Abstract
Background:
Previous preclinical models of multicompartmental injury have investigated its effects for durations of less than 72 hours and the long-term effects have not been defined. We hypothesized that a model of multicompartmental injury would result in systemic inflammation and multi-organ dysfunction that persists at one week.
Materials and Methods:
Male and proestrus female Sprague-Dawley rats (n=16/group) underwent polytrauma (PT) (unilateral right lung contusion, hemorrhagic shock, cecectomy, bifemoral pseudofractures) and were compared to naive controls. Weight, hemoglobin, plasma neutrophil gelatinase-associated lipocalin (NGAL), and plasma toll-like receptor 4 (TLR4) were evaluated on days two and seven. Bilateral lungs were sectioned, stained and assessed for injury at day seven. Comparisons were performed in Graphpad with significance defined as *p<0.05.
Results:
Rats who underwent PT had significant weight loss and anemia at day 2 (p=0.001) compared to naïve rats which persisted at day 7 (p=0.001). PT rats had elevated plasma NGAL at day 2 compared to naïve (p<0.0001) which remained elevated at day 7 (p<0.0001). Plasma TLR4 was elevated in PT compared to naïve at day 2 (p=0.03) and day 7 (p=0.01). Bilateral lungs showed significant injury in PT cohorts at day 7 compared to naïve (p<0.0004). PT males had worse renal function at day seven compared to females (p=0.02).
Conclusions:
Multicompartmental trauma induces systemic inflammation and multi-organ dysfunction without recovery by day seven. However, females demonstrate improved renal recovery compared to males. Long-term assessment of preclinical polytrauma models are crucial to better understand and evaluate future therapeutic immunomodulatory and anti-inflammatory treatments.
Keywords: trauma, polytrauma, multiorgan injury
INTRODUCTION
Multiple, severe injuries are known to impact outcomes after trauma, resulting in worse morbidity and mortality with multifactorial etiologies including multiorgan failure and sepsis (1). However, there remains a need for a translational preclinical animal model to understand the pathophysiology not only acutely after such severe injury, but also recovery long-term. Rodents have been shown to be appropriate models to study responses to trauma in the preclinical setting (2). However, previous rodent studies of polytrauma include multiple injuries but only evaluate acute changes within 72 hours of injury and tend to only evaluate male subjects (3–8). Multiple biomarkers of systemic inflammation and subsequent activation of the immune system after trauma can be utilized to evaluate the systemic response to injury. Toll-like receptors, such as toll-like receptor 4 (TLR4), have been implicated in contributing to lung injury and even multiorgan failure after trauma (9–14). Plasma neutrophil gelatinase-associated lipocalin (NGAL) is a known biomarker of acute renal injury (15). However, the complex mechanisms of the systemic inflammatory response and sequelae postinjury in severe trauma remains to be fully understood.
Sex differences after trauma have been shown in many studies of isolated or less severe injury with or without hemorrhagic shock. Preclinical studies have demonstrated that females have better outcomes after traumatic brain injury with regards to lesion volume and neuronal death, attenuated postinjury immunosuppression, decreased pulmonary and renal injury after hemorrhagic shock, and improved overall survival (16–18). Most of these improved outcomes after injury have been attributed to the protective effects of estrogen (16, 18–21). Retrospective analyses have even demonstrated that females are less likely to develop inpatient complications compared to males postinjury (22). Despite this evidence in isolated or less severe injury, there also remains a paucity of data regarding female response and outcomes after severe polytrauma.
We utilized a preclinical model of severe multicompartmental injury to study multiorgan dysfunction in both male and female subjects. Our aim was to characterize acute changes in weight, systemic inflammation, renal injury, anemia, and lung injury at two days postinjury and evaluate recovery from severe trauma after seven days. We hypothesized that both males and females would demonstrate signs of systemic inflammation and organ dysfunction, but that females would have improved recovery by day seven.
METHODS
Animals
Male and female Sprague-Dawley rats (Charles River, Wilmington, MA) aged 9–11 weeks weighing 168 to 386 grams were housed in pairs on arrival in conventional rodent housing with ad lib access to standard irradiated pelleted diet and water. Prior to initiation of the experiment, rats were acclimated to a 12-hour light-dark cycle for at least 72 hours and remained in their same cage for at least 10 days, with housing room temperature range kept at 68–79°F with 30–70% humidity. Rats were separated after enrollment and housed individually for the duration of the study. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC Protocol 202011247) and carried out in accordance with the national Research Council’s Guide for the Care and Use of Laboratory Animals (23). The ARRIVE guidelines were utilized to ensure proper reporting of methods, results, and discussion (see Supplementary Material 1) (24).
Experimental Design
Rodents were randomly assigned to the following cohorts (n=16/group, with exactly 8 males and 8 females within each group): naïve or polytrauma (PT) for a duration of either two or seven days similar to previously described (25, 26). A total of 72 rats were studied: 64 were assigned to groups and 8 were donors for pseudofracture. A power analysis demonstrated that a minimum of eight rats are needed per group to identify a 30% change, assuming greater than 80% incidence in control rats at baseline. Injuries were chosen to represent blunt chest trauma, hemorrhage, intestinal injury, and lower extremity fractures (PT). Cohorts were unable to be blinded during animal care due to the presence of incisions on experimental groups. Animals were euthanized on postoperative day two or seven unless a humane endpoint was met prior; rats were evaluated and scored twice daily based on set criteria developed with animal care veterinarians (Supplementary Material 2).
