Abstract
Nanoparticles are emerging as transformative agents in endodontics, addressing challenges in treating the dentin-pulp complex. This scoping review aims to explore multifunctional applications of nanoparticles in endodontics, with a focus on their roles in promoting tissue regeneration through therapeutic effects, enhancing material properties, and serving a carrier function. Following PRISMA-Scoping Review guidelines, a comprehensive literature search was conducted across Web of Science, PubMed, and Scopus. A total of 490 articles were initially identified, of which 92 met the preliminary eligibility criteria. Following full-text screening, 70 studies were included in the qualitative synthesis. Key findings from both in vitro and in vivo studies are summarized in tabular form. Results reveal a notable imbalance in the types of nanomaterials studied: inorganic nanomaterials were reported in 77% of the studies, while only 23% investigated organic nanomaterials. Despite their lower representation, organic nanomaterials demonstrated considerable relevance. Chitosan was reported in 29% of the carrier studies, while extracellular vesicles were featured in 22% of the therapeutic applications. Among inorganic materials, bioactive glass was frequently reported, appearing in 31% of enhancer-related studies, 26% of therapeutic studies, and 13% of those investigating carrier functions. The end applications of these nanoparticles were in 69% of the studies either (direct) pulp capping or root canal filling, highlighting the need for innovative materials in these applications. Regarding experimental models, 75% of the studies conducted in vitro research on relevant cell lines, while 25% employed animal models. Of those 25% in vivo studies, 18% also reported in vitro findings. Nanoparticles hold significant promise for transforming endodontics, offering enhanced antibacterial efficacy and bioactivity while addressing critical limitations of conventional materials. However, challenges remain regarding their long-term biocompatibility, scalability, and integration into clinical workflows. This review emphasizes the need for translational research to bridge the gap between laboratory innovations and clinical practice.
Graphical abstract
Keywords: Nanoparticles, Endodontics, Dental Materials, Biocompatible materials, Regeneration
Introduction
Biomaterials play a critical role in modern endodontics, serving as replacements for damaged tissues, delivery systems for bioactive molecules, and scaffolds for tissue regeneration [1, 2]. However, despite advances in materials science, the development of biomaterials that interact effectively with the intricate dentin-pulp complex remains a challenge. This complex microenvironment consists of multiple layers with different mechanical and physiological properties, requiring materials that balance durability, biocompatibility, and bioactivity [3]. Unfortunately, many conventional endodontic biomaterials fail to achieve this balance, limiting their clinical efficacy.
For example, while calcium silicate-based cements are widely used due to their bioactivity, they have drawbacks such as slow setting time, tooth discoloration, inconsistent mechanical properties, and suboptimal handling properties [4]. Similarly, current pulp capping agents and root canal sealers can promote tissue healing but lack sufficient antibacterial efficacy to prevent reinfection, a primary cause of endodontic treatment failure [5]. The limitations of these materials highlight the urgent need for innovative solutions that address not only mechanical and biological requirements, but also antimicrobial and regenerative potential.
Nanoparticles, defined as colloidal particles ranging between 1 and 100 nm, have already shown immense potential in several medical fields, including cancer treatment, wound healing, and endodontics [6–10]. They can be synthesised from a variety of materials and are often divided into two distinct groups: organic and inorganic nanoparticles [11]. In endodontics, nanoparticles could enhance the functional properties of biomaterials by improving mechanical strength, providing antibacterial effects to combat biofilm formation, and promoting stem cell differentiation [12, 13]. Their ability to act as carriers for therapeutic agents also enables innovative applications such as localised drug delivery and regenerative therapies. Antibacterial effects are critical in endodontics, where biofilm formation can compromise treatment outcomes [14]. These unique properties make nanoparticles valuable tools for addressing the complexities of the dentin-pulp complex and advancing endodontic biomaterials.
Even though the potential of nanoparticles has attracted considerable attention, existing reviews have mainly focused on their antibacterial properties [15, 16]. While studies have highlighted their efficacy in reducing biofilm-associated infections, their role in material reinforcement, regenerative endodontics, and drug delivery has been less systematically reviewed [17–20]. This gap highlights the need for a broader, more integrative perspective on nanoparticle applications in endodontics.
This scoping review aims to fill this gap by providing a comprehensive overview of the multiple roles of nanoparticles in the advancement of biomaterials in endodontics. It will explore their applications as material enhancers, carriers of therapeutic agents, and facilitators of tissue regeneration. The review synthesises findings from in vitro and in vivo studies, identifies limitations in current research, and outlines directions for future investigation. By addressing the challenges of the dentin-pulp complex, this scoping review aims to pave the way for the next generation of endodontic biomaterials and contribute to the wider adoption of nanoparticle-based therapies in clinical practice.
Methods
Protocol
Our protocol was drafted using the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA) [21].
Eligibility criteria
Published original research articles in English on applying nanoparticles in endodontics were considered eligible. Clinical studies were excluded due to the review’s focus on basic and preclinical research. Conference proceedings, recommendations, expert statements, technical reports, reviews, case reports, and non-original papers were excluded as well.
Information sources
The databases used were Web of Science (WoS), PubMed, and Scopus. The primary literature search was performed on the 3rd of August 2023, and to ensure the inclusion of the most relevant and up-to-date literature, the databases were continuously monitored for newly published studies, and eligible articles meeting the inclusion criteria were manually added until the 13th of February 2025. This approach allowed for a comprehensive and current synthesis of the available evidence.
