Abstract
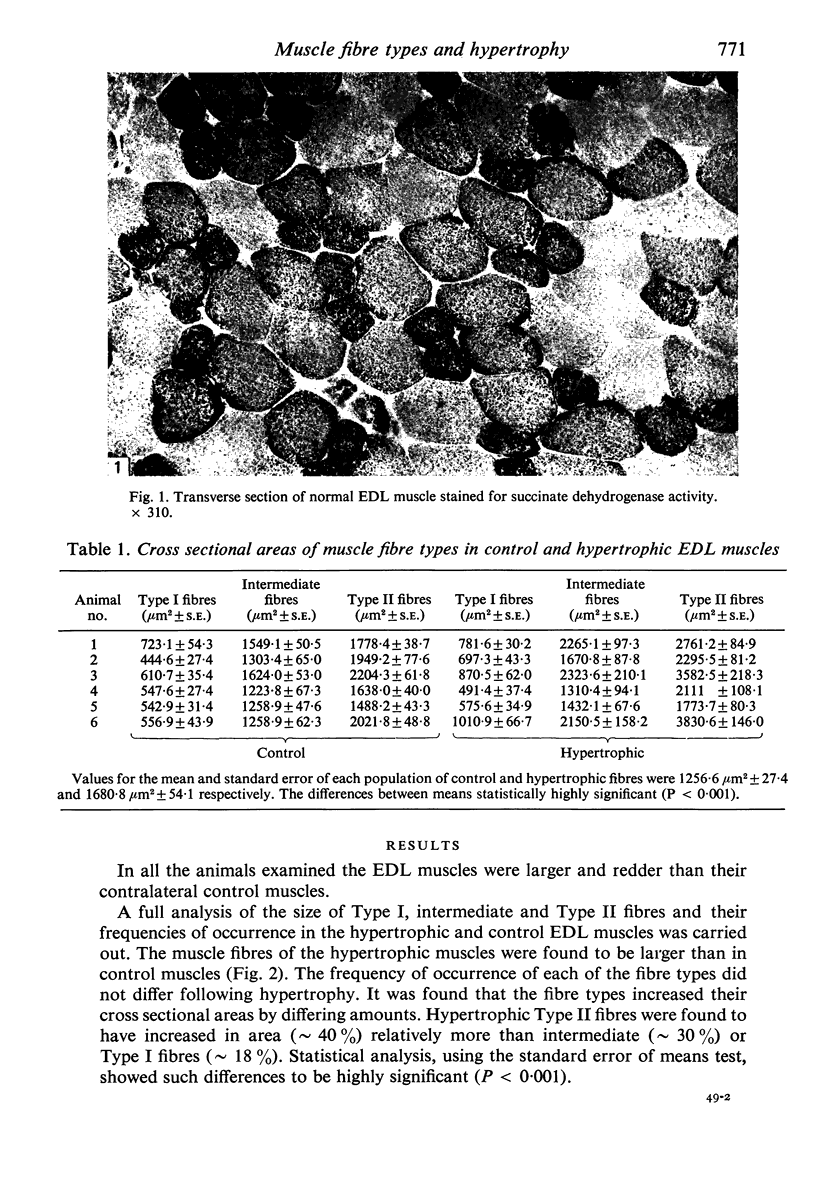
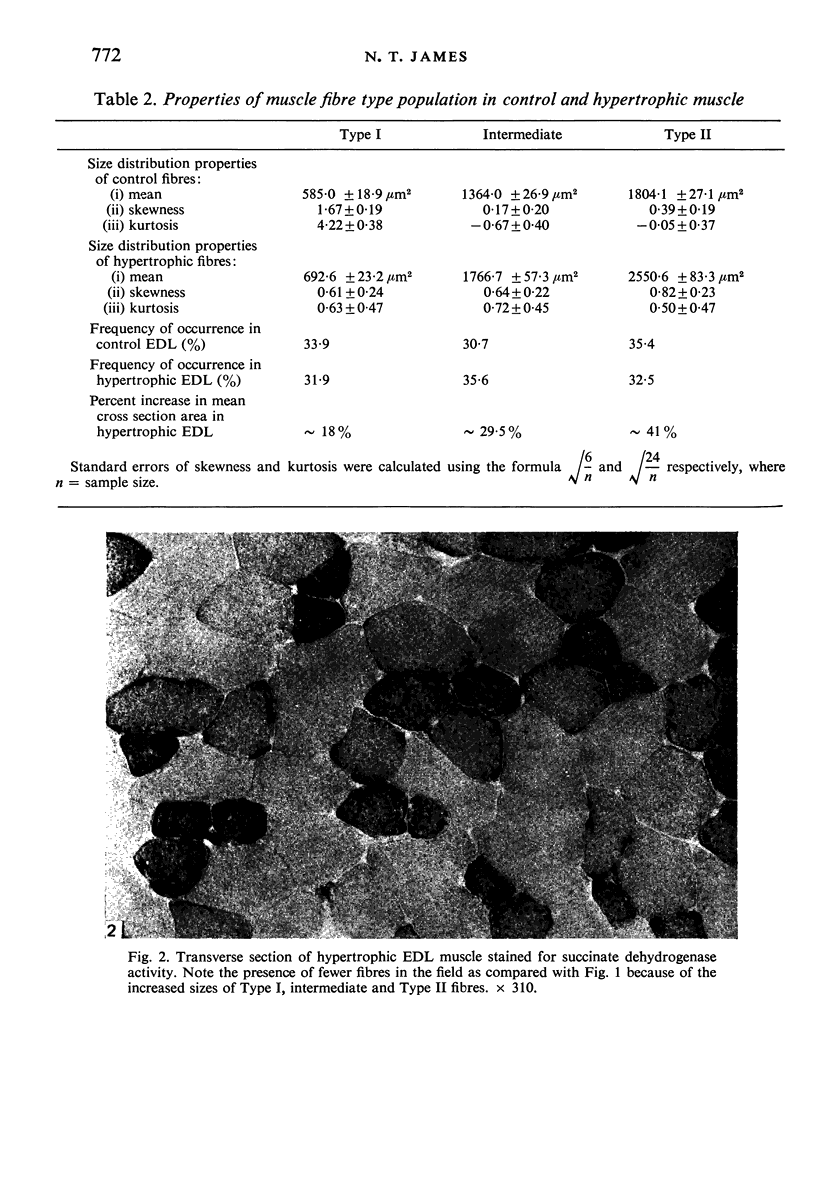
The sizes of the different types of muscle fibre in the extensor digitorum longus (EDL) muscles of mice have been measured, EDL muscles showing compensatory hypertrophy following the removal of the tibialis anterior muscle 116 days previously being compared with normal contralateral controls. Contrary to previous findings, the hypertrophy was well maintained after 116 days and Type II fibres were enlarged preferentially.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Henriksson J. Training induced changes in the subgroups of human type II skeletal muscle fibres. Acta Physiol Scand. 1977 Jan;99(1):123–125. doi: 10.1111/j.1748-1716.1977.tb10361.x. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Klinkerfuss G. H., Terjung R. L., Molé P. A., Holloszy J. O. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972 Feb;222(2):373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- Bass A., Macková E., Vítek V. Activity of some enzymes of the energy-supplying metabolism in the rat soleus after tenotomy of synergistic muscles and in the contralateral "control" muscle. Physiol Bohemoslov. 1973;22(6):613–621. [PubMed] [Google Scholar]
- Costill D. L., Daniels J., Evans W., Fink W., Krahenbuhl G., Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol. 1976 Feb;40(2):149–154. doi: 10.1152/jappl.1976.40.2.149. [DOI] [PubMed] [Google Scholar]
- DUBOWITZ V., PEARSE A. G. A comparative histochemical study of oxidative enzyme and phosphorylase activity in skeletal muscle. Z Zellforch Microsk Anat Histochem. 1960;2:105–117. doi: 10.1007/BF00744575. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Barnard R. J., Peter J. B., Gillespie C. A., Simpson D. R. Overloaded skeletal muscles of a nonhuman primate (Galago senegalensis). Exp Neurol. 1972 Nov;37(2):322–339. doi: 10.1016/0014-4886(72)90077-5. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Gerchman L., Carrow R. Histochemical changes in rat skeletal muscle after exercise. Exp Neurol. 1969 May;24(1):110–123. doi: 10.1016/0014-4886(69)90009-0. [DOI] [PubMed] [Google Scholar]
- Eriksson E., Myrhage R. Microvascular dimensions and blood flow in skeletal muscle. Acta Physiol Scand. 1972 Oct;86(2):211–222. doi: 10.1111/j.1748-1716.1972.tb05327.x. [DOI] [PubMed] [Google Scholar]
- Faulkner J. A., Maxwell L. C., Lieberman D. A. Histochemical characteristics of muscle fibers from trained and detrained guinea pigs. Am J Physiol. 1972 Apr;222(4):836–840. doi: 10.1152/ajplegacy.1972.222.4.836. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Saltin B., Saubert C. W., 4th, Sembrowich W. L., Shepherd R. E. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973 Jan;34(1):107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- Gonyea W. J., Ericson G. C. An experimental model for the study of exercise-induced skeletal muscle hypertrophy. J Appl Physiol. 1976 Apr;40(4):630–633. doi: 10.1152/jappl.1976.40.4.630. [DOI] [PubMed] [Google Scholar]
