through high-speed centrifugation, followed by sonication and extrusion through a 100 nm polycarbonate membrane to get definite vesicles [121,122]. However, in the case of other eukaryotic cells such as cancer cells, WBCs and stem cells, the membrane extraction processes are quite complex due to their intricate composition, which mainly involves the culture of isolated cells, further treating them to hypotoniclysis, mechanical rupture and discontinuous sucrose gradient centrifugation to remove cytoplasm and nuclei for pure membrane isolation [62,123]. Subsequent centrifugation, sonication, and membrane extrusion remain consistent [124]. Once the membranes are isolated, core NPs are synthesized for the fusion process [125]. The major fusion methods include the co-extrusion process, sonication, microfluidic electroporation and cell membrane-templated polymerization. In the co-extrusion method, vesicles along with a NP solution are co-extruded, and the mixture is sonicated for a few minutes. Extrusion is responsible for generating the force that disrupts the integrity of the membrane and enabling it to coat the NPs [126]. The major challenge with this method is scaling up production. On the other hand, sonication utilizes ultrasonic energy at certain frequencies for nanovesicles. The amplitude of the ultrasound plays a critical role in the fusion of the co-incubated vesicles and NP core [127]. Microfluidic electroporation is carried out in a microfluidic chip, where pores are created in the cell membrane by the generated electromagnetic energy. This method is particularly advantageous for preserving NP stability [128]. A relatively newer technique, cell membrane-templated polymerization, exploits the interfacial interactions between the cell membrane and the polymeric NP core. Here polymeric cores are used to manipulate the NP size and carry out the efficient coating [129]. Although sonication and extrusion are commonly used, recent studies have revealed limitations in these conventional methods. For instance, investigation has demonstrated that the final fusion product often contains partially coated NPs, and source cells internalize only 40% of these partially covered NPs, reducing targeting and therapeutic efficacy. To address these drawbacks, recently an investigation reported ways to ratiometrically enhance the full cell membrane coating for better targeting efficiency. They found that limited membrane fluidity results in the formation of cell membrane patches and fails to fuse properly. Thus, understanding the crucial role of cell membrane fluidity for the final fusion product, the investigators utilized the external phospholipid to tune the membrane fluidity. This led to better internalization and targeting of the biomimetic NPs [130].
To characterize cell membrane-coated NPs, standard physicochemical methods are carried out, such as dynamic light scattering (DLS) and transmission electron microscopy (TEM). For instance, RBCs coated NPs illustrate ∼8 nm increase in size due to the thickness of the RBCMs. Additionally, surface charge is also an essential indicator of these NPs [131]. Usually, zeta potential depicts the surface charge of the respective vesicle membrane [125]. Antibody tagging for cell membrane-coated NPs is often analysed with the help of flow-cytometric analysis [131]. Other confirmatory analysis methods are also performed, including western blotting and SDS-PAGE, depending on the type of membrane used [132]. Most of the time, universal procedures for characterization and preparation are adapted which might sometimes require minor modifications.
2.4. Significance of cell membrane coated biomimetics in targeted therapies
The brain serves as the body's central control system, regulating essential functions including respiration, heartbeat, cognitive functions, and emotional tasks. As one of the most versatile organs, the brain's intricate architecture and diverse functionalities make its protection critically important. The BBB plays a pivotal role in this protective mechanism. This physiological marvel defends the neural environment from environmental toxins and pathogens. But the characteristics that make BBB a safeguard also create significant therapeutic challenges [133]. BBB is formed by tightly packed endothelial cells with tight junctions, which restrict paracellular transport and prevent the passage of large or hydrophilic molecules into the brain. While only small and lipophilic molecules pass freely, essential nutrients like amino acids and glucose are transported via specialized carrier proteins. The BBB exhibits plasticity, adjusting its permeability in response to physiological changes or pathological conditions. This dynamic nature influences drug delivery efficacy [134]. Over 98% of small-molecule drugs and nearly all large-molecule therapeutics struggle to cross BBB effectively, necessitating innovative strategies to enhance drug delivery [134].
The intricacies of BBB restrict the entry of anti-PD medications. As a result, despite many drugs, such as matrine and curcumin, showing promising anti-PD effects in pre-clinical settings, they have not demonstrated clinical efficacy at clinical levels. Significant efforts are underway to develop strategies that facilitate drug passage across the BBB [135]. However, in chronic conditions like PD, alterations in BBB integrity occur [136], posing opportunities in drug delivery. Cellular components, including stem cells and immune cells, are activated and recruited in the region of post-injury lesion. Inside the lesion vicinity, a chemokine concentration gradient was developed, and the deformation of the recruited cells enables them to cross the endothelial cell slit and enter the brain region [137]. Various receptor-ligand interactions occur for the arrest step, to attain high affinity conformation for the deformation process. Furthermore, matrix metalloproteinases are secreted by cells to degrade extracellular matrix and basement membrane for invasion and BBB crossing through TJs fenestrations [138]. When BBB integrity is compromised, there is a notable increase in pinocytotic vesicles within brain microvascular endothelial cells (BMECs). On average, injured BMECs contain 25 pinocytotic vesicles, which range in size from 70 nm to 200 nm [139]. Additionally, the capacity for phagocytosis and transcytosis of damaged BMECs rather than through the compromised tight junctions [140]. Recent studies indicate that endocytosis and transport mechanisms in injured BMECs may be the primary pathways for cell membrane-based biomimetic vehicles to traverse the BBB. However, it has been observed that these vehicles struggle to cross the BBB even when it is temporarily opened using hypertonic solutions. Therefore, the processes of recruitment, cell recognition, intracellular transport, and BBB crossing may operate through different mechanisms. Currently, research on targeting mechanisms of cell membrane-based biological vehicles focuses primarily on their recognition by BMECs, transport processes, and targeting interventions for diseased cells [141].
Acute encephalopathies associated with disorders such as glioblastoma (GBM) and ischemic stroke are responsible for the disruption of dynamic BBB integrity [142]. However, chronic encephalopathies associated with disorders such as PD, Huntington’s disease (HD) and Alzheimer’s disease (AD) degrade the BBB integrity very slowly and do not present targets with major variations, which generates hurdles in designing target-specific delivery systems [143]. Different mechanisms adopted by these biomimetics to enter the biological interfaces have been illustrated in Fig. 3. Cell-membrane-based biomimetics cannot sense changes in chemokine concentrations and may arrive at the lesion site through passive transport after systemic administration. Major cellular events for CMNPs include recruitment, cell recognition, intracellular transport and BBB crossing. Currently, brain targeting mainly focuses on cell memory-based bio-vehicles. Although RBCs are commonly used biomimetic vehicles, they lack intrinsic brain-targeting capability, so their applications are dependent on the modification by targeting moieties [144]. In contrast, the targeting properties of stem cells and immune cells depend on their systemic homing ability, which follows a cascade of tethering, rolling, activation, arrest and transendothelial migration. Certain factors play an essential role in the trafficking and recruitment of WBCs and stem cells, such as CXCR4/stromal cell-derived factor. Recently, a study demonstrated that upregulated CXCR4 expression on MSC membranes was converted to cell-membrane derived biomimetic, which showed enhanced brain-targeting efficiency in the mice model of MCAO [145,146]. However, protein translation and intracellular signal transduction are necessary for cell migration following the interaction of CXCR4 and SDF1 molecules on the cell membrane. The intracellular process is not necessarily complete even if the cell membrane-based biomimetic over expresses the CXCR4. Thus, more investigations are required to explore its targeting mechanism [147]. Cell membrane-based biomimetics are at the nano-meter size level and mostly transferred via endothelial cell transport. Some investigations suggest that this process might depend on the injured BMECs by recognition, internalization, and finally transportation [148]. Adhesion molecules including vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) are majorly expressed in the injured BMECs which provides a suitable target for inflamed BBB [149]. Very late antigen-4 (VLA-4) is the naturally expressed ligand for VCAM-1. Therefore, a target delivery vehicle is grafted after the fusion of liposomes to the stem cell membrane. These biomimetics are mainly taken up via clathrin-mediated endocytosis and micropinocytosis, which is dominant for the uptake of BMECs post-inflammatory insult. However, the fate of cell membrane-based biomimetics remains unclear, and further research is needed to validate their brain-targeting capacity. Most existing studies have employed the synthetic membrane or in vitro evidence, but physiological evidence is still limited. A recent investigation demonstrated a dual-targeting strategy for tuberculosis by encapsulating polymeric cores with mycobacterium-stimulated macrophage membranes with a photothermal agent excitable with a 1064 nm laser. This specific biomimetic expressed mycobacterium tuberculosis-related receptors enabling them to target granuloma, which demonstrated alleviation in pathological damage and inflamed lungs resulting in improved therapeutic efficacy [150].
Fig. 3.
Brain targeting mechanism of cell membrane-based biomimetics which includes lipophilic transcellular pathway, receptor-mediated transcytosis, transporter-mediated transcytosis, adsorptive-mediated transcytosis, functionalized-carrier mediated pathway. Recent advancement and biomedical application of bioinspired nanoplatforms.
Renowned physicist Richard Feynman first introduced the concept of nanotechnology in 1959 during his presentation [151]. NPs have emerged as crucial components in modern medicine, finding applications as targeted delivery in neurodegenerative diseases, contrast agents in imaging, and carriers for drug and gene delivery into tumors. Their utilization enables novel analyses and therapeutic interventions that were previously unfeasible [152]. The NPs are designed to control surface properties and particle size, and to target specific delivery to achieve the desired therapeutic response [13]. The disadvantage of NPs in targeted drug delivery is the potential for unintended toxicity while the goal of targeted delivery is to enhance the specificity of drug action, the unique properties of NPs, such as their small size and increased surface area, may lead to unexpected interactions with biological systems. These interactions could trigger immune responses or cause adverse effects on healthy tissues, compromising the overall safety of the delivery system. The challenge lies in carefully engineering NPs to achieve effective targeting without inducing harmful side effects, emphasizing the need for thorough preclinical and clinical evaluations to ensure the safety and efficacy of NP-based DDSs [153].
Due to the various disadvantages associated with synthetic NPs, biomembrane NPs can be a better approach for targeted delivery. Biomembrane NPs, also known as biomimetic NPs, represent a novel approach in nanotechnology that leverages natural cell membranes' structural and functional attributes. Recently, different cell sources such as blood-derived cells, immune cells, bacterial cells and even cancer cells have been explored for developing biohybrids having versatile functionalities. These NPs are designed to mimic the composition and properties of biological membranes, offering unique advantages in various applications. One notable application is in drug delivery, where biomembrane NPs can enhance biocompatibility and reduce immunogenicity, improving their interactions with biological systems. Additionally, biomembrane NPs may facilitate targeted drug delivery by incorporating specific targeting molecules that recognize and bind to receptors on the surfaces of target cells. This biomimetic approach holds promise in overcoming some of the challenges associated with traditional NPs, such as potential toxicity and immune responses [154]. By utilizing the specific features of cell membranes such as tissue binding affinity, immune escape, prolonged circulation time and anti bioadhesion, the effects of synthetic materials can be masked [155].
