Abstract
Background
Vascular remodeling (VR), such as arterial stiffness and atherosclerosis (AS), is general pathological characters in the progression of hypertension. It is urgent to develop therapeutic strategies for VR in the progression of hypertension. Songling Xuemaikang capsule (SXC), a Chinese patent medicine, has been preliminarily demonstrated the benefits of lowering blood pressure (BP) and associated hypertensive symptoms. We further investigate the efficacy of SXC in treating the early stages of stage 1 hypertension and the mechanism among SXC, BP and VR.
Methods
This study is designed as a prospective, multicenter, double-blinded, randomized controlled trial. One hundred eligible patients with stage 1 hypertension will be randomly allocated 1:1 into the SXC or placebo treatment for 12 weeks. All individuals were required to follow a healthy lifestyle checklist throughout the process. The primary endpoint is 24-hour ambulatory systolic BP. Secondary endpoints include brachial-ankle pulse wave velocity, skin capillary density, 24-h ambulatory diastolic BP, daytime and nighttime average BP, and other efficacy indicators. The gut microbiome-metabolome profile was analyzed to explore therapeutic mechanisms.
Conclusions
This study will determine the clinical efficacy of SXC on stage 1 hypertension and obtain the possible therapeutic mechanism of SXC on BP and VR, supporting the evidence for traditional Chinese medicine intervention in the development of hypertension.
Trial registration
The study was registered on ClinicalTrials.gov (no. NCT06093932) on October 23, 2023.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-025-05038-y.
Keywords: Vascular remodeling, Hypertension, Songling xuemaikang capsule, Randomized controlled trial, Protocol
Introduction
In the recent 30 years, the number of adults aged 30–79 with hypertension worldwide has doubled from 6.3 billion to 12.7 billion, while the control and treatment rates were less than a quarter [1]. The predictive model of China’s cardiovascular disease (CVD) policy from 2015 to 2025 showed that if patients with stage 1 and 2 hypertension were followed up and treated regularly, there would be reduced 0.8 million cases per year and obtained 1.2 million quality-adjusted life years compared with maintaining the current status [2]. It is recommended that controlling blood pressure (BP) in an early stage contributes to better prognosis and less complications, including vascular remodeling (VR) and organs damage. Two well-established interventions, behavioral and pharmacological strategies, are suggested to control BP [3, 4]. Although maintaining a heart-healthy lifestyle is beneficial to lowering BP, the shortcomings of fragile durability and susceptibility to external factors hinder the stabilization of its efficacy [5]. There is less consistency about whether antihypertension drugs should be initiated during early stage of hypertension. Especially, patients with low-moderate cardiovascular risk are generally concerned about the possible side effects rather than the benefits of long-term medication. Therefore, the guidelines indicate that stage 1 hypertension with lower BP range and low CV risk may be initially treated with non-pharmacological methods in 3 months [4, 6].
VR, mainly including structure and function alternation of small and large arteries, has been regarded as the primary marker of risk in hypertension [7]. Several longitudinal studies suggested a strong association between VR and hypertension [8, 9]. In the temporal analysis, VR preceded the increase in BP [10]. Mendelian randomization research revealed the significant bidirectional genetic predisposition between arterial stiffness and BP [11]. Aortic stiffness could damage various target organs through multiple mechanisms, which imposed a greater pulsatile load on the left ventricle (LV) contraction, evoking LV hypertrophy, impaired diastolic function and microcirculation damage of high-flow organs, such as brain and kidneys [12]. Strong evidence indicated evaluating arterial stiffness could perform incremental value to predict future CVD events over traditional risk factors [13]. Adding pulse wave velocity (PWV), a parameter reflecting the stiffness, to the Framingham risk score model resulted in net reclassification index up to over 20% [14]. Small resistance artery damage also leads to impaired vasodilatation, promoting the structural elevation of resistance in hypertension. However, current research on reversing VR relied on treatments of related risk factors, such as antihypertensive therapy [15, 16]. It is still controversial whether antihypertensive drugs have benefits on VR.
Dysbiosis of gut microbiome plays a vital role in the cardiometabolic dysfunction, which triggers the progression of hypertension and VR [17]. It is mainly manifested by dramatically reduction in microbial richness and diversity, the variation of dominant bacterial genera, causing bacterial dysfunction and disturbances in associated metabolites levels [18], such as prevotella dominates gut enterotype, promoting LPS biosynthesis to induce systematic low-grade inflammation [19]. Decreased abundance of Ruminococcaceae is associated with arterial stiffness [20]. Meanwhile, the specialized metabolic profiling in hypertension associated VR is tightly associated with intestinal microflora variation [21]. An increased ratio of Firmicutes to Bacteroidetes is accompanied with reduced production of short-chain fatty acids, causing endothelial dysfunction, reduced vascular compliance, and elevated BP [22]. However, few studies focused on how gut microbiome and related metabolites change during the process of hypertension and the association among hypertension, VR and host-microbial co-metabolism. The role of specific bacteria and associated metabolites in regulating BP and VR during antihypertensive treatment also needs to be clarified.