To control for estrous cycle variability and given known protective effects of estrogen postinjury, only female rats in the proestrus phase were enrolled for the study (18). Female rats were subjected to vaginal lavage with sterile normal saline and cells evaluated on a under a Nikon Eclipse E200 microscope at 40X objective for predominance of nucleated epithelial cells consistent with the proestrus phase on the potential day of enrollment (27).
Pseudofracture solution was prepared to simulate bilateral femur fractures similar to previous (26, 28, 29). Donor rats of the same age, sex and weight were euthanized and bilateral femurs and tibias harvested under aseptic conditions and crushed, with 3mL of normal saline added to produce a pseudofracture solution as previously described and kept at 4°C until use (30). Only females in the proestrus phase were enrolled as donors. One donor rat supplied pseudofracture solution for four rats.
As previously described, after induction of anesthesia with 1–5% isoflurane (Patterson Veterinary, Loveland, CA), 1mg/kg sustained-release buprenorphine (ZooPharm, Laramie, WY) was administered subcutaneously (26, 28, 29). The right lung was contused with a manual nail gun (Arrow, Saddle River, NJ) applied to a 12mm plate on the rodent’s right axilla. Proposed incisions in the right neck, lower abdominal midline, and medial left thigh were injected with 4mg/kg ropivacaine (Akron, Lake Forest, IL) and the left femoral artery and right internal jugular vein cannulated with polyethylene-10 and polyethylene-50 tubing, respectively. The arterial line was connected to a BP-2 Digital Blood Pressure Monitor device (Columbus Instruments, Columbus, OH) for continuous measurement of mean arterial pressure (MAP). Hemorrhagic shock was induced by blood withdrawal from the venous cannula at a rate of 1mL/minute to results in a MAP of 30–35mmHg for 45 minutes. A midline laparotomy and cecectomy was performed by exteriorizing the cecum and ligation with a 2–0 silk tie with subsequent abdominal wall closure in layers. At the conclusion of hemorrhagic shock, half of the shed blood was reinfused and 150μL of pseudofracture bone marrow solution from age-, sex- and weight-matched rats was injected intramuscularly into each medial thigh. Additional resuscitation with 3mL of subcutaneously administered normal saline was performed. Rats were provided with a 3-inch manzanita wood gnawing stick (Bio-Serv, Flemington, NJ) postoperatively for PT groups or immediately after enrollment for naïve rats.
All animals were handled and weighed daily after the start of the study. Percent weight change is represented by change in weight per rat at day of euthanasia compared to enrollment weight. Euthanasia was performed via terminal anesthesia with 1–5% isoflurane, thoracotomy, cardiac puncture, and exsanguination on either day two or day seven. Whole spleens from each rodent were collected on day of euthanasia and weighed; spleen to body weight ratio was calculated from spleen weight and body weight on day of euthanasia.
Blood Studies
Blood was obtained via cardiac puncture on day of euthanasia in a 10mL syringe containing 0.1mL of heparinized saline (1000units/mL). From this, hemoglobin was measured on a VETSCAN® HM5 Hematology Analyzer (Zoetis, Parsippany-Troy Hills, NJ). An aliquot of blood was centrifuged at 800g for ten minutes and plasma stored in a −80°C freezer until further processing. Plasma analytes were measured using standard sandwich enzyme-linked immunosorbent assays (ELISA): neutrophil gelatinase-associated lipocalin (NGAL) (Abcam, Cambridge, United Kingdom) and toll-like receptor 4 (TLR4) (MyBiosource, San Diego, CA according to manufacturer’s protocols.
Histology
Bilateral lungs were obtained on day of euthanasia, flushed and stored in formalin for 24 hours and then transferred to 70% ethanol. Tissues were sectioned, stained in hematoxylin and eosin, and embedded in paraffin. Histological slides of right and left lungs were analyzed by a blinded veterinary pathologist and scored for injury similar to previously described; each category was graded from 0 (no injury) to 4 (severe changes) (Supplementary Material 3) (31, 32). One slide of the right lung and one slide of the left lung were scored per rat; for each slide, the entire tissue was evaluated at 10x and 100x magnification followed by evaluating 20 to 30 representative fields at 200x and 400x overall magnifications.
Statistical Analysis
Analyses of weight, hemoglobin, plasma TLR4, plasma NGAL, spleen to body weight ratio, and lung injury scores were performed in GraphPad Prism version 9.5.0 (GraphPad Software, La Jolla, CA). All data sets of pooled cohorts containing males and females were evaluated for normal distribution with the Shapiro-Wilk test and were not of normal distribution; thus, either Mann-Whitney tests or Kruskal-Wallis tests with Dunn’s multiple comparisons test with correction were performed. Comparisons between males and females were performed either with student’s t-test with Welch’s correction for unequal standard deviations for data with normal distribution or Mann-Whitney test for data without a normal distribution. Data without a normal distribution are presented as median with interquartile range along with minimum and maximum values; *p<0.05 was considered statistically significant.