CMNPs are expected to offer advantages in drug delivery tools, bioimaging, and cancer therapeutics. However, always using the conventional systems of cell membrane coating may limit functional diversity. To address this, the incorporation of mixed types of cell membranes or new membrane sources, such as different cell or bacterial membranes, is suggested [156].
3. Cell-membrane camouflaged biomimetics for PD
PD is an age-related neurodegenerative disorder attributed to the selective loss of dopaminergic neurons in substantia nigra causing significant brain inflammation, secretion of neurotoxins and microglial activation [154]. Abnormal functioning of dopaminergic neurons in the brain results in hallmark symptoms of PD including tremor, postural imbalance, bradykinesia and rigidity, primarily neuronal death in the substantia nigra. Dopamine, a crucial neurotransmitter for movement, transmits messages within the brain for smooth functioning of muscles and purposeful movements. Another fundamental factor believed to play a crucial role in the development of PD is alpha-synuclein located in the presynaptic terminals and is important for the normal functioning of the brain. The build-up of abnormally folded protein aggregates results in the development of PD [157]. Neuroinflammation associated with PD is characterized by an inflammatory response within the brain. Pro-inflammatory cytokines are released by the hyperactivated microglia causing neuronal death and enhancing neurodegeneration within the CNS [158]. Clinically, controlling the disease mainly includes drugs such as dopaminergic agonists, glutamate antagonists and anticholinergics [[159], [160], [161], [162], [163]]. Levodopa is the first-line treatment for PD used to restore dopamine levels. To prevent peripheral metabolism, it is usually given along with carbidopa. However, resistance to levodopa has also been reported frequently. Other commonly used therapies include dopamine agonists such as ropinirole and pramipexole, which are taken alone or in conjunction with levodopa. However, their long-term use also comes with several side effects. In conjunction with MAO inhibitors (e.g. selegiline, rasagiline) are also commonly used in the early stages of PD, which are often along with the levodopa. But limited efficacy is reported in their case as well. Recently, surgical interventions have been employed involving implanting electrodes in specific regions of the brain which are known to modulate the neural activity and often used in advanced PD stages where the medications become less effective. Reversible therapy often requires careful patient selection and management [164]. Magnetic resonance-guided focused ultrasound ablation has surfaced as a newer approach for PD where tremors are the predominant symptom. This is a non-invasive technique for more precise targeting of lesions in the thalamus [165]. However, current treatments only halt the progression of the disease; none therapy primarily act on the disease modification strategy or addresses the underlying disease mechanism. The major obstacle is the BBB whose compact physiological structure restricts drug delivery (Fig. 4). To overcome this, various strategies have been laid down in the last few years. Intrathecal and intracerebroventricular administration of drugs and maintaining concentrations in the brain are adopted, but limited due to their invasive nature [166].
Fig. 4.
The existence of the blood-brain-barrier poses challenges in treating CNS disorders, as the majority of chemical drugs and biopharmaceuticals encounter obstacles in crossing this protective barrier to enter the brain.
Nanocarriers have commonly been employed to reduce peripheral degradation and improve the specific targeting of the brain. Recently, extracellular vesicles have emerged as an effective drug carrier. These are the lipid-membrane-enclosed nanometer-sized vesicles secreted by most cells composed of various nucleic acids, proteins and lipids of the parent cells, which are heterogeneous and mainly classified as microvesicles, exosomes and apoptotic antibodies [167]. Certain lipids such as sphingomyelins, glycosphingolipids, phosphatidylserines, phosphatidylethanolamines, cholesterol and phosphatidylcholines are more abundantly present in the extracellular vesicles than the plasma membrane [168]. Furthermore, various proteins like annexin II, integrins, heat shock proteins (HSPs) and tetraspanins are also more enriched in the extracellular vesicles [169]. These cell-specific proteins may vary depending on the source of the parent cell. Therefore, by mimicking the natural cellular processes, these vesicles can be engineered to deliver therapeutic agents directly to the affected neurons, enhancing DDSs and improving treatment outcomes. Table 2 summarizes the different cell membrane-based biomimetics for drug delivery in PD. The major drawback in drug delivery is the lack of specificity and rapid clearance by the immune system. A broad range of strategies for systemic delivery of therapeutic moieties have been explored, such as targeting sequence conjugation including aptamers, peptides and antibodies [32]. These targeting motifs are based on the structure of unique components of cellular compartments of interest. But another issue arises during large scale-ups especially when more than one targeting sequence is employed, in which case specificity can be hindered [32]. Additionally, it is critical to identify the targeting molecules for various cell types, making drug delivery not a straight-forward process.
Table 2.
Summary of cell membrane-based biomimetics used for drug delivery in PD.
| System | Cell type of membrane | Brain penetration and targeting efficiency | Drug loading | PD model Brain targeting strategy |
Ref. | |
|---|---|---|---|---|---|---|
| Curcumin nanocrystals | RVG-modified RBC | Cyanine 5 (Cy5) tagged Cy5@RVG 29-RBCm/ CurN CS primarily accumulated in brain in comparison to liver& kidneys -Long circulation time (15.236±1.340 h); sustained release of curcumin levels (7.500 ± 1.000 h); high peak curcumin levels (0.125±0.015 µg/g); high AUC0-t values (3.550±0.332µg·h/g); high mean residence duration (21.841±1.777 h) |
5.13% ± 0.31% | Mice MPTP; SH-SY5Y MPP+ model |
RVG-29 | [117] |
| Curcumin loaded liposome | Natural killer cell | Signal in the brain after administration remained in the brain until 36 h higher relative to liposomal curcumin | 12.6%±0.3% | Mice MPTP, SH-SY5Y MPP+ model | MLV | [118] |
| Mesoporous silicon-loaded S-Nitro glutathione NPs | RBC | Compared to the UCMG group, RBCM/UCMG group efficiently crossed BBB within 2 h and was able to concentrate in brain | ∼12.50% | Mice MPTP model | CD47 protein | [2] |
| Quercetin loaded-PVP NPs (CSPQ) | Neuronal cell | Staining and focused ultrasound demonstrated the accumulation of NPs in the substantia nigra and striatum of the brain | 17.6% | Mice MPTP, SH-SY5Y MPP+ model | Ultrasound | [119] |
| Curcumin loaded-PVP NPs (CSCCT) | Macrophage | Efficiently accumulated in neuronal mitochondria with the assistance of focused ultrasound | 16.5% | Mice MPTP, SH-SY5Y MPP+ model | DSPE-PEG2000-TPP | [120] |
| SC79 loaded Cu2−xSe-PVP NPs (CSS@CM NPs) | RAW 264.7 cell | Staining and focused ultrasound demonstrated accumulation of NPs in substantia nigra of the mice brain | 7.1% | Mice MPTP model | Cu2−xSe NPs | [141] |
Recent investigations have focused on the coating drug-delivery scaffolds with the physiological cell membranes in which the whole scaffold corresponds to the lipids, carbohydrates and protein composition of the cell membrane. These membrane-cloaked drug delivery materials exhibit properties of the source cell [32,44,[170], [171], [172]], and have exhibited better and prolonged residence time and disease-relevant specificity when conjugated with the desired targets [67]. It is interesting and holds significant potential to coat NPs with mammalian cell membranes. These mini physiological moieties can selectively recognize and target specific cell compartments, certain cell types, and even tissues. These specialized NPs can reduce toxicity, allergic reactions, and immune reactions for PD associated drug delivery [173]. In a recently developed targeted therapy for PD, to by-pass the limitation of BBB, a group of researchers proposed the delivery of curcumin liposomes encapsulated in a membrane of natural killer cells, forming a biomimetic nano-complex named BLIPO—CUR. This complex was delivered by meningeal lymphatic vessel (MLV) route via subcutaneous injection in the neck region of MPTP mice. Through this route, the biomimetic liposomes escape the macrophage-based phagocytosis and effectively recognize the damaged neurons. Moreover, BLIPO—CUR efficiently cleared α-synuclein and ROS and enhanced the expression of toll-like receptor 4 (TLR4) on the natural killer cell surface. Moreover, in vivo, images illustrated the delivery of BLIPO—CUR to the mice's brain and compared subcutaneous and intravenous routes. This highlights the importance of the natural killer cell membrane for effective amelioration of PD signs, with better effects observed when the meningeal lymphatic route was adopted for the delivery [118]. In another investigation, researchers modified the UCNPs surface with the RBCM for effective brain targeting. UCNPs were used to coat the mesoporous silicon and S-nitrosoglutathione as a source of NO donor. NO at appropriate concentrations can promote neuronal growth and decrease cell apoptosis and inflammation [174]. Thus, in this study, it was hypothesized that Ln3+-doped UCNPs could be used for the efficient conversion of NIR light to higher-energy green light, causing excitation at 540 nm of NO donor as shown in Fig. 4. This resulted in the light-mediated anti-inflammatory response and a decrease in the levels of pro-inflammatory factors in brain [2].
In a more recent report, investigators used a 29-amino acid-based rabies virus polypeptide-modified RBCM. These nanodecoys were designed to encapsulate curcumin nanocrystals. The RVG29-RBCm/Cur-NCs nanodecoys efficiently bypassed the reticuloendothelial system (RES) uptake, effectively improving the BBB penetration and residence time, with improved dopamine levels, decreased aggregated α-synuclein and mitigation of mitochondrial dysfunction associated with PD in mice model. The illustrated overview is shown in Fig. 5 [117].
Fig. 5.
(a) Schematic illustration of the synthesis of RBCM/UCMG; (b) By utilizing the surface CD47 protein that modifies the RBCM to exert immune evasion, UCNPs could rapidly pass through the BBB and target brain lesions. Adapted with permission from [2], Copyright© 2023 American Chemical Society.
