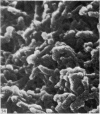
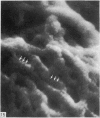
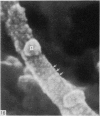
Abstract
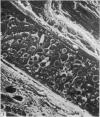
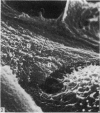
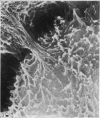
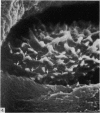
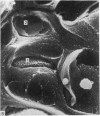
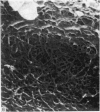
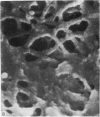
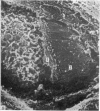
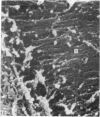
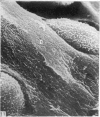
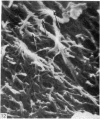
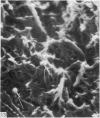
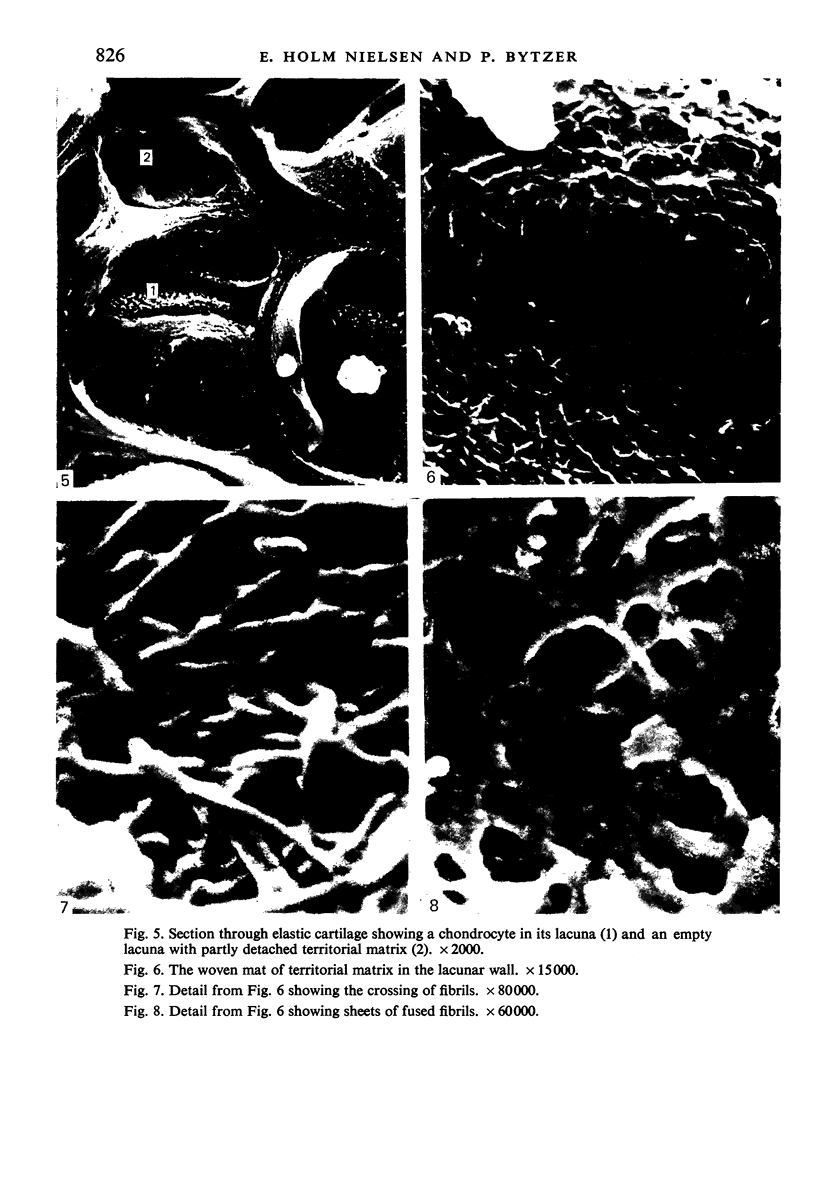
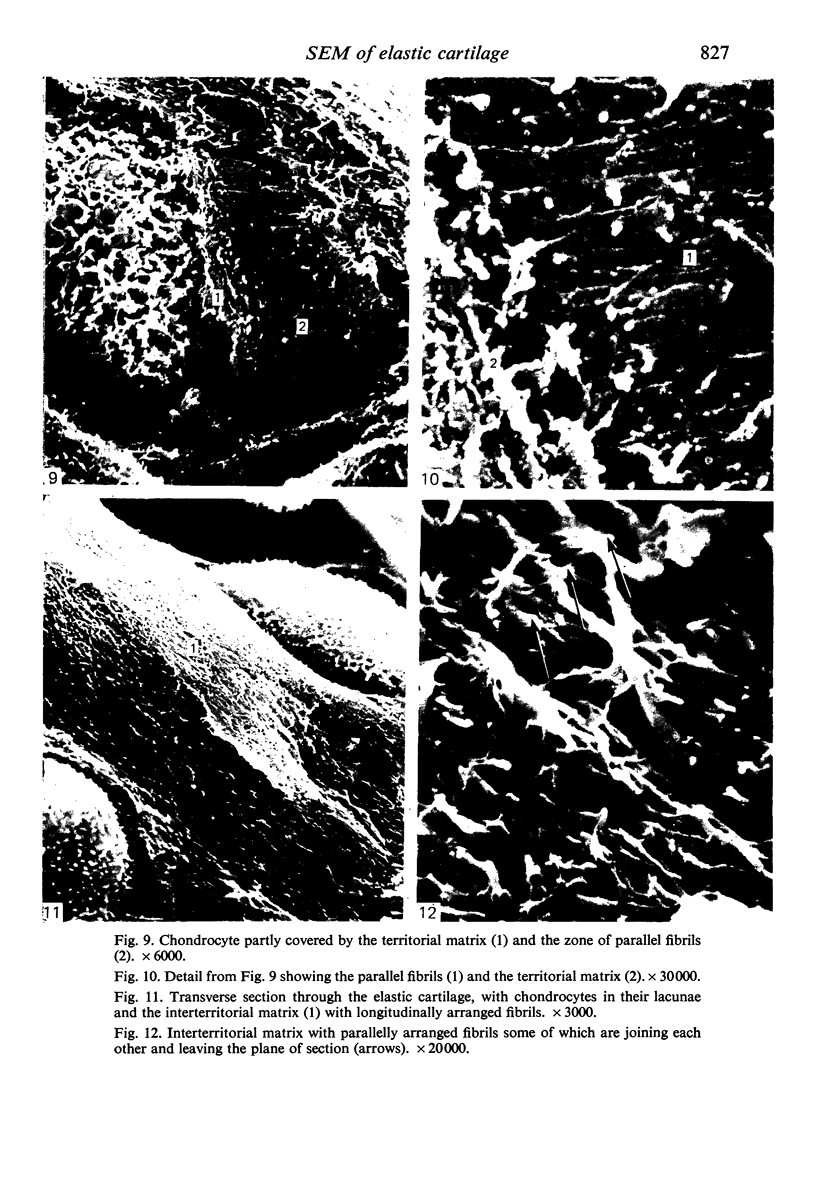
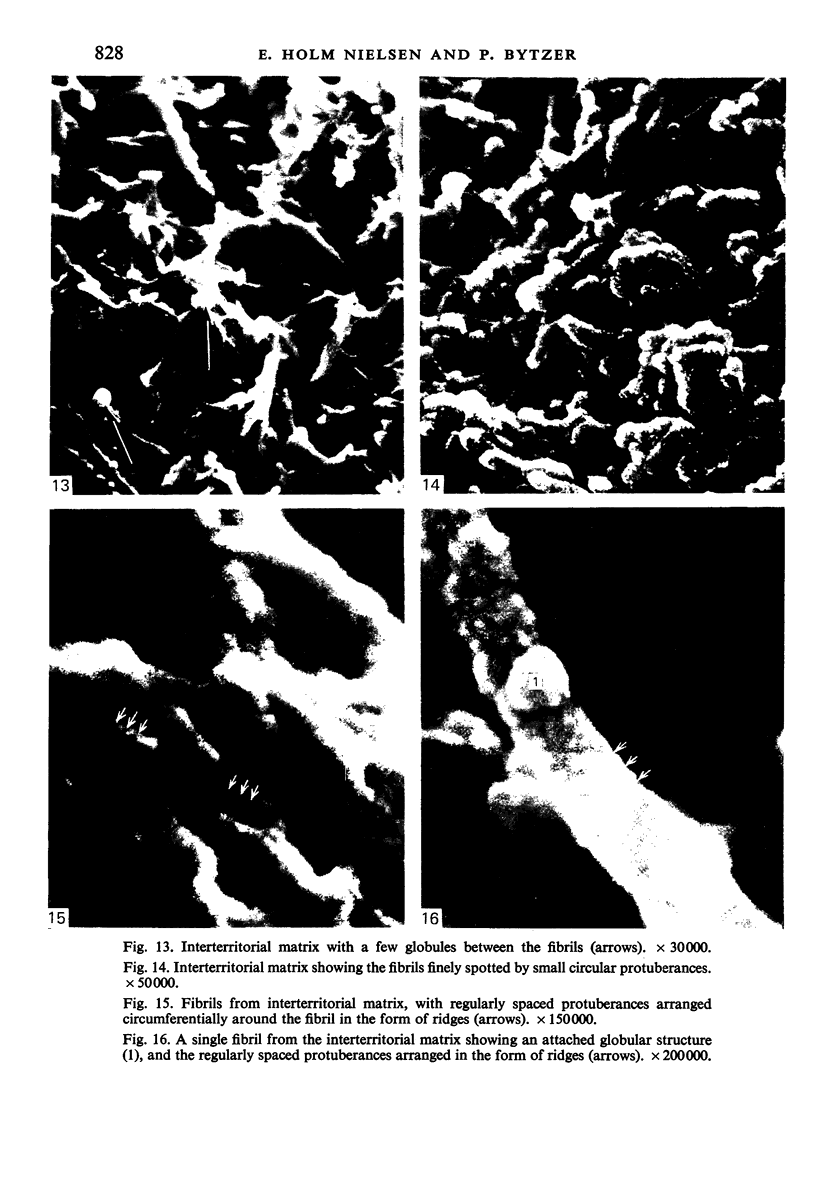
The elastic cartilage of the rat epiglottis was studied with a high-resolution SEM technique. The chondrocytes were found to be anchored in their lacunae by fibrils running in from the territorial matrix. This matrix exhibited a dense network of fibrils arranged tangentially around the lacunar cavity. The fibrils of the inter-territorial matrix however, formed a three dimensional network of sheets with interconnecting fibrils. The SEM has shown up for the first time a substructure in the fibrils in the form of circular protuberances arranged circumferentially around the fibrils and forming ridges 12--19 nm apart. We suggest that the fibrils are collagen, and the protuberances are the proteoglycans attached to the collagen fibrils. Globules seen attached to the fibrils are most probably 'matrix granules' as observed in other kinds of cartilage. The total inability to visualize elastin with the high resolution SEM is puzzling.
