Abstract
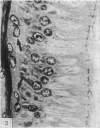
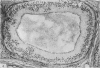
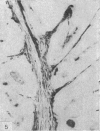
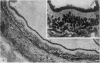
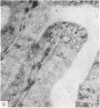
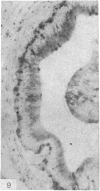
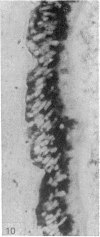
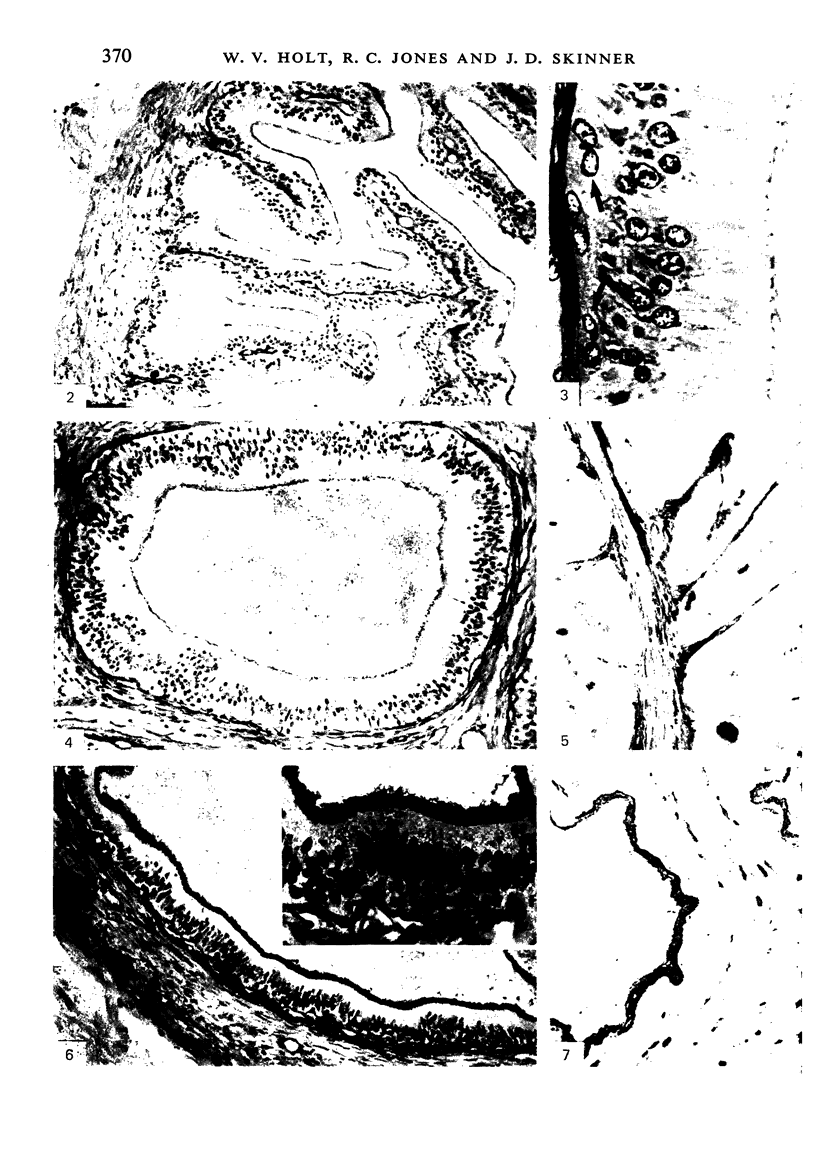
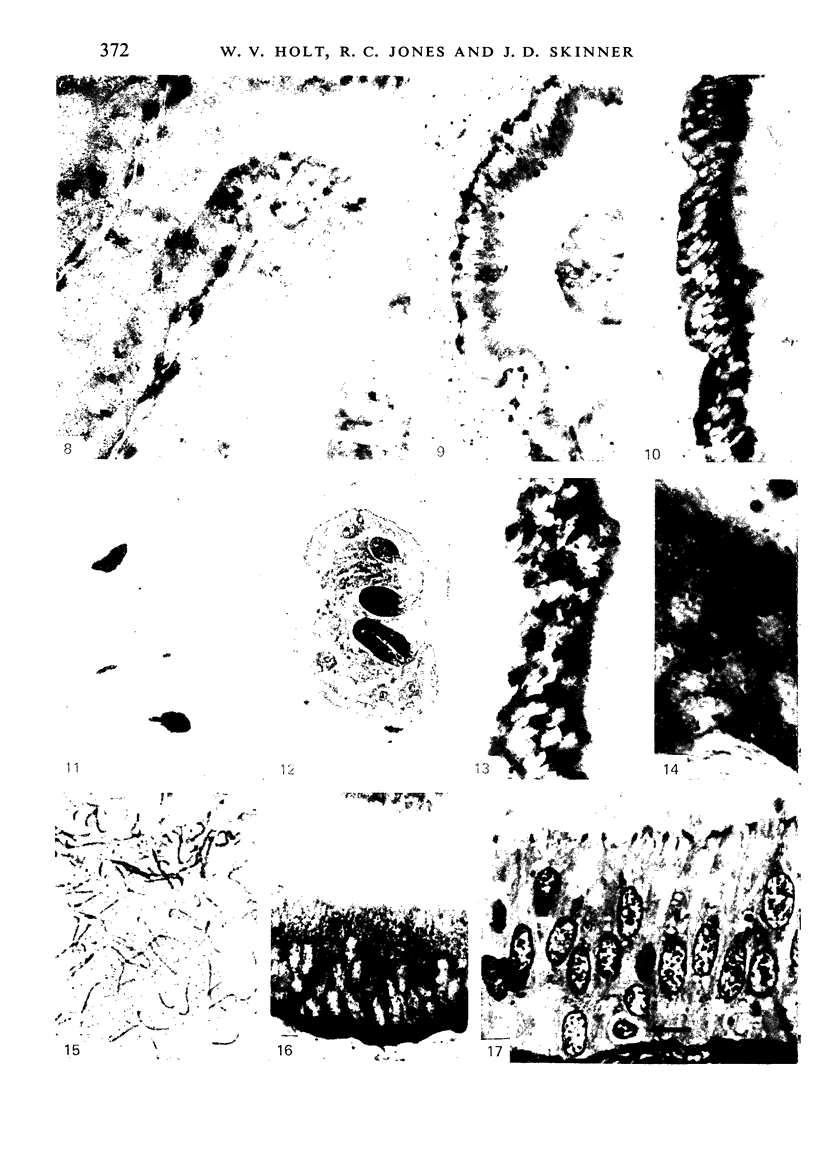
The three main segments of the elephant epididymis were examined for the occurrence, in the spermatozoa and lining epithelium, of carbohydrates, neutral lipids and phospholipids, ATPase, alkaline phosphatase, succinic dehydrogenase, glucose-6-phosphate dehydrogenase, diaphorases, hydroxysteroid dehydrogenases, acid phosphatase and non-specific esterase. The most distinct feature of the carbohydrate content of the epididymis was a layer of acidic, alcian blue-positive glycoprotein over the luminal surface of the epithelium, particularly in the terminal segment. PAS-positive, diastase-resistant inclusions were also found throughout the epdidymis. Neutral lipid occurred as droplets above and below the nucleus in the epithelium of the middle segment, and as supranuclear accumulations in the terminal segment. All the enzymes except the steroid dehydrogenases were detected in the epididymal epithelium, and all except the steroid dehydrogenases and acid phosphatase were detected in the spermatozoa. There was considerable variation in the intensity of the cytochemical reactions in the epithelium, but not in the spermatozoa, in different regions of the epididymis. In general, the enzymes involved in active transport showed strongest reactions in the initial and terminal segments, the reactions in the stereocilia being the most intense. The enzymes involved in energy metabolism showed strongest reactions in the middle and terminal segments, with the activity being fairly evenly distributed throughout the cytoplasm of the principal cells. However, the two lysosomal enzymes which were studied showed quite different distributions: the reactions for acid phosphatase were strongest in the