In another study, novel biomimetic NPs (Cu-xSe-PVP-Qe) were designed containing quercetin loaded poly (vinylpyrrolidone) (PVP) NPs cloaked with neuronal cell membrane denoted by CSPQ@CM NPs for ameliorating PD by effectively targeting and modulating microglia. This investigation revealed that biomimetic CSPQ@CM NPs were directed toward the microglia via precise interactions between α4β1 integrin and vascular cells present on the surface of the membranes adhering to microglia-expressed molecule-1. MES23.5 substantia nigra dopaminergic neuron cell membrane was used to camouflage NPs. The mean size as per the TEM image was found to be 24.7 nm, while the hydrodynamic size was found to be 78.8 nm as shown in Fig. 6b. In addition, the protein profile showed efficient retention of proteins. As demonstrated by BV2 cells, coating of NPs not only improves biocompatibility but also enhances endocytosis of the formulation. Fig. 6d–6e demonstrates that FITC-labelled NPs were phagocytosed better by BV2 cells than NIH-3T3 cells. Additionally, enhanced expression of VCAM-1 and α4β1 integrin shows their strong binding affinity, high accumulation and better targeting efficiency in BV2 cells. Quantification of the amount of NPs in the cells further proved the targeting capacity and retention in cells as shown in Fig. 6i. This highlights the advantage of biomimetic NPs for brain-targeting therapy in PD [119].
Fig. 6.
Modification of CSPQ NPs with MES23.5 cell membrane (i.e., CSPQ@CM) for targeting microglia. (a) Schematic illustration of the preparation of CSPQ@CM NPs. (b) TEM image of CSPQ@CM NPs. (c) Proteins determined by SDS-PAGE electrophoresis assay of (1) MES23.5 cell lysates, (2) MES23.5 cell membrane and (3) CSPQ@CM NPs. (d) CLSM images and (e) fluorescence distribution and intensity in NIH-3T3 and BV2 cells after incubation with different concentrations of CSPQ@CM NPs. The cell membrane was labeled as FITC. (f, g) Integrin α4 expressed by BV2 cells and (h) VCAM-1 expressed by MES23.5 cells detected by immunofluorescence staining and observed under a laser scanning confocal microscope. (i) Cu concentration in the same number of cells after culturing with 0.2 mM CSPQ or CSPQ@CM NPs. Adapted with permission from [119], Copyright© 2020 American Chemical Society.
Similarly, an investigation developed a macrophage cell membrane cloaked Cu2−xSe-SC79 nano biomimetic (denoted as CSS@CM NPs) where the source of the macrophage cell membrane was RAW 264.7 macrophage cells, and SC79 is a specific unique agonist of Akt phosphorylation. SC79 was used to potentially target the neuronal damage by reducing neuron excitotoxicity. However, its precise brain delivery is a barrier to its therapeutic efficacy. Thus, Cu2−xSe-SC79 was used as a carrier for SC79 due to its ultrasmall nature, and the release of copper ions can significantly facilitate neuronal proliferation and migration, which activates the Akt and ERK pathway. Therefore, CSS@CM NPs could significantly enhance neurite outgrowth and interactions of α4β1-VCAM-1, and reduce neuronal apoptosis and synaptic dysfunction in PD mice brain regions, such as substantia nigra and striatum [175].
Thus, there is a wide application of cell membrane-cloaked biomimetics for PD. This highlights a new era in targeted delivery systems. These biological deliveries are attractive candidates for precise delivery in the brain. The only drawback we are facing is related to the clinical translation of these bioscaffolds, which includes the scale-up processes, integrity and membrane coverage of NPs that needs to be addressed at its earliest.
4. Biomedical applications in neurodegenerative diseases
Biomimetic NPs represent a cutting-edge approach to biomedical applications, offering innovative solutions for various medical challenges. One particularly promising avenue is their application in the realm of neurodegenerative disorders, where the need for targeted and effective therapeutic interventions is paramount. Neurodegenerative diseases, such as AD and PD, pose significant challenges due to their complex etiology and the limited success of conventional treatments. Biomimetic NPs, designed to mimic biological entities' structural and functional aspects, present a revolutionary strategy to address these challenges [176]. These NPs, inspired by the intricate design of biological systems, exhibit unique properties that enhance their interaction with biological entities. In the context of neurodegenerative disorders, the biomimetic approach holds great promise for delivering therapeutic agents to specific regions of the brain with precision and efficacy. One noteworthy application involves using biomimetic NPs for drug delivery, enabling the targeted release of neuroprotective agents to combat the progression of neurodegeneration. Mimicking the natural mechanisms of cellular uptake, these NPs can traverse the BBB, ensuring that therapeutic payloads reach the affected areas of the brain [177].
Biomimetic NPs can be engineered to replicate the functions of endogenous cells, offering a multifaceted approach to neurodegenerative disorders. For instance, synthetic NPs can mimic the behavior of glial cells, playing a crucial role in maintaining neuronal health and function. By integrating features such as surface receptors and signaling molecules, these NPs can modulate the neuroinflammatory response, a key contributor to the progression of neurodegenerative diseases. This biomimetic modulation of the immune system can help attenuate the damaging effects of chronic inflammation on neural tissues. Various neurodegenerative disorders, including PD, AD, HD and multiple sclerosis (MS), will be elucidated. The exploration will delve into their distinct pathophysiological mechanisms and potential biomarkers, fostering a comprehensive understanding of each disease [141]. Not only in neurodegenerative disorders, but these biomimetics have also shown promise in other neurological disorders. For instance, recent research has focused on the role of targeting miRNAs in GBM. In this study, miRNA-nanosponge was developed and coated with BV2 cell membrane for crossing BBB which specifically targets miRNA-221, miRNA-215, miRNA-21 and miRNA-9 to modulate the immune system and inhibit GBM progression [178]. This highlights the importance of biomimetics in neurological disorders.
4.1. Parkinson's disease (PD)
PD is a progressive neurodegenerative disorder characterized by the selective loss of dopaminergic neurons in the substantia nigra pars compacta, a critical region of the brain responsible for motor control [179]. First described by James Parkinson in 1817, the pathophysiology involves the accumulation of misfolded alpha-synuclein protein in neuronal cytoplasmic inclusions, known as Lewy bodies. The resultant dopamine depletion in the basal ganglia leads to the cardinal motor symptoms of tremor, bradykinesia, rigidity and postural instability. While the etiology remains multifactorial, genetic predisposition and environmental factors are implicated in the complex interplay contributing to the disease's onset. Present therapeutic approaches focus on alleviating Parkinson’s symptoms through a combination of pharmacotherapy and surgical interventions. Furthermore, the existing treatment options continue to pose significant challenges, prompting the exploration of novel approaches for DDS development [180].
Investigation done by Liu, et al. on a novel therapeutic approach for PD was explored through the development of brain-targeted biomimetic nanodecoys, specifically RVG29-RBCm/Cur-NCs. The goal of this investigation was to increase the residence time in the systemic circulation, cross the BBB, and increase the anti-PD drug’s bioavailability. RVG29-RBCm/Cur-NCs were successfully introduced in the BBB, accumulated on neurons by binding to nAChR receptors, and demonstrated effective neuroprotective effects in a PD mouse model induced by MPTP/MPP+. These effects manifested as improvements in motor behavior, mitigation of the pathological decrease in TH+ neurons, inhibition of abnormal α-synuclein aggregation, elevation of dopamine levels, and reversal of mitochondrial dysfunction in the brain. Additionally, RVG29-RBCm/Cur-NCs exhibited commendable biocompatibility. The outcomes of this study highlight RVG29-RBCm/Cur-NCs as a promising strategy for targeted drug delivery to the brain in the context of PD and other neurodegenerative diseases [117].
In another study, the investigator engineered a therapeutic platform utilizing UCNP responsive to 980 nm NIR light, allowing controlled release of NO for the treatment of inflammatory PD. This system, enclosed within a cell membrane, demonstrated efficient penetration of the BBB and precise delivery to the inflamed region in the brain. Activation of UCNPs by NIR prompted the release of NO, swiftly diminishing proinflammatory factors in the affected area and achieving a therapeutic outcome [118].
Another study done by Liu et al. created biomimetic NPs based on ultrasmall Cu2–xSe and demonstrated their efficacy in targeting and modulating microglia to enhance PD therapy. These Cu2–xSe NPs, termed CSPQ, were endowed with exceptional multienzyme-like activities through modification with quercetin. Furthermore, they encapsulate them with the membrane of neuronal cells (MES23.5 cells), resulting in CSPQ-CM NPs with superior targeting capabilities and specificity. Studies reveal that the abundant expression of VCAM-1 on MES23.5 cell membranes facilitated the specific targeting of CSPQ-CM NPs to microglial α4β1 integrin through robust α4β1-VCAM-1 interactions. Additionally, these NPs induced microglial polarization toward the M2-like phenotype, exerting an anti-inflammatory effect by reducing neurotoxic IL-6 and increasing neuroprotective IL-10. Utilizing focused ultrasound assistance, CSPQ-CM NPs could efficiently and precisely reach the substantia nigra and striatum of the brain, effectively modulating microglia, and the brain environment by eliminating ROS. This led to increased dopamine levels in the cerebrospinal fluid, elevated expression of tyrosine hydroxylase, and reduced expression of IBA-1. The significant alleviation of PD symptoms, along with improvements in dyskinesia and cognition impairment in PD mice [119].
4.2. Alzheimer’s disease (AD)
AD is a progressive and incurable brain condition that gets worse over time. It causes problems with memory, thinking and behavior. People with AD may struggle with short-term memory, everyday activities, and how they see things and make decisions. There are also rare types of AD, like primary progressive aphasia, posterior cortical atrophy and the frontal variant of AD, which have different symptoms but still affect memory [181]. AD stands as the predominant form of dementia, constituting 60%–70% of all cases. Its incidence rises with age, affecting 10% of individuals aged 65 and older, and ∼50% of those aged 85 and above. Although less common, AD can also manifest in younger individuals, occasionally in their 20 s, often linked to genetic abnormalities. Projections estimate that the global prevalence of AD will reach 131 million by 2050, with the highest burden expected in middle-income and low-income countries. AD poses a significant societal and economic challenge, with annual healthcare costs rivalling those of cardiovascular disorders and exceeding those of cancer. It ranks as the 4th to 5th leading cause of death. To date, there is no curative intervention capable of slowing disease progression [182].
