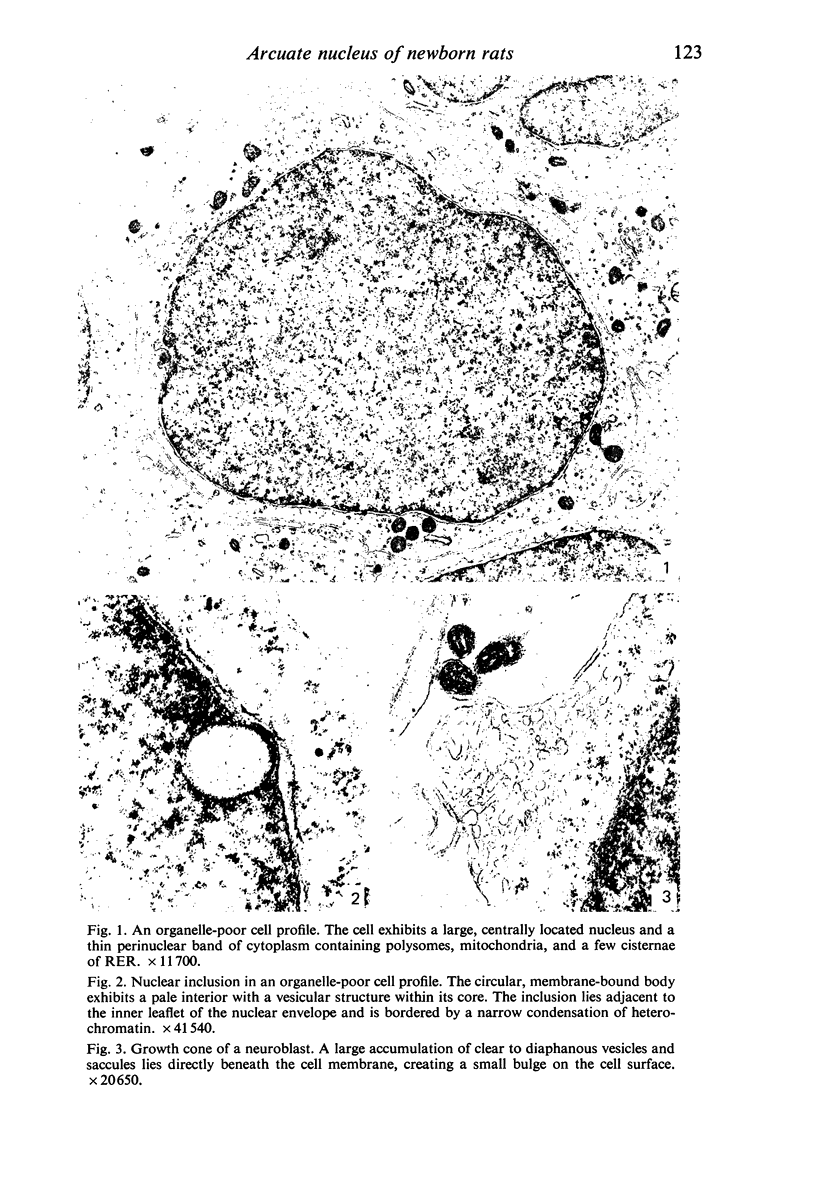
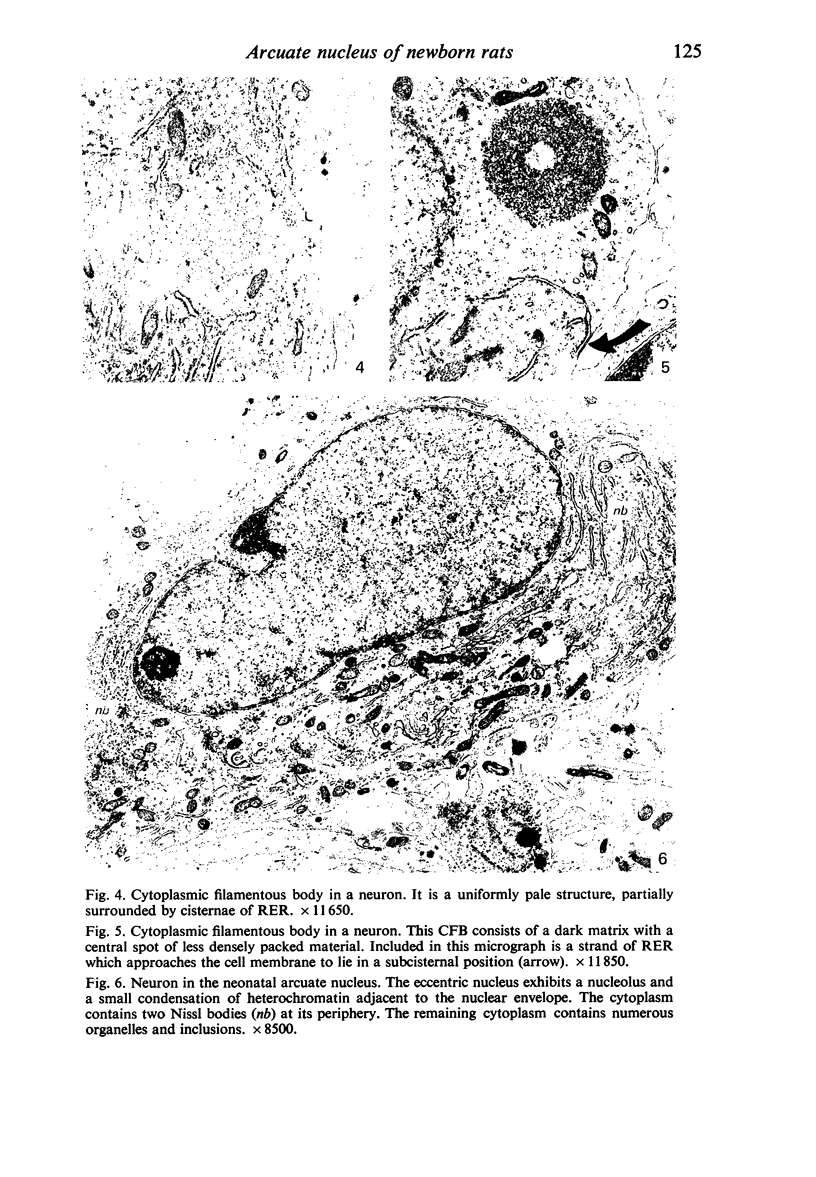
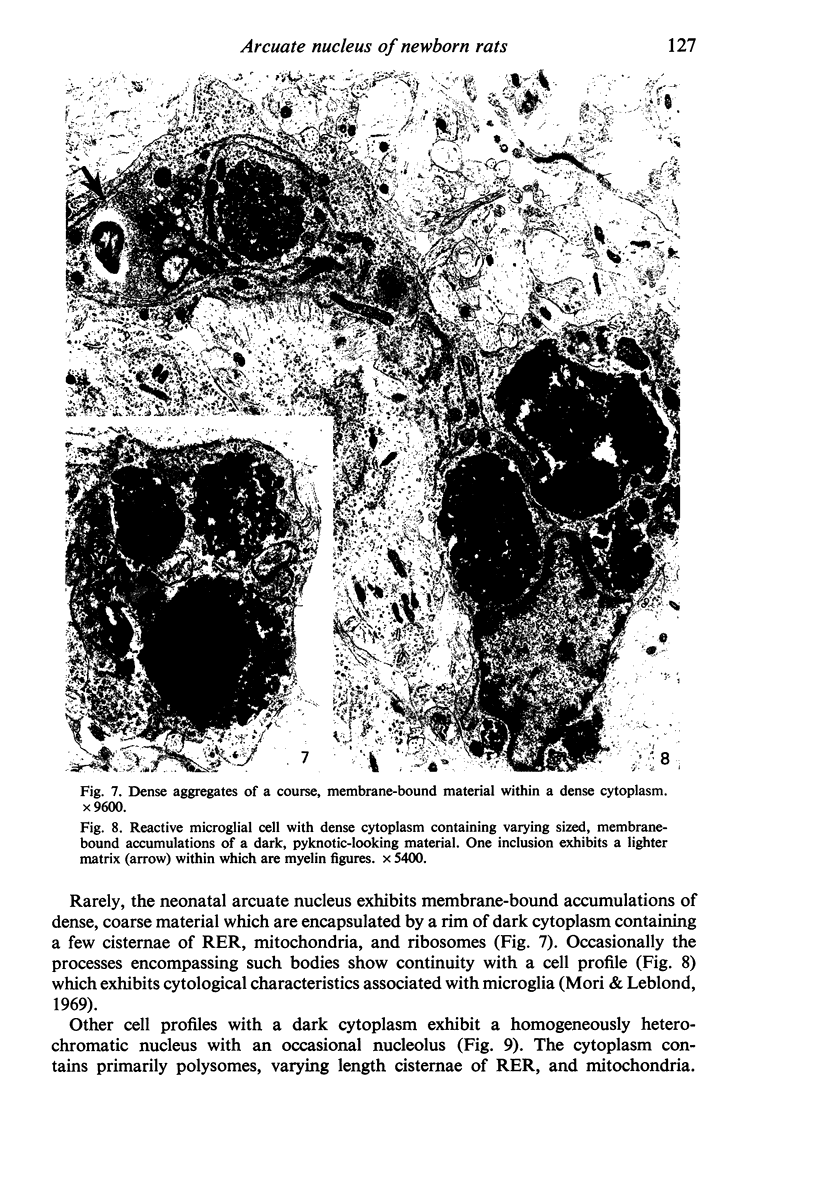
Abstract
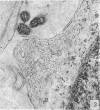
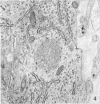
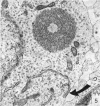
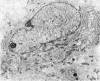
Five coronal levels of the arcuate nuclei in newborn male and female rats were examined with the transmission electron microscope. The nuclei from male and female neonates appear similar in all respects. All levels exhibit a significant population of round to oval cell profiles with large centrally located nuclei and scant cytoplasm which contains predominantly ribosomes, sparse mitochondria, and a few short cisternae of rough endoplasmic reticulum. These organelle-poor cell profiles resemble neuroblasts in other parts of the developing CNS. The arcuate nuclei of neonates also exhibit some cell profiles with the variety and quantity of organelles characteristic of mature neurons in the arcuate nuclei of adult rats. In addition, the neonatal arcuate nuclei show a paucity of synapses with apparent immaturity of those present, and numerous structures identified as growth cones. Definitive macroglia are not present in the arcuate nuclei of newborn rats.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Toran-Allerand C. D., Ferin M., Vande Wiele R. L. Immunoreactive gonadotropin-releasing hormone (Gn-RH) during maturation in the rat: Ontogeny of regional hypothalamic differences. Endocrinology. 1975 Sep;97(3):693–697. doi: 10.1210/endo-97-3-693. [DOI] [PubMed] [Google Scholar]
- Brawer J. R. The role of the arcuate nucleus in the brain-pituitary-gonad axis. J Comp Neurol. 1971 Dec;143(4):411–445. doi: 10.1002/cne.901430403. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. An electron microscopic study of neurons during postnatal development of the rat cerebral cortex. J Comp Neurol. 1968 May;133(1):17–44. doi: 10.1002/cne.901330103. [DOI] [PubMed] [Google Scholar]
- Caley D. W., Maxwell D. S. An electron microscopic study of the neuroglia during postnatal development of the rat cerebrum. J Comp Neurol. 1968 May;133(1):45–70. doi: 10.1002/cne.901330104. [DOI] [PubMed] [Google Scholar]