RESULTS
Systemic changes after polytrauma
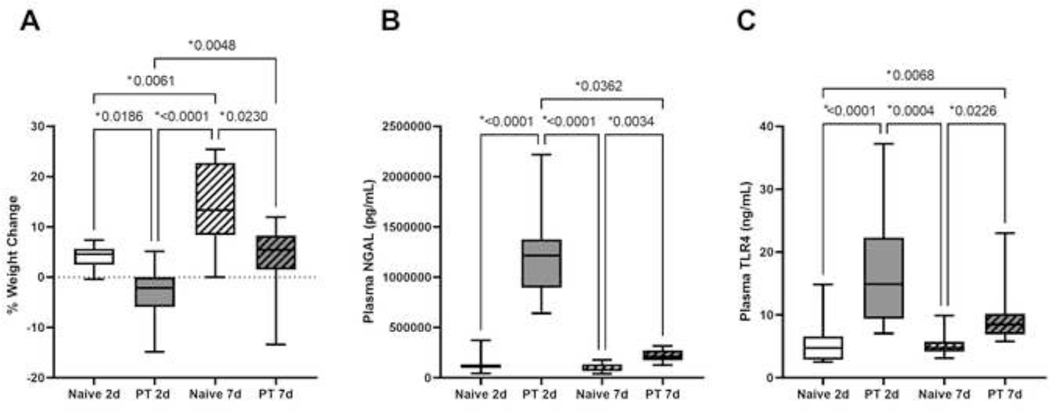
When assessing both males and females in each cohort two days after injury, polytrauma induced severe weight loss (−2.2%, −5.9% to 0%) compared to their naïve counterparts who gained weight (4.6%, 2.5% to 5.7%; p=0.02) (Fig. 1A). By day seven, rats who underwent polytrauma began to show small weight gains compared to enrollment weight (5.4%, 1.5% to 8.3%); however, this was still significantly less than naïve counterparts (13.3%, 8.4% to 22.7%; p=0.02) (Fig. 1A).
Figure 1A-C.
Naïve and polytrauma (PT) cohorts at two-day (2d) and seven-day (7d) study duration A) percent weight change, B) plasma neutrophil gelatinase-associated lipocalin (NGAL), and C) plasma toll-like receptor 4 (TLR4). Only statistically significant comparisons displayed (*p<0.05).
Plasma NGAL on day two demonstrated severe renal injury in rats subjected to polytrauma (Naïve 2d: 109361pg/mL, 100440pg/mL to 133949pg/mL; PT 2d: 1214893pg/mL, 895015pg/mL to 1376083pg/mL; p<0.0001) (Fig. 1B). Seven days after enrollment, the polytrauma cohort showed improvement in NGAL compared to two-day counterparts (PT 7d: 208686pg/mL, 173351pg/mL to 274471pg/mL; p=0.04), but did not exhibit full recovery compared to naïve (Naïve 7d: 78267pg/mL, 67895pg/mL to 134909pg/mL; p=0.003) (Fig. 1B).
Systemic inflammation as measured by plasma TLR4 increased two days after polytrauma compared to controls (Naïve 2d: 4.7ng/mL, 2.9ng/mL to 6.6ng/mL; PT 2d: 14.9ng/mL, 9.4ng/mL to 22.3ng/mL; p<0.0001) (Fig. 1C). One-week postinjury, rats who underwent PT continued to have significantly elevated plasma TLR4 compared to naïve counterparts (Naïve 7d: 4.8ng/mL, 4.2ng/mL to 5.8ng/mL; PT 7d: 8.5ng/mL, 6.9ng/mL to 10.2ng/mL; p=0.02) (Fig. 1C). Together these data suggest not only significant loss in body weight after injury without full recovery after one week, but also evidence of renal injury and systemic inflammation acutely and at seven days postinjury.
Hematologic changes after polytrauma
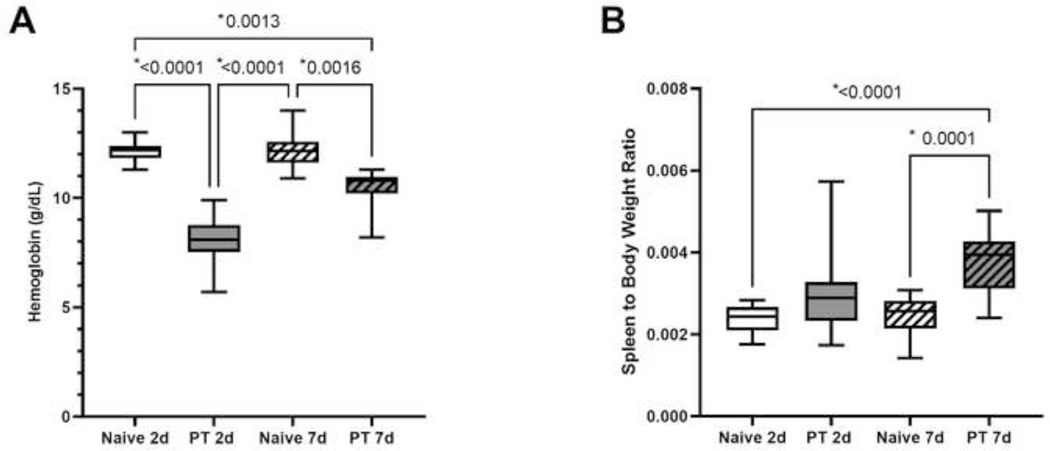
Severe injury induced by polytrauma resulted in a significant decline in hemoglobin within 48 hours of injury despite resuscitation (Naïve 2d: 12.2g/dL, 11.8g/dL to 12.4d/gL; PT 2d: 8.1g/dL, 7.5g/dL to 8.8d/gL; p<0.0001) (Fig. 2A). Despite an increase in hemoglobin seven days after PT compared to two-days (PT 7d: 10.8g/dL, 10.2g/dL to 11.0g/dL), this did not reach statistical significance (p=0.1) (Fig. 2A). In addition, despite some recovery of hemoglobin in the polytrauma group, hemoglobin was still significantly lower one-week after injury compared to naïve counterparts (Naïve 7d: 12.2g/dL, 11.6g/dL to 12.6g/dL; p=0.002) (Fig. 2A).
Figure 2A-B.
