Abstract
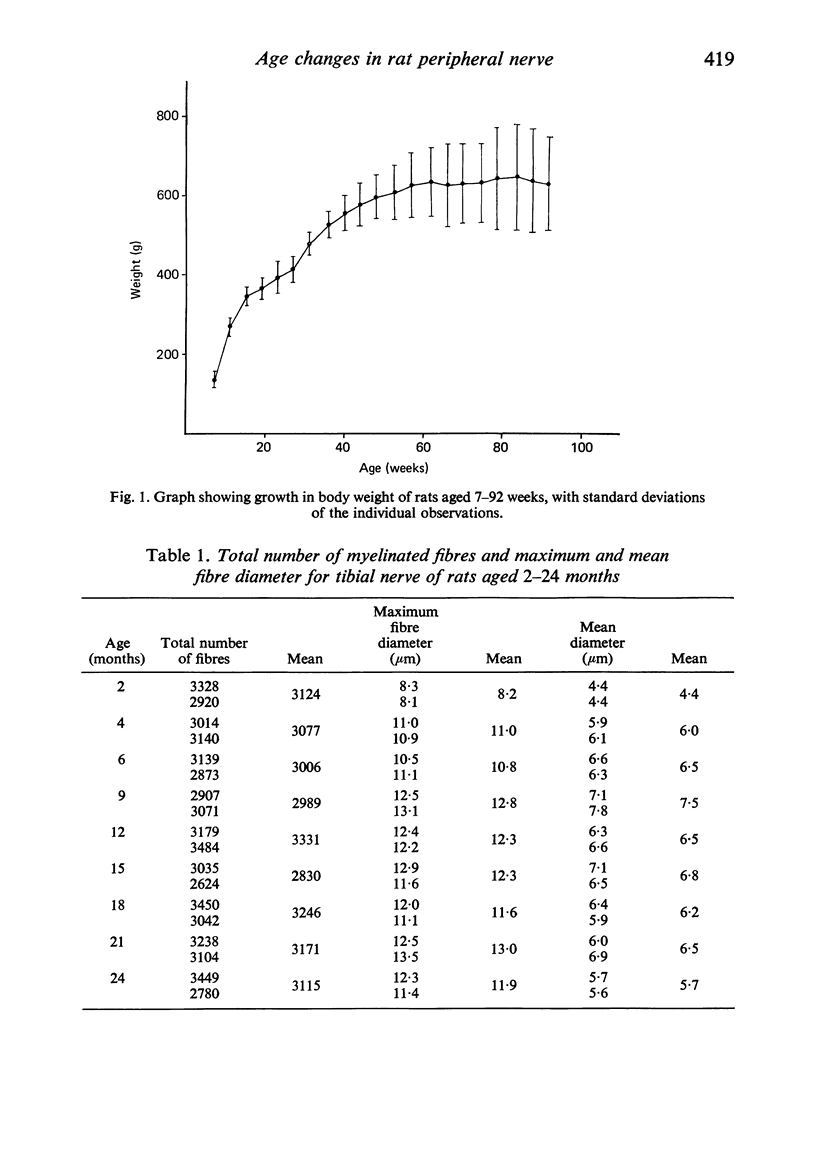
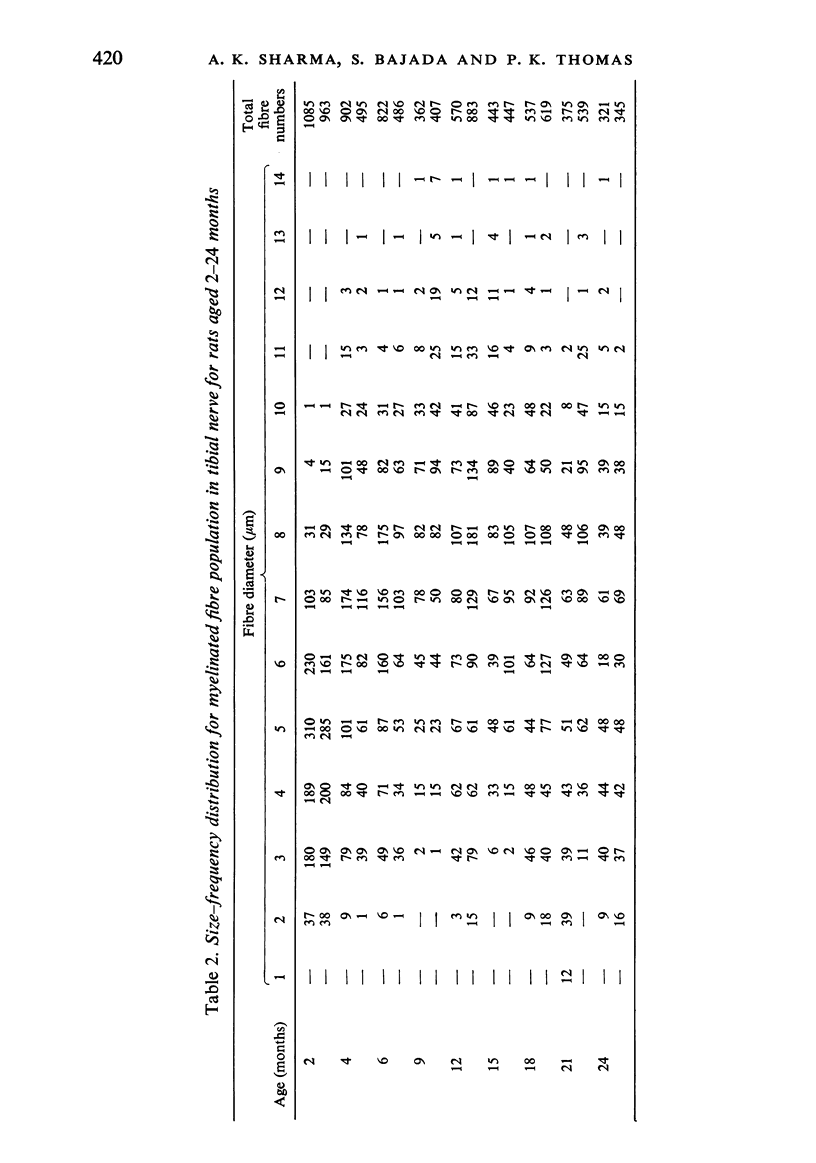
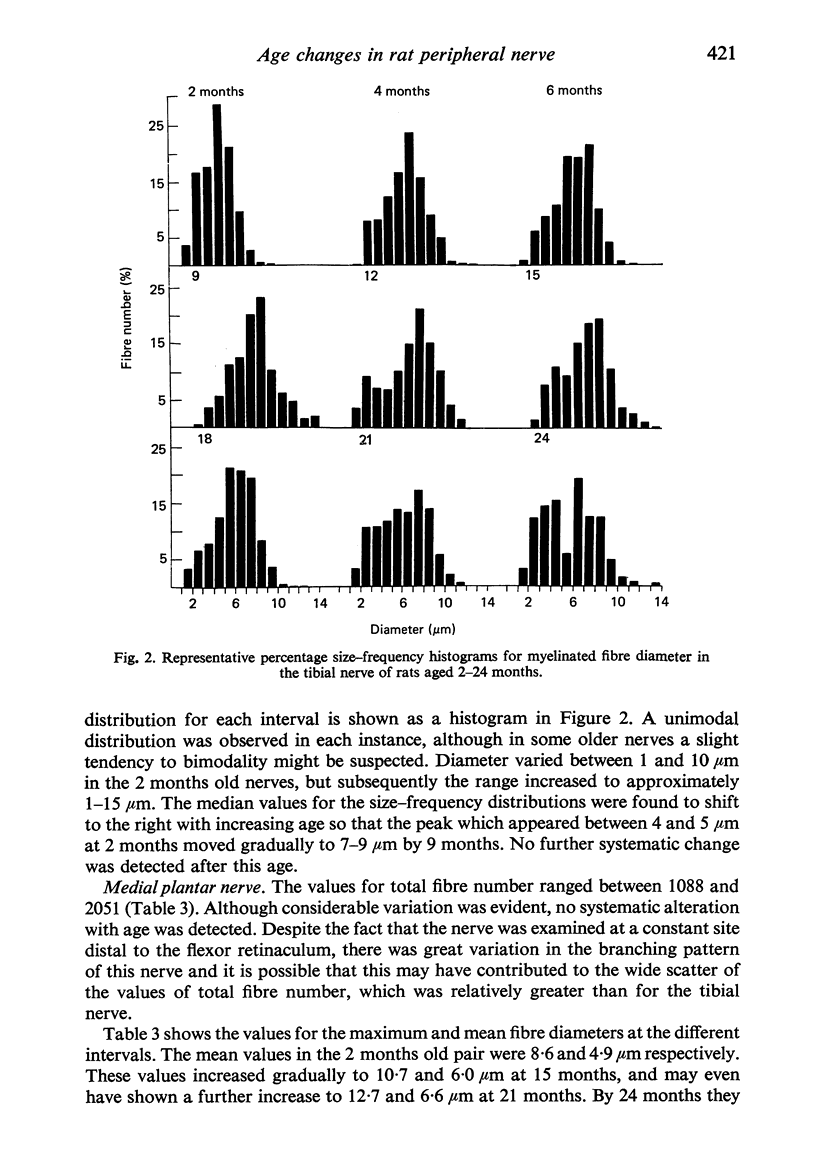
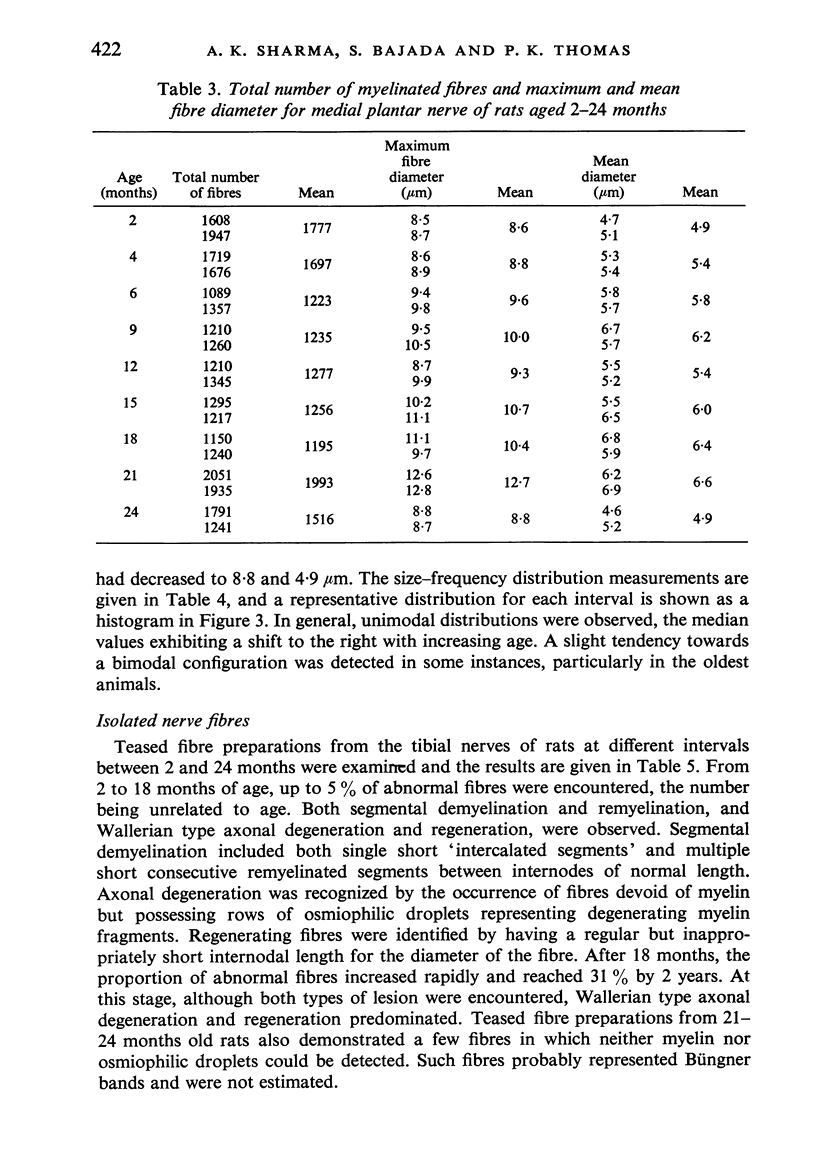
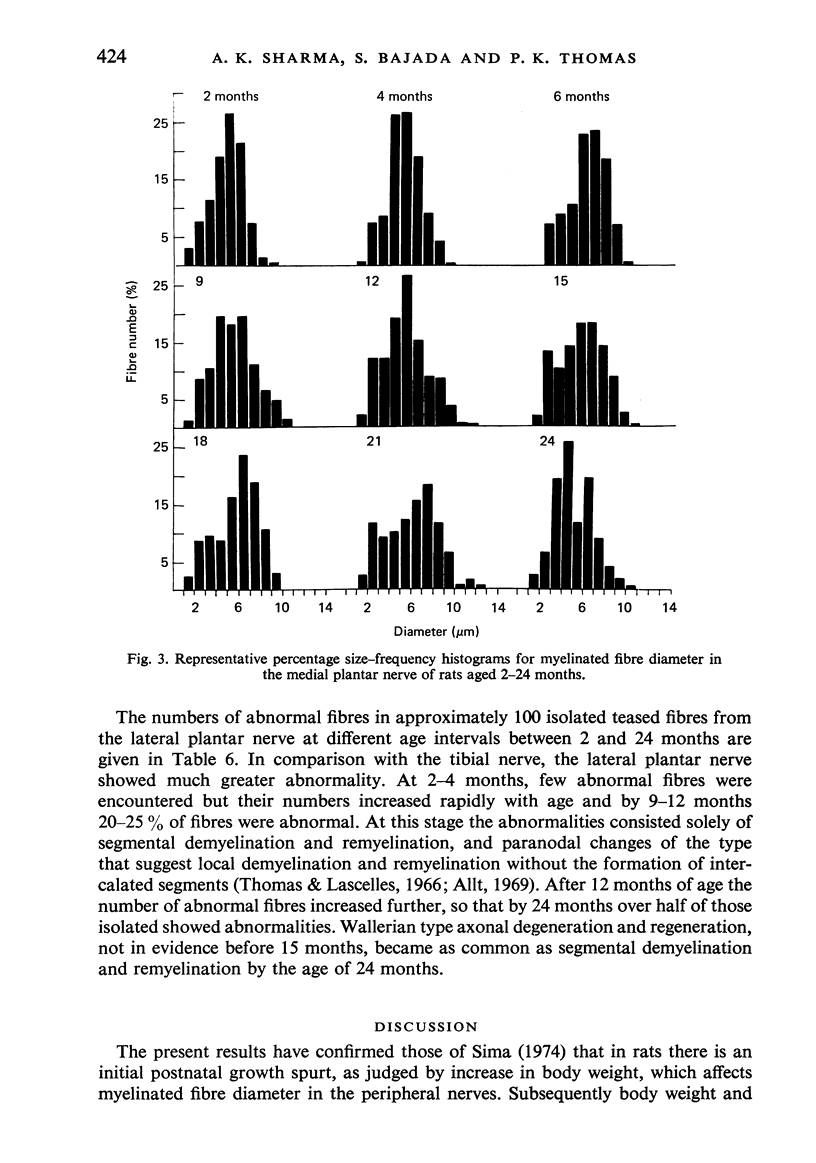
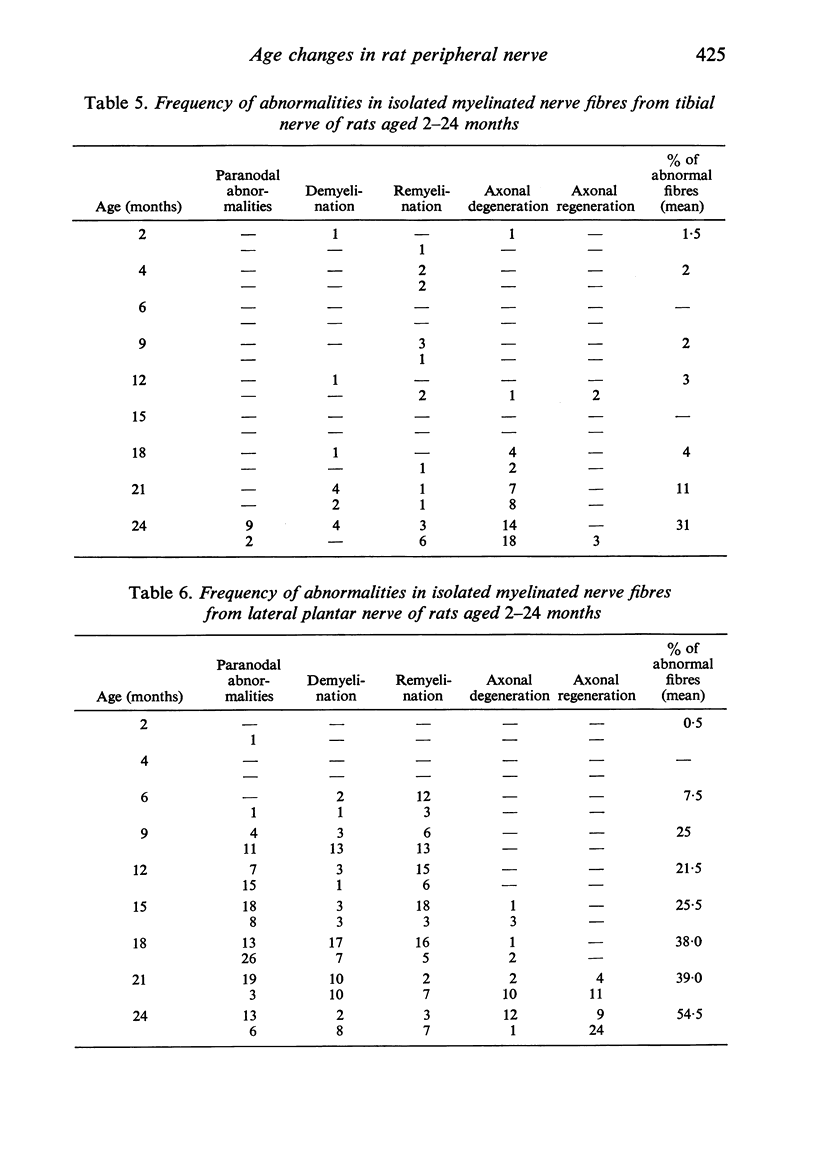
Observations have been made on the changes in the myelinated fibres of the rat tibial and plantar nerves between 2 and 24 months of age. There is an initial rapid increase in fibre diameter followed by a later more gradual increase, which ceases after approximately 9 months of age in the tibial nerve but which continues for longer in the medial plantar nerve. The fibre size distribution remains substantially unimodal throughout. In both nerves maximal and average fibre diameter become reduced by 24 months. Total fibre number shows considerable variability between animals, but no definite systematic alteration with age is detectable. Teased fibre preparations demonstrate a low level