initial and middle segments, whilst the reactions for non-specific esterase were strongest in the middle and terminal segments. It is suggested that the initial segment is involved in absorptive and anabolic activity, the middle segment in anabolic activity, and the terminal segment (where spermatozoa are stored ready for ejaculation) in considerable metabolic activity and active transport of substrates across the epithelium.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALOGH K., Jr, COHEN R. B. A CYTOCHEMICAL TECHNIC FOR STUDYING OXIDATIVE ENZYME SYSTEMS OF MAMMALIAN SPERMATOZOA IN SEMEN SMEARS. Fertil Steril. 1964 Jan-Feb;15:35–39. doi: 10.1016/s0015-0282(16)35105-6. [DOI] [PubMed] [Google Scholar]
- BEDFORD J. M. CHANGES IN THE ELECTROPHORETIC PROPERTIES OF RABBIT SPERMATOZOA DURING PASSAGE THROUGH THE EPIDIDYMIS. Nature. 1963 Dec 21;200:1178–1180. doi: 10.1038/2001178a0. [DOI] [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical demonstration of phosphatases in frozen sections with naphthol AS-phosphates. J Histochem Cytochem. 1961 Mar;9:146–153. doi: 10.1177/9.2.146. [DOI] [PubMed] [Google Scholar]
- Darin-Bennet A., Morris S., Jones R. C., White I. G. Proceedings: The glycerylphosphorylcholine and phospholipid pattern of the genital duct and spermatozoa of the African elephant, Loxodonta africana. J Reprod Fertil. 1976 Mar;46(2):506–507. doi: 10.1530/jrf.0.0460506. [DOI] [PubMed] [Google Scholar]
- Dym M., Romrell L. J. Intraepithelial lymphocytes in the male reproductive tract of rats and rhesus monkeys. J Reprod Fertil. 1975 Jan;42(1):1–7. doi: 10.1530/jrf.0.0420001. [DOI] [PubMed] [Google Scholar]
- EDWARDS R. G., VALENTINE R. C. CYTOCHEMICAL DEMONSTRATION OF SUCCINIC DEHYDROGENASE IN INTACT RABBIT SPERMATOZOA. Exp Cell Res. 1963 Sep;31:508–516. doi: 10.1016/0014-4827(63)90398-7. [DOI] [PubMed] [Google Scholar]
- Elleder M., Lojda Z. Studies in lipid histochemistry. XI. New, rapid, simple and selective method for the demonstration of phospholipids. Histochemie. 1973;36(2):149–166. doi: 10.1007/BF00304390. [DOI] [PubMed] [Google Scholar]
