Abstract
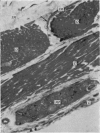
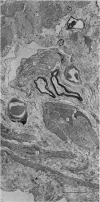
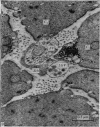
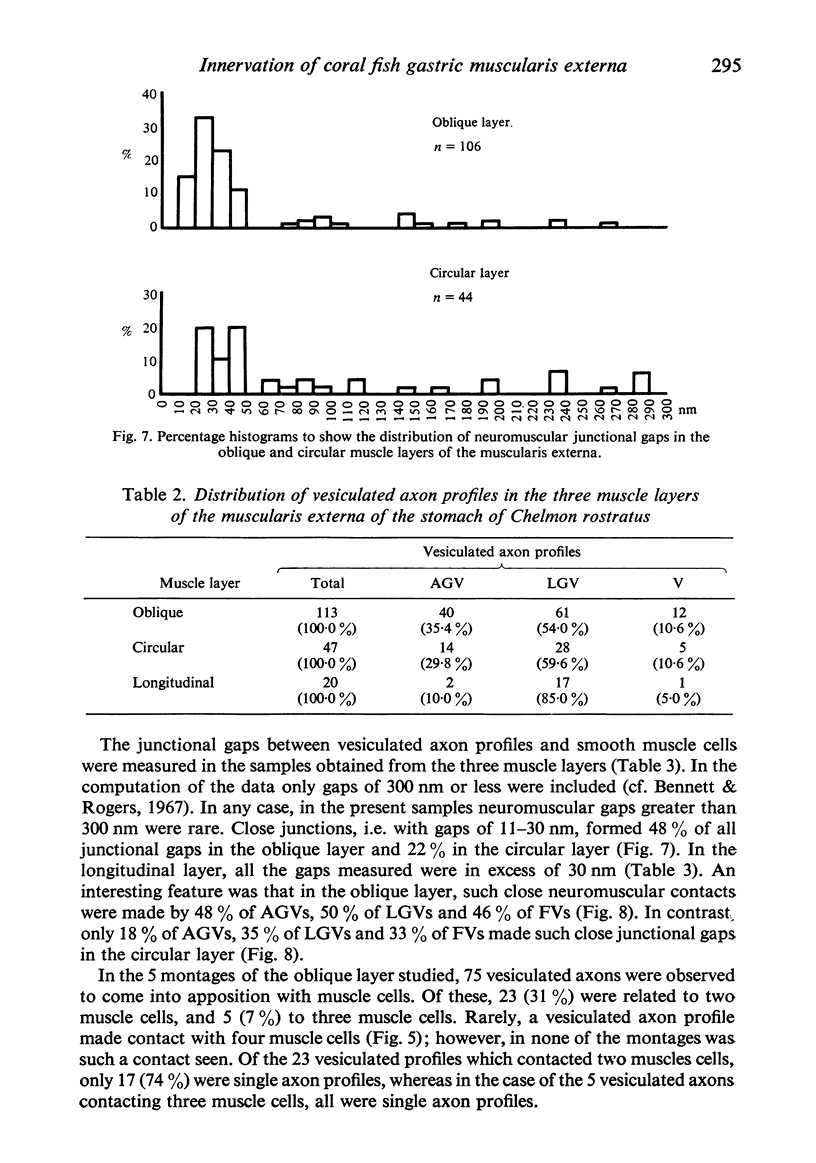
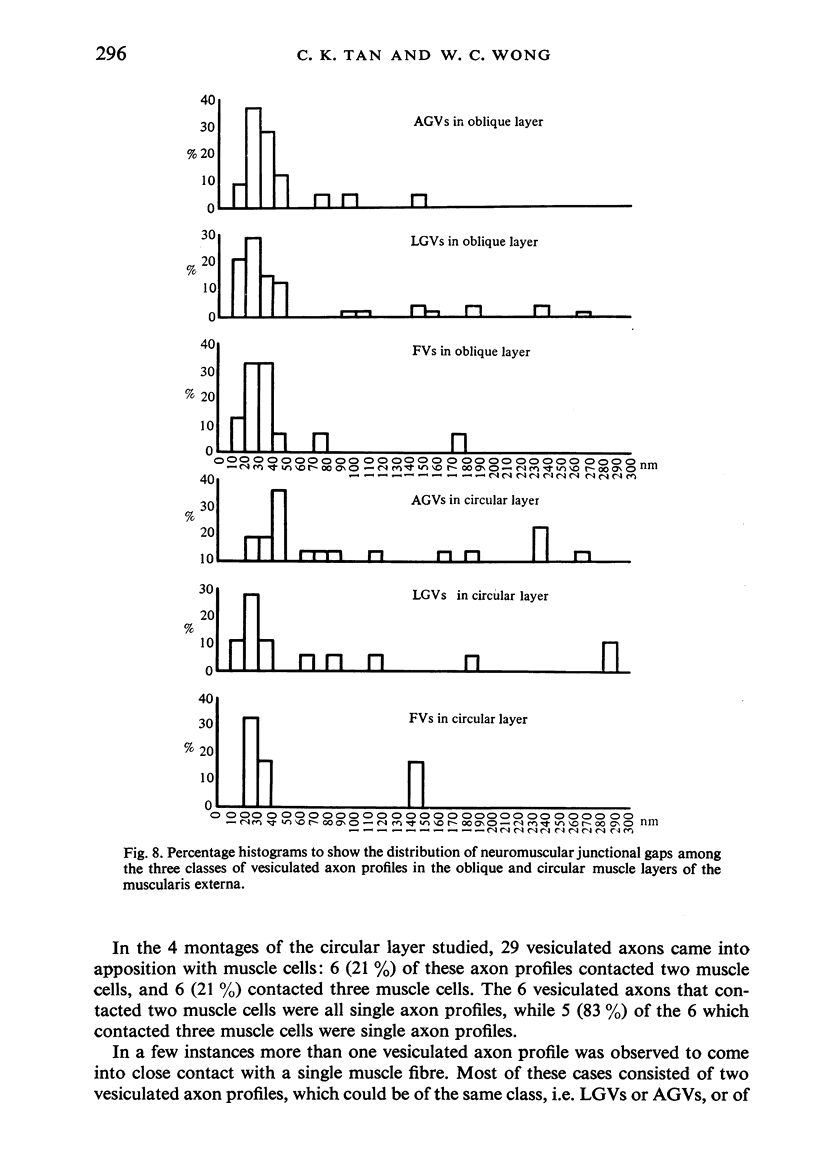
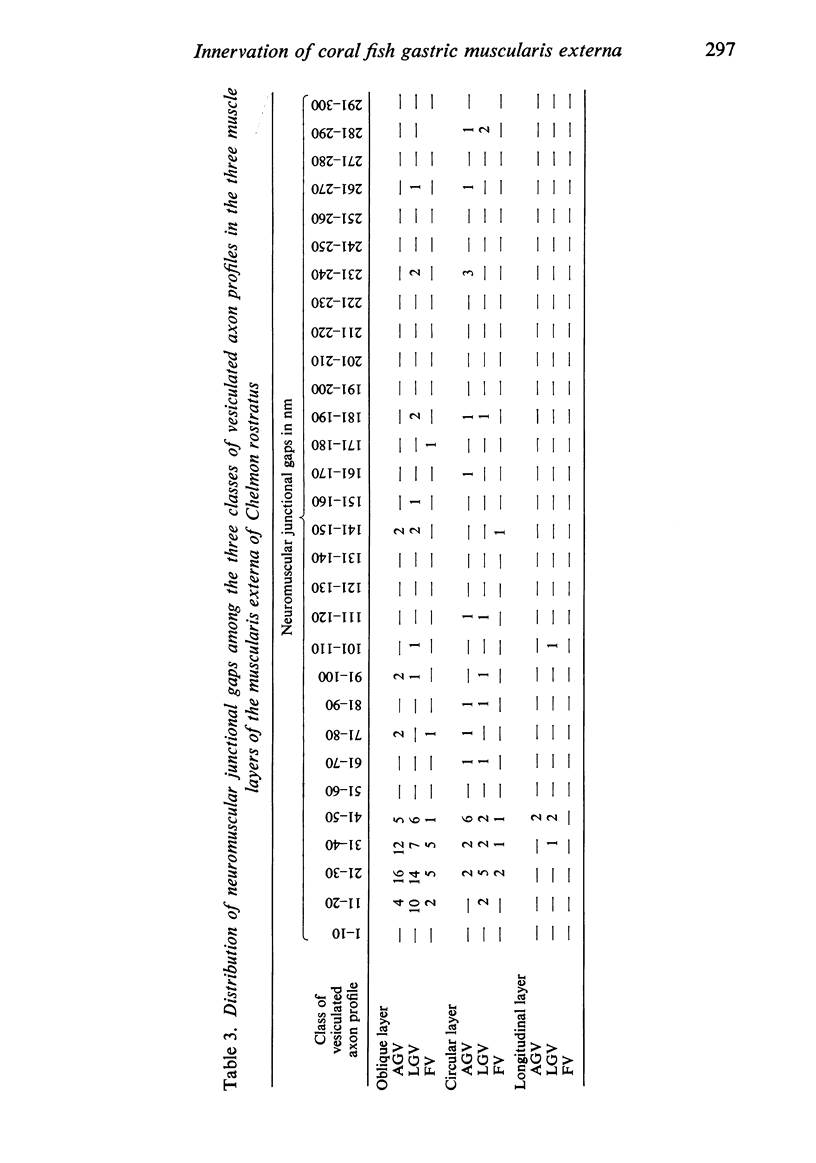
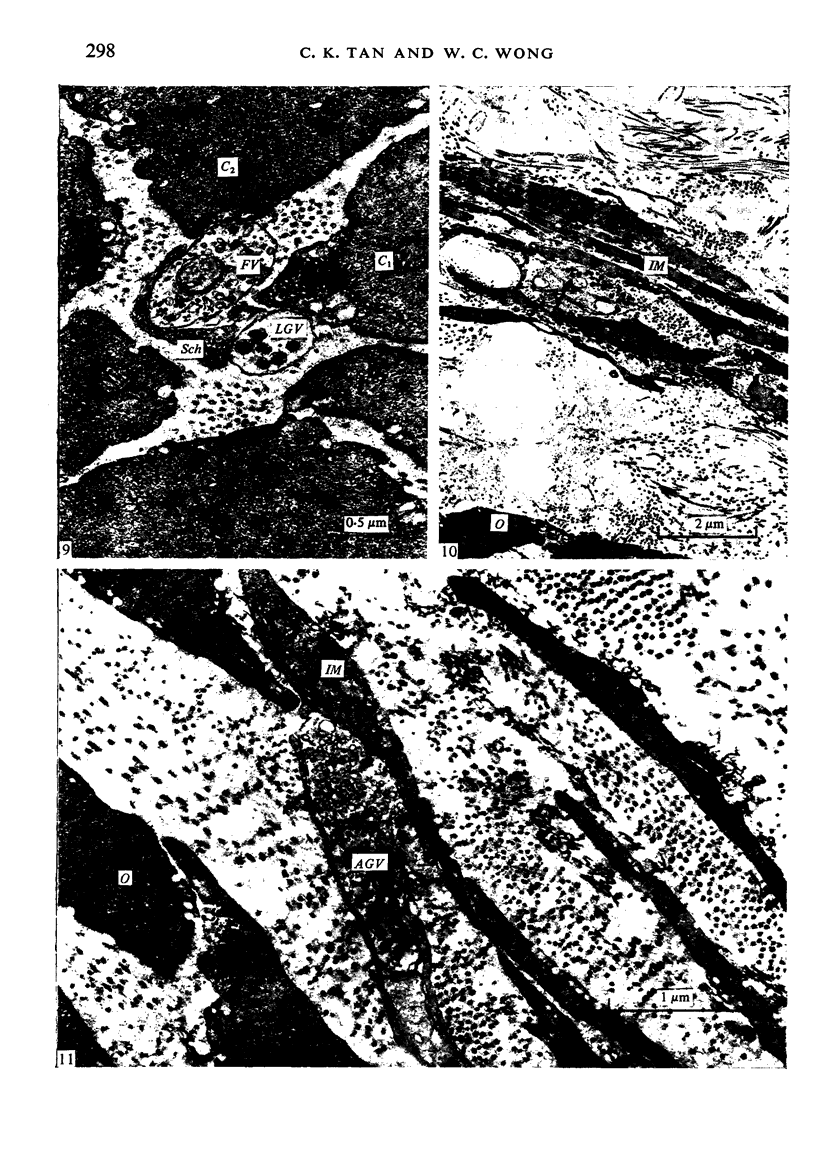
The muscularis externa of the stomach of the coral fish, Chelmon rostratus Cuvier, consists of four discrete layers - an outermost longitudinal layer followed by a circular, an oblique and an innermost layer. The muscle bundles of the oblique layer appear to be surrounded by thin prolongations of the innermost layer. In longitudinal sections of the innermost layer only rarely were small bundles of unmyelinated axons seen to course between the muscle cells. Vesiculated axon profiles within such nerve bundles occasionally came into apposition with the surface of muscle cells. The number of cross sectioned muscle fibres counted in the oblique, circular and