Some studies have shown the therapeutic use of biomimetic NPs. A study by Gao et al. focused on biomimetic engineered nanosystems in AD mice. They prepared biomimetic NPs (T807/TPP-RBC—NPs) that can deliver antioxidants into neuronal mitochondria. The biocompatibility and sustained circulation of T807/TPP-RBC—NPs result from the optimal physicochemical properties of the "inner core" in conjunction with the unique biological functions of the "outer shell." The incorporation of T807 and TPP on the "outer shell" facilitates the biomimetic-engineered nanosystem’s efficient traversal across the BBB and specific binding to neurons, ultimately reaching the mitochondria. While the current research data is preliminary, it collectively implies that T807/TPP-RBC—NPs hold promise as an intravenous delivery platform tailored for targeted mitochondrial delivery in AD treatment [183]. In another study, investigators engineered and synthesized CuxO-EM-K, a nanomaterial with active Aβ-targeting capabilities, aimed at enhancing protein adsorption resistance and minimizing immunogenicity. This design facilitates the selective and effective clearance of peripheral Aβ. The incorporation of RBC membrane coating, in conjunction with DSPE-PEG-K, serves as a nanocaptor for selectively capturing Aβ in the bloodstream. Simultaneously, the CuxO enzyme plays a crucial role in alleviating Aβ-induced membrane oxidative damage and stabilizing the RBC membrane, ensuring prolonged blood circulation essential for Aβ adsorption. Notably, the RBC membrane coating prevents the formation of protein coronas, preserving the Aβ-targeting capability of CuxO-EM-K in the bloodstream. Consequently, CuxO-EM-K effectively reduces Aβ burden in both the blood and the brain, ultimately reversing learning and memory impairments in 3xTg-AD mice. Importantly, blood biochemistry and histology analyses revealed no significant systemic toxicity associated with CuxO-EM-K. In summary, the biomimetic CuxO-EM-K presented in this study provides a safe and effective approach for the peripheral clearance of Aβ associated with AD [184]. Huo et al. developed a novel approach using exosomes loaded with silibinin (Exo-Slb) to simultaneously target Aβ aggregation and astrocyte activation, aiming to alleviate cognitive impairment in mice with AD. In this investigation, Exo-Slb exhibited high selectivity in binding to Aβ1–42, effectively inhibiting its aggregation. Moreover, Exo-Slb demonstrated the ability to suppress astrocyte activation, leading to a reduction in the secretion of inflammatory cytokines. Ultimately, Exo-Slb reversed neuronal damage and mitigated cognitive impairment in the AD mouse model induced by Aβ by modulating the NF-κB pathway. In summary, the design of silibinin-loaded exosomes derived from macrophages, serving as a multifunctional DDS, holds promise as a potentially beneficial treatment for AD in the future [185].
4.3. Multiple sclerosis (MS)
Inflammation subsequently causes demyelination and gliosis-associated neurodegeneration are the two key pathological characteristics of MS. However, the damage caused due to MS is limited to the CNS [186]. Several treatments are there like ocrelizumab, ofatumumab, natalizumab and mitoxantrone, but they have side effects including infusion-related reactions, upper respiratory tract infections, oral herpes infections, and urinary tract infections are frequently reported. FNb-1a (Rebif), IFNb-1a (Avonex) and PegIFNb-1a (Plegridy) are some more drugs that are used for MS but their mechanism of action has not been discovered yet, and here the biomimetic NPs play a crucial role in the treatment of MS [187].
MS is a serious condition affecting young individuals, and dimethyl fumarate (DMF) is among the oral treatment options for this disease. While DMF effectively reduces inflammatory conditions, its various side effects pose challenges to patient adherence and increase economic burdens. In this study, a novel approach was employed, using platelet membranes (PNs) and platelet membrane-coated nanoparticles (PCCN), to create a biomimetic DDS for managing MS. In vivo, results demonstrated that these prepared NPs significantly increased DMF concentrations in the brain compared to the free drug. Moreover, notable differences were observed between conventional nanoparticles (CN) and PCCN in brain concentration and AUC0-t from the concentration-time curve. PNs exhibited a higher brain uptake clearance value than CN and PCCN, suggesting that PNs could enhance DMF penetration and accumulation in the brain. This study concludes that PNs present a promising cell-based biological approach for effectively delivering drugs used in the treatment of MS [188]. In another study, the interaction between myelin basic protein (MBP) and large unilamellar vesicles (LUV) with both native and experimental autoimmune encephalomyelitis (EAE) modified lipid compositions, investigating the subsequent liposomal assembly process. The affinity of LUV towards MBP with EAE-modified membranes was observed to be more vigorous than with innate membranes, leading to a heightened inclination for aggregation in EAE-mimicking LUV compared to native-like liposomes. While EAE-modified membranes remained stable in this aggregated state, native vesicles underwent a secondary reordering process over several days, resulting in LUV fusion and the formation of a significant amount of multilamellar structure. In investigations involving bending fluctuations of the LUV, liposomes with a native lipid composition exhibited higher bending rigidity than EAE-modified membranes. Additional experiments with oriented bilayers revealed the formation of a concentrated protein layer on top of the membranes for both lipid compositions. The surface excess of MBP on EAE lipid composition membranes exceeded that on native lipid composition membranes, indicating stronger MBP interactions with diseased membranes that caused structural perturbations. This manifested as membrane swelling and buffer insertion in the hydrocarbon section of the membrane [189]. The application and limitation of different cell membranes used to synthesize biomimetics is illustrated in Table 3.
Table 3.
Application and limitation of different cell membranes used to synthesize biomimetics.
| Membrane type | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| RBCs | Long circulation, simple approach for membrane functionalization. | Purification is time-consuming; there are proper protocols for preparation and purification | [190] |
| WBC | Regulation of inflammatory response, selectivity at specific disease site. | Complexity of WBC membrane | [191] |
| Platelets | Promising properties in treating hemorrhage and targeted delivery. | Complex purification routes | [190] |
| Exosomes | Favorable for long-distance cell-to-cell communications. | No proper standardized method to produce and isolate in the desired amount | [192] |
| Macrophage | Long circulation time, BBB penetrating ability, target specific. | Limited capacity for drug incorporation; Unfavorable or inappropriate drug release; Degradation of the drug within cells | [192] |
| T-cell | Long circulation time, targeting the specific site. | MHC restriction, targeted to limited site | [192] |
5. Challenges and future perspectives
A key challenge in utilizing biomimetic nanomaterials stems from their inherent complexity. Understanding the significance of specific molecules in shaping nanomaterial behavior is challenging due to the multitude of molecules in the entire membrane, potentially numbering in the hundreds. Few studies have endeavored to block single membrane proteins using antibodies to observe changes in particle behavior without their functional contribution. However, redundancy among proteins with similar functions or unexpected contributions from other proteins may complicate the interpretation of such studies. Investigating this heightened complexity may necessitate future efforts involving innovative techniques for a more comprehensive understanding [53]. Efforts to expand knowledge and identify essential molecules crucial for desired particle functions could pave the way for producing nanomaterials that combine single proteins found in different cell types on a single platform. This approach would eliminate the need to include excessive unwanted cellular material, resulting in simpler formulations with more reproducible features and improved scalability. Despite the predominant focus on surface proteins in current research, it is crucial to recognize that the biological function of cell membranes is not solely attributed to surface proteins. The phospholipid bilayer and the complex sugars associated with proteins and lipids also play crucial roles. Surprisingly, there is limited research on these specific membrane components, and further exploration could unveil additional possibilities for innovative biomimetic nanosystems [53]. In a recent study by Liu et al. [37], an important issue was raised regarding the coating of NPs. The study demonstrated how processes such as extrusion, sonication, and the common ratios between particles and membranes used for coating can result in formulations with only a very small percentage of particles being completely coated. Interestingly, the authors found that cells internalize partially coated NPs using different mechanisms compared to fully coated ones. This discovery holds significance for improving current approaches to biomimetic membrane-coated nanovectors. Understanding this, researchers should aim to optimize NP coverage with cell membranes. This optimization could potentially lead to better stability, a prolonged presence of NPs in the body, and increased effectiveness due to the higher biological content carried by these NPs. Most studies so far have relied on qualitative techniques like TEM imaging, zeta potential assessment, or SDS-PAGE to confirm the presence of membrane proteins on NPs. Looking forward, researchers must incorporate quantitative methods in future formulations to accurately measure the extent of NP membrane coverage.
Furthermore, cell membrane-based biomimetics come with certain key concerns in terms of stability, storage, maintaining the functionality of embedded membrane proteins, potential issues with in vivo circulation and immune response, and ultimately, translating these systems to clinical applications, which can be significantly hindered by challenges related to production, scalability and consistent quality control [193]. Membrane proteins are extremely sensitive to environmental alterations and vulnerable to losing their native structure and function when extracted from the cell membrane, requiring optimization for stabilization during storage [194]. Lipid bilayer membranes are vulnerable to degradation over a long period, which also affects the integrity and stability of the biomimetic system. Cell membranes also tend to fuse or aggregate impacting the size distribution causing aggregation and disturbing their overall functionality [193]. Another important feature is maintaining the correct orientation and conformation of the membrane proteins within the biomimetic system, which is crucial for its overall function and can certainly challenge the fabrication process [193]. Another important issue is the ligand binding and signaling. Ensuring that receptors and proteins on the membrane retain the ability to interact with specific ligands and initiate downstream signaling pathways is crucial [195]. When the source of the cell membrane is different from the host source, immune responses play a major role. The foreign membrane can trigger immune responses leading to rapid clearance. Rapid clearance from the bloodstream may also occur by the RES due to their size and surface properties [196]. Many more modifications are required to address these critical challenges. Moreover, translating the pre-clinical evidence to the clinical level has various challenges including bio-compatibility issues, scalability, and regulatory hindrances [42]. On-going research is required to address the efficacy and safety profiles of these promising therapeutic tools.
Conflicts of interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
We wish to acknowledge the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Centre of Excellence (CoE), NDDS, NIPER-Raebareli. Communication Number/737.
