Abstract
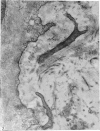
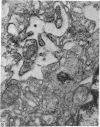
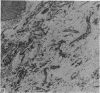
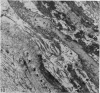
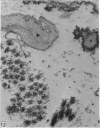
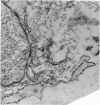
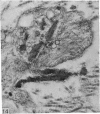
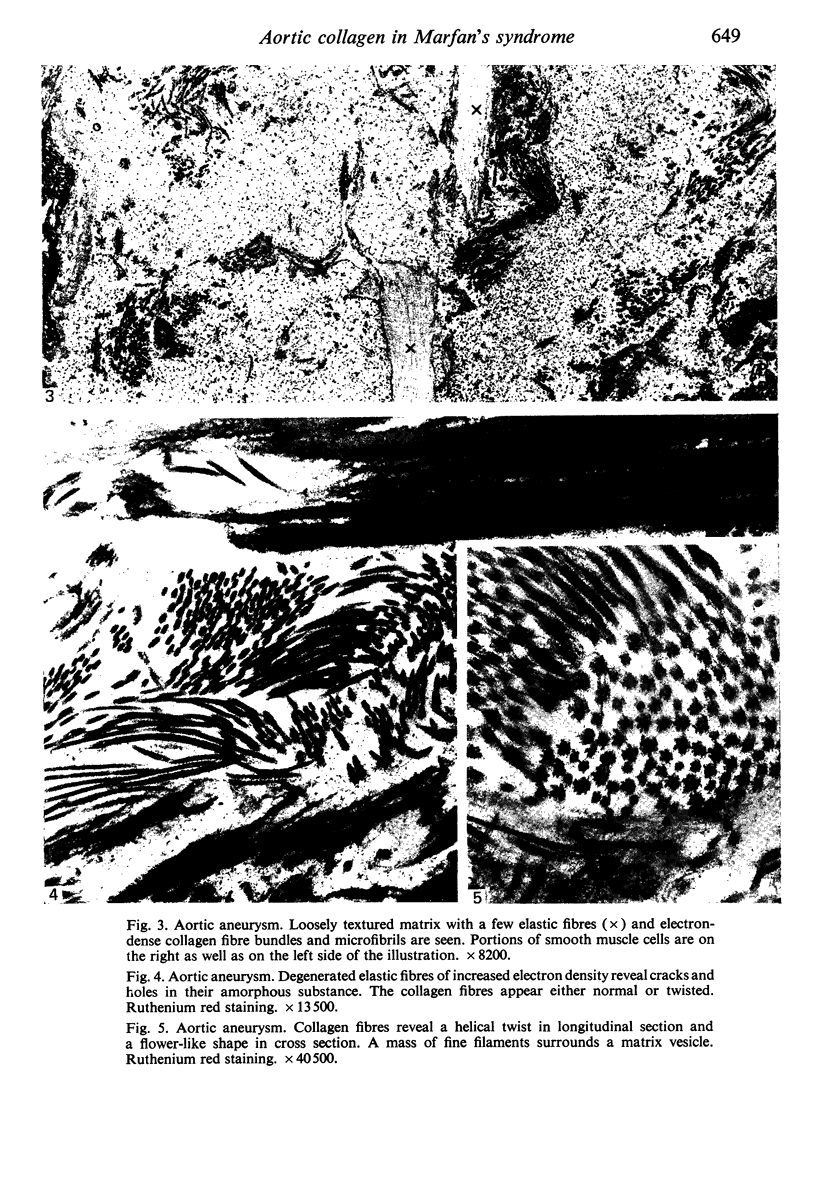
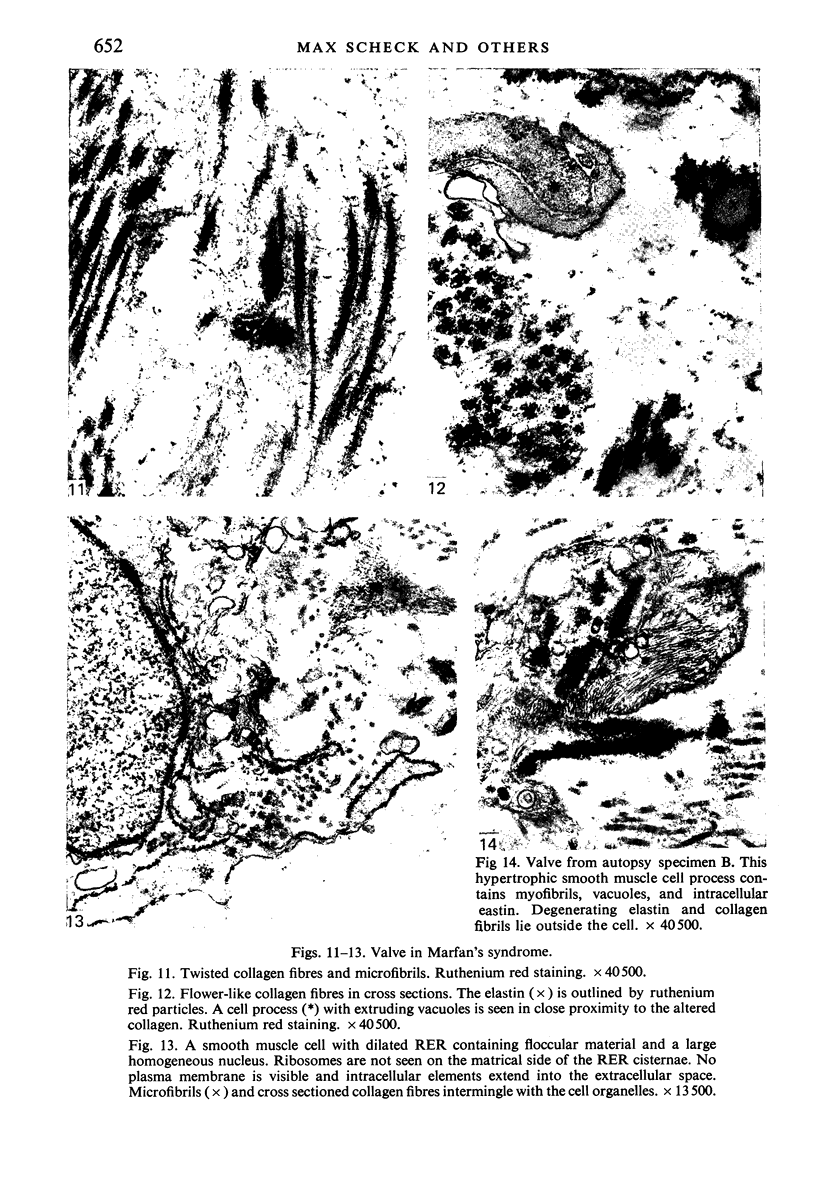
Aneurysmal aortic tissue and the mitral valve of a patient with Marfan's syndrome were examined. Biochemical analysis of the tissue showed a qualitative and quantitative defect in alpha 2 chain production of Type I collagen. On polyacrylamide gel electrophoresis of the aortic extract, two separate bands in the alpha 2 region and an increase of the alpha 1 to alpha 2 ratio were found. Examination by electron microscopy revealed elastic fibre degeneration, helical collagen fibres, and metabolically active modified smooth muscle cells. The formation of helical collagen fibres is attributed to a defect in the development of chains and cross-links of collagen precursors produced by the hypertrophic smooth muscle cells. Elastic fibre disintegration is believed to be due to a lack of support by Type I collagen fibres, which have decreased tensile strength. A scheme for the pathogenesis of aortic aneurysm and other connective tissue abnormalities in Marfan's syndrome is proposed as follows. Type I collagen fibres have decreased tensile strength because of a defect in the alpha 2 chain biosynthesis and decreased cross-linking. Over many years the wall of the ascending aorta is subjected to cyclic stresses and it dilates. Elastic fibres disintegrate. The attempt at repair by metabolically activated modified smooth muscle cells is abortive, and rupture is likely to occur.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Danielsen L., Asboe-Hansen G. Biosynthesis of collagen in pseudoxanthoma elasticum. Acta Derm Venereol. 1973;53(6):429–434. [PubMed] [Google Scholar]
- Bouteille M., Pease D. C. The tridimensional structure of native collagenous fibrils, their proteinaceous filaments. J Ultrastruct Res. 1971 May;35(3):314–338. doi: 10.1016/s0022-5320(71)80161-2. [DOI] [PubMed] [Google Scholar]
- Danielsen L., Kobayasi T. Degeneration of dermal elastic fibres in relation to age and light-exposure. Preliminary report on electron microscopic studies. Acta Derm Venereol. 1972;52(1):1–10. [PubMed] [Google Scholar]