Traditional Chinese medicine (TCM) has a long history in China and other Asiatic countries. Reliable evidence from current studies supports TCM could be considered a complementary and alternative approach to treating and preventing of CVD [23, 24]. TCM has multi-component and multi-target properties, which could inevitably interact with gut microbiome after oral administration, regulating the composition and function of the bacterial colony to exert therapeutic effects [25]. Songling Xuemaikang capsule (SXC), a Chinese patent medicine, contains Pueraria lobata, fresh pine needles, and pearl powder. It has been approved by the National Medical Products Administration of China to anti-hypertension and improve hypertension-related symptoms. A meta-analysis study revealed the BP-lowering benefits of SXC, especially in combination with antihypertensive agents [26]. SXC has reliable safety in patients with mild hypertension, and the BP-lower efficacy of its monotherapy is not inferior to losartan [27]. Both SXC single and combination therapy could effectively improve hypertension-related symptoms and raise life quality of patients [28]. In addition, SXC has shown effects on preventing vascular endothelial damage during hypertension, alleviating arterial remodeling, and reversing myocardial structural remodeling [29, 30]. Given the clinical evidence of SXC in improving early hypertension phenotype and related VR was insufficient, and it remains unclear the mechanisms on how SXC regulates intestinal flora and metabolism to treat vascular remodeling in hypertension. Therefore, we designed a randomized, double-blind, multicenter, placebo-controlled trial to investigate the efficacy and mechanisms of SXC in treating stage 1 hypertension and VR.
Methods
Study design and setting
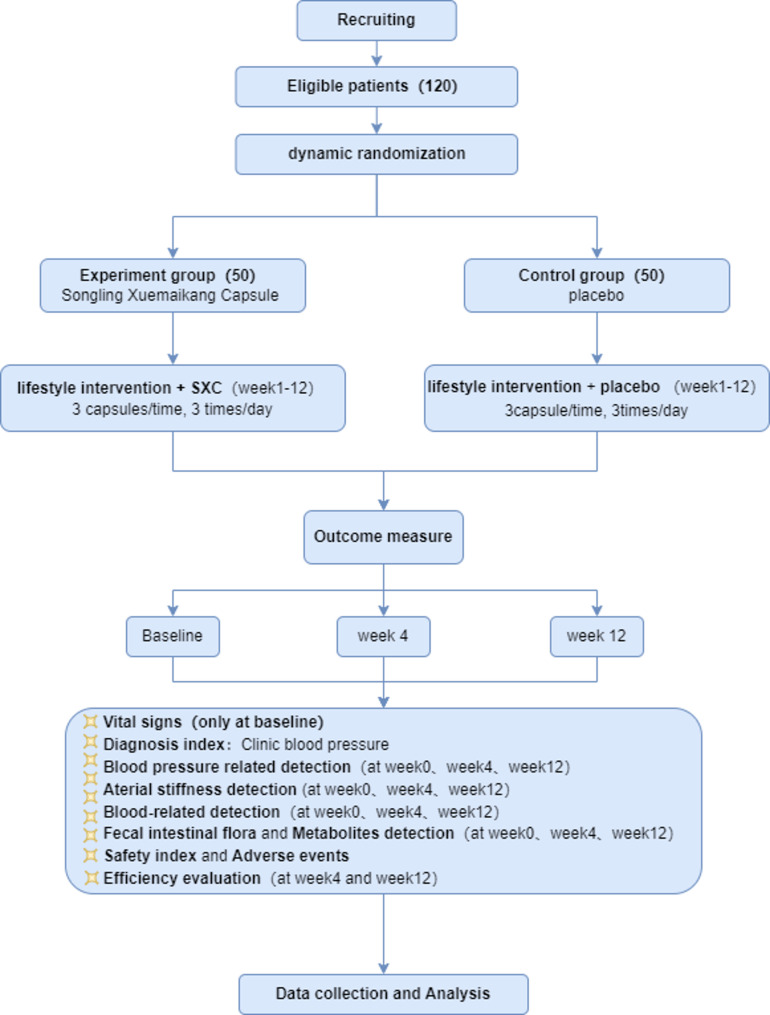
This study is a prospective, multicenter, double-blinded, pragmatic randomized controlled trial. Five clinical centers carry out recruitment, treatments, and assessments. Informed consent will be obtained after screening for eligibility, and baseline assessments will be conducted before randomization. One hundred eligible patients will be randomly allocated 1:1 into the treatment of SXC or placebo for 12 weeks. All individuals were required to follow a healthy lifestyle. During this period, antihypertensive effects of SXC and the vascular function and structural remodeling will be measured, the mechanisms of SXC in treating BP and VR will also be revealed. This study has been approved by the central Independent Ethics Committee (IEC) of Xiyuan Hospital of China Academy of Chinese Medical Sciences (Approval No. 2023.09.14v3.0). All sites obtained admission from their own ethics committees. This presentation is in accordance with the Standard Protocol Items: Recommendations for Interventional Trials 2013 (SPIRIT 2013) guidelines. This study flowchart is presented in Fig. 1.