References
- 1.Gudmundsdottir H., Habermann E.B., Vierkant R.A., Starlinger P., Thiels C.A., Warner S.G. Survival and symptomatic relief after cytoreductive hepatectomy for neuroendocrine tumor liver metastases-long-term follow-up of over 500 patients. Ann Surg Oncol. 2023;30(8):4854–4855. doi: 10.1245/s10434-023-13372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Z., Hu B., Xiang B., Fang H., Zhang B., Wang Y., et al. Biomimetic biomembrane encapsulation and targeted delivery of a nitric oxide release platform for therapy of parkinson's disease. ACS Biomater Sci Eng. 2023;9(5):2545–2557. doi: 10.1021/acsbiomaterials.3c00146. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan L., Chow B.W., Gu C. Neuronal regulation of the blood-brain barrier and neurovascular coupling. Nat Rev Neurosci. 2020;21(8):416–432. doi: 10.1038/s41583-020-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montagne A., Nation D.A., Sagare A.P., Barisano G., Sweeney M.D., Chakhoyan A., et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581(7806):71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nation D.A., Sweeney M.D., Montagne A., Sagare A.P., D'Orazio L.M., Pachicano M., et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvanitis C.D., Ferraro G.B., Jain R.K. The blood-brain barrier and blood-tumor barrier in brain tumors and metastases. Nat Rev Cancer. 2020;20(1):26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardridge W.M. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens DE 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y., Li C., Chen Q., Liu P., Guo Q., Zhang Y., et al. Microthrombus-targeting micelles for neurovascular remodeling and enhanced microcirculatory perfusion in acute ischemic stroke. Adv Mater. 2019;31(21) doi: 10.1002/adma.201808361. [DOI] [PubMed] [Google Scholar]
- 11.Chapman A.P. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54(4):531–545. doi: 10.1016/s0169-409x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- 12.Lubich C., Allacher P., de la Rosa M., Bauer A., Prenninger T., Horling F.M., et al. The mystery of antibodies against polyethylene glycol (PEG) - what do we know? Pharm Res. 2016;33(9):2239–2249. doi: 10.1007/s11095-016-1961-x. [DOI] [PubMed] [Google Scholar]
- 13.Najahi-Missaoui W., Arnold R.D., Cummings B.S. Cummings, safe nanoparticles: are we there yet? Int J Mol Sci. 2020;22(1):385. doi: 10.3390/ijms22010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie J., Shen Z., Anraku Y., Kataoka K., Chen X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224 doi: 10.1016/j.biomaterials.2019.119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klyachko N.L., Haney M.J., Zhao Y., Manickam D.S., Mahajan V., Suresh P., et al. Macrophages offer a paradigm switch for CNS delivery of therapeutic proteins. Nanomedicine (Lond) 2014;9(9):1403–1422. doi: 10.2217/nnm.13.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brynskikh A.M., Zhao Y.L., Mosley R.L., Li S., Boska M.D., Klyachko N.L., et al. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson's disease. Nanomedicine (Lond) 2010;5(3):379–396. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J., Chu D., Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J Control Release. 2016;224:208–216. doi: 10.1016/j.jconrel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathias N.R., Hussain M.A. Non-invasive systemic drug delivery: developability considerations for alternate routes of administration. J Pharm Sci. 2010;99(1):1–20. doi: 10.1002/jps.21793. [DOI] [PubMed] [Google Scholar]
- 19.Song Y., Day C.M., Afinjuomo F., Tan J.E., Page S.W., Garg S., et al. Advanced strategies of drug delivery via oral, topical, and parenteral administration routes: where do equine medications stand? Pharmaceutics. 2023;15(1):186. doi: 10.3390/pharmaceutics15010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A.P., Biswas A., Shukla A., Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct Target Ther. 2019;4:33. doi: 10.1038/s41392-019-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., Wang Q., Xia G., Adilijiang N., Li Y., Hou Z., et al. Recent advances in targeted drug delivery strategy for enhancing oncotherapy. Pharmaceutics. 2023;15(9):2233. doi: 10.3390/pharmaceutics15092233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dash P., Piras A.M., Dash M. Cell membrane coated nanocarriers - an efficient biomimetic platform for targeted therapy. J Control Release. 2020;327:546–570. doi: 10.1016/j.jconrel.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Narain A., Asawa S., Chhabria V., Patil-Sen Y. Cell membrane coated nanoparticles: next-generation therapeutics. Nanomedicine (Lond) 2017;12(21):2677–2692. doi: 10.2217/nnm-2017-0225. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 25.Shen Z., Ye H., Kröger M., Li Y. Aggregation of polyethylene glycol polymers suppresses receptor-mediated endocytosis of PEGylated liposomes. Nanoscale. 2018;10(9):4545–4560. doi: 10.1039/c7nr09011k. [DOI] [PubMed] [Google Scholar]
- 26.Atha D.H., Ingham K.C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem. 1981;256(23):12108–12117. [PubMed] [Google Scholar]
- 27.Garay R.P., El-Gewely R., Armstrong J.K., Garratty G., Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 2012;9(11):1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Fuentes M., Prego C., Torres D., Alonso M.J.A. A comparative study of the potential of solid triglyceride nanostructures coated with chitosan or poly(ethylene glycol) as carriers for oral calcitonin delivery. Eur J Pharm Sci. 2005;25(1):133–143. doi: 10.1016/j.ejps.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Ishida T., Maeda R., Ichihara M., Irimura K., Kiwada H. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J Control Release. 2003;88(1):35–42. doi: 10.1016/s0168-3659(02)00462-5. [DOI] [PubMed] [Google Scholar]
- 30.Vandorpe J., Schacht E., Stolnik S., Garnett M.C., Davies M.C., Illum L., et al. Poly(organo phosphazene) nanoparticles surface modified with poly(ethylene oxide) Biotechnol Bioeng. 1996;52(1):89–95. doi: 10.1002/(SICI)1097-0290(19961005)52:1<89::AID-BIT8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci USA. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30(23) doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J., Kroll A.V., Holay M., Fang R.H., Zhang L. Biomimetic nanotechnology toward personalized vaccines. Adv Mater. 2020;32(13) doi: 10.1002/adma.201901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang L.L., Nie W., Zhang J., Xie H.Y. Cell-membrane-based biomimetic systems with bioorthogonal functionalities. Acc Chem Res. 2020;53(1):276–287. doi: 10.1021/acs.accounts.9b00559. [DOI] [PubMed] [Google Scholar]
- 35.Liu H., Su Y.Y., Jiang X.C., Gao J.Q. Cell membrane-coated nanoparticles: a novel multifunctional biomimetic drug delivery system. Drug Deliv Transl Res; 2023;13(3):716–737. doi: 10.1007/s13346-022-01252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo C., Fang H., Zhou M., Li J., Zhang X., Liu S., et al. Biomimetic open porous structured core-shell microtissue with enhanced mechanical properties for bottom-up bone tissue engineering. Theranostics. 2019;9(16):4663–4677. doi: 10.7150/thno.34464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L., Bai X., Martikainen M.V., Kårlund A., Roponen M., Xu W., et al. Cell membrane coating integrity affects the internalization mechanism of biomimetic nanoparticles. Nat Commun. 2021;12(1):5726. doi: 10.1038/s41467-021-26052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L., Yu W., Seitsonen J., Xu W., Lehto V.P., et al. Correct identification of the core-shell structure of cell membrane-coated polymeric nanoparticles. Chemistry (Easton) 2022;28(68) doi: 10.1002/chem.202200947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oldenborg P.A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 40.Le Q.V., Lee J., Lee H., Shim G., Oh Y.K. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm Sin B. 2021;11(8):2096–2113. doi: 10.1016/j.apsb.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fazal S., Lee R. Biomimetic bacterial membrane vesicles for drug delivery applications. Pharmaceutics. 2021;13(9):1430. doi: 10.3390/pharmaceutics13091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li R., He Y., Zhang S., Qin J., Wang J. Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B. 2018;8(1):14–22. doi: 10.1016/j.apsb.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan Z., Li P.Y., Deng J., Bady S.C., Cheng H. Cell membrane coating for reducing nanoparticle-induced inflammatory responses to scaffold constructs. Nano Res. 2018;11(10):5573–5583. doi: 10.1007/s12274-018-2084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang R.H., Hu C.M., Luk B.T., Gao W., Copp J.A., Tai Y., et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tietjen G.T., Bracaglia L.G., Saltzman W.M., Pober J.S. Focus on fundamentals: achieving effective nanoparticle targeting. Trends Mol Med. 2018;24(7):598–606. doi: 10.1016/j.molmed.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moyano D.F., Liu Y., Peer D., Rotello V.M. Modulation of immune response using engineered nanoparticle surfaces. Small. 2016;12(1):76–82. doi: 10.1002/smll.201502273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deirram N., Zhang C., Kermaniyan S.S., Johnston A.P.R. Such GK. pH-responsive polymer nanoparticles for drug delivery. Macromol Rapid Commun. 2019;40(10) doi: 10.1002/marc.201800917. [DOI] [PubMed] [Google Scholar]
- 48.Franklin Robin J.M., Ffrench-Constant C. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat Rev Neurosci. 2017;18(12):753–769. doi: 10.1038/nrn.2017.136. [DOI] [PubMed] [Google Scholar]
- 49.Chooi W.H., Chew S.Y. Modulation of cell-cell interactions for neural tissue engineering: potential therapeutic applications of cell adhesion molecules in nerve regeneration. Biomaterials. 2019;197:327–344. doi: 10.1016/j.biomaterials.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 50.Zhang N., Lin J., Chew S.Y. Neural cell membrane-coated nanoparticles for targeted and enhanced uptake by central nervous system cells. ACS Appl Mater Interfaces. 2021;13(47):55840–55850. doi: 10.1021/acsami.1c16543. [DOI] [PubMed] [Google Scholar]
- 51.Huang S., Liu S., Wang K., Yang C., Luo Y., Zhang Y., et al. Highly fluorescent and bioresorbable polymeric nanoparticles with enhanced photostability for cell imaging. Nanoscale. 2015;7(3):889–895. doi: 10.1039/c4nr05576d. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y., Wang J. Research progress of cell membrane biomimetic nanoparticles for circulating tumor cells. Front Oncol. 2024;14 doi: 10.3389/fonc.2024.1389775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rampado R., Caliceti P., Agostini M. Latest advances in biomimetic cell membrane-coated and membrane-derived nanovectors for biomedical applications. Nanomaterials (Basel) 2022;12(9):1543. doi: 10.3390/nano12091543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong X., Na Y., Yin S., Yan C., Gu J., Zhang N., et al. Cell membrane biomimetic nanoparticles with potential in treatment of Alzheimer's disease. Molecules. 2023;28(5):2336. doi: 10.3390/molecules28052336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iurisci I., Cumashi A., Sherman A.A., Tsvetkov Y.E., Tinari N., Piccolo E., et al. Synthetic inhibitors of galectin-1 and -3 selectively modulate homotypic cell aggregation and tumor cell apoptosis. Anticancer Res. 2009;29(1):403–410. [PubMed] [Google Scholar]