Naïve and polytrauma (PT) cohorts at two-day (2d) and seven-day (7d) study duration A) hemoglobin and B) spleen to body weight ratio. Only statistically significant comparisons displayed (*p<0.05).
Spleen weight to body weight ratio was not significantly elevated two days after polytrauma compared to naïve subjects (p=0.1) (Fig. 2B). However, by day seven, rats subjected to polytrauma showed enlargement of splenic tissue as evidenced by increased spleen to body weight ratio compared to naïve controls (p=0.0001) (Fig. 2B). This demonstrates a persistent anemia one week after injury with extramedullary hematopoiesis as evidenced by splenic tissue enlargement.
Lung injury after polytrauma
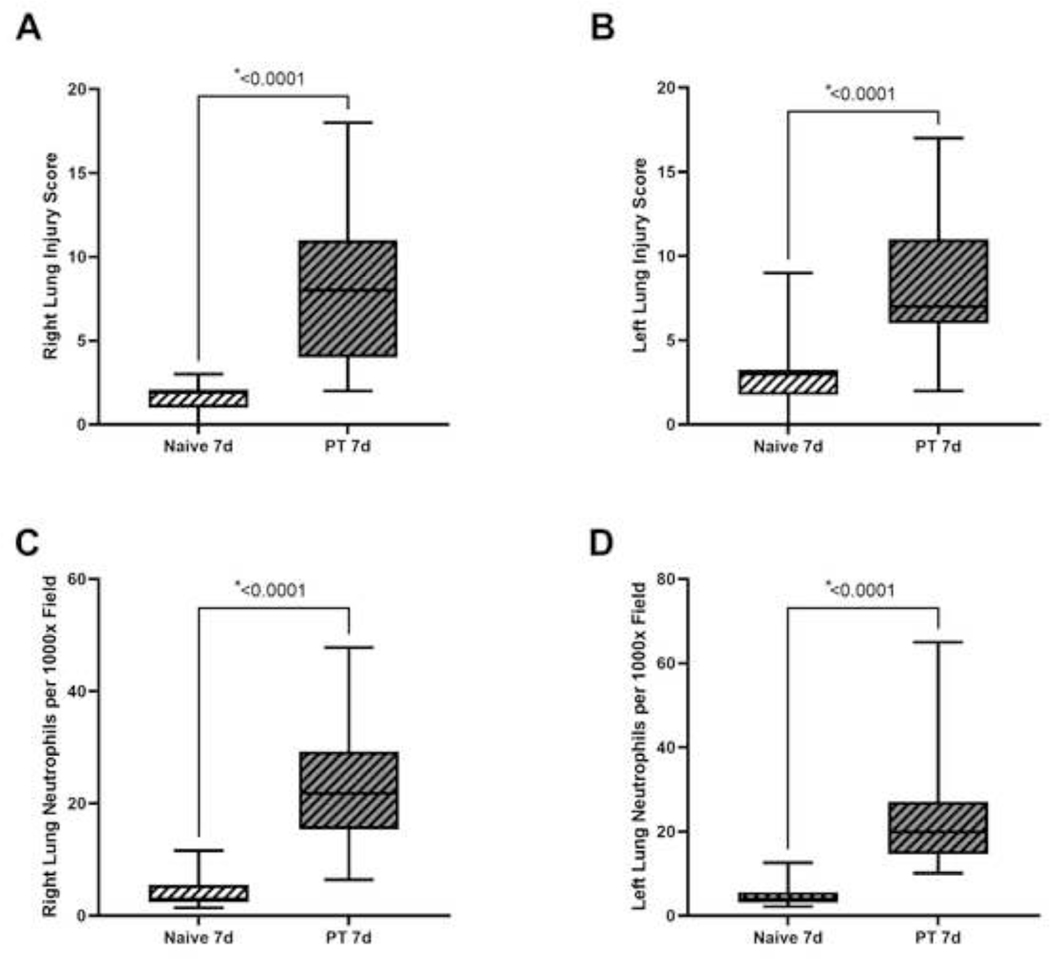
Representative right lung tissue at day seven from naïve and polytrauma cohorts can be found in Figure 3A-B. Lung injury scores for the right lung, which underwent contusion, demonstrated significantly elevated lung injury scores compared to naïve (Naïve 7d: 2.0, 1.0 to 2.0; PT 7d: 8.0, 4.0 to 11.0; p<0.0001) (Fig. 4A). Similarly, lung injury scores of the left lung were elevated compared to naïve (Naïve 7d: 3.0, 1.8 to 3.3; PT 7d: 7.0, 6.0 to 11.0; p<0.0001) (Fig. 4B). Histology of lungs from the PT cohort showed evidence of poor alveolar wall integrity, interstitial histiocytosis, apoptotic bodies, and neutrophils (Fig. 3B). Right lung histology showed elevated numbers of neutrophils per 1000x field (Naïve 7d: 3.0, 2.4 to 5.5; PT 7d: 21.8, 15.4 to 29.3; p<0.0001) (Fig. 4C). The left lung also demonstrated the presence of neutrophils compared to naïve counterparts (Naïve 7d: 4.1, 3.1 to 5.6; PT 7d: 19.9, 14.6 to 27.1; p<0.0001) (Fig. 4D). This suggests that despite unilateral lung contusion, polytrauma results in a systemic response to multiple injuries with histologic evidence of lung changes bilaterally.
Figure 3A-B.
Sample right lung histology from seven-day male cohorts for A) naïve with a lung injury score of 1 and B) polytrauma (PT) with a lung injury score of 14. Scale bar represents 100μm. Black arrow demonstrates neutrophil presence and white stars show expanded and thickened alveolar walls with increased cellularity.
Figure 4A-D.