Fig. 1.
Study flowchart
Ethical issues
The overall supervision of our trial will be in charge of the central IEC of Xiyuan Hospital of China Academy of Chinese Medical Sciences; any change in the protocol will be submitted to and decided by the Ethics Committee.
Participants
We intend to recruit 100 eligible patients from (1) Xiyuan Hospital of China Academy of Chinese Medical Sciences, (2) Xiyuan Hospital Shanxi Hospital of China Academy of Chinese Medical Sciences, (3) Suzhou Hospital of Traditional Chinese Medicine, (4) Affiliated Hospital of Shandong University of Traditional Chinese Medicine, (5) Xiyuan Hospital Jining Hospital of China Academy of Chinese Medical Sciences (Jining Hospital of Traditional Chinese Medicine). The eligible patients must satisfy the criteria defined below.
Inclusion criteria
Patients will be further screened for eligibility if they fulfill the following inclusion criteria: (1) Age 35 to 65 years, male or female. (2) Meets diagnostic criteria for essential hypertension and is classified as stage 1 hypertension [3]. (3) Have not taken any antihypertensive medicine in the past or not taken antihypertensive medicine regularly. (4) Voluntary participation in the trial with consent forms signed.
Exclusion criteria
Patients will be excluded if they have any one of the following: (1) Significant liver and kidney dysfunction, alanine and aspartate aminotransferase upper the twice of the normal range, or serum creatinine ascension (≥ 2.0 mg/dl), or estimated glomerular filtration rate reduction [<60 ml/(min·1. 73m2)]. (2) Be allergic to the ingredients of clinical trial medicine. (3) Women who are pregnant or breastfeeding, men who plan to give birth within half a year. (4) Smoking. (5) Abdominal obesity ( waist circumference: male ≥ 90 cm, female ≥ 85 cm ) or obesity (body mass index (BMI) ≥ 28 kg/m2) (BMI = weight (kg) / height 2 (m2). (6) Fasting serum uric acid levels were higher than 420 µmol/L in men and 360 µmol/L in women. (7) Abnormal fasting blood glucose (6.1 ~ 6.9 mmol/L) or impaired glucose tolerance (2-h postprandial blood glucose 7.8 ~ 11.0 mmol/L). (8) Participants with hyperlipidemia or treated with lipid-lowering drugs. (9) Combined with LV hypertrophy; carotid intima-media thickness (CIMT) ≥ 0.9 mm or atherosclerotic plaque; ankle-brachial index < 0.9. (10) Hypertensive comorbidities (cerebrovascular disease, other CVD, kidney disease, peripheral vascular disease, retinopathy, diabetes). (11) Gastrointestinal diseases, which may affect drug absorption. (12) Participants with other liver, kidney, hematopoietic systems and other serious illnesses. (13) Have a history of alcohol or drug abuse or other serious conditions which is not fit for the trial.
Randomization and blinding
This study applied the central randomization system (Nanjing Hitech Medical Information System Co., Ltd.) to conduct stratified block randomization. The stratification rules were divided into two strata according to the brachial-ankle PWV (baPWV) cutoff value of 1800 cm/s. Eligible patient information was entered into the central randomization system to generate a random number in a double-blind manner, and each enrolled patient was divided into the grouping of experiment or placebo at a 1:1 ratio. The system automatically associates each patient’s randomization number with the package number of the study drug. For drug distribution, investigators should deliver the corresponding packages for enrolled patients according to the random number.