- 56.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Guo Q., Wang S., Xu R., Tang Y., Xia X. Cancer cell membrane-coated nanoparticles: a promising anti-tumor bionic platform. RSC Adv. 2024;14(15):10608–10637. doi: 10.1039/d4ra01026d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui X., Pertile R., Liu P., Eyles D.W. Vitamin D regulates tyrosine hydroxylase expression: n-cadherin a possible mediator. Neuroscience. 2015;304:90–100. doi: 10.1016/j.neuroscience.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 59.Lelièvre E.C., Plestant C., Boscher C., Wolff E., Mège R.M., Birbes H. N-cadherin mediates neuronal cell survival through Bim down-regulation. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0033206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun H., Su J., Meng Q., Yin Q., Chen L., Gu W., et al. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv Mater. 2016;28(43):9581–9588. doi: 10.1002/adma.201602173. [DOI] [PubMed] [Google Scholar]
- 61.Zhu J.Y., Zheng D.W., Zhang M.K., Yu W.Y., Qiu W.X., Hu J.J., et al. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16(9):5895–5901. doi: 10.1021/acs.nanolett.6b02786. [DOI] [PubMed] [Google Scholar]
- 62.Yang R., Xu J., Xu L., Sun X., Chen Q., Zhao Y., et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12(6):5121–5129. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 63.Xiao Y., Xu R.H., Dai Y. Nanoghosts: harnessing mesenchymal stem cell membrane for construction of drug delivery platforms via optimized biomimetics. Small. 2024;20(1) doi: 10.1002/smll.202304824. [DOI] [PubMed] [Google Scholar]
- 64.Lei T., Li C., Liu Y., Cui Z., Deng S., Cao J., et al. Microfluidics-enabled mesenchymal stem cell-derived neuron-like cell membrane-coated nanoparticles inhibit inflammation and apoptosis for Parkinson's Disease. J Nanobiotechnology. 2024;22(1):370. doi: 10.1186/s12951-024-02587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao Z., Zhang L., Hu J., Sun Y. Mesenchymal stem cells: a potential targeted-delivery vehicle for an anti-cancer drug, loaded nanoparticles. Nanomedicine. 2013;9(2):174–184. doi: 10.1016/j.nano.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Hu Y.L., Fu Y.H., Tabata Y., Gao J.Q. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J Control Release. 2010;147(2):154–162. doi: 10.1016/j.jconrel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 67.Yang N., Ding Y., Zhang Y., Wang B., Zhao X., Cheng K., et al. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy. ACS Appl Mater Interfaces. 2018;10(27):22963–22973. doi: 10.1021/acsami.8b05363. [DOI] [PubMed] [Google Scholar]
- 68.Tang J., Shen D., Caranasos T.G., Wang Z., Vandergriff A.C., Allen T.A., et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat Commun. 2017;8 doi: 10.1038/ncomms13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y., Zhao J., Jiang J., Chen F., Fang X. Doxorubicin delivered using nanoparticles camouflaged with mesenchymal stem cell membranes to treat colon cancer. Int J Nanomedicine. 2020;15:2873–2884. doi: 10.2147/IJN.S242787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bose R.J., Kim B.J., Arai Y., Han I.B., Moon J.J., Paulmurugan R., et al. Bioengineered stem cell membrane functionalized nanocarriers for therapeutic targeting of severe hindlimb ischemia. Biomaterials. 2018;185:360–370. doi: 10.1016/j.biomaterials.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 71.Robin A.M., Zhang Z.G., Wang L., Zhang R.L., Katakowski M., Zhang L., et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(1):125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 72.Xue L., Wang J., Wang W., Yang Z., Hu Z., Hu M., et al. The effect of stromal cell-derived factor 1 in the migration of neural stem cells. Cell Biochem Biophys. 2014;70(3):1609–1616. doi: 10.1007/s12013-014-0103-5. [DOI] [PubMed] [Google Scholar]
- 73.Farjadian F., Moghoofei M., Mirkiani S., Ghasemi A., Rabiee N., Hadifar S., et al. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol Adv. 2018;36(4):968–985. doi: 10.1016/j.biotechadv.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shao J., Xuan M., Zhang H., Lin X., Wu Z., He Q. Chemotaxis-guided hybrid neutrophil micromotors for targeted drug transport. Angew Chem Int Ed Engl. 2017;56(42):12935–12939. doi: 10.1002/anie.201706570. [DOI] [PubMed] [Google Scholar]
- 75.Gao F., Xu L., Yang B., Fan F., Yang L. Kill the real with the fake: eliminate intracellular Staphylococcus aureus using nanoparticle coated with its extracellular vesicle membrane as active-targeting drug carrier. ACS Infect Dis. 2019;5(2):218–227. doi: 10.1021/acsinfecdis.8b00212. [DOI] [PubMed] [Google Scholar]
- 76.Xia Q., Zhang Y., Li Z., Hou X., Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9(4):675–689. doi: 10.1016/j.apsb.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemarchand C., Gref R., Couvreur P. Polysaccharide-decorated nanoparticles. Eur J Pharm Biopharm. 2004;58(2):327–341. doi: 10.1016/j.ejpb.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 78.Ren H., Liu J., Li Y., Wang H., Ge S., Yuan A., et al. Oxygen self-enriched nanoparticles functionalized with erythrocyte membranes for long circulation and enhanced phototherapy. Acta Biomater. 2017;59:269–282. doi: 10.1016/j.actbio.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 79.Luk B.T., Hu C.M., Fang R.H., Dehaini D., Carpenter C., Gao W., et al. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale. 2014;6(5):2730–2737. doi: 10.1039/c3nr06371b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lv W., Xu J., Wang X., Li X., Xu Q., Xin H. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano. 2018;12(6):5417–5426. doi: 10.1021/acsnano.8b00477. [DOI] [PubMed] [Google Scholar]
- 81.Hong H.Y., Choi J.S., Kim Y.J., Lee H.Y., Kwak W., Yoo J., et al. Detection of apoptosis in a rat model of focal cerebral ischemia using a homing peptide selected from in vivo phage display. J Control Release. 2008;131(3):167–172. doi: 10.1016/j.jconrel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Y., Jiang Y., Lv W., Wang Z., Lv L., Wang B., et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J Control Release. 2016;233:64–71. doi: 10.1016/j.jconrel.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 83.Estelrich J., Busquets M.A. Iron oxide nanoparticles in photothermal therapy. Molecules. 2018;23(7):1567. doi: 10.3390/molecules23071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren X., Zheng R., Fang X., Wang X., Zhang X., Yang W., et al. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials. 2016;92:13–24. doi: 10.1016/j.biomaterials.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 85.Wu Y.W., Goubran H., Seghatchian J., Burnouf T. Smart blood cell, and microvesicle-based Trojan horse drug delivery: merging expertise in blood transfusion and biomedical engineering in the field of nanomedicine. Transfus Apher Sci. 2016;54(2):309–318. doi: 10.1016/j.transci.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 86.Ihler G.M., Glew R.H., Schnure F.W. Enzyme loading of erythrocytes. Proc Natl Acad Sci USA. 1973;70(9):2663–2666. doi: 10.1073/pnas.70.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aryal S., Hu C.M., Fang R.H., Dehaini D., Carpenter C., Zhang D.E., et al. Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomedicine (Lond) 2013;8(8):1271–1280. doi: 10.2217/nnm.12.153. [DOI] [PubMed] [Google Scholar]
- 88.Gao W., Hu C.M., Fang R.H., Luk B.T., Su J., Zhang L. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25(26):3549–3553. doi: 10.1002/adma.201300638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piao J.G., Wang L., Gao F., You Y.Z., Xiong Y., Yang L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8(10):10414–10425. doi: 10.1021/nn503779d. [DOI] [PubMed] [Google Scholar]
- 90.Skorokhod O.A., TTs Garmaeva, Vitvitsky V.M., Isaev V.G., Parovichnikova E.N., Savchenko V.G., Ataullakhanov F.I. Pharmacokinetics of erythrocyte-bound daunorubicin in patients with acute leukemia. Med Sci Monit. 2004;10(4):PI55–PI64. [PubMed] [Google Scholar]
- 91.Batlle A.M., Bustos N.L., Stella A.M., Wider E.A., Conti H.A., Mendez A. Enzyme replacement therapy in porphyrias–IV. First successful human clinical trial of delta-aminolevulinate dehydratase-loaded erythrocyte ghosts. Int J Biochem. 1983;15(10):1261–1265. doi: 10.1016/0020-711x(83)90216-1. [DOI] [PubMed] [Google Scholar]
- 92.Eichler H.G., Rameis H., Bauer K., Korn A., Bacher S., Gasić S. Survival of gentamicin-loaded carrier erythrocytes in healthy human volunteers. Eur J Clin Invest. 1986;16(1):39–42. doi: 10.1111/j.1365-2362.1986.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 93.Friedl P., Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9(9):960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 94.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 95.Parodi A., Quattrocchi N., van de Ven A.L., Chiappini C., Evangelopoulos M., Martinez J.O., et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R., et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krishnamurthy S., Gnanasammandhan M.K., Xie C., Huang K., Cui M.Y., Chan J.M. Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale. 2016;8(13):6981–6985. doi: 10.1039/c5nr07588b. [DOI] [PubMed] [Google Scholar]
- 98.Wan S.W., Wu-Hsieh B.A., Lin Y.S., Chen W.Y., Huang Y., Anderson R. The monocyte-macrophage-mast cell axis in dengue pathogenesis. J Biomed Sci. 2018;25(1):77. doi: 10.1186/s12929-018-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baek S.K., Makkouk A.R., Krasieva T., Sun C.H., Madsen S.J., Hirschberg H. Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. J Neurooncol. 2011;104(2):439–448. doi: 10.1007/s11060-010-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang L., Li R., Chen H., Wei J., Qian H., Su S., et al. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer. Int J Nanomedicine. 2017;12:2129–2142. doi: 10.2147/IJN.S126016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feng L., Dou C., Xia Y., Li B., Zhao M., Yu P., et al. Neutrophil-like cell-membrane-coated nanozyme therapy for ischemic brain damage and long-term neurological functional recovery. ACS Nano. 2021;15(2):2263–2280. doi: 10.1021/acsnano.0c07973. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Y., Haney M.J., Klyachko N.L., Li S., Booth S.L., Higginbotham S.M., et al. Polyelectrolyte complex optimization for macrophage delivery of redox enzyme nanoparticles. Nanomedicine (Lond) 2011;6(1):25–42. doi: 10.2217/nnm.10.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J., Ai Y., Wang L., Bu P., Sharkey C.C., Wu Q., et al. Targeted drug delivery to circulating tumor cells via platelet membrane-functionalized particles. Biomaterials. 2016;76:52–65. doi: 10.1016/j.biomaterials.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu L., Gao F., Fan F., Yang L. Platelet membrane coating coupled with solar irradiation endows a photodynamic nanosystem with both improved antitumor efficacy and undetectable skin damage. Biomaterials. 2018;159:59–67. doi: 10.1016/j.biomaterials.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 105.Hu C.M., Fang R.H., Wang K.C., Luk B.T., Thamphiwatana S., Dehaini D., et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jing L., Qu H., Wu D., Zhu C., Yang Y., Jin X., et al. Platelet-camouflaged nanococktail: simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics. 2018;8(10):2683–2695. doi: 10.7150/thno.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu C., Pan Y., Zhang H., Sun Y., Cao Y., Qi P., et al. Platelet-membrane-coated polydopamine nanoparticles for neuroprotection by reducing oxidative stress and repairing damaged vessels in intracerebral hemorrhage. Adv Healthc Mater. 2023;12(26) doi: 10.1002/adhm.202300797. [DOI] [PubMed] [Google Scholar]