Search
The search conducted was: ("Dental pulp" [Mesh] OR "Dental Pulp Cavity" [Mesh] OR "Dentin" [Mesh] OR "Endodontics" [Mesh] OR "Dental pulp exposure" [Mesh] OR "Dental Pulp Diseases" [Mesh] OR "Dental Pulp Capping" [Mesh] OR endodont* OR “pulp exposure” OR “pulp capping” OR “tooth” OR “dentine” OR “dental pulp” OR “dentistry”) AND ("Regenerative medicine" [Mesh] OR "Endodontic Regeneration" OR "Regenerative Endodontics" [Mesh] OR "Odontogenic differentiation" OR "Odontoblasts" [Mesh] OR "Cell Differentiation" [Mesh] OR “Pulp Regeneration” OR “dentinogenesis” OR “dentin formation”) AND ("Nanoparticles" [Mesh] OR "Nanoparticle Drug Delivery System" [Mesh] OR nanoparticle*) NOT (“Crowns” [Mesh] OR "Denture, Partial, Fixed" [Mesh] OR “Resin Cement” [Mesh] OR “Dental Cement” [Mesh] OR “Ceramics” [Mesh] OR “Dental Bonding” [Mesh] OR “Composite Resins” [Mesh] OR “post”). This search strategy was adapted to fulfill the requirements of different search engines.
Selection of sources of evidence
After removing duplicates, both reviewers (U.I. and C.M.R.) screened the same publications independently. The articles were first screened on titles and abstracts. Following, a full-text screening was executed, where following exclusion criteria were used:
Not referring to nanoparticles or unclear whether they do
Applications other than cell differentiation, regeneration, or antimicrobial
Studies related to bone
No full text is available
Poor quality
Retracted article
Disagreements on study selection and data extraction were resolved by consensus and discussion.
Data charting process
Two reviewers (U.I. and C.M.R.) jointly developed a data-charting form to determine which variables to extract. The two reviewers independently charted the data, discussed the results, and continuously updated the data-charting form in an iterative process.
Data items
Data was abstracted on article characteristics (e.g., dental application), nanomaterial (e.g., bioactive glass, calcium phosphate, chitosan, silver, gold), used cell types, and results of the nanoparticle application (e.g., antibacterial activity, dental material enhancement or regeneration potential).
Synthesis of the results
The studies were grouped by the function the nanoparticles fulfilled. They summarized the type of nanoparticles, cell types used, and experimental models for each paper, along with a concise effect of the nanoparticles. In the context of drug delivery, a carrier refers to nanoparticles designed to transport and release pharmaceutical compounds within the body. These nanosystems can target specific cells or tissues, ensuring that the drug is delivered precisely where needed, thereby increasing the efficacy of the treatment and reducing side effects. When functioning as an enhancer, nanoparticles are utilized to improve the properties of existing materials. By integrating nanoparticles, the material gains additional attributes such as increased strength, enhanced thermal stability, improved electrical conductivity, or better biocompatibility, which are beneficial for the intended application. As therapeutic agents, nanoparticles themselves exert a direct therapeutic effect on tissues. These nanoparticles can influence biological processes such as cellular differentiation, regeneration, or inflammation modulation, providing therapeutic benefits independent of any drug they might carry.
Results
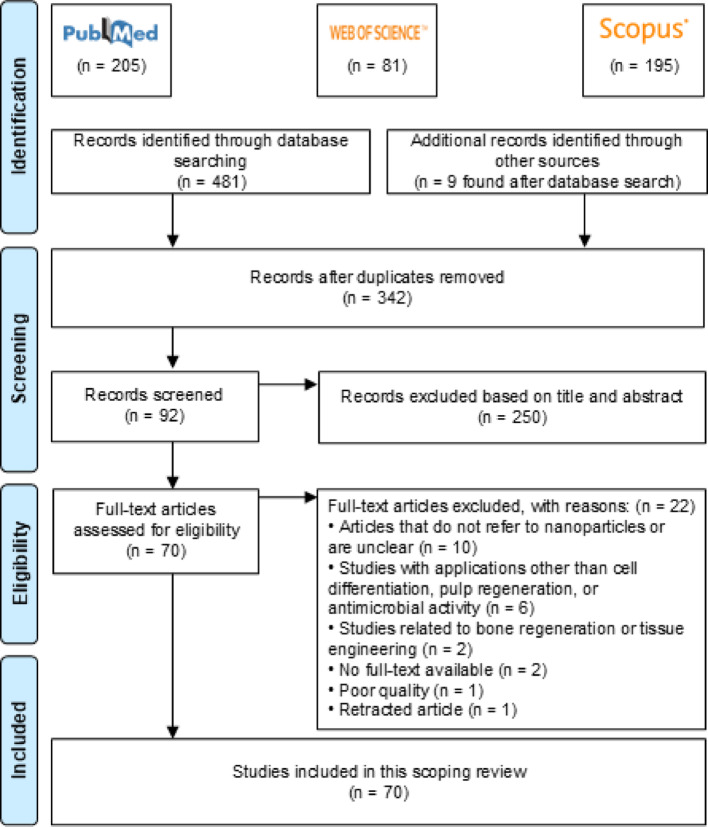
A total of 490 papers were identified from database searches, with one additional paper identified manually. After removing duplicates, 342 papers were screened based on titles and abstracts. Of these, 92 full-text articles were assessed for eligibility, and 70 studies were ultimately included in the scoping review (Fig. 1). All included studies were published within the past two decades.
Fig. 1.
Overview of studies included in a PRISMA flow chart
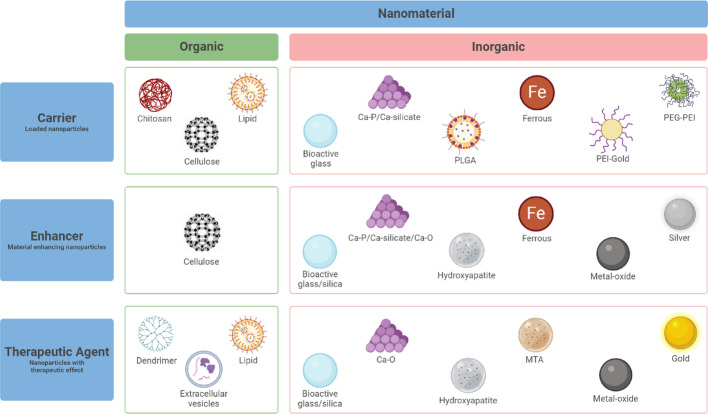
The studies were categorized based on the primary functions of the nanoparticles: carriers (n = 24), enhancers (n = 26), and therapeutic agents (n = 23). Notably, three studies reported dual functions, classifying nanoparticles as carriers and enhancers [22–24] or both carriers and therapeutic agents [24].