Interventions
Eligible patients will be randomly assigned to oral administration of SXC or placebo for 12 weeks, produced by Kanghong Pharmaceutical Co. Ltd., Chengdu, China, and are almost identical in color, smell, dosage form, and appearance. All individuals will take the usual oral dose of 1.5 g (3 capsules, the usual dose) of the study drug three times daily and fill out a Medication Log Card for feedback (supplementary file 1). All subjects will receive guidance on healthy lifestyles throughout the process, including reducing sodium intake, dietary management, weight control, regular exercise, quitting smoking, limiting alcohol intake, and regulating emotions (Table 1), and fill out the Lifestyle Weekly Card (supplementary file 1). Any other drugs that affect efficacy evaluation are prohibited during the entire trial.
Table 1.
Hypertension lifestyle intervention item
| Content | Objective |
|---|---|
| 1 Reduce sodium intake and increase potassium intake | The daily salt intake per person should not exceed 6 g (one beer bottle cap), and pay attention to the intake of hidden salt (pickles, chicken essence, soy sauce, ham, pickled products, etc.) |
| 2 Reasonable meals | A nutritionally balanced, and DASH diet is recommended |
| 3 Weight control | BMI: 18.5–23.9 kg/m2; Waist circumference < 90 cm (male); Waist circumference < 85 cm (female) |
| 4 Regular exercise | 30–60 min of moderate-intensity exercise, mainly aerobic, 4–7 days a week |
| 5 Quit smoking | Quit smoking scientifically and avoid passive smoking |
| 6 Limit alcohol consumption | Daily drinking limit: liquor < 50 ml (1 tael), wine < 100 ml, or beer < 250 ml |
| 7 Psychological balance | Reduce mental stress and keep mood happy |
DASH = Dietary Approaches to Stop Hypertension
Efficacy evaluations
At baseline, 4-week and 12-week after randomization, the results of physical examination, metabolomics detection, gut microbiome omics measurements, BP-related and VR-related tests in each patient were collected. The diagram of data collection is shown in Table 2. The primary efficacy variable was the change in 24-h ambulatory systolic BP (SBP) after 4-week and 12-week treatment. Secondary efficacy variables included the change in ba-PWV, skin capillary density (SCD) and CIMT after 4-week and 12-week treatment; the change in 24-h ambulatory diastolic BP (DBP) and pulse pressure (PP), BP load, clinic, home, daytime, nighttime ambulatory SBP, DBP and PP after 4-week and 12-week treatment; the change in Hypertension Symptoms Scale score, The Patient Health Questionnaire-9 (PHQ9) and Generalized Anxiety Disorder(GAD) scale score after 4-week and 12-week treatment. In addition, the change in the gut microbiome-metabolome profile was analyzed to explore therapeutic mechanisms.
Table 2.
Diagram of data collection
| Item | Run-in period | Baseline | Treatment period | |
|---|---|---|---|---|
| Days − 14 to -3 | Days − 3 to 0 | Days 28 ± 7 | Days 84 ± 7 | |
| Informed consent | ● | |||
| History | ● | |||
| Inclusion/exclusion criteria | ● | |||
| Physical examination | ● | ● | ● | |
| Randomization | ● | |||
| Study drugs distribution | ● | ● | ||
| Comorbid medications | ● | ● | ● | |
| 24-h ambulatory BP | ● | ● | ● | |
| clinic BP | ● | ● | ● | ● |
| home BP | ● | ● | ● | |
| Progress to stage 2 or above hypertension or target organ damage | ● | |||
| ba-PWV | ● | ● | ● | |
| CIMT | ● | ● | ● | |
| SCD | ● | ● | ● | |
| Hypertension Symptoms Scale | ● | ● | ● | |
| PHQ-9 scale | ● | ● | ● | |
| GAD scale | ● | ● | ● | |
| Blood glucose test | ● | ● | ● | |
| Blood lipid tast | ● | ● | ● | |
| Hematology | ● | ● | ● | |
| Four hormones related to hypertension | ● | ● | ● | |
| Metabolomics detection | ● | ● | ● | |
| Proteomics detection | ● | ● | ● | |
| Gut microbiome omics measurements | ● | ● | ● | |
| Blood routine | ● | ● | ● | |
| Liver and renal function | ● | ● | ● | |
| Electrocardiogram | ● | ● | ● | |
| Adverse events | ● | ● | ||
BP = blood pressure; ba-PWV = brachial-ankle pulse wave velocity; CIMT = carotid intima-media thickness; SCD = skin capillary density; PHQ-9 = The Patient Health Questionnaire-9; GAD = Generalized Anxiety Disorder
Safety evaluations include adverse events and changes in physical and biochemical examinations (blood routine, liver, renal function test, and electrocardiogram).