- 108.Silva A.K., Di Corato R., Pellegrino T., Chat S., Pugliese G., Luciani N., et al. Cell-derived vesicles as a platform for the encapsulation of theranostic nanomaterials. Nanoscale. 2013;5(23):11374–11384. doi: 10.1039/c3nr01541f. [DOI] [PubMed] [Google Scholar]
- 109.Keaney J., Campbell M. The dynamic blood-brain barrier. FEBS J. 2015;282(21):4067–4079. doi: 10.1111/febs.13412. [DOI] [PubMed] [Google Scholar]
- 110.Sun J., Huang Y., Gong J., Wang J., Fan Y., Cai J., et al. Transplantation of hPSC-derived pericyte-like cells promotes functional recovery in ischemic stroke mice. Nat Commun. 2020;11(1):5196. doi: 10.1038/s41467-020-19042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei W., Cheng W., Dai W., Lu F., Cheng Y., Jiang T., et al. A nanodrug coated with membrane from brain microvascular endothelial cells protects against experimental cerebral malaria. Nano Lett. 2022;22(1):211–219. doi: 10.1021/acs.nanolett.1c03514. [DOI] [PubMed] [Google Scholar]
- 112.Lennartz F., Adams Y., Bengtsson A., Olsen R.W., Turner L., Ndam N.T., et al. Structure-guided identification of a family of dual receptor-binding PfEMP1 that is associated with cerebral malaria. Cell Host Microbe. 2017;21(3):403–414. doi: 10.1016/j.chom.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hao W., Cui Y., Fan Y., Chen M., Yang G., Wang Y., et al. Hybrid membrane-coated nanosuspensions for multi-modal anti-glioma therapy via drug and antigen delivery. J Nanobiotechnol. 2021;19(1):378. doi: 10.1186/s12951-021-01110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu L., Li Q., Deng J., Shen J., Xu W., Yang W., et al. Platelet-tumor cell hybrid membrane-camouflaged nanoparticles for enhancing therapy efficacy in glioma. Int J Nanomed. 2021;16:8433–8446. doi: 10.2147/IJN.S333279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi W., Cao X., Liu Q., Zhu Q., Liu K., Deng T., et al. Hybrid membrane-derived nanoparticles for isoliquiritin enhanced glioma therapy. Pharmaceuticals (Basel) 2022;15(9):1059. doi: 10.3390/ph15091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin R.R., Jin L.L., Xue Y.Y., Zhang Z.S., Huang H.F., Chen D.F., et al. Hybrid membrane-coated nanoparticles for precise targeting and synergistic therapy in Alzheimer's disease. Adv Sci (Weinh) 2024;11(24) doi: 10.1002/advs.202306675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y., Luo J., Liu Y., Liu W., Yu G., Huang Y., et al. Brain-targeted biomimetic nanodecoys with neuroprotective effects for precise therapy of Parkinson's disease. ACS Cent Sci. 2022;8(9):1336–1349. doi: 10.1021/acscentsci.2c00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu J., Gao D., Hu D., Lan S., Liu Y., Zheng H., et al. Delivery of biomimetic liposomes via meningeal lymphatic vessels route for targeted therapy of Parkinson's disease. Research (Wash DC) 2023;6:0030. doi: 10.34133/research.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu H., Han Y., Wang T., Zhang H., Xu Q., Yuan J., et al. Targeting microglia for therapy of Parkinson's disease by using biomimetic ultrasmall nanoparticles. J Am Chem Soc. 2020;142(52):21730–21742. doi: 10.1021/jacs.0c09390. [DOI] [PubMed] [Google Scholar]
- 120.Zheng Q., Liu H., Zhang H., Han Y., Yuan J., Wang T., et al. Ameliorating mitochondrial dysfunction of neurons by biomimetic targeting nanoparticles mediated mitochondrial biogenesis to boost the therapy of Parkinson's disease. Adv Sci (Weinh) 2023;10(22) doi: 10.1002/advs.202300758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu C.M., Fang R.H., Copp J., Luk B.T., Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8(5):336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Su J., Sun H., Meng Q., Zhang P., Yin Q., Li Y. Enhanced blood suspensibility and laser-activated tumor-specific drug release of theranostic mesoporous silica nanoparticles by functionalizing with erythrocyte membranes. Theranostics. 2017;7(3):523–537. doi: 10.7150/thno.17259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cao H., Dan Z., He X., Zhang Z., Yu H., Yin Q. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10(8):7738–7748. doi: 10.1021/acsnano.6b03148. [DOI] [PubMed] [Google Scholar]
- 124.Rao L., He Z., Meng Q.F., Zhou Z., Bu L.L., Guo S.S., et al. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J Biomed Mater Res A. 2017;105(2):521–530. doi: 10.1002/jbm.a.35927. [DOI] [PubMed] [Google Scholar]
- 125.Harris J.C., Scully M.A., Day E.S. Cancer cell membrane-coated nanoparticles for cancer management. Cancers (Basel) 2019;11(12):1836. doi: 10.3390/cancers11121836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhai Y., Su J., Ran W., Zhang P., Yin Q., Zhang Z. Preparation and application of cell membrane-camouflaged nanoparticles for cancer therapy. Theranostics. 2017;7(10):2575–2592. doi: 10.7150/thno.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Copp J.A., Fang R.H., Luk B.T., Hu C.M., Gao W., Zhang K. Clearance of pathological antibodies using biomimetic nanoparticles. Proc Natl Acad Sci U S A. 2014;111(37):13481–13486. doi: 10.1073/pnas.1412420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rao L., Cai B., Bu L.L., Liao Q.Q., Guo S.S., Zhao X.Z., et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11(4):3496–3505. doi: 10.1021/acsnano.7b00133. [DOI] [PubMed] [Google Scholar]
- 129.Zhang J., Gao W., Fang R.H., Dong A., Zhang L. Synthesis of nanogels via cell membrane-templated polymerization. Small. 2015;11(34):4309–4313. doi: 10.1002/smll.201500987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu L., Pan D., Chen S., Martikainen M.V., Kårlund A., Ke J., et al. Systematic design of cell membrane coating to improve tumor targeting of nanoparticles. Nat Commun. 2022;13(1):6181. doi: 10.1038/s41467-022-33889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ben-Akiva E., Meyer R.A., Yu H., Smith J.T., Pardoll D.M., Green J.J., et al. Biomimetic anisotropic polymeric nanoparticles coated with red blood cell membranes for enhanced circulation and toxin removal. Sci Adv. 2020;6(16):eaay9035. doi: 10.1126/sciadv.aay9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang H., Wu J., Williams G.R., Fan Q., Niu S., Wu J., et al. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J Nanobiotechnol. 2019;17(1):60. doi: 10.1186/s12951-019-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Niazi SK. Non-invasive drug delivery across the blood-brain barrier: a prospective analysis. Pharmaceutics. 2023;15(11):2599. doi: 10.3390/pharmaceutics15112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chaulagain B., Gothwal A., Lamptey R.N.L., Trivedi R., Mahanta A.K., Layek B., et al. Experimental models of in vitro blood-brain barrier for CNS drug delivery: an evolutionary perspective. Int J Mol Sci. 2023;24(3):2710. doi: 10.3390/ijms24032710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cheng G., Liu Y., Ma R., Cheng G., Guan Y., Chen X. Anti-parkinsonian therapy: strategies for crossing the blood-brain barrier and nano-biological effects of nanomaterials. Nanomicro Lett. 2022;14(1):105. doi: 10.1007/s40820-022-00847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Storck S.E., Hartz A.M.S., Pietrzik C.U. The blood-brain barrier in Alzheimer's disease. Handb Exp Pharmacol. 2022;273:247–266. doi: 10.1007/164_2020_418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nitzsche F., Müller C., Lukomska B., Jolkkonen J., Deten A., Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35(6):1446–1460. doi: 10.1002/stem.2614. [DOI] [PubMed] [Google Scholar]
- 138.Huang Y., Wu Q., Tam P.K.H. Immunomodulatory mechanisms of mesenchymal stem cells and their potential clinical applications. Int J Mol Sci. 2022;23(17) doi: 10.3390/ijms231710023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shibata S. Ultrastructure of capillary walls in human brain tumors. Acta Neuropathol. 1989;78(6):561–571. doi: 10.1007/BF00691283. [DOI] [PubMed] [Google Scholar]
- 140.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu H., Zhang T., Li N., Gao J. Cell membrane-based biomimetic vehicles for effective central nervous system target delivery: insights and challenges. J Control Release. 2023;360:169–184. doi: 10.1016/j.jconrel.2023.06.023. [DOI] [PubMed] [Google Scholar]
- 142.Shlosberg D., Benifla M., Kaufer D., Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zenaro E., Piacentino G., Constantin G. The blood-brain barrier in Alzheimer's disease. Neurobiol Dis. 2017;107:41–56. doi: 10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zenaro E., Piacentino G., Constantin G. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale. 2013;5(19):8884–8888. doi: 10.1039/c3nr03064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luo L., Zang G., Liu B., Qin X., Zhang Y., Chen Y., et al. Bioengineering CXCR4-overexpressing cell membrane functionalized ROS-responsive nanotherapeutics for targeting cerebral ischemia-reperfusion injury. Theranostics. 2021;11(16):8043–8056. doi: 10.7150/thno.60785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang D., Jiang S., Zhang F., Ma S., Heng B.C., Wang Y., et al. Cell membrane vesicles with enriched CXCR4 display enhances their targeted delivery as drug carriers to inflammatory sites. Adv Sci (Weinh) 2021;8(23) doi: 10.1002/advs.202101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wysoczynski M., Reca R., Ratajczak J., Kucia M., Shirvaikar N., Honczarenko M., et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood; 2005;105(1):40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 148.Dong X., Gao J., Zhang C.Y., Hayworth C., Frank M., Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019;13(2):1272–1283. doi: 10.1021/acsnano.8b06572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chauhan P.S., Kumarasamy M., Carcaboso A.M., Sosnik A., Danino D. Multifunctional silica-coated mixed polymeric micelles for integrin-targeted therapy of pediatric patient-derived glioblastoma. Mater Sci Eng C Mater Biol Appl. 2021;128 doi: 10.1016/j.msec.2021.112261. [DOI] [PubMed] [Google Scholar]
