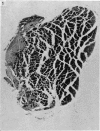
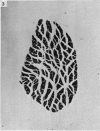
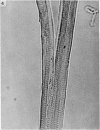
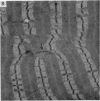
Abstract
Soleus muscles of male and female mice were overloaded by surgical resection of parts of gastrocnemius and plantaris. The effects of overloading were examined histologically after 7, 55 and 208 post-operative days, and also in teased preparations. Animals studied after 7 post-operative days showed a marked increase in muscle weight, but no significant change in mean fibre diameter or fibre number. Animals studied after 55 and 208 post-operative days showed an increase in soleus muscle weight, with fibre hypertrophy (but no increase in fibre number) proximally, while distally there was an increase in the number of fibre profiles in cross sections, some being wider, some thinner than normal. The small diametered fibres seem to persist indefinitely. From the evidence, both direct and indirect, it was concluded that surgically overloaded fibres split longitudinally into unequal parts, and that this explains the increase in fibre profiles in distal cross sections as well as their variation in size. It is clear that, because of the splitting, a surgically overloaded muscle is a difficult model on which to study fibre hypertrophy.
Full text
PDF



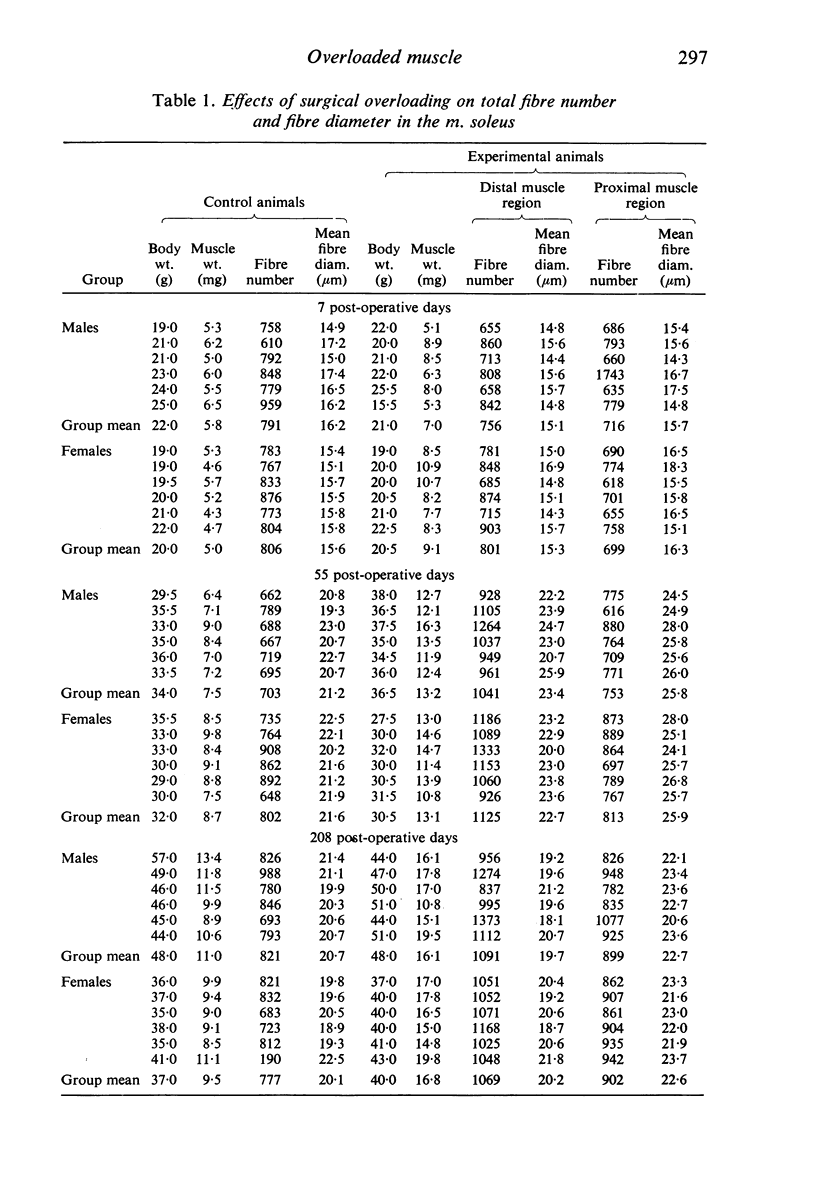
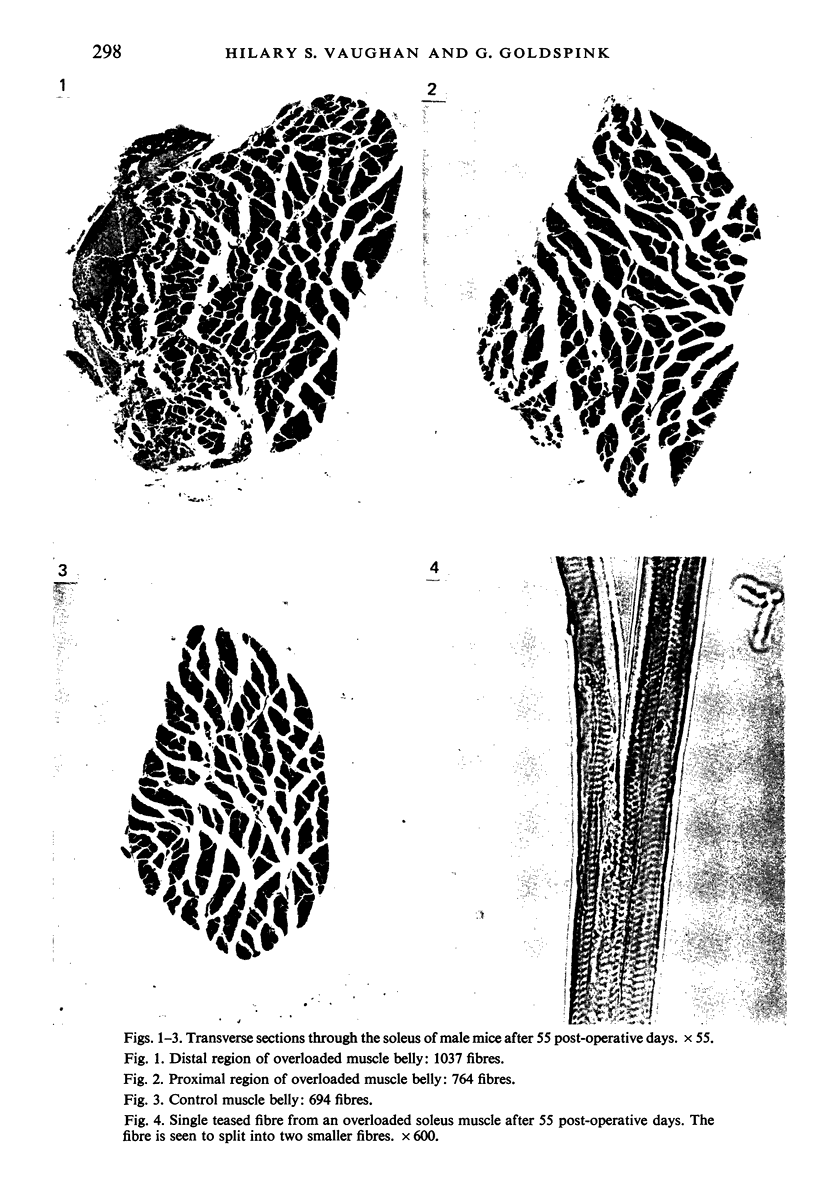

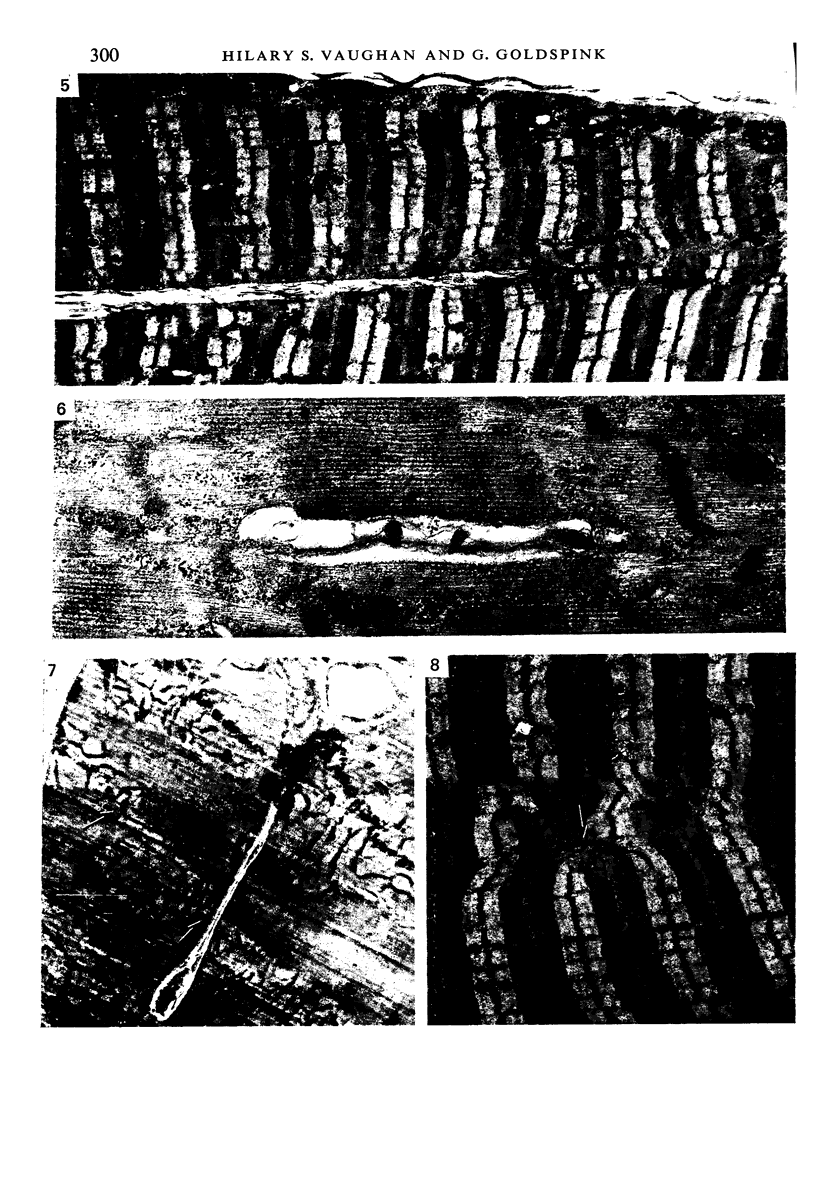



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binkhorst R. A. The effect of training on some isometric contraction characteristics of a fast muscle. Pflugers Arch. 1969;309(3):193–202. doi: 10.1007/BF00586797. [DOI] [PubMed] [Google Scholar]