- Danielsen L., Kobayasi T., Larsen H. W., Midtgaard K., Christensen H. E. Pseudoxanthoma elasticum. A clinico-pathological study. Acta Derm Venereol. 1970;50(5):355–373. [PubMed] [Google Scholar]
- Danielsen L., Kobayasi T. Pseudoxanthoma elasticum. An ultrastructural study of scar tissue. Acta Derm Venereol. 1974;54(2):121–128. [PubMed] [Google Scholar]
- Fabre M. T., Duret J. C., Broustet A., Bouissou H. Ultrastructure du derme dans le syndrome de Marfan. Presse Med. 1968 Feb 21;76(9):419–422. [PubMed] [Google Scholar]
- Greenlee T. K., Jr, Ross R., Hartman J. L. The fine structure of elastic fibers. J Cell Biol. 1966 Jul;30(1):59–71. doi: 10.1083/jcb.30.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Klingmüller G., Rodermund O. E. Hyalinosis cutis et mucosae. An electron microscopic study. Acta Derm Venereol. 1972;52(3):179–195. [PubMed] [Google Scholar]
- Krieg T., Müller P. K. The marfan's syndrome. In vitro study of collagen metabolism in tissue specimens of the aorta. Exp Cell Biol. 1977;45(3-4):207–221. [PubMed] [Google Scholar]
- Lillie J. H., MacCallum D. K., Scaletta L. J., Occhino J. C. Collagen structure: evidence for a helical organization of the collagen fibril. J Ultrastruct Res. 1977 Feb;(2):134–143. doi: 10.1016/s0022-5320(77)90025-9. [DOI] [PubMed] [Google Scholar]
- Macek M., Hurych J., Chvapil M., Kadlecová V. Study of fibroblasts in Marfan's syndrome. Humangenetik. 1966;3(2):87–97. doi: 10.1007/BF00291289. [DOI] [PubMed] [Google Scholar]
- Merker H. J., Shibolet S., Sohar E., Gafni J., Heler H. Alterations in fine structure of collagen in amyloidosis. Naturwissenschaften. 1966 Oct;53(20):527–527. doi: 10.1007/BF00600646. [DOI] [PubMed] [Google Scholar]
- Priest R. E., Moinuddin J. F., Priest J. H. Letter: Collagen of Marfan syndrome is abnormally soluble. Nature. 1973 Oct 5;245(5423):264–266. doi: 10.1038/245264a0. [DOI] [PubMed] [Google Scholar]
- Puchtler H., Meloan S. N., Pollard G. R. Light microscopic distinction between elastin, pseudo-elastica (type III collagen?) AND INTERSTITIAL COLLAGEN. Histochemistry. 1976 Oct 7;49(1):1–14. doi: 10.1007/BF00490121. [DOI] [PubMed] [Google Scholar]
- ROARK J. W. The Marfan syndrome: report of one case with autopsy, special histological study, and review of the literature. AMA Arch Intern Med. 1959 Jan;103(1):123–132. doi: 10.1001/archinte.1959.00270010129017. [DOI] [PubMed] [Google Scholar]