- 150.Beckman H. Laser iridotomy. Trans New Orleans Acad Ophthalmol. 1985;33:177–181. [PubMed] [Google Scholar]
- 151.Mohajerani A., Burnett L., Smith J.V., Kurmus H., Milas J., Arulrajah A. Nanoparticles in construction materials and other applications, and implications of nanoparticle use. Materials (Basel) 2019;12(19):3052. doi: 10.3390/ma12193052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013;2013 doi: 10.1155/2013/238428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Missaoui W.N., Arnold R.D., Cummings B.S. Toxicological status of nanoparticles: what we know and what we don't know. Chem Biol Interact. 2018;295:1–12. doi: 10.1016/j.cbi.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu Y., Wan S., Yang S., Hu H., Zhang C., Lai J., et al. Macrophage cell membrane-based nanoparticles: a new promising biomimetic platform for targeted delivery and treatment. J Nanobiotechnol. 2022;20(1):542. doi: 10.1186/s12951-022-01746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nguyen-Thi P.T., Nguyen T.T., Phan H.L., Ho T.T., Vo T.V., Vo G.V., et al. Cell membrane-based nanomaterials for therapeutics of neurodegenerative diseases. Neurochem Int. 2023;170 doi: 10.1016/j.neuint.2023.105612. [DOI] [PubMed] [Google Scholar]
- 156.Xuan M., Shao J., Li J. Cell membrane-covered nanoparticles as biomaterials. Natl Sci Rev. 2019;6(3):551–561. doi: 10.1093/nsr/nwz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rizek P., Kumar N., Jog M.S. An update on the diagnosis and treatment of Parkinson's disease. CMAJ. 2016;188(16):1157–1165. doi: 10.1503/cmaj.151179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu F.D., Hu Y.J., Yu L., Zhou X.G., Wu J.M., Tang Y., et al. Nanoparticles: a hope for the treatment of inflammation in CNS. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.683935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kalia L.V., Lang A.E. Parkinson's disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 160.Mantri S., Fullard M., Gray S.L., Weintraub D., Hubbard R.A., Hennessy S., et al. Patterns of dementia treatment and frank prescribing errors in older adults with Parkinson disease. JAMA Neurol. 2019;76(1):41–49. doi: 10.1001/jamaneurol.2018.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Charvin D., Medori R., Hauser R.A., Rascol O. Therapeutic strategies for Parkinson disease: beyond dopaminergic drugs. Nat Rev Drug Discov. 2018;17(11):804–822. doi: 10.1038/nrd.2018.136. [DOI] [PubMed] [Google Scholar]
- 162.Margolesky J., Singer C. Extended-release oral capsule of carbidopa-levodopa in Parkinson disease. Ther Adv Neurol Disord. 2018;11 doi: 10.1177/1756285617737728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Montastruc J.L., Rascol O., Senard J.M. Glutamate antagonists and Parkinson's disease: a review of clinical data. Neurosci Biobehav Rev. 1997;21(4):477–480. doi: 10.1016/s0149-7634(96)00035-8. [DOI] [PubMed] [Google Scholar]
- 164.Xu D.C., Chen Y., Xu Y., Shen Tu CY, Peng L.H. Signaling pathways in Parkinson's disease: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):73. doi: 10.1038/s41392-023-01353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martínez-Fernández R., Rodríguez-Rojas R., Del Álamo M, Hernández-Fernández F., Pineda-Pardo J.A., Dileone M., et al. Focused ultrasound subthalamotomy in patients with asymmetric Parkinson's disease: a pilot study. Lancet Neurol. 2018;17(1):54–63. doi: 10.1016/S1474-4422(17)30403-9. [DOI] [PubMed] [Google Scholar]
- 166.Pandit R., Chen L., Götz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 2020:165–166. doi: 10.1016/j.addr.2019.11.009. 1-14. [DOI] [PubMed] [Google Scholar]
- 167.Wiklander O.P.B., Brennan M.Á., Lötvall J., Breakefield X.O., El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492):8521. doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van der Koog L., Gandek T.B., Nagelkerke A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv Healthc Mater. 2022;11(5) doi: 10.1002/adhm.202100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Anand S., Samuel M., Kumar S., Mathivanan S. Ticket to a bubble ride: cargo sorting into exosomes and extracellular vesicles. Biochim Biophys Acta Proteins Proteom. 2019;1867(12) doi: 10.1016/j.bbapap.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 170.Chen W., Zhang Q., Luk B.T., Fang R.H., Liu Y., Gao W., et al. Coating nanofiber scaffolds with beta cell membrane to promote cell proliferation and function. Nanoscale. 2016;8(19):10364–10370. doi: 10.1039/c6nr00535g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fang R.H., Jiang Y., Fang J.C., Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83. doi: 10.1016/j.biomaterials.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hu C.M., Fang R.H., Luk B.T., Chen K.N., Carpenter C., Gao W., et al. Marker-of-self' functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale. 2013;5(7):2664–2668. doi: 10.1039/c3nr00015j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kumar J., Karim A., Sweety U.H., Sarma H., Nurunnabi M., Narayan M. Bioinspired approaches for central nervous system targeted gene delivery. ACS Appl Bio Mater. 2024;7(8):4975–4997. doi: 10.1021/acsabm.3c00842. [DOI] [PubMed] [Google Scholar]
- 174.Jiang Y., Fu P., Liu Y., Wang C., Zhao P., Chu X., et al. Near-infrared light-triggered NO release for spinal cord injury repair. Sci Adv. 2020;6(39):3513. doi: 10.1126/sciadv.abc3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu L., Han Y., Jiang Z., Qing Z., Gao Y., et al. Boosting neurite outgrowth and anti-oxidative stress for treatment of Parkinson's disease by biomimetic ultrasmall nanoparticles. Sustain Mater Technol. 2024;39 J.Y. [Google Scholar]
- 176.Vashist A., Manickam P., Raymond A.D., Arias A.Y., Kolishetti N., Vashist A., et al. Recent advances in nanotherapeutics for neurological disorders. ACS Appl Bio Mater. 2023;6(7):2614–2621. doi: 10.1021/acsabm.3c00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mistretta M., Farini A., Torrente Y., Villa C. Multifaceted nanoparticles: emerging mechanisms and therapies in neurodegenerative diseases. Brain. 2023;146(6):2227–2240. doi: 10.1093/brain/awad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yin Y., Tian N., Deng Z., Wang J., Kuang L., Tang Y., et al. Targeted microglial membrane-coated microRNA nanosponge mediates inhibition of glioblastoma. ACS Nano. 2024;18(42):29089–29105. doi: 10.1021/acsnano.4c10509. [DOI] [PubMed] [Google Scholar]
- 179.Kaur J., Singh H., Naqvi S. Intracellular DAMPs in neurodegeneration and their role in clinical therapeutics. Mol Neurobiol. 2023;60(7):3600–3616. doi: 10.1007/s12035-023-03289-9. [DOI] [PubMed] [Google Scholar]
- 180.Cannon J.R., Tapias V., Na H.M., Honick A.S., Drolet R.E., Greenamyre J.T. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 2009;34(2):279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alonso Vilatela ME, López-López M., Yescas-Gómez P. Genetics of Alzheimer's disease. Arch Med Res. 2012;43(8):622–631. doi: 10.1016/j.arcmed.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 182.Zvěřová M. Clinical aspects of Alzheimer's disease. Clin Biochem. 2019;72:3–6. doi: 10.1016/j.clinbiochem.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 183.Sacks D., Baxter B., Campbell B.C.V., Carpenter J.S., Cognard C., Dippel D., et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 184.Ma M., Liu Z., Gao N., Pi Z., Du X., Ren J., et al. Self-protecting biomimetic nanozyme for selective and synergistic clearance of peripheral amyloid-beta in an Alzheimer's disease model. J Am Chem Soc. 2020;142(52):21702–21711. doi: 10.1021/jacs.0c08395. [DOI] [PubMed] [Google Scholar]
- 185.Huo Q., Shi Y., Qi Y., Huang L., Sui H., Zhao L. Biomimetic silibinin-loaded macrophage-derived exosomes induce dual inhibition of Abeta aggregation and astrocyte activation to alleviate cognitive impairment in a model of Alzheimer's disease. Mater Sci Eng C Mater Biol Appl. 2021;129 doi: 10.1016/j.msec.2021.112365. [DOI] [PubMed] [Google Scholar]
- 186.Klineova S., Lublin F.D. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(9) doi: 10.1101/cshperspect.a028928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hauser S.L., Cree B.A.C. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380–1390. doi: 10.1016/j.amjmed.2020.05.049. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mehdi-Alamdarlou S., Ahmadi F., Azadi A., Shahbazi M.A., Heidari R., Ashrafi H. A cell-mimicking platelet-based drug delivery system as a potential carrier of dimethyl fumarate for multiple sclerosis. Int J Pharm. 2022;625 doi: 10.1016/j.ijpharm.2022.122084. [DOI] [PubMed] [Google Scholar]
- 189.Krugmann B., Radulescu A., Appavou M.S., Koutsioubas A., Stingaciu L.R., Dulle M., Förster S., et al. Membrane stiffness and myelin basic protein binding strength as the molecular origin of multiple sclerosis. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-73671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ai X., Hu M., Wang Z., Zhang W., Li J., Yang H., et al. Recent advances of membrane-cloaked nanoplatforms for biomedical applications. Bioconjug Chem. 2018;29(4):838–851. doi: 10.1021/acs.bioconjchem.8b00103. [DOI] [PubMed] [Google Scholar]
- 191.Khatoon N., Zhang Z., Zhou C., Chu M. Macrophage membrane coated nanoparticles: a biomimetic approach for enhanced and targeted delivery. Biomater Sci. 2022;10(5):1193–1208. doi: 10.1039/d1bm01664d. [DOI] [PubMed] [Google Scholar]
- 192.Gong P., Wang Y., Zhang P., Yang Z., Deng W., Sun Z., et al. Immunocyte membrane-coated nanoparticles for cancer immunotherapy. Cancers (Basel) 2020;13(1):77. doi: 10.3390/cancers13010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Waeterschoot J., Gosselé W., Lemež Š., Casadevall I Solvas X. Casadevall, artificial cells for in vivo biomedical applications through red blood cell biomimicry. Nat Commun. 2024;15(1):2504. doi: 10.1038/s41467-024-46732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ryu H., Fuwad A., Yoon S., Jang H., Lee J.C., Kim S.M., et al. Biomimetic membranes with transmembrane proteins: state-of-the-art in transmembrane protein applications. Int J Mol Sci. 2019;20(6):1437. doi: 10.3390/ijms20061437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhu L., Yu X., Cao T., Deng H., Tang X., Lin Q., et al. Immune cell membrane-based biomimetic nanomedicine for treating cancer metastasis. Acta Pharm Sin B. 2023;13(6):2464–2482. doi: 10.1016/j.apsb.2023.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xia Z., Mu W., Yuan S., Fu S., Liu Y., Zhang N. Cell membrane biomimetic nano-delivery systems for cancer therapy. Pharmaceutics. 2023;15(12):2770. doi: 10.3390/pharmaceutics15122770. [DOI] [PMC free article] [PubMed] [Google Scholar]