- De Cerro M. P., Snider R. S. Studies on the developing cerebellum. Ultrastructure of the growth cones. J Comp Neurol. 1968 Jul;133(3):341–362. doi: 10.1002/cne.901330305. [DOI] [PubMed] [Google Scholar]
- Dyer R. G., Cross B. A. Antidromic identification of units in the preoptic and anterior hypothalamic areas projecting directly to the ventromedial and arcuate nuclei. Brain Res. 1972 Aug 11;43(1):254–258. doi: 10.1016/0006-8993(72)90291-0. [DOI] [PubMed] [Google Scholar]
- Gerall A. A., Dunlap J. L. Evidence that the ovaries of the neonatal rat secrete active substances. J Endocrinol. 1971 Jul;50(3):529–530. doi: 10.1677/joe.0.0500529. [DOI] [PubMed] [Google Scholar]
- Gorski R. A. Modification of ovulatory mechanisms by postnatal administration of estrogen to the rat. Am J Physiol. 1963 Nov;205(5):842–844. doi: 10.1152/ajplegacy.1963.205.5.842. [DOI] [PubMed] [Google Scholar]
- HARRIS G. W. SEX HORMONES, BRAIN DEVELOPMENT AND BRAIN FUNCTION. Endocrinology. 1964 Oct;75:627–648. doi: 10.1210/endo-75-4-627. [DOI] [PubMed] [Google Scholar]
- Hannah R. S., Nathaniel J. H. Ultrastructural studies on postnatal differentiation of neurons in the substantia gelatinosa of rat cervical spinal cord. Anat Rec. 1975 Oct;183(2):323–337. doi: 10.1002/ar.1091830208. [DOI] [PubMed] [Google Scholar]
- Hinds J. W., Hinds P. L. Reconstruction of dendritic growth cones in neonatal mouse olfactory bulb. J Neurocytol. 1972 Sep;1(2):169–187. doi: 10.1007/BF01099183. [DOI] [PubMed] [Google Scholar]
- Holtzman E., Novikoff A. B., Villaverde H. Lysosomes and GERL in normal and chromatolytic neurons of the rat ganglion nodosum. J Cell Biol. 1967 May;33(2):419–435. doi: 10.1083/jcb.33.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Armstrong-James M. Morphology of superficial postnatal cerebral cortex with special reference to synapses. Z Zellforsch Mikrosk Anat. 1970;110(4):540–558. doi: 10.1007/BF00330103. [DOI] [PubMed] [Google Scholar]