- CRAWFORD G. N. Experimentally induced hypertrophy of growing voluntary muscle. Proc R Soc Lond B Biol Sci. 1961 Apr 18;154:130–138. doi: 10.1098/rspb.1961.0024. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R. Morphology and histochemistry of the soleus muscle from normal and exercised rats. Am J Anat. 1970 Jan;127(1):81–87. doi: 10.1002/aja.1001270107. [DOI] [PubMed] [Google Scholar]
- GOLDSPINK G. THE COMBINED EFFECTS OF EXERCISE AND REDUCED FOOD INTAKE ON SKELETAL MUSCLE FIBERS. J Cell Physiol. 1964 Apr;63:209–216. doi: 10.1002/jcp.1030630211. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Protein synthesis during work-induced growth of skeletal muscle. J Cell Biol. 1968 Mar;36(3):653–658. doi: 10.1083/jcb.36.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. I. Protein catabolism during work-induced hypertrophy and growth induced with growth hormone. J Biol Chem. 1969 Jun 25;244(12):3217–3222. [PubMed] [Google Scholar]
- Goldberg A. L. Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol. 1967 Nov;213(5):1193–1198. doi: 10.1152/ajplegacy.1967.213.5.1193. [DOI] [PubMed] [Google Scholar]
- Gordon E. E. Anatomical and biochemical adaptations of muscle to different exercises. JAMA. 1967 Sep 4;201(10):755–758. [PubMed] [Google Scholar]
- Gutmann E., Schiaffino S., Hanzliková V. Mechanism of compensatory hypertrophy in skeletal muscle of the rat. Exp Neurol. 1971 Jun;31(3):451–464. doi: 10.1016/0014-4886(71)90248-2. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs E. C., Lawrence C. A. Longitudinal fibre division in skeletal muscle: a light- and electronmicroscopic study. Z Zellforsch Mikrosk Anat. 1970;109(4):481–494. doi: 10.1007/BF00343963. [DOI] [PubMed] [Google Scholar]
- Hall-Craggs E. C. The longitudinal division of fibres in overloaded rat skeletal muscle. J Anat. 1970 Nov;107(Pt 3):459–470. [PMC free article] [PubMed] [Google Scholar]