longitudinal layers was 1365, 1408 and 745 respectively. The number of nerve bundles (single and multiple axon profiles) per 1000 muscle fibres was 62 (oblique layer), 30 (circular layer) and 28 (longitudinal layer). Single axon profiles comprised 51% (oblique layer), 36% (circular layer) and 10% (longitudinal layer). The number of axon profiles in the nerve bundles varied between 2 and 43. Nerve bundles containing 2-5 axon profiles formed 43% (oblique layer), 55% (circular layer) and 38% (longitudinal layer) of all nerve bundles. Vesiculated axon profiles within the nerve bundles made up approximatey 55% (oblique layer), 41% (circular layer) and 28% (longitudinal layer). Axon profiles containing round agranular vesicles (AGVs) comprised 35% (oblique layer), 30% (circular layer) and 10% (longitudinal layer). Axon profiles containing large granulated vesicles (LGVs) comprised 54% (oblique layer), 60% (circular layer) and 85% (longitudinal layer). Axon profiles containing flattened vesicles comprised 11% (oblique and circular layers) and 5% (longitudinal layer). The neuromuscular junctional gaps between vesiculated axon profiles and the surfaces of muscle cells varied between 11-270 nm (oblique layer), 11-290 nm (circular layer) and 31-110 nm (longitudinal layer). Gaps of 11-30 nm formed 48% and 22% in the oblique and circular layers, respectively. In the oblique layer such gaps were made up of 48% of AGVs, 50% of LGVs and 46% of FVs. The corresponding figures in the circular layer were 18%, 35% and 33%. The majority of vesiculated axon profiles contacted one muscle cell. Vesiculated axon profiles contacting two muscle cells comprised 31% (oblique layer) and 21% (circular layer). Vesiculated profiles contacting three muscle cells comprised 7% (oblique layer) and 21% (circular layer). Rarely, an axon profile contacted four muscle cells. An innervated muscle cell was usually contacted by a single axon profile but there were cases where two or even three axon profiles made contact with a single muscle cell.