Adverse events
Adverse events (AEs) are defined as any adverse medical event during the study period, regardless of the association with the study drug. All AEs will be assessed for their severity and relation with the research drug, then recorded in original records and electronic case report forms (eCRFs). Severe adverse events (SAE), including necessary hospitalization, prolonged hospitalization, disability, affecting workability, life-threatening or death, and causing congenital malformations, new adverse drug reactions, or severe adverse drug reactions, must be reported within 24 h according to the corresponding procedures.
Sample size
A sample size of the study was calculated using PASS 15.0 software based on the expected reduction in 24-h ambulatory SBP [31–33]. According to the previous studies [34, 35], the assumptions included a 24-h ambulatory SBP reduction value of 2.64 ± 1.22 mmHg in the SXC group and 1.7 ± 1.33 mmHg in the placebo group. Therefore, an estimated sample size 40 in each group would meet 90% (2 sides α = 0.05, β = 0.1) statistical power to detect a significant difference. Accounting a dropout rate of 20%, a total of 100 patients are needed to be allocated to attain the required number of patients for the efficacy analysis.
Statistical methods
The analysis will be performed at the Clinical Pharmacology Research Institute, Xiyuan Hospital, China Academy of Chinese Medical Sciences, combining data from all participating centers for statistical analysis of efficacy variables and AEs. Statistical analyses will be carried out using SAS 9.2 software, and the significant threshold was considered as 2-sided P < 0.05. Measurement data are described as mean and SD, which will be compared using paired t-test, analysis of variance or rank sum test. The Chi-square test, Fisher’s exact test or other methods will be used appropriately for enumeration data, described as frequencies and percentages.
Data management and quality control
This study applies the clinical data management system of Xiyuan Hospital, China Academy of Chinese Medical Sciences. The project data manager writes the data management plan, establishes the project database, and produces the CRF draft. The CRF, the first draft and the final version of the research medical record are completed by the research undertaking unit. The research unit and CRO provide standard operating procedures (SOP) to standardize operating procedures. All investigators should receive training and permission from the principal investigator to carry out the specific trial procedures. The research unit is responsible for the audit of this trial and provides the researcher with an audit certificate. The main unit has access to the final trial dataset and will eventually communicate the experimental results in a published form.
Discussion
VR is an important pathological character for hypertensive damage and other CVD. Early interventions in VR and BP may improve the prognosis of diseases. Although recent studies have demonstrated that TCM can effectively lower BP and improve related VR [36, 37], the interactions among SXC, BP, and VR, as well as the underlying mechanisms remain unclear.
Recent evidence has demonstrated the benefits of SXC in BP lowering, alleviating hypertension-related symptoms, and improving blood lipid levels [26, 27, 38]. While, there are insufficient high-quality RCTs evaluating the role of SXC in improving the vascular structure and function. This study is a multicenter, randomized, double-blind, placebo-controlled clinical trial aiming to investigate the efficacy and mechanism of the SXC on stage1 hypertension. Considering the significant association between VR and hypertension, this study evaluates the changes in vascular structure and function during the entire intervention process. This study utilized baPWV to evaluate arterial stiffness, explicitly focusing on individuals with stage 1 hypertension from 35 to 65 years of age to reduce the influence of age and BP classification on the findings. Moreover, to investigate the potential effects of the study drug on a population of stage 1 hypertensive with different degrees of arterial stiffness, the study was randomly stratified according to baPWV < 1800 cm/s or ≥ 1800 cm/s.
Microvascular rarefaction (MVR), a reduction in the number of multilayered capillaries and small arterioles, is a typical pathological lesion in the early stage of elevated BP. Previous studies have shown that patients with hypertension have a 12–20% reduction in capillary number compared with normal BP [39–41]. MVR has been shown to affect tissue pressure and flow patterns, resulting in increased peripheral resistance, inadequate tissue perfusion and altered body metabolism [42, 43]. Previous studies have yet to explore whether the antihypertensive effect of SXC partially attributes to the enhancement of microcirculation and the decrease of peripheral vascular resistance. Therefore, the novelty of this study is the evaluation of the efficacy of SXC on MVR as assessed by SCD examination.