Naïve and polytrauma (PT) cohorts for seven-day (7d) study duration A) right lung injury score, B) left lung injury score, C) right lung neutrophils per 1000x field, and D) left lung neutrophils per 1000x field. Only statistically significant comparisons displayed (*p<0.05).
Sex differences in recovery after polytrauma
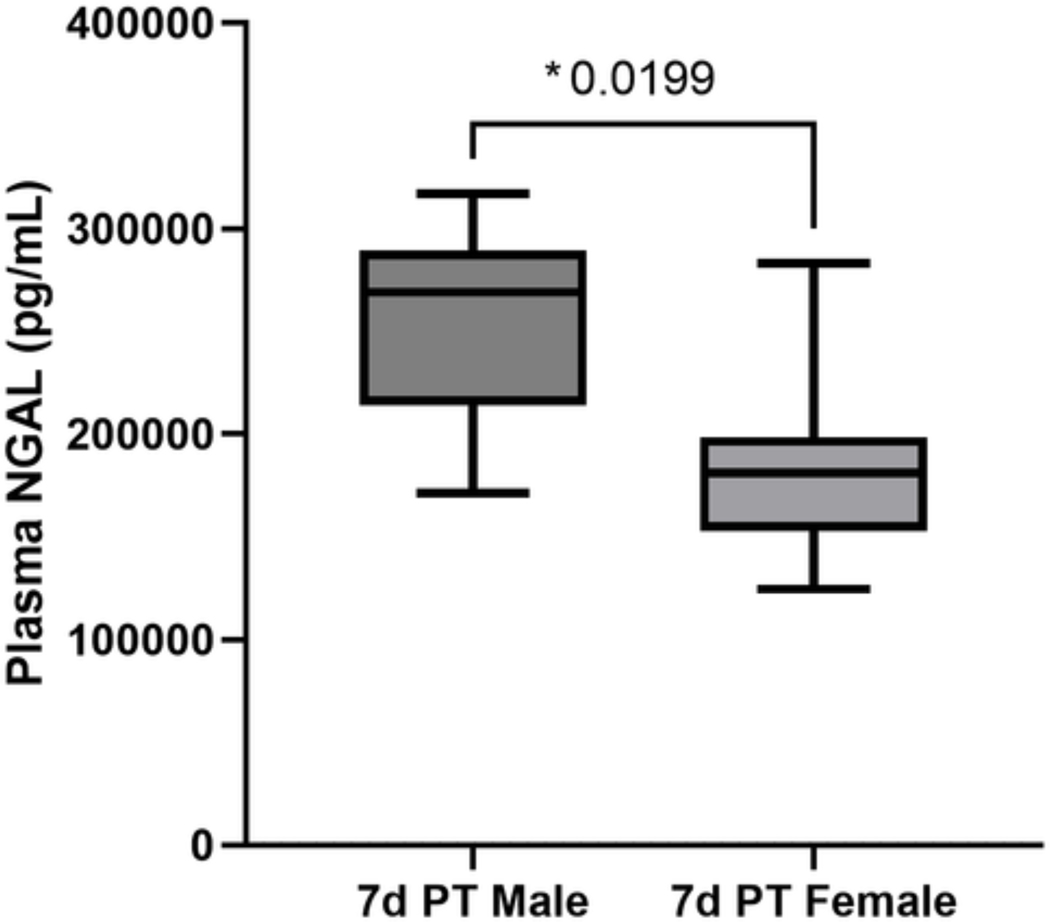
On subgroup analysis between males and females in polytrauma for two-day duration, there were no differences in weight loss, plasma NGAL, plasma TLR4, hemoglobin, or spleen to body weight ratio between sexes. On subgroup analysis of the seven-day PT cohort, while there were no sex differences in plasma TLR4, lung injury score, hemoglobin or spleen to body weight ratio. However, males had significantly elevated plasma NGAL at day seven compared to females (p=0.02) (Fig. 5). This suggests that females have improved renal recovery after severe injury compared to males.
Figure 5A-B.
Polytrauma seven-day cohort (PT 7d) comparing plasma neutrophil gelatinase-associated lipocalin (NGAL) between males and females. Only statistically significant comparisons displayed (*p<0.05).
DISCUSSION
Multicompartmental injury acutely results in drastic systemic changes, characterized by weight loss, renal injury, and systemic inflammation. These findings were persistent one-week after injury without recovery to baseline. Despite resuscitation of subjects after injury, anemia was present both two- and seven-days post-injury at levels still significantly decreased compared to controls. By day seven, there was evidence of extramedullary hematopoiesis with an increase in spleen to body weight ratio. Despite unilateral lung contusion of the right chest, seven days after injury there was evidence of lung injury bilaterally consisting of poor alveolar wall integrity, interstitial histiocytosis, apoptotic bodies, and neutrophils. On subgroup analysis by sex, males had worse renal injury at day seven compared to female counterparts, suggesting a slower recovery after severe injury. Together, these results demonstrate acute and subacute changes in physiology after severe injury with sexual dimorphism in renal recovery.
Both males and females demonstrated significant weight loss, persistent anemia, and renal injury both acutely at two days and at seven days after polytrauma. Other preclinical studies of less severe injury patterns do demonstrate similar acute weight loss with slow recovery similar to our results, although these studies were only in male animals (33, 34). Our findings of persistent anemia after severe injury and enlarged splenic tissue is also consistent with other groups who studied less severe injury as evidence of extramedullary hematopoiesis (35, 36). Finally, renal injury is commonly identified postinjury in other models of less severe injury as well as a result of complex underlying inflammatory and metabolic pathways (37, 38).