- Gonyea W., Bonde-Petersen F. Alterations in muscle contractile properties and fiber composition after weight-lifting exercise in cats. Exp Neurol. 1978 Mar;59(1):75–84. doi: 10.1016/0014-4886(78)90202-9. [DOI] [PubMed] [Google Scholar]
- Gordon E. E., Kowalski K., Fritts M. Adaptations of muscle to various exercises. Studies in rats. JAMA. 1967 Jan 9;199(2):103–108. [PubMed] [Google Scholar]
- Guth L., Wells J. B. Physiological and histochemical properties of the soleus muscle after denervation of its antagonists. Exp Neurol. 1972 Sep;36(3):463–471. doi: 10.1016/0014-4886(72)90006-4. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Hájek I. Differential reaction of muscle to excessive use in compensatory hypertrophy and increased phasic activity. Physiol Bohemoslov. 1971;20(3):205–212. [PubMed] [Google Scholar]
- Gutmann E., Schiaffino S., Hanzliková V. Mechanism of compensatory hypertrophy in skeletal muscle of the rat. Exp Neurol. 1971 Jun;31(3):451–464. doi: 10.1016/0014-4886(71)90248-2. [DOI] [PubMed] [Google Scholar]
- Guy P. S., Snow D. H. The effect of training and detraining on muscle composition in the horse. J Physiol. 1977 Jul;269(1):33–51. doi: 10.1113/jphysiol.1977.sp011891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Craggs E. C., Lawrence C. A. Longitudinal fibre division in skeletal muscle: a light- and electronmicroscopic study. Z Zellforsch Mikrosk Anat. 1970;109(4):481–494. doi: 10.1007/BF00343963. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs E. C. The longitudinal division of fibres in overloaded rat skeletal muscle. J Anat. 1970 Nov;107(Pt 3):459–470. [PMC free article] [PubMed] [Google Scholar]
- Hall-Craggs E. C. The significance of longitudinal fibre division in skeletal muscle. J Neurol Sci. 1972;15(1):27–33. doi: 10.1016/0022-510x(72)90119-0. [DOI] [PubMed] [Google Scholar]
- James N. T. Compensatory hypertrophy in the extensor digitorum longus muscle of the rat. J Anat. 1973 Oct;116(Pt 1):57–65. [PMC free article] [PubMed] [Google Scholar]
- James N. T. Compensatory muscular hypertrophy in the extensor digitorum longus muscle of the mouse. J Anat. 1976 Sep;122(Pt 1):121–131. [PMC free article] [PubMed] [Google Scholar]
- James N. T. The distribution of muscle fibre types in fasciculi and their analysis. J Anat. 1971 Dec;110(Pt 3):335–342. [PMC free article] [PubMed] [Google Scholar]