This study also innovatively focuses on the co-metabolism between the host and gut microbes to explore the mechanism among SXC, VR and hypertension. Current research has confirmed that human-microbial co-metabolism is closely associated to the development of various diseases [44, 45] Together, gut bacteria modulate host metabolic processes and continually shape the immune system [46]. Convincing evidence revealed the disorders of gut barrier function, gut microbiome profile, and metabolites may be crucial factors in the development of hypertension [47]. Human fecal metagenomic sequencing revealed a significantly low abundance and centrality of F.plautii in the microbial community of subjects with elevated arterial stiffness. Moreover, FMT from donors with elevated PWV was sufficient to augment arterial stiffness in mice [48]. Antibiotic treatment improves endothelial cell function due to a diminished NADPH oxidase-dependent ROS production and increases Treg infiltration and IL-10 in the vascular wall in mineralocorticoid excess-treated rats [49]. Additionally, cis-aconitic acid (CAA), a major intermediate metabolite involved in glycolysis, improved elastic fiber network and reversed increased PWV through the suppression of MMP-2 and inhibition of MCP-1 and NF-κB activation in both AngII-induced and humanized model of arterial stiffness [48]. Furthermore, studies have shown that puerarin, the active ingredient of SXC, can downregulate the expression of α-SMA, IL-6, and IL-8 via the miR-29b-3p/IGF1 signaling pathway, thereby suppressing the proliferation, migration, and inflammatory response of vascular smooth muscle cells [50]. However, the causal role of gut microbiota in the progression of VR and the molecular mechanisms underlying the actions of SXC remain largely unknown. Based on the above, we will collected blood, urine, and fecal samples for metabolomics, genomics, and VR-related cytokines tests to investigate the potential effects of SXC on restoring homeostasis of the gut microbes and metabolic to improve VR.
This study has several limitations. As the intervention period was only 12 weeks, further research may be necessary to determine the long-term prognosis of SXC for treating stage 1 hypertension. In addition, a real-world study may be considered to assess the effectiveness of SXC in a real-life diagnostic setting and its impact on cardiovascular events over an extended period.
In conclusion, this study is designed to reveal the clinical efficacy and mechanisms of SXC compared with placebo in the treatment of stage 1 hypertension, and illuminatesthe interaction among SXC, hypertension and VR from the perspective of co-metabolism between the host and gut microbes. This work will provide a high level of clinical evidence on early intervention of TCM on treating hypertension and associated VR.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
AW, QL, LY contributed substantially to the conception, design, and writing of the manuscript. ZZ, ZZ, WT prepared figures and wrote the manuscript. AW and HX contributed substantially to the design. All the authors have read and agreed to the submitted version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China ( No. 82230125).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study has been approved by the central IEC of Xiyuan Hospital of China Academy of Chinese Medical Sciences (Approval No. 2023.09.14v3.0). Participants will be asked to sign an informed consent(s) before participating in this study.
Consent for publication
Not applicable. No data are presented in this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiyu Liu and Linghua Yu contributed equally to this work.
Contributor Information
Anlu Wang, Email: wanganlu@bucm.edu.cn.
Hao Xu, Email: xuhaotcm@hotmail.com.
References
- 1.Worldwide trends in. Hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398(10304):957–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Ma L, Liu M, Fan J, Hu S. Summary of the 2022 report on cardiovascular health and diseases in China. Chin Med J (Engl). 2023;136(24):2899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Algharably EAE, et al. 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the International Society Of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens. 2023;41(12):1874–2071. [DOI] [PubMed] [Google Scholar]
- 5.Pazoki R, Dehghan A, Evangelou E, Warren H, Gao H, Caulfield M, Elliott P, Tzoulaki I. Genetic predisposition to high blood pressure and lifestyle factors: associations with midlife blood pressure levels and cardiovascular events. Circulation. 2018;137(7):653–61. [DOI] [PubMed] [Google Scholar]
