Abstract
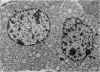
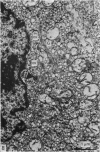
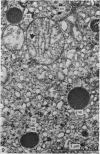
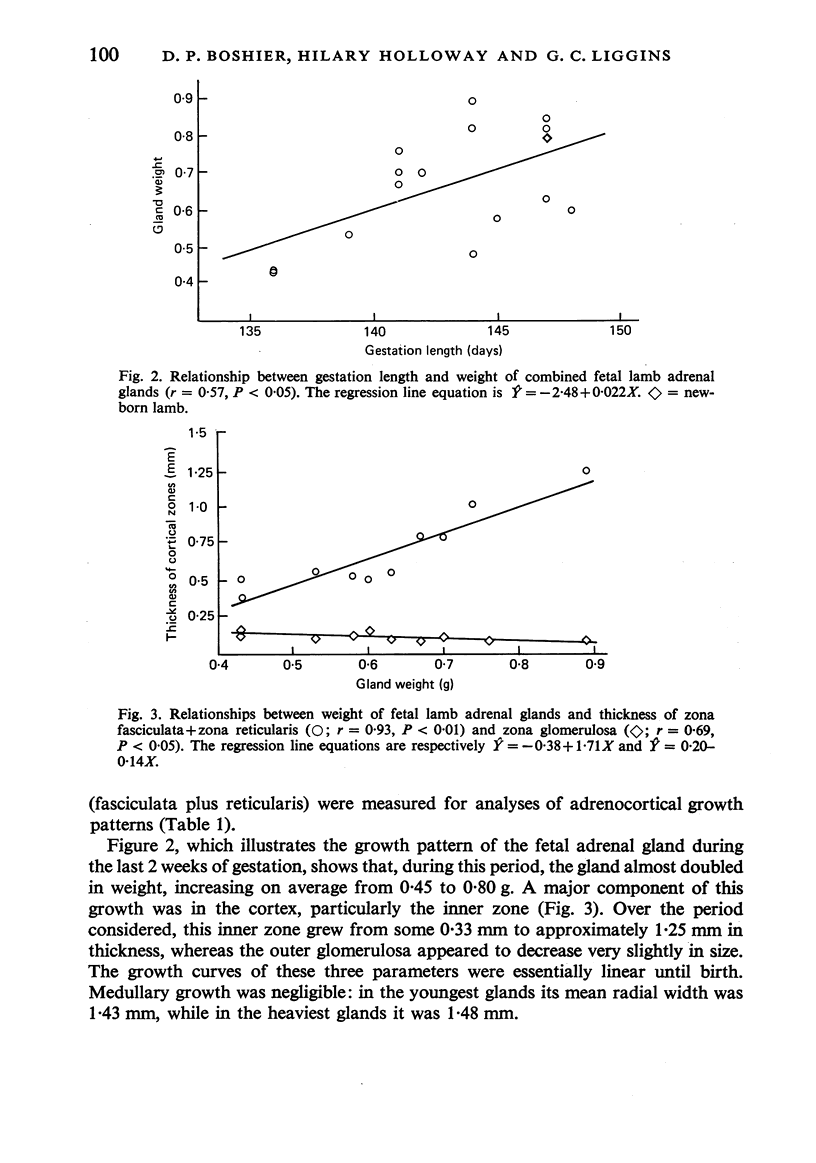
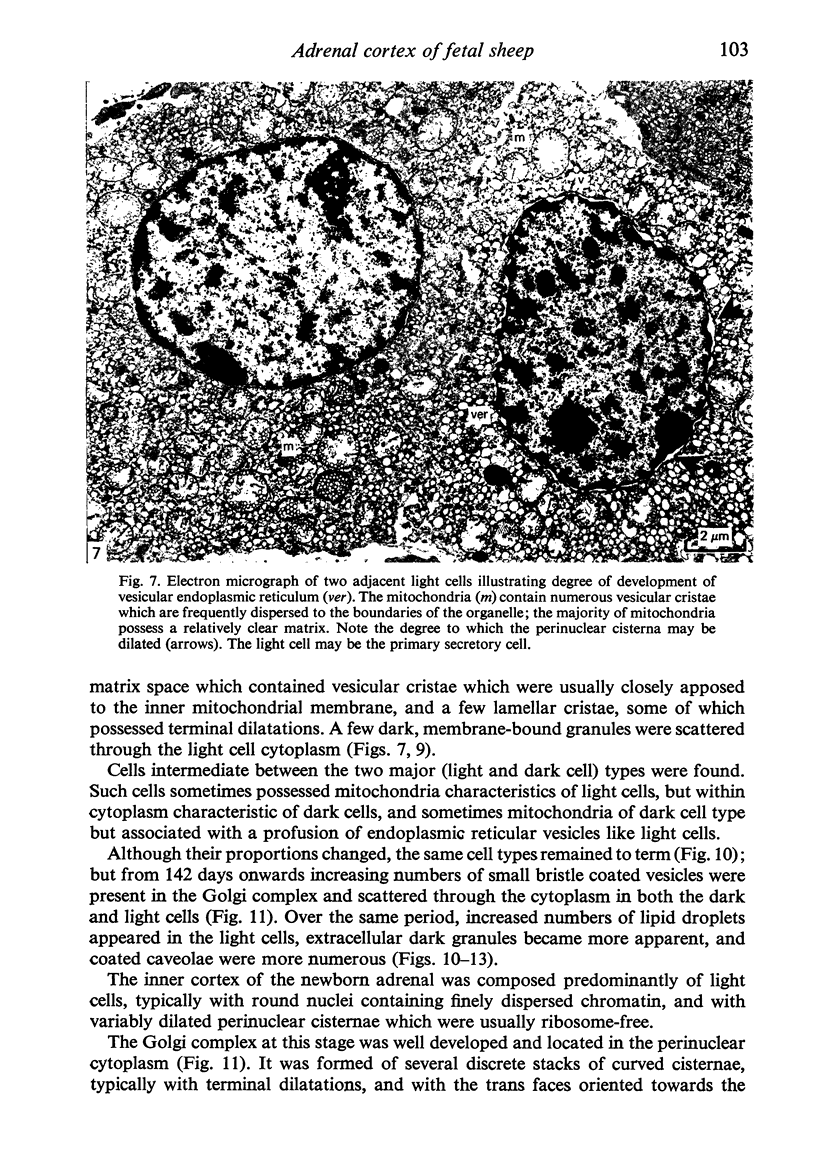
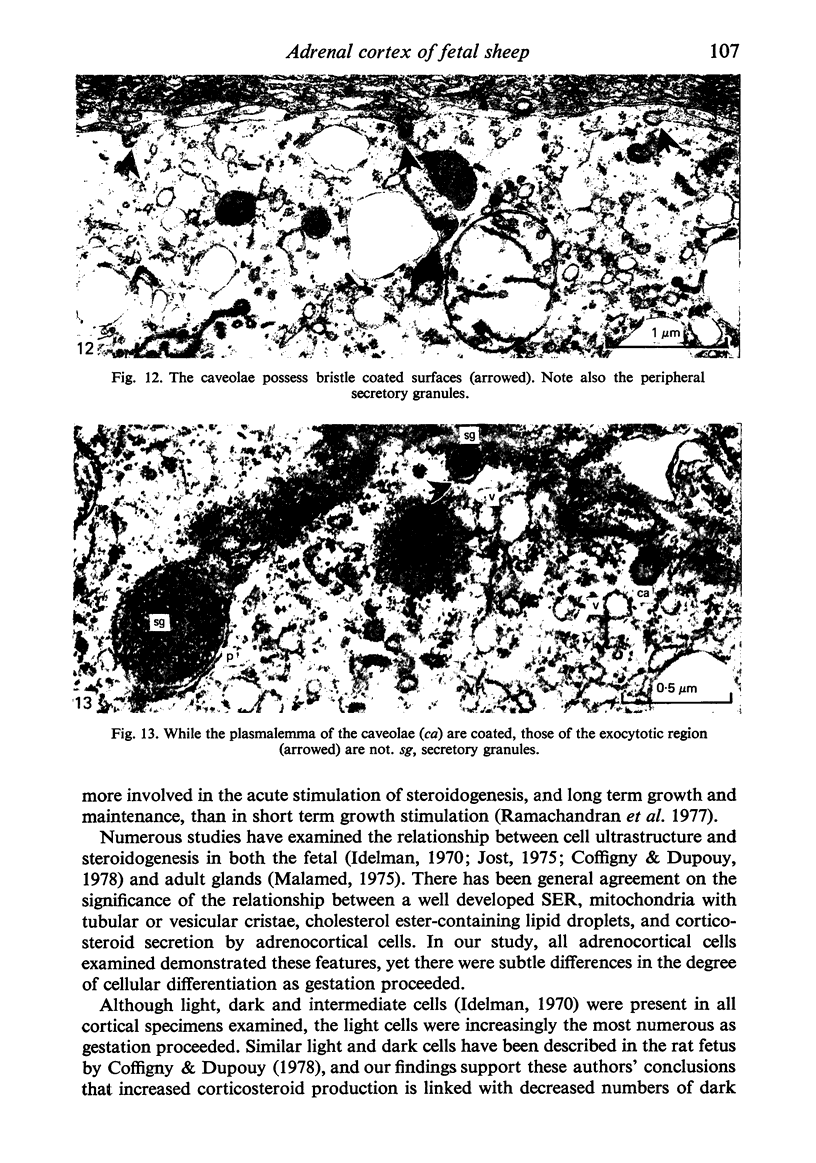
Growth patterns and cytodifferentiation of the fetal lamb adrenal cortex, from 136 days of gestation to birth at normal term, have been examined. The gland almost doubles in weight over this period (0.45--0.80 g) and the inner cortical zone (zona fasciculata+zona reticularis) nearly quadruples in thickness (0.33--1.25 mm). The phase of rapid growth has begun by 136 days, and the growth patterns are essentially linear. The inner cortical zone consists of light and dark cells, the former increasing in number proportionately more than the dark cells. This change in the cell ratio is linked with increased fetal production of cortisol. As growth and differentiation proceed the light cells increasingly possess a more vesicular SER; mitochondria of the orthodox configuration, with predominantly vesicular cristae; a well developed Golgi complex with numerous small, bristle coated vesicles; and dark granules near the Golgi complex, in the peripheral cytoplasm, and external to the cells. The sixfold increase in the volume of the inner cortical zone and the ultrastructural evidence of cytodifferentiation appear adequate to account for the known increase in the fetal synthesis of cortisol during this stage of pregnancy.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. P., Britton H. G., James V. H., Nixon D. A., Parker R. A., Wintour E. M., Wright R. D. Steroid secretion by the adrenal gland of foetal and neonatal sheep. J Endocrinol. 1968 Jan;40(1):1–13. doi: 10.1677/joe.0.0400001. [DOI] [PubMed] [Google Scholar]