- Hall-Craggs E. C. The significance of longitudinal fibre division in skeletal muscle. J Neurol Sci. 1972;15(1):27–33. doi: 10.1016/0022-510x(72)90119-0. [DOI] [PubMed] [Google Scholar]
- Hamosch M., Lesch M., Baron J., Kaufman S. Enhanced protein synthesis in a cell-free system from hypertrophied skeletal muscle. Science. 1967 Aug 25;157(3791):935–937. doi: 10.1126/science.157.3791.935. [DOI] [PubMed] [Google Scholar]
- Jablecki C., Kaufman S. Myosin adenosine triphosphatase activity during work-induced growth of slow and fast skeletal muscle in the normal rat. J Biol Chem. 1973 Feb 10;248(3):1056–1062. [PubMed] [Google Scholar]
- James N. T. Compensatory hypertrophy in the extensor digitorum longus muscle of the rat. J Anat. 1973 Oct;116(Pt 1):57–65. [PMC free article] [PubMed] [Google Scholar]
- James N. T. Compensatory muscular hypertrophy in the extensor digitorum longus muscle of the mouse. J Anat. 1976 Sep;122(Pt 1):121–131. [PMC free article] [PubMed] [Google Scholar]
- Lesch M., Parmley W. W., Hamosh M., Kaufman S., Sonnenblick E. H. Effects of acute hypertrophy on the contractile properties of skeletal muscle. Am J Physiol. 1968 Apr;214(4):685–690. doi: 10.1152/ajplegacy.1968.214.4.685. [DOI] [PubMed] [Google Scholar]
- Macková E., Hník P. 'Compensatory' muscle hypertrophy in the rat induced by tenotomy of synergistic muscles. Experientia. 1971 Sep 15;27(9):1039–1040. doi: 10.1007/BF02138867. [DOI] [PubMed] [Google Scholar]
- Macková E., Hník P. Compensatory muscle hypertrophy induced by tenotomy of synergists is not true working hypertrophy. Physiol Bohemoslov. 1973;22(1):43–49. [PubMed] [Google Scholar]