As shown in Fig. 2, the research landscape remains predominantly centred on inorganic nanomaterials (77%), while organic alternatives are investigated in 23% of the reported studies. Despite this disparity, organic nanomaterials demonstrate significant potential. Chitosan, for example, is featured in 29% of the studies focusing on drug delivery carriers, and extracellular vesicles are utilized in 22% of those targeting therapeutic applications. Among inorganic materials, bioactive glass stands out, appearing in 31% of enhancer-related studies, 26% of therapeutic studies, and 13% of those involving carrier functions.
Fig. 2.
Overview of the primary functions of the nanoparticles and the reported nanomaterials
The included studies reported a broad range of nanoparticle sizes, typically between 10 and 500 nm. However, Raddi et al. (2024) reported a substantially larger size range of 145 to 4200 nm, making it an outlier among the reviewed studies [25].
Out of the studies included, 75% reported in vitro experiments investigating various effects of nanoparticles on stem cells. These effects were validated based on experiments on differentiation, mineralisation capacities, cell viability, and regeneration occurrence. All studies reported odontogenesis to be the main dental application, except a few studies that had amelogenesis [26–28], antibacterial activity [29, 30], and angiogenesis [22] as the main dental application. Human dental pulp stem cells (hDPSCs) were the most frequently studied cell type, followed by stem cells of the apical papilla (SCAPs) [5, 31–35], stem cells from human exfoliated deciduous teeth (SHEDs) [36, 37], and periodontal ligament stem cells (PDLSCs) [38, 39]. Additional cell types included endometrial stem cells (EnSCs) [40], osteosarcoma stem cell lines such as SaOS2 cells [26] and MG63 [27, 41], bone marrow stem cells (BMSCs) [42], and periodontal ligament fibroblasts (PDLF) in co-culture with macrophages (Mφ) [43]. Four studies utilized rodent-derived stem cells, including rat DPSCs [44, 45], rat dental epithelial (HAT-7) cells [28], and RAW 264.7, an adherent cell line isolated from a mouse tumor [46].
Nanoparticles were also investigated for their antibacterial properties, with five studies focusing on their effects against common oral pathogens. These included Escherichia coli (E. coli) [41], Enterococcus faecalis (E. faecalis) [29, 47, 48], Fusobacterium nucleatum (F. nucleatum) [30], Staphylococcus aureus (S. aureus) [41], and Streptococcus mutans (S. mutans) [49].
Next to the in vitro studies, 25% demonstrated the effects of nanoparticles through in vivo investigations, and 18% of these studies combined in vitro and in vivo methodologies [23, 40, 43, 49–63]. Rat models were the most used, examining infected dental pulp tissue defects [50], pulp capping [54, 62], pulpotomy [58], pulpitis [63], diabetic pulp injury [61], and reimplantation [43]. Minipig models were used to study pulp injury [57] and capping [59]. Due to size restrictions to study the dental tissues, mouse models investigated subcutaneous ectopic implantation [51]. Other methods included ectopic implantation of human dentin in rat oral cavities [49], ectopic odontogenesis model and a tooth defect model in rats [23], and dorsum ectopic implantation [55, 60]. One study has reported an ex vivo design where the nanoparticle function was tested on the chick embryo chorioallantoic membrane (CAM) model [22].
The suggested end applications are summarised as well, where the future clinical application of the nanoparticles is elucidated (Table 1, column 7). Predominantly reported end applications are pulp capping materials, regeneration scaffolds, root canal fillings, and dentin conditioners. Fourteen studies had no unambiguous clinical application.
Table 1.
Studies reporting nanoparticles in endodontics
| References | Material | Average particle size (nm) | Application | Load | Dose (mg/ml) | Suggested end application | Experimental model | Effect |
|---|---|---|---|---|---|---|---|---|
| (A) Nanoparticles as carriers | ||||||||
| [64] | Bioactive glass | 63 | Odontogenesis | Dexamethasone | 5–30 | Scaffold (regeneration) | hDPSCs | Odontogenesis ↑ |
| [50] | Bioactive glass | 60 | Odontogenesis | Tetracycline | 1.25–10 | Antimicrobial scaffold | hDPSCs | Biofilm formation ↓ |
| Antibacterial | Chlorhexidine digluconate | 25 | Rat | Odontogenesis ↑ | ||||
| [49] | Bioactive glass | 400 | Antibacterial | Epigallocatechin-3-gallate | 2 | Protection of exposed dentin | S. mutans | Biofilm formation ↓ |
| Odontogenesis | hDPSCs | Odontogenesis ↑ | ||||||
| Rat | Biomineralisation ↑ | |||||||
| [23] | Bioactive glass | – | Odontogenesis | Simvastatin | 0.25–4 | Pulp capping | hDPSCs | High loading capacity ✓ |
| *See also Table B | Rat | |||||||
| [22] | CNC | 57–121 | Angiogenesis | Platelet-derived GF | 0.001–0.008 | Regeneration hydrogel | hDPSCs | Sustained release ✓ |
| *See also Table B | Vascular endothelial GF | Fertilized chicken eggs | Neovascularization ↑ | |||||
| [51] | Calcium phosphate | 75–100 | Odontogenesis | BMP-2 | – | Pulp capping | hDPSCs | Cell viability ↑ |