- Jaweed M. M., Herbison G. J., Ditunno J. F. Myosin ATPase activity after strengthening exercise. J Anat. 1977 Nov;124(Pt 2):371–381. [PMC free article] [PubMed] [Google Scholar]
- Kowalski K., Gordon E. E., Martinez A., Adamek J. Changes in enzyme activities of various muscle fiber types in rat induced by different exercises. J Histochem Cytochem. 1969 Sep;17(9):601–607. doi: 10.1177/17.9.601. [DOI] [PubMed] [Google Scholar]
- Macková E., Hník P. Compensatory muscle hypertrophy induced by tenotomy of synergists is not true working hypertrophy. Physiol Bohemoslov. 1973;22(1):43–49. [PubMed] [Google Scholar]
- Maxwell L. C., Faulkner J. A., Lieberman D. A. Histochemical manifestations of age and endurance training in skeletal muscle fibers. Am J Physiol. 1973 Feb;224(2):356–361. doi: 10.1152/ajplegacy.1973.224.2.356. [DOI] [PubMed] [Google Scholar]
- Reitsma W. Skeletal muscle hypertrophy after heavy exercise in rats with surgically reduced muscle function. Am J Phys Med. 1969 Oct;48(5):237–258. [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Surgically induced hypertrophy in skeletal muscles of the laboratory mouse. Anat Rec. 1968 May;161(1):69–75. doi: 10.1002/ar.1091610107. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Bormioli S. P. Adaptive changes in developing rat skeletal muscle in response to functional overload. Exp Neurol. 1973 Jul;40(1):126–137. doi: 10.1016/0014-4886(73)90129-5. [DOI] [PubMed] [Google Scholar]
- Sola O. M., Christensen D. L., Martin A. W. Hypertrophy and hyperplasia of adult chicken anterior latissimus dorsi muscles following stretch with and without denervation. Exp Neurol. 1973 Oct;41(1):76–100. doi: 10.1016/0014-4886(73)90182-9. [DOI] [PubMed] [Google Scholar]
- Suominen H., Heikkinen E., Parkatti T. Effect of eight weeks' physical training on muscle and connective tissue of the M. vastus lateralis in 69-year-old men and women. J Gerontol. 1977 Jan;32(1):33–37. doi: 10.1093/geronj/32.1.33. [DOI] [PubMed] [Google Scholar]
- Tomanek R. J. A histochemical study of postnatal differentiation of skeletal muscle with reference to functional overload. Dev Biol. 1975 Feb;42(2):305–314. doi: 10.1016/0012-1606(75)90337-1. [DOI] [PubMed] [Google Scholar]
- Tomanek R. J., Woo Y. K. Compensatory hypertrophy of the plantaris muscle in relation to age. J Gerontol. 1970 Jan;25(1):23–29. doi: 10.1093/geronj/25.1.23. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973 Oct;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- Yellin H. Changes in fiber types of the hypertrophying denervated hemidiaphragm. Exp Neurol. 1974 Feb;42(2):412–428. doi: 10.1016/0014-4886(74)90035-1. [DOI] [PubMed] [Google Scholar]