- Reye R. D., Bale P. M. Elastic tissue in pulmonary emphysema in Marfan syndrome. Arch Pathol. 1973 Dec;96(6):427–431. [PubMed] [Google Scholar]
- Ross R., Fialkow P. J., Altman L. K. Fine structure alterations of elastic fibers in pseudoxanthoma elasticum. Clin Genet. 1978 Feb;13(2):213–223. doi: 10.1111/j.1399-0004.1978.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Ross R., Klebanoff S. J. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J Cell Biol. 1971 Jul;50(1):159–171. doi: 10.1083/jcb.50.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Klingmüller G. Elektronenmikroskopische Untersuchungen zur Morphogenese elastischer Fasern bei der senilen Elastose und dem Pseudoxanthoma elasticum. Arch Dermatol Res. 1977 Dec 27;260(3):179–191. doi: 10.1007/BF00561412. [DOI] [PubMed] [Google Scholar]
- Saruk M., Eisenstein R. Aortic lesion in Marfan syndrome: the ultrastructure of cystic medial degeneration. Arch Pathol Lab Med. 1977 Feb;101(2):74–77. [PubMed] [Google Scholar]
- Siegel R. C., Chen K. H., Greenspan J. S., Aguiar J. M. Biochemical and immunochemical study of lysyl oxidase in experimental hepatic fibrosis in the rat. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2945–2949. doi: 10.1073/pnas.75.6.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Staubesand J. Matrix Vesikel und Mediadysplasie. Ein neues Konzept zur formalen Pathogenese der Varikose (Kurzbericht). Med Welt. 1977 Dec 2;28(48):1943–1946. [PubMed] [Google Scholar]
- Steinmann B., Gitzelmann R., Vogel A., Grant M. E., Harwood R., Sear C. H. Ehlers-Danlos syndrome in two siblings with deficient lysyl hydroxylase activity in cultured skin fibroblasts but only mild hydroxylysine deficit in skin. Helv Paediatr Acta. 1975 Oct;30(3):255–274. [PubMed] [Google Scholar]
- Takebayashi S., Kubota I., Takagi T. Ultrastructural and histochemical studies of vascular lesions in Marfan's syndrome, with report of 4 autopsy cases. Acta Pathol Jpn. 1973 Nov;23(4):847–866. doi: 10.1111/j.1440-1827.1973.tb02780.x. [DOI] [PubMed] [Google Scholar]
- Volpin D., Pasquali-Ronchetti I., Castellani I., Giro M. G., Peserico A., Mori G. Ultrastructural and biochemical studies on a case of elastosis perforans serpiginosa. Dermatologica. 1978;156(4):209–223. doi: 10.1159/000250919. [DOI] [PubMed] [Google Scholar]