| DMP-1 | No adverse effects ✓ | |||||||
| Mouse | Odontogenesis ↑ | |||||||
| [44] | Calcium phosphate | 200 | Odontogenesis | Bmp2 | – | – | rDPSCs | Non-viral vector ✓ |
| Odontogenesis ↑ | ||||||||
| [47] | Calcium silicate | 130–154 | Antibacterial | Gentamicin | 1.1 | Root canal filling | E. faecalis | Antibacterial activity ✓ |
| Odontogenesis | Fibroblast GF-2 | hDPSCs | Odontogenic-related protein ↑ | |||||
| [31] | Chitosan | 112–180 | Odontogenesis | BSA | 1.5 | – | SCAPs | Temporal-controlled release ✓ |
| Dentin-pulp regeneration ↑ | ||||||||
| [5] | Chitosan | 59 | Odontogenesis | Dexamethasone | 0.005 | – | SCAPs | Sustained release ✓ |
| Odontogenesis ↑ | ||||||||
| [32] | Chitosan | 112–179 | Odontogenesis | TGF-β1 | 1.5 | Regeneration scaffold | SCAPs | Release ✓ |
| Migration + differentiation SCAPs ↑ | ||||||||
| [33] | Chitosan | 112–179 | Odontogenesis | Dexamethasone | 1.5 | Dentin conditioner | SCAPs | Sustained release ✓ |
| Odontogenesis ↑ | ||||||||
| [34] | Chitosan | 197–262 | Odontogenesis | Dexamethasone | – | Dentin conditioner | SCAPs | Sustained release ✓ |
| Cell viability, migration, differentiation ↑ | ||||||||
| Inflammation ↓ | ||||||||
| [43] | Chitosan | – | Odontogenesis | Dexamethasone | 0.03 | Root modification agents | PDLF, Mφ | Clastic differentiation ↓ |
| Rose Bengal | 0.1 | Rat | Resorption and ankylosis ↓ | |||||
| [65] | Chitosan-polylactic acid | 100–300 | Odontogenesis | Fluocinolone acetonide/coumarin-6 | 5.8 | Pulp capping | DPSCs | Inflammation ↓ |
| BSA/BMP-2 | 8 | Odontogenesis ↑ | ||||||
| [28] | Lipid | – | Amelogenesis | Tbx1 | 0.2 | – | rHAT-7 | Amelogenesis ↑ |
| Enamel-like tissue formation ↑ | ||||||||
| Efficient delivery method | ||||||||
| [25] | Lipid | 145–4200 | Antibacterial | Chlorhexidine | 4–10% w/v | Pulp capping | L929 Murine Fibroblasts | Sustained release ✓ |
| Cell Viability ✓ | ||||||||
| Antibacterial activity ✓ | ||||||||
| [66] | Methacrylic acid | – | Osteogenesis | Tideglusib | 0.0017 | Pulp capping | DPSCs | Osteogenensis ↑ |
| Mineralisation ↑ | ||||||||
| [67] | Ferrous material | – | Dentinogensis | miR-218 | – | – | DPSCs | miR-218 ↓ dentinogenesis of DPSCs |
| miR-218 ❌ → mineralisation ↑ | ||||||||
| [62] | Cerium oxide | 30 | Odontogenesis | DMP-1 | 0.001 | Pulp capping | DPSCs | Reparative dentin formation ✓ |
| Rat | ||||||||
| [68] | PEG-PEI | 150–360 | Odontogenesis | miR146a/bFGF | – | Vital pulp therapy | hDPSCs | Sustained release ✓ |
| Cell proliferation ↑ | ||||||||
| Odontogenesis ↑ | ||||||||
| [52] | PEI-Gold | 30 | Odontogenesis | AntagomiR-3074-3p | – | Pulp capping | hDPSCs | Restorative dentin ↑ |
| Rat | Odontogenesis ↑ | |||||||
| [53] | PLGA | – | Odontogenesis | Lovastatin | 0.1 | Direct pulp capping | hDPSCs | Cytotoxicity lovastatin ↓ |
| Rat | Tubular reparative dentin ↑ | |||||||
| [24] | EV | 30–150 | Odontogenesis | RUNX3 | – | – | DPSCs | Odontogenic differentiation ↑ |
| *See also Table C | ||||||||
| Reference | Material | Average particle size (nm) | Application | Enhanced material | Suggested end application | Experimental model | Effect |
|---|---|---|---|---|---|---|---|
| (B) Nanoparticles as enhancers | |||||||
| [23] | Bioactive glass | – | Odontogenesis | Cement | Regeneration scaffold | hDPSCs | Biocompatibility ↑ |
| *See also Table A | Rat | Odontogenic potential ↑ | |||||
| [69] | Bioactive glass | – | Odontogenesis | PCL/gelatine composite | Scaffold | hDPSCs | Odontogenesis ↑ |
| [70] | Bioactive glass | 80 | Odontogenesis | Calcium phosphate cements | Pulp capping | hDPSCs | Odontogenesis ↑ |
| Angiogenesis ↑ | |||||||
| [71] | Bioactive glass | – | Odontogenesis | Cellulose acetate/oxidized pullulan/gelatine-based scaffolds | Pulp capping | hDPSCs | Cell attachment ↑ |
| Odontogenesis ↑ | |||||||
| [72] | Bioactive glass | 300–600 | Odontogenesis | Graphene oxide composites | Composite filling | hDPSCs | Odontogenesis ↑ |
| [73] | Bioactive glass | – | Odontogenesis | Biodentine (Calcium silicate cement) | Root canal preparation | hDPSCs | Attachment and proliferation ↑ |
| ALP expression ↑ | |||||||
| Mineralisation ↑ | |||||||
| [41] | Bioactive glass | 148–172 | Antibacterial | Composite or cement | – | S. aureus | MBGN antimicrobial activity ↑ |
| Calcium silicate | Agglomerates | Odontogenesis | E. coli | TCS bioactivity ↑ | |||
| MG63 | |||||||
| [74] | Bioactive glass | – | Odontogenesis | Calcium phosphate cement | Pulp capping | hDPSCs | Cell proliferation & adhesion ↑ |
| Angiogenesis | Odontogenesis ↑ | ||||||
| ALP activity & expression odontogenic genes ↑ | |||||||
| [75] | Calcium phosphate | 20–30 | Odontogenesis | Resin-modified glass ionomer cements | Pulp capping | hMSCs | Biocompatibility ↑ |
| ALP activity ↑ | |||||||
| Odontogenesis ↑ | |||||||
| [54] | Calcium phosphate | 116 (refers to previous study [76]) | Antimicrobial | Composite | – | Rat | Pulpal inflammation ↓ |
| Odontogenesis | Adhesive | Tertiary dentin formation ↑ | |||||
| [63] | Calcium phosphate-Zinc phosphate | 200 | Anti-inflammation | Sodium alginate | Pulp capping | DPSCs | Inflammation resolution↑ |
| Odontogenesis | Rat | Dentin mineralization ↑ | |||||