- Kawana E., Sandri C., Akert K. Ultrastructure of growth cones in the cerebellar cortex of the neonatal rat and cat. Z Zellforsch Mikrosk Anat. 1971;115(2):284–298. doi: 10.1007/BF00391129. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Arai Y. Developmental changes in synaptic formation in the hypothalamic arcuate nucleus of female rats. Cell Tissue Res. 1976 Jun 14;169(2):143–156. doi: 10.1007/BF00214204. [DOI] [PubMed] [Google Scholar]
- McDonald P. G., Doughty C. Effect of neonatal administration of different androgens in the female rat: correlation between aromatization and the induction of sterilization. J Endocrinol. 1974 Apr;61(1):95–103. [PubMed] [Google Scholar]
- Mori S., Leblond C. P. Identification of microglia in light and electron microscopy. J Comp Neurol. 1969 Jan;135(1):57–80. doi: 10.1002/cne.901350104. [DOI] [PubMed] [Google Scholar]
- Nosal G., Radouco-Thomas C. Ultrastructural study on the differentiation and development of the nerve cell: the "nucleus-ribosome" system. Adv Cytopharmacol. 1971 May;1:433–456. [PubMed] [Google Scholar]
- Novikoff P. M., Novikoff A. B., Quintana N., Hauw J. J. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J Cell Biol. 1971 Sep;50(3):859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannese E. Developmental changes of the endoplasmic reticulum and ribosomes in nerve cells of the spinal ganglia of the domestic fowl. J Comp Neurol. 1968 Feb;132(2):331–364. doi: 10.1002/cne.901320207. [DOI] [PubMed] [Google Scholar]
- ROSENBLUTH J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol. 1962 Jun;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G., Field P. M. Sexual dimorphism in the neuropil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Res. 1973 May 17;54:1–29. doi: 10.1016/0006-8993(73)90030-9. [DOI] [PubMed] [Google Scholar]
- Sheridan P. J., Sar M., Stumpf W. E. Autoradiographic localization of 3H-estradiol or its metabolites in the central nervous system of the developing rat. Endocrinology. 1974 May;94(5):1386–1390. doi: 10.1210/endo-94-5-1386. [DOI] [PubMed] [Google Scholar]