Despite unilateral lung contusion, we discovered evidence of lung injury bilaterally on histology in this model of severe polytrauma. This aligns with other studies which demonstrate secondary lung injury which can occur after trauma and be attributed to complex inflammatory and immune mechanisms, with histology consisting of interstitial edema and neutrophil presence (39, 40). In fact, TLR2 and TLR4 have been linked to a MyD88 pathway which contributes to this lung injury observed after blunt chest trauma; this aligns with our findings of increased plasma TLR4 after polytrauma (11–14). Therapeutics such as non-selective beta blockade and vincristine have been implicated in reducing the severity of lung injury after contusion after less severe trauma models, but remains to be further studied in the setting of polytrauma (41, 42).
Subgroup analysis of differences between males and females demonstrated that subjects demonstrated delayed renal recovery compared to females. These findings are similar to that of other studies in ischemia and reperfusion of the kidneys, although no studies to date have evaluated renal injury after polytrauma in preclinical studies of males and females (43, 44). We postulate that estrogen may play a role in improved renal recovery after injury in females based on evidence provided in other studies that estrogen may have protective effects after trauma, however this needs to be further studied in the setting of polytrauma (18).
We were surprised find an absence of sex differences in weight change, anemia, spleen size, systemic inflammation and lung injury by day seven. Most preclinical studies which report change in body weight postinjury only studied male subjects (33, 34). While other preclinical studies have not evaluated spleen weight after trauma specifically, they suggest that estradiol plays a role in the maintenance of immunocompetence after trauma with hemorrhage (45–47). Anemia recovery after severe polytrauma including hemorrhage has not been evaluated previously; however, preclinical studies in hemorrhage in the setting of trauma show that females have less alterations in cardiac and immune function and increased survival compared to males and ovariectomized females (18, 48). Additional studies are needed to evaluate anemia recovery after trauma including hemorrhage to investigate the presence of sexual dimorphism. Few preclinical studies have evaluated sexual dimorphism in postinjury inflammation, but one of trauma-hemorrhage demonstrated that estradiol attenuates tumor necrosis factor alpha levels (49). Others have demonstrated that females are protected against lung injury in trauma and hemorrhagic shock, although these models did not include lung contusion; these groups have associated estrogen the mediator of this protection (50–52). While we did not identify similar sex differences, a longer duration of study may demonstrate similar results to these regarding recovery from such severe injury.
This study has limitations. While rats subjected to polytrauma began to show recovery by day seven, the full course of recovery back to baseline was not elucidated; future studies of severe trauma for longer periods of time are necessary. Pseudofracture was used in this study to take the place of femur fractures in animals; while this has been demonstrated to induce similar physiologic changes as a femur fracture, they are not equivalent (30). However, this limited the suffering of animals to allow for an ethical longer duration of study in a model of polytrauma. In addition, we utilized spleen to body weight ratio to represent extramedullary hematopoiesis. Splenomegaly can be attributed to a variety of etiologies such as increased splenic function, infiltration, or congestion; given the model; in this study, increased size was presumed to represent extramedullary hematopoiesis (53). Further study of splenic tissue with flow cytometry should be pursued to evaluate for the presence of hematopoietic progenitor cells. Finally, only one age-group of animals was evaluated; it has been shown that age is associated with worse outcomes, such as hospital length of stay and mortality, after trauma (54). Therefore, additional preclinical studies of the response of different age groups to severe trauma is warranted.
In summary, this preclinical multicompartmental trauma model provides insight into the severe systemic change which both females and males undergo after injury. This study suggests that sex may play a role in renal recovery after injury, although longer durations of study are indicated to better understand the course to full recovery and how this may differ between males and females.
CONCLUSIONS
Severe traumatic injury induces weight loss, systemic inflammation, renal injury, lung injury, anemia and extramedullary hematopoiesis which do not fully recover after one week. Male subjects demonstrate prolonged renal recovery compared to females. Future studies should include longer durations of study to evaluate recovery from severe injury and the potential for therapeutic immunomodulatory and anti-inflammatory treatments to improve outcomes.
Supplementary Material
HIGHLIGHTS.
Multicompartmental injury induces system inflammation and multi-organ dysfunction
Multiple injuries in Sprague-Dawley rats results in bilateral lung histological changes
Males have prolonged renal recovery after injury compared to females
ACKNOWLEDGEMENTS
The authors would also like to acknowledge the Animal Care Services for their guidance and input regarding the design of this protocol along with their assistance with rodent care for this study.
These findings have been presented at the 18th Annual Academic Surgical Congress in Houston, Texas on February 8, 2023.
Funding:
This work was supported by the National Institutes of Health. AMM was supported by NIH NIGMS R01 GM105893. JAM, GSG and LSK were supported by postgraduate training grant NIH NIGMS T32 GM-008721 in burns, trauma, and perioperative injury.
Footnotes
No reprints will be requested.