| [36] | Calcium silicate | 100 | Odontogenesis | Biodentine (calcium silicate cement) | Pulp capping | SHEDs | Odontogenesis ↑ |
| [77] | Calcium silicate | – | Odontogenesis | iRoot FS (calcium silicate cement) | Root canal filling | hSCAPs | Cell migration ↑ |
| Osteo/odontogenesis ↑ | |||||||
| [22] | CNC | 57–121 | Angiogenesis | Hyaluronic acid | Regeneration hydrogel | hDPSCs | Material stability ↑ |
| *see also Table A | Fertilized chicken eggs | ||||||
| [78] | Hydroxyapatite | W 17–23 | Odontogenesis | PCL nanofibrous composite scaffold | Pulp capping | DPSCs | Cell viability and adhesion ↑ |
| H 93–146 | Odontogenesis ↑ | ||||||
| [79] | Ferrous material | 10 | Odontogenesis | Magnetic nanofiber scaffold | Regeneration scaffold | hDPSCs | Cell growth ↑ |
| Angiogenesis | Odontogenic differentiation ↑ | ||||||
| Pro-angiogenesis ↑ | |||||||
| [80] | Iron oxide | 38–246 | Odontogenesis | GelMA/PEGDA composite hydrogel | Regeneration hydrogel | DPSCs | Osteo/odontogenic differentiation ↑ |
| [81] | Ferrous material | – | Odontogenesis | Magnetic nanofiber scaffold | Regeneration scaffold | hDPSCs | Cell migration ↑ |
| Odontogenesis ↑ | |||||||
| [82] | Calcium oxide | 200–340 | Odontogenesis | SAPO-34 zeolite and chitosan composite | Regeneration scaffold | hDPSCs | Cell proliferation ↑ |
| Iron oxide | Osteogenic differentiation ↑ | ||||||
| [83] | Silica | 30–60 | Odontogenesis | Calcium silicate cement | Pulp capping | hDPSCs | Cell viability ↑ |
| Odontogenic marker level ↑ | |||||||
| [30] | Silver | 10 | Antibacterial | Calcium hydroxide | Irrigant/ intracanal treatment | F. nucleatum | Combined effect is more efficient |
| [38] | Silver | – | Odontogenesis | Calcined tooth powder | Pulp capping | Canine PDLSCs | Odontogenic & neuronal differentiation ↑ |
| [29] | Silver | 25 | Antibacterial | Methylcellulose gel | Intracanal medicament | E. faecalis | Biofilm reduced and eliminated |
| [48] | Silver | – | Antibacterial | Methacrylated gelatine | Pulp capping | E. faecalis | Antibacterial activity ✓ |
| Angiogenesis | DPSCs | Angiogenesis ↑ | |||||
| [40] | Titanium oxide | < 50 | Odontogenesis | Chitosan | Direct pulp capping | EnSCs | Dentin repair ↑ |
| Rat | |||||||
| [84] | Zinc-oxide | – | Odontogenesis | Gutta-Percha | Root canal filling | hDPSCs | Hydroxyapatite deposition ↑ |
| Reference | Material | Average particle size (nm) | Application | Suggested end application | Experimental model | Effect |
|---|---|---|---|---|---|---|
| (C) Nanoparticles as therapeutic agents | ||||||
| [85] | Bioactive glass | – | Odontogenesis | Protection of exposed dentin | hDPSCs | Odontogenesis ↑ |
| [55] | Bioactive glass | 20 | Odontogenesis | Pulp capping | hDPSCs | Odontogenesis ↑ |
| Mouse | Dentin formation ↑ | |||||
| [86] | Bioactive glass | 24–44 | Odontogenesis | – | hDPSCs | Odontogenesis ↑ |
| [37] | Bioactive glass | 160 | Odontogenesis | – | SHEDs | Odontogenic & dentin regeneration ↑ |
| [45] | Bioactive glass | 300–500 | Odontogenesis | Pulp capping | rDPSCs | Odontogenesis ↑ |
| [26] | Bio-silica | 30 | Amelogenesis | – | SaOS-2 cells | Hydroxyapatite deposition ↑ |
| Biomineralisation ↑ | ||||||
| [87] | Calcium phosphate lipid | 100–500 | Odontogenesis | Pulp capping | hDPSCs | Inflammation ↓ |
| Odontogenesis ↑ | ||||||
| [56] | Calcium oxide | 15–65 | Odontogenesis | Tooth & pulp-dentin complex development | Rat | Predentin & periodontal ligament thickness ↑ |
| Vascularization in pulp tissue ↓ | ||||||
| [46] | Calcium oxide | – | Odontogenesis | Intracanal medicament | RAW 264.7 | Osteoclast |
| Calcium oxide loaded PLGA | differentiation ↓ | |||||
| [35] | Copper-oxide | – | Odontogenesis | Regenerative endodontics | SCAPs | Osteogenic/Odontogenic differentiation ↑ |
| [57] | EV | 50–150 | Odontogenesis | Pulp capping | Minipig | Odontoblast related protein ↑ |
| Formation continuous reparative dentin ↑ | ||||||
| [88] | EV | 80–200 | Odontogenesis | Regeneration hydrogel | DPSCs | Angiogenesis ↑ |
| [39] | EV | < 200 | Odontogenesis | – | PDLSCs | Osteo/odontogenic differentiation ↑ |
| [60] | EV | 100–150 | Odontogenesis | Regeneration hydrogel | DPSCs | Odontoblastic differentiation ↑ |
| Rat | ||||||
| [24] | EV | 30–150 | Odontogenesis | – | DPSCs | Odontogenic differentiation ↑ |
| *See also Table A | ||||||
| [42] | Gold | 34 | Odontogenesis | Dental tissue engineering and odontoblastic differentiation | DPSCs | Endogenous stem cells ↑ |
| BMSCs | Dental pulp regeneration ↑ | |||||
| [58] | Hydroxyapatite | – | Odontogenesis | Direct pulp capping | Rat | Formation dentinal bridge containing dentinal tubules ↑ |
| [59] | Hydroxyapatite | – | Odontogenesis | Direct pulp capping/ Pulpotomy | Pig | Inflammatory reaction ❌ |
| [27] | Hydroxyapatite | – | Amelogenesis | – | MG63 | Enamel remineralisation ↑ |
| Hydroxyapatite | W 17–23 | Odontogenesis | Pulp capping | DPSCs | Immunomodulatory genes ↑ | |
| H 93–146 | ||||||
| [89] | MTA powder | 41 | Odontogenesis | Pulp capping | DPSCs | Odontogenesis ↑ |
| [90] | PAMAM | – | Odontogenesis | – | DPCs | Odontogenesis ↑ |