- Glover T. D., Nicander L. Some aspects of structure and function in the mammalian epididymis. J Reprod Fertil Suppl. 1971 May;13(Suppl):39–50. [PubMed] [Google Scholar]
- HOLT S. J. Indigogenic staining methods for esterases. Gen Cytochem Methods. 1958;1:375–398. [PubMed] [Google Scholar]
- HRUDKA F. CYTOCHEMICAL DEMONSTRATION OF CATABOLIC ACTIVITY IN SPERMATOZOA BY THE FORMAZAN TEST. J Reprod Fertil. 1965 Aug;10:15–20. doi: 10.1530/jrf.0.0100015. [DOI] [PubMed] [Google Scholar]
- Hoffer A. P., Hamilton D. W., Fawcett D. W. The ultrastructure of the principal cells and intraepithelial leucocytes in the initial segment of the rat epididymis. Anat Rec. 1973 Feb;175(2):169–201. doi: 10.1002/ar.1091750205. [DOI] [PubMed] [Google Scholar]
- Jones R. C., Holt W. V. The effects of washing on the ultrastructure and cytochemistry of ram spermatozoa. J Reprod Fertil. 1974 Nov;41(1):159–167. doi: 10.1530/jrf.0.0410159. [DOI] [PubMed] [Google Scholar]
- Jones R. C., Rowlands I. W., Skinner J. D. Spermatozoa in the genital ducts of the African elephant, Loxodonta africana. J Reprod Fertil. 1974 Nov;41(1):189–192. doi: 10.1530/jrf.0.0410189. [DOI] [PubMed] [Google Scholar]
- Kempenich-Pinto O., Schindler H., Bornstein S., Baroutchieva M. The fertilization rate of domestic hens after intramagnal or intra-uterine inseminations with turkeyspermatozoa. J Reprod Fertil. 1970 Mar;21(2):353–354. doi: 10.1530/jrf.0.0210353. [DOI] [PubMed] [Google Scholar]
- Mathur R. S. Histo-enzymological observations on spermatozoa of inbred strains of mice. J Reprod Fertil. 1971 Oct;27(1):5–11. doi: 10.1530/jrf.0.0270005. [DOI] [PubMed] [Google Scholar]
- Mercado E., Rosado A. Structural properties of the membrane of intact human spermatozoa. A study with fluorescent probes. Biochim Biophys Acta. 1973 Mar 29;298(3):639–652. doi: 10.1016/0005-2736(73)90080-1. [DOI] [PubMed] [Google Scholar]
- Moniem K. A., Glover T. D. Alkaline phosphatase in the cytoplasmic droplet of mammalian spermatozoa. J Reprod Fertil. 1972 Apr;29(1):65–69. doi: 10.1530/jrf.0.0290065. [DOI] [PubMed] [Google Scholar]