- 6.Liu J. Highlights of the 2018 Chinese hypertension guidelines. Clin Hypertens. 2020;26:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116(6):1007–21. [DOI] [PubMed] [Google Scholar]
- 8.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore longitudinal study of aging. J Am Coll Cardiol. 2008;51(14):1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Li S, Fernandez C, Sun D, Lai CC, Zhang T, Bazzano L, Urbina EM, Deng HW. Temporal relationship between elevated blood pressure and arterial stiffening among middle-aged black and white adults: the Bogalusa heart study. Am J Epidemiol. 2016;183(7):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecelja M, Keehn L, Ye L, Spector TD, Hughes AD, Chowienczyk P. Genetic aetiology of blood pressure relates to aortic stiffness with bi-directional causality: evidence from heritability, blood pressure polymorphisms, and Mendelian randomization. Eur Heart J. 2020;41(35):3314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128(7):864–86. [DOI] [PubMed] [Google Scholar]
- 13.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698–722. [DOI] [PMC free article] [PubMed]
- 14.Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension. 2017;69(6):1045–52. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Boutouyrie P. Dose-dependent arterial destiffening and inward remodeling after olmesartan in hypertensives with metabolic syndrome. Hypertension. 2014;64(4):709–16. [DOI] [PubMed] [Google Scholar]
- 16.Protogerou A, Blacher J, Stergiou GS, Achimastos A, Safar ME. Blood pressure response under chronic antihypertensive drug therapy: the role of aortic stiffness in the REASON (Preterax in regression of arterial stiffness in a controlled double-blind) study. J Am Coll Cardiol. 2009;53(5):445–51. [DOI] [PubMed] [Google Scholar]
- 17.Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol. 2018;15(1):20–32. [DOI] [PubMed] [Google Scholar]
- 18.Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR Jr., Shikany JM, Lloyd-Jones DM, Launer LJ, Fodor AA, et al. Gut microbiota composition and blood pressure. Hypertension. 2019;73(5):998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menni C, Lin C, Cecelja M, Mangino M, Matey-Hernandez ML, Keehn L, Mohney RP, Steves CJ, Spector TD, Kuo CF, et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur Heart J. 2018;39(25):2390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang F, Chen H, Gao Y, An N, Li X, Pan X, Yang X, Tian L, Sun J, Xiong X, et al. Gut microbiota-derived short-chain fatty acids and hypertension: mechanism and treatment. Biomed Pharmacother. 2020;130:110503. [DOI] [PubMed] [Google Scholar]
- 23.Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. 2017;69(24):2952–66. [DOI] [PubMed] [Google Scholar]
- 24.Zhang DY, Cheng YB, Guo QH, Shan XL, Wei FF, Lu F, Sheng CS, Huang QF, Yang CH, Li Y, et al. Treatment of masked hypertension with a Chinese herbal formula: a randomized, placebo-controlled trial. Circulation. 2020;142(19):1821–30. [DOI] [PubMed] [Google Scholar]
- 25.Feng W, Ao H, Peng C, Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–91. [DOI] [PubMed] [Google Scholar]
- 26.Meng T, Wang P, Xie X, Li T, Kong L, Xu Y, Cao K, Gao Y, He Q, Lai X. Efficacy and safety of Songling Xuemaikang capsule for essential hypertension: a systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2022;107:154459. [DOI] [PubMed] [Google Scholar]
- 27.Lai X, Dong Z, Wu S, Zhou X, Zhang G, Xiong S, Wu W, Cao R, Wang X, Hua Q, et al. Efficacy and safety of Chinese herbal medicine compared with losartan for mild essential hypertension: a randomized, multicenter, double-blind, noninferiority trial. Circ Cardiovasc Qual Outcomes. 2022;15(3):e007923. [DOI] [PubMed] [Google Scholar]
- 28.Chen WQ, Chen FR. Effect of Songling Xuemaikang capsule combined with captopril on quality of life in primary hypertension patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21(9):660–2. [PubMed] [Google Scholar]
- 29.Qi J, Tan Y, Fan D, Pan W, Yu J, Xu W, Wu J, Zhang M. Songling Xuemaikang capsule inhibits isoproterenol-induced cardiac hypertrophy via CaMKIIδ and ERK1/2 pathways. J Ethnopharmacol. 2020;253:112660. [DOI] [PubMed] [Google Scholar]
- 30.Liu LT, Liang L, Wang W, Yan CQ, Zhang J, Xiao YC, Ye L, Zhao MX, Huang QS, Bian JJ, et al. Isolariciresinol-9’-O-α-L-arabinofuranoside protects against hydrogen peroxide–induced apoptosis of human umbilical vein endothelial cells via a PI3K/Akt/Bad–dependent pathway. Mol Med Rep. 2018;17(1):488–94. [DOI] [PubMed] [Google Scholar]
- 31.White WB, Weber MA, Sica D, Bakris GL, Perez A, Cao C, Kupfer S. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57(3):413–20. [DOI] [PubMed] [Google Scholar]