- Anderson A. B., Pierrepoint C. G., Griffiths K., Turnbull A. C. Steroid metabolism in the adrenals of fetal sheep in relation to natural and corticotrophin-induced parturition. J Reprod Fertil Suppl. 1972 Apr;16(Suppl):25–37. [PubMed] [Google Scholar]
- Anderson R. G., Goldstein J. L., Brown M. S. Localization of low density lipoprotein receptors on plasma membrane of normal human fibroblasts and their absence in cells from a familial hypercholesterolemia homozygote. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2434–2438. doi: 10.1073/pnas.73.7.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett J. M., Thorburn G. D. Foetal plasma corticosteroids and the initiation of parturition in sheep. J Endocrinol. 1969 Jun;44(2):285–286. doi: 10.1677/joe.0.0440285. [DOI] [PubMed] [Google Scholar]
- COMLINE R. S., SILVER M. The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. J Physiol. 1961 May;156:424–444. doi: 10.1113/jphysiol.1961.sp006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffigny H., Dupouy J. P. The fetal adrenals of the rat: correlations between growth, cytology, and hormonal activity, with and without ACTH deficiency. Gen Comp Endocrinol. 1978 Mar;34(3):312–322. doi: 10.1016/0016-6480(78)90254-x. [DOI] [PubMed] [Google Scholar]
- Daikoku S., Kinutani M., Sako M. Ultrastructural study on the hypothalamic-hypophysial-adrenal axis in fetal rats. Cell Tissue Res. 1976 May 26;168(4):549–559. doi: 10.1007/BF00216002. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Long J. A., Jones A. L. The ultrastructure of endocrine glands. Recent Prog Horm Res. 1969;25:315–380. doi: 10.1016/b978-0-12-571125-8.50010-7. [DOI] [PubMed] [Google Scholar]