Declaration of Interest: None
REFERENCES
- 1.Probst C, Zelle BA, Sittaro NA, Lohse R, Krettek C, et al. Late death after multiple severe trauma: when does it occur and what are the causes? J Trauma 2009:66:1212–1217. [DOI] [PubMed] [Google Scholar]
- 2.Weber B, Lackner I, Haffner-Luntzer M, Palmer A, Pressmar J, et al. Modeling trauma in rats: similarities to humans and potential pitfalls to consider. J Transl Med 2019:17:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weckbach S, Perl M, Heiland T, Braumuller S, Stahel PF, et al. A new experimental polytrauma model in rats: molecular characterization of the early inflammatory response. Mediators Inflamm 2012:2012:890816. [DOI] [PMC free article] [PubMed]
- 4.Gentile LF, Nacionales DC, Cuenca AG, Armbruster M, Ungaro RF, et al. Identification and description of a novel murine model for polytrauma and shock. Crit Care Med 2013:41:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denk S, Weckbach S, Eisele P, Braun CK, Wiegner R, et al. Role of Hemorrhagic Shock in Experimental Polytrauma. Shock 2018:49:154–163. [DOI] [PubMed] [Google Scholar]
- 6.Denk S, Wiegner R, Hones FM, Messerer DA, Radermacher P, et al. Early Detection of Junctional Adhesion Molecule-1 (JAM-1) in the Circulation after Experimental and Clinical Polytrauma. Mediators Inflamm 2015:2015:463950. [DOI] [PMC free article] [PubMed]
- 7.Akscyn RM, Franklin JL, Gavrikova TA, Schwacha MG, Messina JL A rat model of concurrent combined injuries (polytrauma). Int J Clin Exp Med 2015:8:20097–20110. [PMC free article] [PubMed] [Google Scholar]
- 8.Darlington DN, Craig T, Gonzales MD, Schwacha MG, Cap AP, et al. Acute coagulopathy of trauma in the rat. Shock 2013:39:440–446. [DOI] [PubMed] [Google Scholar]
- 9.McGhan LJ, Jaroszewski DE The role of toll-like receptor-4 in the development of multi-organ failure following traumatic haemorrhagic shock and resuscitation. Injury 2012:43:129–136. [DOI] [PubMed] [Google Scholar]
- 10.Frink M, Hsieh YC, Thobe BM, Choudhry MA, Schwacha MG, et al. TLR4 regulates Kupffer cell chemokine production, systemic inflammation and lung neutrophil infiltration following trauma-hemorrhage. Mol Immunol 2007:44:2625–2630. [DOI] [PubMed] [Google Scholar]
- 11.Hoth JJ, Wells JD, Brownlee NA, Hiltbold EM, Meredith JW, et al. Toll-like receptor 4-dependent responses to lung injury in a murine model of pulmonary contusion. Shock 2009:31:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoth JJ, Hudson WP, Brownlee NA, Yoza BK, Hiltbold EM, et al. Toll-like receptor 2 participates in the response to lung injury in a murine model of pulmonary contusion. Shock 2007:28:447–452. [DOI] [PubMed] [Google Scholar]
- 13.Zou F, Su X, Pan P Toll-Like Receptor-4-Mediated Inflammation is Involved in Intermittent Hypoxia-Induced Lung Injury. Lung 2020:198:855–862. [DOI] [PubMed] [Google Scholar]
- 14.Reino DC, Palange D, Feketeova E, Bonitz RP, Xu DZ, et al. Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock 2012:38:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haase M, Bellomo R, Haase-Fielitz A Neutrophil gelatinase-associated lipocalin. Curr Opin Crit Care 2010:16:526–532. [DOI] [PubMed] [Google Scholar]
- 16.Choudhry MA, Bland KI, Chaudry IH Trauma and immune response--effect of gender differences. Injury 2007:38:1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villapol S, Loane DJ, Burns MP Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 2017:65:1423–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, et al. Gender differences in acute response to trauma-hemorrhage. Shock 2005:24 Suppl 1:101–106. [DOI] [PubMed] [Google Scholar]
- 19.Weniger M, Angele MK, Chaudry IH The Role and Use of Estrogens Following Trauma. Shock 2016:46:4–11. [DOI] [PubMed] [Google Scholar]
- 20.Angele MK, Schwacha MG, Ayala A, Chaudry IH Effect of gender and sex hormones on immune responses following shock. Shock 2000:14:81–90. [DOI] [PubMed] [Google Scholar]
- 21.Bosch F, Angele MK, Chaudry IH Gender differences in trauma, shock and sepsis. Mil Med Res 2018:5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haider AH, Crompton JG, Oyetunji T, Stevens KA, Efron DT, et al. Females have fewer complications and lower mortality following trauma than similarly injured males: a risk adjusted analysis of adults in the National Trauma Data Bank. Surgery 2009:146:308–315. [DOI] [PubMed] [Google Scholar]
- 23., Guide for the Care and Use of Laboratory Animals. Washington (DC): 2011. [Google Scholar]
- 24.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMJ Open Sci 2020:4:e100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munley JA, Kelly LS, Pons EE, Kannan KB, Coldwell PS, et al. Multicompartmental Traumatic Injury and the Microbiome: Shift to a Pathobiome. J Trauma Acute Care Surg 2022. [DOI] [PMC free article] [PubMed]
- 26.Munley JA, Kelly LS, Park G, Gillies GS, Pons EE, et al. Multicompartmental Traumatic Injury Induces Sex-Specific Alterations in the Gut Microbiome. J Trauma Acute Care Surg 2023. [DOI] [PMC free article] [PubMed]
- 27.Marcondes FK, Bianchi FJ, Tanno AP Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 2002:62:609–614. [DOI] [PubMed] [Google Scholar]
- 28.Munley JA, Kelly LS, Pons EE, Kannan KB, Coldwell PS, et al. Multicompartmental traumatic injury and the microbiome: Shift to a pathobiome. J Trauma Acute Care Surg 2023:94:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly LS, Munley JA, Pons EE, Coldwell PS, Kannan KB, et al. Multicompartmental trauma alters bone marrow erythroblastic islands. J Trauma Acute Care Surg 2023:94:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darwiche SS, Kobbe P, Pfeifer R, Kohut L, Pape HC, et al. Pseudofracture: an acute peripheral tissue trauma model. J Vis Exp 2011. [DOI] [PMC free article] [PubMed]