Full text
PDF

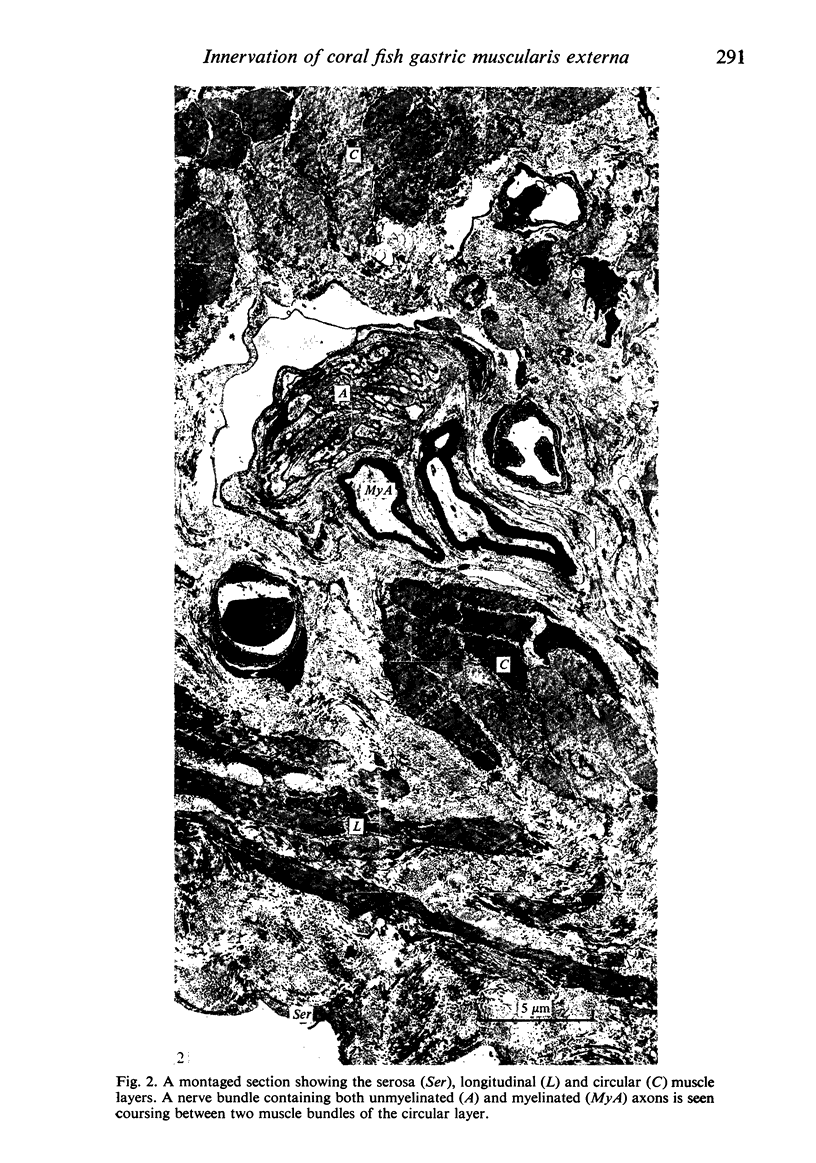
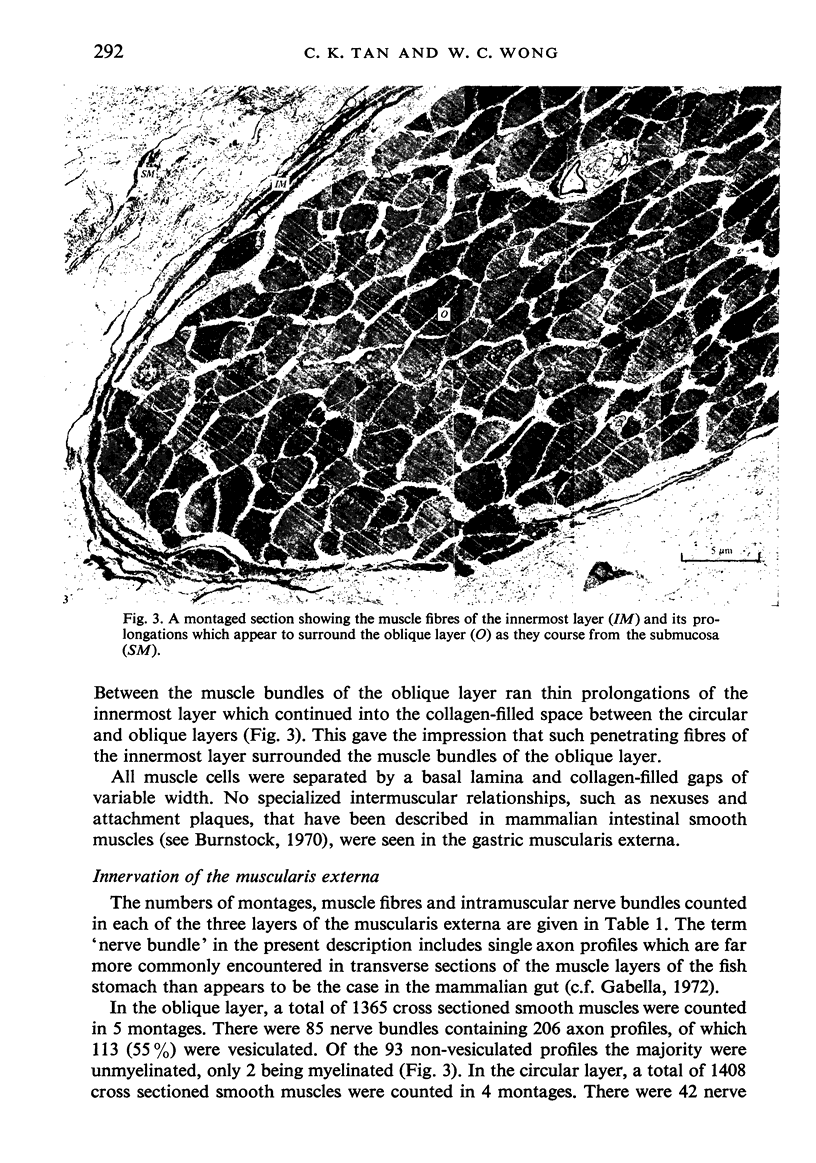
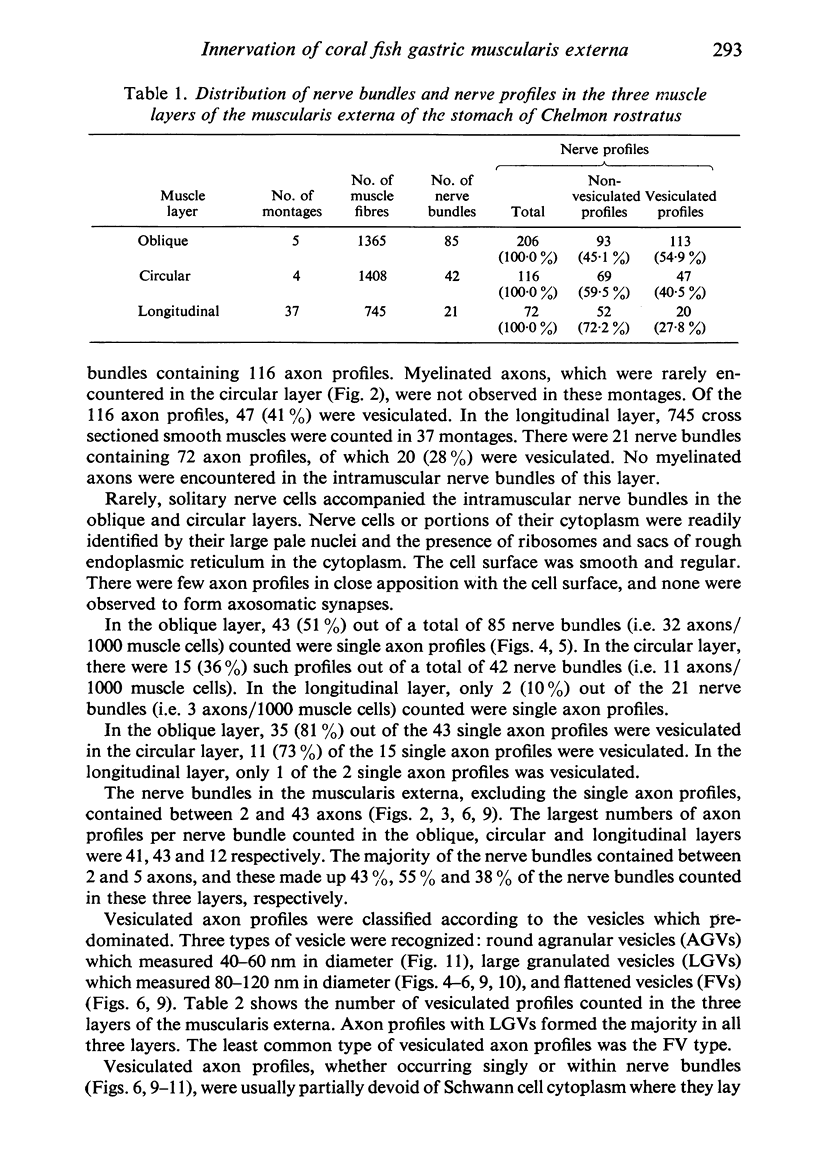
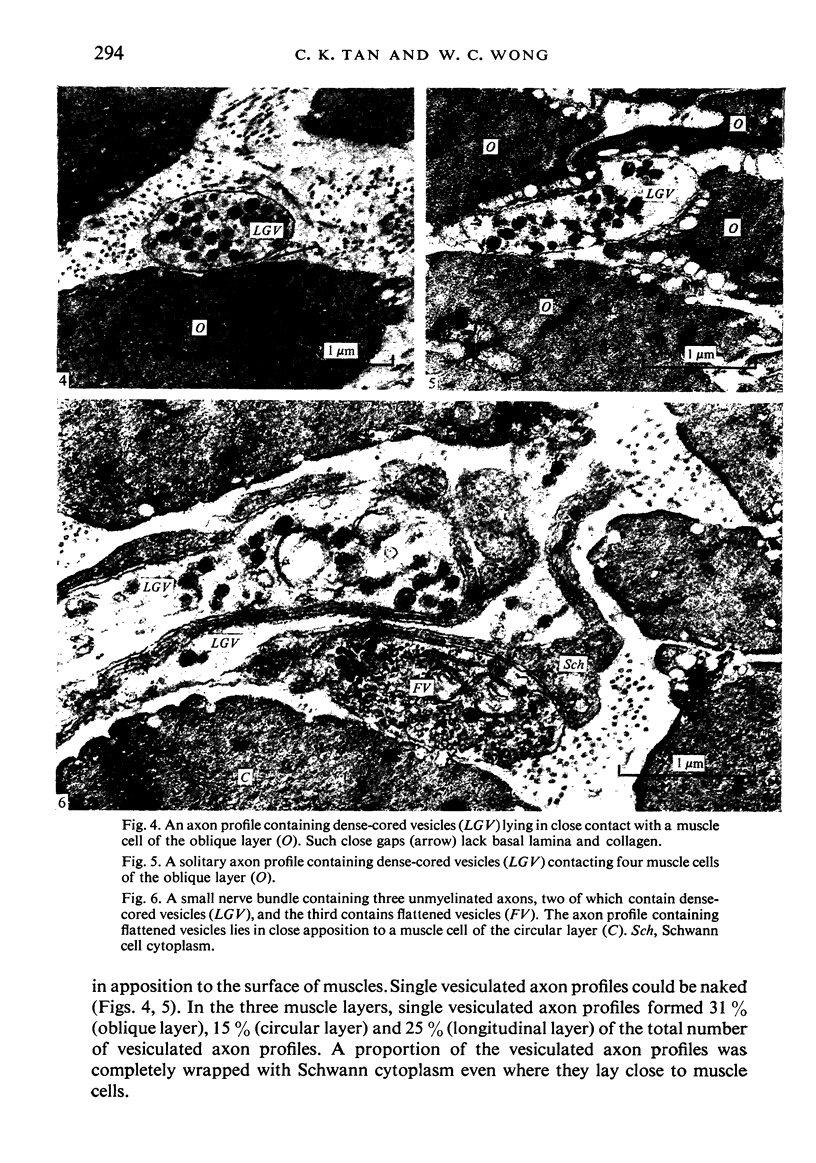





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD H., BURNSTOCK G., ROGERS D. INNERVATION OF THE LARGE INTESTINE OF THE TOAD (BUFO MARINUS). Br J Pharmacol Chemother. 1964 Aug;23:151–163. doi: 10.1111/j.1476-5381.1964.tb01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten H. G., Holstein A. F., Owman C. Auerbach's plexus of mammals and man: electron microscopic identification of three different types of neuronal processes in myenteric ganglia of the large intestine from rhesus monkeys, guinea-pigs and man. Z Zellforsch Mikrosk Anat. 1970;106(3):376–397. doi: 10.1007/BF00335780. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Rogers D. C. A study of the innervation of the taenia coli. J Cell Biol. 1967 Jun;33(3):573–596. doi: 10.1083/jcb.33.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G., Gannon B. J. The splanchnic nerve supply to the stomach of the trout, Salmo trutta and S. gairdneri. Comp Biochem Physiol C. 1976;55(1):51–53. doi: 10.1016/0306-4492(76)90011-3. [DOI] [PubMed] [Google Scholar]
- DE ROBERTIS E., PELLEGRINO DE IRALDIA Plurivesicular secretory processes and nerve endings in the pineal gland of the rat. J Biophys Biochem Cytol. 1961 Jul;10:361–372. doi: 10.1083/jcb.10.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Innervation of the intestinal muscular coat. J Neurocytol. 1972 Dec;1(4):341–362. doi: 10.1007/BF01102939. [DOI] [PubMed] [Google Scholar]
- Gabella G. Special muscle cells and their innervation in the mammalian small intestine. Cell Tissue Res. 1974;153(1):63–77. doi: 10.1007/BF00225446. [DOI] [PubMed] [Google Scholar]
- Howard E. R., Garrett J. R. The intrinsic myenteric innervation of the hind-gut and accessory muscles of defaecation in the cat. Z Zellforsch Mikrosk Anat. 1973;136(1):31–44. doi: 10.1007/BF00307678. [DOI] [PubMed] [Google Scholar]
- Hörtnagl H., Winkler H. Bovine splenic nerve: characterization of noradrenaline-containing vesicles and other cell organelles by density gradient centrifugation. J Physiol. 1969 Nov;205(1):103–114. doi: 10.1113/jphysiol.1969.sp008954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Kuriyama H. Nervous control of the motility of the alimentary canal of the silver carp. J Exp Biol. 1971 Oct;55(2):469–487. doi: 10.1242/jeb.55.2.469. [DOI] [PubMed] [Google Scholar]
- LANE B. P., RHODIN J. A. CELLULAR INTERRELATIONSHIPS AND ELECTRICAL ACTIVITY IN TWO TYPES OF SMOOTH MUSCLE. J Ultrastruct Res. 1964 Jun;10:470–488. doi: 10.1016/s0022-5320(64)80023-x. [DOI] [PubMed] [Google Scholar]