- Friend D. S., Farquhar M. G. Functions of coated vesicles during protein absorption in the rat vas deferens. J Cell Biol. 1967 Nov;35(2):357–376. doi: 10.1083/jcb.35.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell R. T., Laychock S. G., Rubin R. P. Ultrastructural and biochemical evidence for a steroid-containing secretory organelle in the perfused cat adrenal gland. J Cell Biol. 1977 Jan;72(1):209–215. doi: 10.1083/jcb.72.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelman S. Ultrastructure of the mammalian adrenal cortex. Int Rev Cytol. 1970;27:181–281. doi: 10.1016/s0074-7696(08)61248-6. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Boddy K., Robinson J. S. Changes in the concentration of adrenocorticotrophin and corticosteroid in the plasma of foetal sheep in the latter half of pregnancy and during labour. J Endocrinol. 1977 Mar;72(3):293–300. doi: 10.1677/joe.0.0720293. [DOI] [PubMed] [Google Scholar]
- Jost A. Le rôle des hormones foetales dans la croissance du foetus. J Physiol (Paris) 1977;73(6):877–890. [PubMed] [Google Scholar]
- Liggins G. C., Fairclough R. J., Grieves S. A., Kendall J. Z., Knox B. S. The mechanism of initiation of parturition in the ewe. Recent Prog Horm Res. 1973;29:111–159. doi: 10.1016/b978-0-12-571129-6.50007-5. [DOI] [PubMed] [Google Scholar]
- Liggins G. C. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969 Dec;45(4):515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- Liggins G. C. Premature parturition after infusion of corticotrophin or cortisol into foetal lambs. J Endocrinol. 1968 Oct;42(2):323–329. doi: 10.1677/joe.0.0420323. [DOI] [PubMed] [Google Scholar]
- Madill D., Bassett J. M. Corticosteroid release by adrenal tissue from foetal and newborn lambs in response to corticotrophin stimulation in a perifusion system in vitro. J Endocrinol. 1973 Jul;58(1):78–87. doi: 10.1677/joe.0.0580075. [DOI] [PubMed] [Google Scholar]