- 31.Loftus TJ, Thomson AJ, Kannan KB, Alamo IG, Millar JK, et al. Clonidine restores vascular endothelial growth factor expression and improves tissue repair following severe trauma. Am J Surg 2017:214:610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loftus TJ, Thomson AJ, Kannan KB, Alamo IG, Ramos HN, et al. Effects of trauma, hemorrhagic shock, and chronic stress on lung vascular endothelial growth factor. J Surg Res 2017:210:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi L, Cui X, Dong W, Barrera R, Nicastro J, et al. Ghrelin attenuates brain injury after traumatic brain injury and uncontrolled hemorrhagic shock in rats. Mol Med 2012:18:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuura H, Ohnishi M, Yoshioka Y, Togami Y, Hosomi S, et al. Original experimental rat model of blast-induced mild traumatic brain injury: a pilot study . Brain Inj 2021:35:368–381. [DOI] [PubMed] [Google Scholar]
- 35.Alamo IG, Kannan KB, Loftus TJ, Ramos H, Efron PA, et al. Severe trauma and chronic stress activates extramedullary erythropoiesis. J Trauma Acute Care Surg 2017:83:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alamo IG, Kannan KB, Bible LE, Loftus TJ, Ramos H, et al. Daily propranolol administration reduces persistent injury-associated anemia after severe trauma and chronic stress. J Trauma Acute Care Surg 2017:82:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiang L, Calderon AS, Klemcke HG, Hudson IL, Hinojosa-Laborde C, et al. Extremity trauma exacerbates acute kidney injury following prolonged hemorrhagic hypotension. J Trauma Acute Care Surg 2021:91:S113–S123. [DOI] [PubMed] [Google Scholar]
- 38.Burmeister DM, Gomez BI, Dubick MA Molecular mechanisms of trauma-induced acute kidney injury: Inflammatory and metabolic insights from animal models. Biochim Biophys Acta Mol Basis Dis 2017:1863:2661–2671. [DOI] [PubMed] [Google Scholar]
- 39.Perl M, Lomas-Neira J, Venet F, Chung CS, Ayala A Pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med 2011:5:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, et al. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock 2009:32:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baranski GM, Sifri ZC, Cook KM, Alzate WD, Livingston DH, et al. Is the sympathetic system involved in shock-induced gut and lung injury? J Trauma Acute Care Surg 2012:73:343–350; discussion 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raghavendran K, Davidson BA, Woytash JA, Helinski JD, Marschke CJ, et al. The evolution of isolated bilateral lung contusion from blunt chest trauma in rats: cellular and cytokine responses. Shock 2005:24:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawasaki T, Chaudry IH The effects of estrogen on various organs: therapeutic approach for sepsis, trauma, and reperfusion injury. Part 2: liver, intestine, spleen, and kidney. J Anesth 2012:26:892–899. [DOI] [PubMed] [Google Scholar]
- 44.Satake A, Takaoka M, Nishikawa M, Yuba M, Shibata Y, et al. Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int 2008:73:308–317. [DOI] [PubMed] [Google Scholar]
- 45.Knoferl MW, Angele MK, Schwacha MG, Bland KI, Chaudry IH Preservation of splenic immune functions by female sex hormones after trauma-hemorrhage. Crit Care Med 2002:30:888–893. [DOI] [PubMed] [Google Scholar]
- 46.Hildebrand F, Hubbard WJ, Choudhry MA, Thobe BM, Pape HC, et al. Are the protective effects of 17beta-estradiol on splenic macrophages and splenocytes after trauma-hemorrhage mediated via estrogen-receptor (ER)-alpha or ER-beta? J Leukoc Biol 2006:79:1173–1180. [DOI] [PubMed] [Google Scholar]
- 47.Angele MK, Ayala A, Cioffi WG, Bland KI, Chaudry IH Testosterone: the culprit for producing splenocyte immune depression after trauma hemorrhage. Am J Physiol 1998:274:C1530–1536. [DOI] [PubMed] [Google Scholar]
- 48.Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, et al. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med 1997:25:106–110. [DOI] [PubMed] [Google Scholar]
- 49.Akabori H, Moeinpour F, Bland KI, Chaudry IH Mechanism of the anti-inflammatory effect Of 17beta-estradiol on brain following trauma-hemorrhage. Shock 2010:33:43–48. [DOI] [PubMed] [Google Scholar]
- 50.Doucet D, Badami C, Palange D, Bonitz RP, Lu Q, et al. Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PLoS One 2010:5:e9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caruso JM, Xu DZ, Lu Q, Dayal SD, Deitch EA The female gender protects against pulmonary injury after trauma hemorrhagic shock. Surg Infect (Larchmt) 2001:2:231–240. [DOI] [PubMed] [Google Scholar]
- 52.Caruso JM, Deitch EA, Xu DZ, Lu Q, Dayal SD Gut injury and gut-induced lung injury after trauma hemorrhagic shock is gender and estrus cycle specific in the rat. J Trauma 2003:55:531–539. [DOI] [PubMed] [Google Scholar]
- 53.McKenzie CV, Colonne CK, Yeo JH, Fraser ST Splenomegaly: Pathophysiological bases and therapeutic options. Int J Biochem Cell Biol 2018:94:40–43. [DOI] [PubMed] [Google Scholar]
- 54.Fu CY, Bajani F, Bokhari M, Starr F, Messer T, et al. Age itself or age-associated comorbidities? A nationwide analysis of outcomes of geriatric trauma. Eur J Trauma Emerg Surg 2022:48:2873–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.