| [61] | Cerium oxide (hyaluronic acid modified) | 182.8 | – | Pulp capping | DPSCs | Diabetes-induced dental pulp damage ↓ |
| Mouse | ||||||
| [91] | Titanium dioxide | 29 | Odontogenesis | – | DPSCs | Biomineralisation ↑ |
CNC: cellulose nanocrystals; GF: Growth Factor; BMP: Bone Morphogenetic Protein; DMP: dentin matrix protein; BSA: bovine serum albumin; TGF: Transforming growth factor; PDLF: periodontal ligament fibroblasts; Mφ: macrophages; Tbx: T-box gene; PEG-PEI: polyethylene glycol-polyethylene imine; PEI, polyethylene imine; PLGA: poly(lactic-co-glycolic acid); PCL: polycaprolactone; GelMA: Methacrylated gelatine, PEGDA: Poly(ethylene glycol) diacrylate, MTA: mineral trioxide aggregate; W: width; H: height; nHA(EA): nanohydroxyapatite (from Elaeagnus angustifolia); EV: extracellular vesicles. Experimental model: Bold, in vitro; Italic, in vivo; Underline, ex vivo
The results were summarized in three tables according to the primary function of the nanoparticles described. In Table 1A, nanoparticles that exhibit a carrier function were summarized. In Table 1B, nanoparticles enhancing existing dental materials were elaborated, while in Table 1C nanoparticle therapeutic properties were shown. These tables provide detailed insights into the materials, experimental models, and effects in vitro/in vivo reported across the studies, offering a comprehensive overview of nanoparticle applications in endodontics.
Discussion
This review comprehensively explored the versatile roles of nanoparticles in endodontics, emphasizing their potential to enhance cell differentiation, improve antimicrobial activity, and address the challenges posed by the complex dentin-pulp structure. Nanoparticles, classified as drug carriers, material enhancers, or therapeutic agents, offer significant promise in advancing endodontic treatments and paving the way for innovative regenerative approaches.
Nanoparticles provide a highly effective platform for delivering therapeutic agents by encapsulating, adsorbing, or dissolving drugs within their matrix [6]. These therapeutic agents include antimicrobials such as gentamicin [47] and tetracycline [50], while growth factors like fibroblast growth factor 2 (FGF-2) [47], platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) [22], have shown potential to promote angiogenesis and tissue regeneration. Moreover, the gene Tbx1 [28] is essential for ameloblast differentiation, ensuring normal tooth development, while gene BMP-2 was encapsulated for its ability to stimulate the differentiation of hDPSCs into odontoblasts [44, 51]. Polymeric nanoparticles have prominently been reported in the function of a carrier in dental applications. Chitosan nanoparticles have gained prominence thanks to their cationic nature, which facilitates spontaneous assembly and protects sensitive agents from degradation [92]. Beyond endodontics, chitosan nanoparticles have shown potential in other fields, such as anti-diabetes therapy [93], Crohn’s disease management [94], and oncological treatment [95]. These findings highlight their versatility and promise in clinical applications.
Enhancing the properties of dental materials represents another critical application of nanoparticles. Bioactive glass, widely studied for its ionic dissolution of phosphorus, calcium, and silicate ions [96] and its ability to promote hydroxyapatite formation, has proven effective in integrating hard and soft tissues [69, 97]. Bioactive glass also has antibacterial effects when doped in metal ions such as silver, making it a popular choice for endodontic materials [98]. Additionally, green-synthesised nanohydroxyapatite, created using plant extracts, has demonstrated the potential to control nanoparticle morphology while promoting angiogenesis and odontogenesis [78, 99]. These plant-derived hydroxyapatite nanoparticles offer antioxidant and anti-inflammatory properties, further enhancing their therapeutic benefits and making them a promising option for sustainable biomaterial development. Moreover, they stimulate odontogenic differentiation of DPSCs, as an upregulation of odontogenic and osteogenic gene expression was measured. The presence of phenolic compounds and flavonoids in the plant extracts can raise Ca2+ deposition as well as activate osteoblast differentiation [100, 101].
Incorporating antibacterial properties into endodontic materials is critical for preventing infections and ensuring successful treatment outcomes. Silver nanoparticles have been extensively studied for their bactericidal properties, primarily attributed to the release of Ag + ions [8]. However, their clinical application remains limited due to biocompatibility issues and the risk of tooth discoloration [9]. Bioactive glass also demonstrates antibacterial potential, though its mechanisms require further elucidation [102]. Other papers reported that the antibacterial effects come from the increased dissolution tendency, leading to higher pH and alkali ion concentrations, which is harmful to the bacteria [102]. Strengthening the antibacterial properties of nanoparticles while addressing these limitations remains a vital area for future research.