- NICANDER L. On the regional histology and cytochemistry of the ductus epididymidis in rabbits. Acta Morphol Neerl Scand. 1957;1(2):99–118. [PubMed] [Google Scholar]
- NICANDER L. Studies on the regional histology and cytochemistry of the ductus epididymidis in stallions, rams and bulls. Acta Morphol Neerl Scand. 1958;1(4):337–362. [PubMed] [Google Scholar]
- Nicander L., Glover T. D. Regional histology and fine structue of the epididymal duct in the golden hamster (Mesocricetus auratus). J Anat. 1973 Apr;114(Pt 3):347–364. [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Yanagimachi R. Terminal saccharides on sperm plasma membranes: identification by specific agglutinins. Science. 1972 Jul 21;177(4045):276–279. doi: 10.1126/science.177.4045.276. [DOI] [PubMed] [Google Scholar]
- Olson G. E., Hamilton D. W. Characterization of the surface glycoproteins of rat spermatozoa. Biol Reprod. 1978 Aug;19(1):26–35. doi: 10.1095/biolreprod19.1.26. [DOI] [PubMed] [Google Scholar]
- Prasad M. R., Rajalakshmi M., Gupta G., Karkun T. Control of epididymal function. J Reprod Fertil Suppl. 1973 Jul;18:215–222. [PubMed] [Google Scholar]
- Roussel J. D., Stallcup O. T. A histochemical study of the development of alkaline phosphatase in the testicle and epididymis of young bovine males. Int J Fertil. 1966 Apr-Jun;11(2):215–225. [PubMed] [Google Scholar]
- Short R. V., Mann T., Hay M. F. Male reproductive organs of the African elephant, Loxodonta africana. J Reprod Fertil. 1967 Jun;13(3):517–536. doi: 10.1530/jrf.0.0130517. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Glover T. D. Proceedings: The effect of castration on the epididymal epithelium of the golden hamster, Mesocricetus auratus. J Reprod Fertil. 1973 Dec;35(3):584–585. doi: 10.1530/jrf.0.0350584. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E., NIEDZWIEDZ A. Histochemical demonstration of mitochondrial adenosine triphosphatase with the lead-adenosine triphosphate technique. J Histochem Cytochem. 1960 Sep;8:387–388. doi: 10.1177/8.5.387. [DOI] [PubMed] [Google Scholar]