of abnormality in the tibial nerve until after 18 months of age, but by 24 months approximately 30% of fibres display abnormalities. Although both paranodal and segmental demyelination and remyelination, and axonal degeneration and regeneration occur, the latter type of change predominates. By contrast, in the lateral plantar nerve paranodal and segmental demyelination become detectable to a significant extent from 6 months of age. Axonal degeneration and regeneration also become evident after 15 months, and by 24 months of age 55% of fibres show abnormalities. The possible explanation of these changes is discussed, as is their relevance to the frequent use of the tibial nerve for studies on experimental neuropathies.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allt G. Repair of segmental dehyelination in peripheral nerves: an electron microscope study. Brain. 1969;92(3):639–646. doi: 10.1093/brain/92.3.639. [DOI] [PubMed] [Google Scholar]
- BIRREN J. E., WALL P. D. Age changes in conduction velocity, refractory period, number of fibers, connective tissue space and blood vessels in sciatic nerve of rats. J Comp Neurol. 1956 Feb;104(1):1–16. doi: 10.1002/cne.901040102. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. THE CONDUCTION VELOCITY OF REGENERATED PERIPHERAL NERVE FIBRES. J Physiol. 1964 May;171:164–175. doi: 10.1113/jphysiol.1964.sp007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton P. M., Gilliatt R. W. Median and ulnar neuropathy in the guinea-pig. J Neurol Neurosurg Psychiatry. 1967 Oct;30(5):393–402. doi: 10.1136/jnnp.30.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A. P., Morgan-Hughes J. A. The effect of local pressure in diphtheritic neuropathy. J Neurol Neurosurg Psychiatry. 1969 Dec;32(6):614–623. doi: 10.1136/jnnp.32.6.