Beyond their role in enhancing materials, nanoparticles themselves act as therapeutic agents. Bioactive glass, known for its mineralisation-inducing capabilities, is a leading example [103]. Emerging evidence also points to the therapeutic potential of extracellular vesicles (EVs), which are secreted by mesenchymal stem cells and carry bioactive molecules such as growth factors and chemokines [104]. EVs have demonstrated pro-angiogenic effects and the ability to induce odontogenesis and bone regeneration when derived from specific stem cells [105]. Their potential for patient-specific applications positions them as promising agents for personalized regenerative therapies, though ethical and economic challenges must be addressed.
Despite their promise, several critical issues in nanoparticle research require attention. Many studies focus on enhancing the differentiation of human dental pulp stromal/stem cells (DPSCs), given their role in maintaining and repairing the dentin-pulp complex. DPSCs, which have neurovascular and multi-differentiation properties, are a vital target for regenerative approaches, making them a primary focus of nanoparticle research [106]. However, the heterogeneity of this cell population and the complexity of this tissue remains an understudied topic that needs to be considered before the translation of research results [107]. Moreover, for nanoparticles functioning as drug carriers, precise dosage and particle size reporting is essential to ensure therapeutic efficacy and reproducibility [108, 109]. Notably, seven out of 24 studies categorized under drug carriers (Table 1A) did not report dosage, undermining the reliability of their findings [24, 34, 44, 51, 52, 67, 68]. Similarly, accurate classification of nanoparticle morphology is crucial, as non-spherical structures such as nanorods have occasionally been misclassified, leading to potential misinterpretation [78, 99].
The choice of stem cells is critical for ensuring relevance in regenerative studies. Dental-origin stem cells, such as DPSCs, PDLSCs, and SHEDs, are more directly applicable to endodontic research than non-dental stem cells like endometrial [40] or osteosarcoma stem cells [26, 27, 41]. Researchers must provide strong justification for using non-dental stem cells to avoid introducing irrelevant variables and ensure findings are translatable to dental applications. Similarly, the reliance on traditional in vitro models, while useful, often fails to replicate the complexity of the oral environment. Advanced 3D in vitro and ex vivo models, such as whole tooth models, offer more physiologically relevant alternatives [110]. Additionally, in vivo studies using animal models, such as rats or minipigs, are essential for validating findings before transitioning to clinical trials.
The antibacterial properties of nanoparticles remain a focal point in endodontics, yet most studies focus on single bacterial strains, limiting the generalizability of their findings [50]. Expanding antibacterial testing to include multispecies biofilms and microbiome-based approaches would better reflect the complexities of the oral environment and improve the clinical relevance of the results.
Finally, while nanoparticles have been explored in clinical trials for periodontal and orthodontic applications, their use in endodontics remains limited [111–114]. Only two randomized clinical trials have explored the antibacterial properties of nanoparticles in endodontics, demonstrating promising outcomes in bacterial reduction and pain relief [115, 116]. However, further research is needed to expand nanoparticle applications to other therapeutic areas, such as tissue regeneration and personalized endodontic treatments. Future studies must address scalability, long-term safety, and cost-effectiveness challenges to realize the full potential of nanoparticles in clinical practice.
This review highlights nanoparticles' transformative potential in addressing endodontics' challenges, particularly in regenerative approaches. However, bridging the gap between laboratory innovation and clinical application requires sustained efforts to standardize methodologies, optimize therapeutic strategies, and evaluate long-term outcomes in diverse patient populations.
Conclusion and future perspectives
Traditional endodontic treatments often rely on the removal of pulp tissue and its replacement with bio-inert materials, a practice that, while effective in the short term, may lead to long-term complications such as structural weakness and reinfection. The need for biologically compatible, tissue-preserving solutions is becoming increasingly urgent, particularly as the field shifts toward regenerative approaches. A multi-modal strategy that integrates biomaterials capable of mimicking the native properties of the dentin-pulp complex offers significant promise in overcoming these limitations.
Nanoparticles have emerged as pivotal tools in advancing this strategy, offering unique advantages in enhancing the mechanical and biological properties of endodontic materials. Their role as carriers for therapeutic agents, including antimicrobials and regenerative factors, enables targeted and localized treatments, potentially transforming the standard of care. Additionally, their ability to enhance material performance and promote tissue repair makes them invaluable in addressing the challenges of regenerating the complex dentin-pulp structure.
Despite the substantial progress demonstrated by the studies reviewed, significant challenges remain. Many findings are derived from controlled laboratory settings, which often fail to replicate the complexity of the oral environment. Advanced in vitro systems, such as 3D models, and in vivo studies using animal models are essential to validate these promising results. Additionally, microfluidic tissue-on-chips and organ-on-chips models are becoming increasingly important in validating results and providing more physiologically relevant data [117]. Moreover, scaling these findings for clinical application requires addressing issues such as long-term safety, biocompatibility, and cost-effectiveness.
Future research should prioritize bridging the gap between laboratory innovation and clinical application. Key areas of focus include optimizing nanoparticle formulations for specific therapeutic goals, improving the reproducibility of findings through standardized methodologies, and expanding antibacterial testing to reflect the multispecies nature of the oral microbiome. Additionally, clinical trials are needed to evaluate the real-world efficacy of nanoparticle-enhanced materials and their potential to improve patient outcomes.
Incorporating nanoparticles into endodontic practice represents a transformative opportunity to advance the field from bio-inert to bioactive treatments. By fostering tissue preservation, promoting regeneration, and enabling targeted therapies, nanoparticles offer a pathway to revolutionize endodontic care and significantly improve long-term outcomes for patients.