- 32.Pareek AK, Messerli FH, Chandurkar NB, Dharmadhikari SK, Godbole AV, Kshirsagar PP, Agarwal MA, Sharma KH, Mathur SL, Kumbla MM. Efficacy of low-dose chlorthalidone and hydrochlorothiazide as assessed by 24-h ambulatory blood pressure monitoring. J Am Coll Cardiol. 2016;67(4):379–89. [DOI] [PubMed] [Google Scholar]
- 33.Staplin N, de la Sierra A, Ruilope LM, Emberson JR, Vinyoles E, Gorostidi M, Ruiz-Hurtado G, Segura J, Baigent C, Williams B. Relationship between clinic and ambulatory blood pressure and mortality: an observational cohort study in 59 124 patients. Lancet. 2023;401(10393):2041–50. [DOI] [PubMed] [Google Scholar]
- 34.Kawano Y, Sato Y, Yoshinaga K. A randomized trial of the effect of an angiotensin II receptor blocker SR47436 (irbesartan) on 24-hour blood pressure in patients with essential hypertension. Hypertens Res. 2008;31(9):1753–63. [DOI] [PubMed] [Google Scholar]
- 35.Zhu GH, Sun XP, Ding CT, Zhao H, Li J, Hua Q. Effect of Songlingxuemaikang on mild essential hypertension in patients: a randomized parallel-controlled study. J Tradit Chin Med. 2021;41(5):799–805. [DOI] [PubMed] [Google Scholar]
- 36.Yao J, Zhang C, Yang Y, Fang X, Chen Q, Zhong G. Comparative transcriptomic analysis revealed novel potential therapeutic targets of traditional Chinese medicine (Pinggan-Qianyang decoction) on vascular remodeling in spontaneously hypertensive rats. Chin Med. 2021;16(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, Xu S, Liang S, Duan C, Xu Z, Zhao L, Wen F, Li Q, Li Y, Zhang J. Hypertensive vascular and cardiac remodeling protection by allicin in spontaneous hypertension rats via CaMK II/NF-κB pathway. Biomed Pharmacother. 2022;155:113802. [DOI] [PubMed] [Google Scholar]
- 38.Lai X, Fang Z, Dong Z, Wu S, Zhou X, Gao Y. A propensity score matched comparison of blood pressure lowering in essential hypertension patients treated with antihypertensive Chinese herbal medicine: comparing the real-world registry data vs. randomized controlled trial. Clin Exp Hypertens. 2023;45(1):2249269. [DOI] [PubMed] [Google Scholar]
- 39.Prasad A, Dunnill GS, Mortimer PS, MacGregor GA. Capillary rarefaction in the forearm skin in essential hypertension. J Hypertens. 1995;13(2):265–8. [PubMed] [Google Scholar]
- 40.Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GC. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997;99(8):1873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serné EH, Gans RO, ter Maaten JC, ter Wee PM, Donker AJ, Stehouwer CD. Capillary recruitment is impaired in essential hypertension and relates to insulin’s metabolic and vascular actions. Cardiovasc Res. 2001;49(1):161–8. [DOI] [PubMed] [Google Scholar]
- 42.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999;34(4 Pt 1):655–8. [DOI] [PubMed] [Google Scholar]
- 43.Hudetz AG. Percolation phenomenon: the effect of capillary network rarefaction. Microvasc Res. 1993;45(1):1–10. [DOI] [PubMed] [Google Scholar]
- 44.Brial F, Chilloux J, Nielsen T, Vieira-Silva S, Falony G, Andrikopoulos P, Olanipekun M, Hoyles L, Djouadi F, Neves AL, et al. Human and preclinical studies of the host-gut microbiome co-metabolite hippurate as a marker and mediator of metabolic health. Gut. 2021;70(11):2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang K, Zhang Z, Hang J, Liu J, Guo F, Ding Y, Li M, Nie Q, Lin J, Zhuo Y, et al. Microbial-host-isozyme analyses reveal microbial DPP4 as a potential antidiabetic target. Sci (New York NY). 2023;381(6657):eadd5787. [DOI] [PubMed] [Google Scholar]
- 46.Mirji G, Worth A, Bhat SA, El Sayed M, Kannan T, Goldman AR, Tang HY, Liu Q, Auslander N, Dang CV, et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci Immunol. 2022;7(75):eabn0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, Wang Q, Liu Y, Wang L, Ge Z, Li Z, Feng S, Wu C. Gut microbiota and hypertension: association, mechanisms and treatment. Clin Exp Hypertens. 2023;45(1):2195135. [DOI] [PubMed] [Google Scholar]
- 48.Luo S, Zhao Y, Zhu S, Liu L, Cheng K, Ye B, Han Y, Fan J, Xia M. Flavonifractor plautii protects against elevated arterial stiffness. Circ Res. 2023;132(2):167–81. [DOI] [PubMed] [Google Scholar]
- 49.Robles-Vera I, de la Visitación N, Toral M, Sánchez M, Romero M, Gómez-Guzmán M, Vargas F, Duarte J, Jiménez R. Changes in gut microbiota induced by doxycycline influence in vascular function and development of hypertension in DOCA-salt rats. Nutrients. 2021;13(9). [DOI] [PMC free article] [PubMed]
- 50.Li J, Li Y, Yuan X, Yao D, Gao Z, Niu Z, Wang Z, Zhang Y. The effective constituent puerarin, from Pueraria lobata, inhibits the proliferation and inflammation of vascular smooth muscle in atherosclerosis through the miR-29b-3p/IGF1 pathway. Pharm Biol. 2023;61(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.