- Li P. L. The intramural nervous system of the small intestine with special reference to the innervation of the inner subdivision of its circular muscle. J Anat. 1940 Apr;74(Pt 3):348–359. [PMC free article] [PubMed] [Google Scholar]
- Nilsson S., Fänge R. Adrenergic and cholinergic vagal effects on the stomach of a teleost (Gadus morhua). Comp Biochem Physiol. 1969 Aug 15;30(4):691–694. doi: 10.1016/0010-406x(69)92148-3. [DOI] [PubMed] [Google Scholar]
- Nilsson S., Fänge R. Adrenergic receptors in the swimbladder and gut of a teleost (Anguilla anguilla). Comp Biochem Physiol. 1967 Nov;23(2):661–664. doi: 10.1016/0010-406x(67)90417-3. [DOI] [PubMed] [Google Scholar]
- Rogers D. C., Burnstock G. Muliaxonal autonomic junctions in intestinal smooth muscle of the toad (Bufo marinus). J Comp Neurol. 1966 Apr;126(4):625–652. [PubMed] [Google Scholar]
- THAEMERT J. C. The ultrastructure and disposition of vesiculated nerve processes in smooth muscle. J Cell Biol. 1963 Feb;16:361–377. doi: 10.1083/jcb.16.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. C. Degeneration of adrenergic axons in the longitudinal muscle coat of the rat duodenum following treatment with 6-hydroxydopamine. Experientia. 1975 Sep 15;31(9):1080–1082. doi: 10.1007/BF02326972. [DOI] [PubMed] [Google Scholar]
- Wong W. C., Tan C. K. Fine structure of the myenteric and submucous plexuses in the stomach of a coral fish, Chelmon rostratus Cuvier. J Anat. 1978 Jun;126(Pt 2):291–301. [PMC free article] [PubMed] [Google Scholar]
- Wong W. C. The myenteric plexus in the oesophagus of the toad (Bufo melanostictus). Acta Anat (Basel) 1973;85(1):52–62. doi: 10.1159/000143980. [DOI] [PubMed] [Google Scholar]
- Wong W. C. Ultrastructural localization of adrenergic nerve terminals in the circular muscle layer and muscularis mucosae of the rat duodenum after acute treatment with 6-hydroxydopamine. J Anat. 1977 Dec;124(Pt 3):637–642. [PMC free article] [PubMed] [Google Scholar]