- Nathanielsz P. W. Endocrine mechanisms of parturition. Annu Rev Physiol. 1978;40:411–445. doi: 10.1146/annurev.ph.40.030178.002211. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B. The endoplasmic reticulum: a cytochemist's view (a review). Proc Natl Acad Sci U S A. 1976 Aug;73(8):2781–2787. doi: 10.1073/pnas.73.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussdorfer G. G., Mazzocchi G., Neri G., Robba C. Investigations into the mechanism of hormone release by rat adrenocortical cells. Cell Tissue Res. 1978 Jun 8;189(3):403–407. doi: 10.1007/BF00209129. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Rao A. J., Liles S. Studies of the trophic action of ACTH. Ann N Y Acad Sci. 1977 Oct 28;297:336–348. doi: 10.1111/j.1749-6632.1977.tb41865.x. [DOI] [PubMed] [Google Scholar]
- Rees L. H., Jack P. M., Thomas A. L., Nathanielsz P. W. Role of foetal adrenocorticotrophin during parturition in sheep. Nature. 1975 Jan 24;253(5489):274–275. doi: 10.1038/253274a0. [DOI] [PubMed] [Google Scholar]
- Robinson J. S., Challis J. R., Pooley G., Thorburn G. D. Foetal and maternal cortisol and progesterone and maternal oestradiol in prolonged pregnancy after foetal hypophysectomy in sheep. J Endocrinol. 1977 Feb;72(2):241–242. doi: 10.1677/joe.0.0720241. [DOI] [PubMed] [Google Scholar]
- Suyama A. T., Long J. A., Ramachandran J. Ultrastructural changes induced by ACTH in normal adrenocortical cells in culture. J Cell Biol. 1977 Mar;72(3):757–763. doi: 10.1083/jcb.72.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurley D. C. Prenatal growth of the adrenal gland in sheep. N Z Vet J. 1972 Oct;20(10):177–179. doi: 10.1080/00480169.1972.34045. [DOI] [PubMed] [Google Scholar]