- Macková E., Hník P. Time course of compensatory hypertrophy of slow and fast rat muscles in relation to age. Physiol Bohemoslov. 1972;21(1):9–17. [PubMed] [Google Scholar]
- PILGRIM H. I., DEOME K. B. Intraperitoneal pentobarbital anesthesia in mice. Exp Med Surg. 1955;13(4):401–403. [PubMed] [Google Scholar]
- Peachey L. D., Eisenberg B. R. Helicoids in the T system and striations of frog skeletal muscle fibers seen by high voltage electron microscopy. Biophys J. 1978 May;22(2):145–154. doi: 10.1016/S0006-3495(78)85480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma W. Some structural changes in skeletal muscles of the rat after intensive training. Acta Morphol Neerl Scand. 1970;7(3):229–245. [PubMed] [Google Scholar]
- Rowe R. W., Goldspink G. Surgically induced hypertrophy in skeletal muscles of the laboratory mouse. Anat Rec. 1968 May;161(1):69–75. doi: 10.1002/ar.1091610107. [DOI] [PubMed] [Google Scholar]
- Sola O. M., Christensen D. L., Martin A. W. Hypertrophy and hyperplasia of adult chicken anterior latissimus dorsi muscles following stretch with and without denervation. Exp Neurol. 1973 Oct;41(1):76–100. doi: 10.1016/0014-4886(73)90182-9. [DOI] [PubMed] [Google Scholar]
- VAN LINGE B. The response of muscle to strenuous exercise. An experimental study in the rat. J Bone Joint Surg Br. 1962 Aug;44-B:711–721. doi: 10.1302/0301-620X.44B3.711. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Longitudinal growth of striated muscle fibres. J Cell Sci. 1971 Nov;9(3):751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973 Oct;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- Yellin H. Changes in fiber types of the hypertrophying denervated hemidiaphragm. Exp Neurol. 1974 Feb;42(2):412–428. doi: 10.1016/0014-4886(74)90035-1. [DOI] [PubMed] [Google Scholar]