- Sheridan P. J., Sar M., Stumpf W. E. Autoradiographic localization of 3H-testosterone or its metabolites in the neonatal rat brain. Am J Anat. 1974 Aug;140(4):589–594. doi: 10.1002/aja.1001400411. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Changeux J. P. Transsynaptic degeneration 'en cascade' in the cerebellar cortex of staggerer mutant mice. Brain Res. 1974 Mar 8;67(3):519–526. doi: 10.1016/0006-8993(74)90499-5. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. Light microscopic identification of immature glial cells in semithin sections of the developing mouse corpus callosum. J Anat. 1976 Dec;122(Pt 3):521–537. [PMC free article] [PubMed] [Google Scholar]
- Sumi S. M. The extracellular space in the developing rat brain: its variation with changes in osmolarity of the fixative, method of fixation and maturation. J Ultrastruct Res. 1969 Dec;29(5):398–425. doi: 10.1016/s0022-5320(69)90062-8. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. D. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: implications for sexual differentiation. Brain Res. 1976 Apr 23;106(2):407–412. doi: 10.1016/0006-8993(76)91038-6. [DOI] [PubMed] [Google Scholar]
- Walsh R. J., Brawer J. R., Lin P. L. Early postnatal development of ependyma in the third ventricle of male and female rats. Am J Anat. 1978 Mar;151(3):377–407. doi: 10.1002/aja.1001510305. [DOI] [PubMed] [Google Scholar]
- Wechsler W. Elektronenmikroskopischer beitrag zur Nervenzelldifferenzierung und Histogenese der grauen Substanz des Rückenmarks von Hühnerembryonen. Z Zellforsch Mikrosk Anat. 1966;74(3):401–422. [PubMed] [Google Scholar]
- Woodward D. J., Hoffer B. J., Siggins G. R., Bloom F. E. The ontogenetic development of synaptic junctions, synaptic activation and responsiveness to neurotransmitter substances in rat cerebellar purkinje cells. Brain Res. 1971 Nov;34(1):73–97. doi: 10.1016/0006-8993(71)90352-0. [DOI] [PubMed] [Google Scholar]
- Yagimura T., Matsuda A., Murasawa Y., Kobayashi T., Kobayashi T. Presence of hypothalamo-pituitary testicular axis in the early postnatal period. Endocrinol Jpn. 1969 Feb;16(1):5–10. doi: 10.1507/endocrj1954.16.5. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971 Jun;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]