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys J. G., Palmano K. P., Sharma A. K., Thomas P. K. Influence of dietary myoinositol on nerve conduction and inositol phospholipids in normal and diabetic rats. J Neurol Neurosurg Psychiatry. 1978 Apr;41(4):333–339. doi: 10.1136/jnnp.41.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles R. G., Thomas P. K. Changes due to age in internodal length in the sural nerve in man. J Neurol Neurosurg Psychiatry. 1966 Feb;29(1):40–44. doi: 10.1136/jnnp.29.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell H., Knox D., Lee S., Charters A. C., Orloff M., Garrett R., Lampert P. Alloxan diabetic neuropathy: electron microscopic studies. Neurology. 1977 Jan;27(1):60–66. doi: 10.1212/wnl.27.1.60. [DOI] [PubMed] [Google Scholar]
- Samorajski T. Age differences in the morphology of posterior tibial nerves of mice. J Comp Neurol. 1974 Oct 15;157(4):439–445. doi: 10.1002/cne.901570406. [DOI] [PubMed] [Google Scholar]
- Schlaepfer W. W., Myers F. K. Relationship of myelin internode elongation and growth in the rat sural nerve. J Comp Neurol. 1973 Jan 15;147(2):255–266. doi: 10.1002/cne.901470207. [DOI] [PubMed] [Google Scholar]
- Sharma A. K., Thomas P. K., Baker R. W. Peripheral nerve abnormalities related to galactose administration in rats. J Neurol Neurosurg Psychiatry. 1976 Aug;39(8):794–802. doi: 10.1136/jnnp.39.8.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A. K., Thomas P. K., De Molina A. F. Peripheral nerve fiber size in experimental diabetes. Diabetes. 1977 Jul;26(7):689–692. doi: 10.2337/diab.26.7.689. [DOI] [PubMed] [Google Scholar]
- Sharma A. K., Thomas P. K. Peripheral nerve structure and function in experimental diabetes. J Neurol Sci. 1974 Sep;23(1):1–15. doi: 10.1016/0022-510x(74)90136-1. [DOI] [PubMed] [Google Scholar]
- Sima A. Studies on fibre size in developing sciatic nerve and spinal roots in normal, undernourished, and rehabilitated rats. Acta Physiol Scand Suppl. 1974;406:1–55. [PubMed] [Google Scholar]
- Skoglund S., Romero C. Postnatal growth of spinal nerves and roots. A morphological study in the cat with physiological correlations. Acta Physiol Scand Suppl. 1965;260:1–50. [PubMed] [Google Scholar]
- Spencer P. S., Thomas P. K. The examination of isolated nerve fibres by light and electron microscopy, with observations on demyelination proximal to neuromas. Acta Neuropathol. 1970;16(3):177–186. doi: 10.1007/BF00687357. [DOI] [PubMed] [Google Scholar]
- Stanmore A., Bradbury S., Weddell A. G. A quantitative study of peripheral nerve fibres in the mouse following the administration of drugs. 1. Age changes in untreated CBA mice from 3 to 21 months of age. J Anat. 1978 Sep;127(Pt 1):101–115. [PMC free article] [PubMed] [Google Scholar]
- VIZOSO A. D. The relationship between internodal length and growth in human nerves. J Anat. 1950 Oct;84(4):342–353. [PMC free article] [PubMed] [Google Scholar]