Full text
PDF
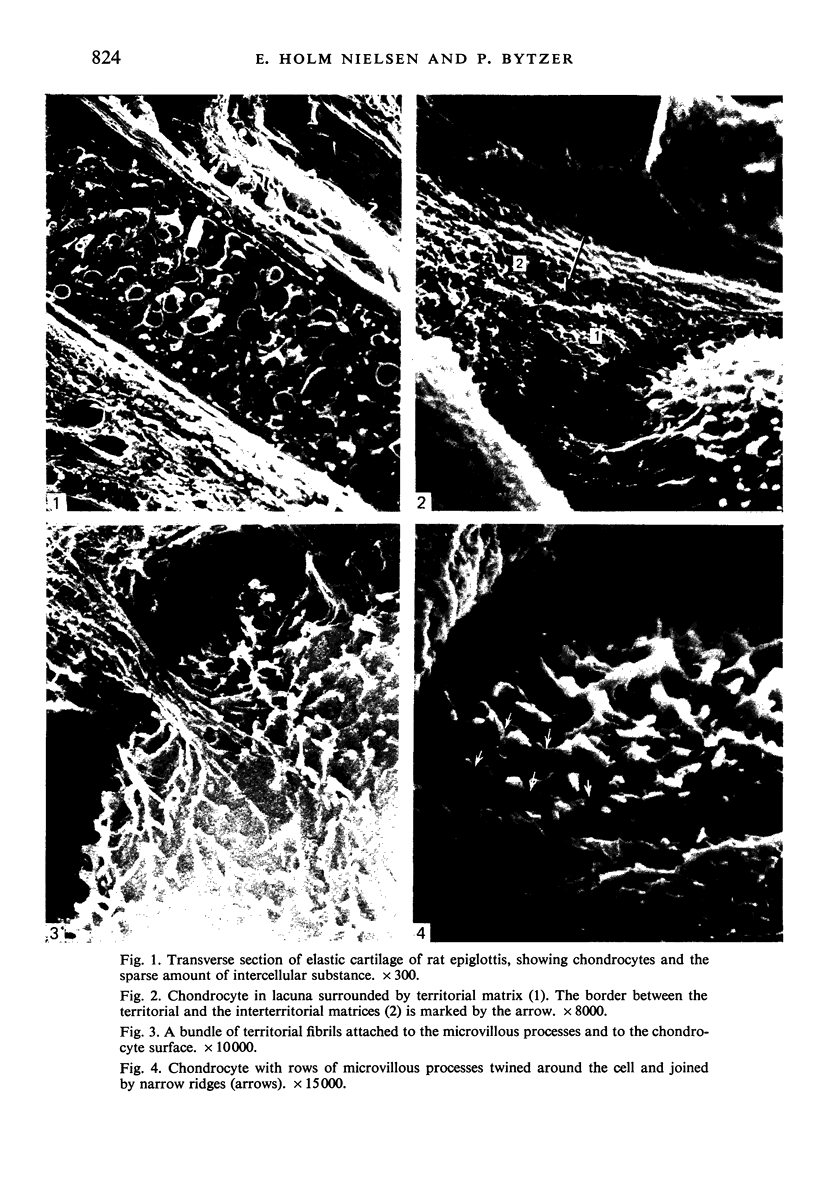




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke I. C. Articular cartilage: a review and scanning electron microscope study. 1. The interterritorial fibrillar architecture. J Bone Joint Surg Br. 1971 Nov;53(4):732–750. [PubMed] [Google Scholar]
- Clarke I. C. Articular cartilage: a review and scanning electron microscope study. II. The territorial fibrillar architecture. J Anat. 1974 Nov;118(Pt 2):261–280. [PMC free article] [PubMed] [Google Scholar]
- Nielsen E. H. The elastic cartilage in the normal rat epiglottis. I. Fine structure. Cell Tissue Res. 1976 Oct 6;173(2):179–191. doi: 10.1007/BF00221374. [DOI] [PubMed] [Google Scholar]
- Ornoy A., Langer Y. Scanning electron microscopy studies on the origin and structure of matrix vesicles in epiphyseal cartilage from young rats. Isr J Med Sci. 1978 Jul;14(7):745–752. [PubMed] [Google Scholar]
- Smith J. W., Peters T. J., Serafini-Fracassini A. Observations on the distribution of the proteinpolysaccharide complex and collagen in bovine articular cartilage. J Cell Sci. 1967 Mar;2(1):129–136. doi: 10.1242/jcs.2.1.129. [DOI] [PubMed] [Google Scholar]
- Thyberg J. Electron microscopy of cartilage proteoglycans. Histochem J. 1977 May;9(3):259–266. doi: 10.1007/BF01004761. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Lohmander S., Friberg U. Electron microscopic demonstration of proteoglycans in guinea pig epiphyseal cartilage. J Ultrastruct Res. 1973 Dec;45(5):407–427. doi: 10.1016/s0022-5320(73)80070-x. [DOI] [PubMed] [Google Scholar]
- Zimmy M. L., Redler I. Scanning electron microscopy of chondrocytes. Acta Anat (Basel) 1972;83(3):398–402. doi: 10.1159/000143874. [DOI] [PubMed] [Google Scholar]