Author contributions
U.I. and C.M.R. contributed to the conception, design, data acquisition, analysis, and interpretation; U.I. drafted, and critically revised the manuscript; A.M., M.E., and R.J., contributed to conception, data acquisition, interpretation, and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the study.
Funding
Open access funding provided by Karolinska Institute. This research was supported by Fonds Wetenschappelijk Onderzoek (FWO, 1SH4N24N) and Research Council of KU Leuven (IDN/23/008).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Share senior authorship: Mostafa EzEldeen and Reinhilde Jacobs.
References
- 1.Kishen A, Hussein H. Bioactive molecule carrier systems in endodontics. Expert Opin Drug Deliv. 2020;17:1093–112. 10.1080/17425247.2020.1777981. [DOI] [PubMed] [Google Scholar]
- 2.Montero-Miralles P, Ibáñez-Barranco R, Cabanillas-Balsera D, Areal-Quecuty V, Sánchez-Domínguez B, Martín-González J, Segura-Egea JJ, Jiménez-Sánchez MC. Biomaterials in periapical regeneration after microsurgical endodontics: a narrative review. J Clin Exp Dent. 2021;13:935–40. 10.4317/jced.58651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EzEldeen M, Moroni L, Nejad ZM, Jacobs R, Mota C. Biofabrication of engineered dento-alveolar tissue. Biomateri Adv. 2023;148:213371. 10.1016/j.bioadv.2023.213371. [DOI] [PubMed] [Google Scholar]
- 4.Vallés M, Mercadé M, Duran-Sindreu F, Bourdelande JL, Roig M. Color stability of white mineral trioxide aggregate. Clin Oral Investig. 2013;17:1155–9. 10.1007/s00784-012-0794-1. [DOI] [PubMed] [Google Scholar]
- 5.Shrestha S, Diogenes A, Kishen A. Temporal-controlled dexamethasone releasing chitosan nanoparticle system enhances odontogenic differentiation of stem cells from apical papilla. J Endod. 2015;41:1253–8. 10.1016/j.joen.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Mei X, Yang M. Drug delivery system for active brain targeting. 2007. 10.1016/B978-1-84569-036-6.50017-8
- 7.Zaimy MA, Saffarzadeh N, Mohammadi A, Pourghadamyari H, Izadi P, Sarli A, Moghaddam LK, Paschepari SR, Azizi H, Torkamandi S, Tavakkoly-Bazzaz J. New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Ther. 2017;24:233–43. 10.1038/cgt.2017.16. [DOI] [PubMed] [Google Scholar]
- 8.Naderi N, Karponis D, Mosahebi A, Seifalian AM. Nanoparticles in wound healing; from hope to promise, from promise to routine. Front Biosci. 2018;23:1038–59. [DOI] [PubMed] [Google Scholar]
- 9.Zakrzewski W, Dobrzyński M, Zawadzka-Knefel A, Lubojański A, Dobrzyński W, Janecki M, Kurek K, Szymonowicz M, Wiglusz RJ, Rybak Z. Nanomaterials application in endodontics. Materials. 2021;14:5296. 10.3390/ma14185296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramaniam Ramachandran V, Radhakrishnan M, Balaraman Ravindrran M, Alagarsamy V, Palanisamy GS, Functionalized nanoparticles: a paradigm shift in regenerative endodontic procedures, Cureus. 2022;14. 10.7759/cureus.32678. [DOI] [PMC free article] [PubMed]
- 11.Rashid EU, Nawaz S, Munawar J, Sarker A, Hussain S, Iqbal HMN, Bilal M. Organic and inorganic nanoparticles. In: Smart polymer nanocomposites: design, synthesis, functionalization, properties, and applications, Elsevier, 2022: pp. 93–119. 10.1016/B978-0-323-91611-0.00014-1.
- 12.Hu D, Tian T, Ren Q, Han S, Li Z, Deng Y, Lu Z, Zhang L. Novel biomimetic peptide-loaded chitosan nanoparticles improve dentin bonding via promoting dentin remineralization and inhibiting endogenous matrix metalloproteinases. Dent Mater. 2023. 10.1016/j.dental.2023.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Hayat F, Sabbir J, Khurshid Z, Zafar MS, Ghabbani HM, Shahbazi MA, Sefat F. Nanoparticles in endodontics. Biomater Endodontics. 2022. 10.1016/B978-0-12-821746-7.00004-8. [Google Scholar]
- 14.Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, Tagami J, Twetman S, Tsakos G, Ismail A. Dental caries. Nat Rev Dis Primers. 2017;3:1–16. 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 15.Zakrzewski W, Dobrzynski M, Zawadzka-Knefel A, Lubojanski A, Dobrzynski W, Janecki M, Kurek K, Szymonowicz M, Wiglusz RJRJ, Rybak Z, Dobrzyński M, Zawadzka-Knefel A, Lubojański A, Dobrzyński W, Janecki M, Kurek K, Szymonowicz M, Wiglusz RJRJ, Rybak Z. Nanomaterials application in endodontics. Materials. 2021;14:5296. 10.3390/ma14185296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramachandran VS, Radhakrishnan M, Ravindrran MB, Alagarsamy GS, Palanisamy GS. functionalized nanoparticles: a paradigm shift in regenerative endodontic procedures, Cureus J. Med Sci. 2022;14. 10.7759/cureus.32678. [DOI] [PMC free article] [PubMed]
- 17.Shrestha A, Kishen A. Antibacterial nanoparticles in endodontics: a review. J Endod. 2016;42:1417–26. 10.1016/j.joen.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Capuano N, Amato A, Dell’Annunziata F, Giordano F, Folliero V, Di Spirito F, More PR, De Filippis A, Martina S, Amato M, Galdiero M, Iandolo A, Franci G. Nanoparticles and their antibacterial application in endodontics. Antibiotics. 2023;12:1690. 10.3390/antibiotics12121690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raura N, Garg A, Arora A, Roma M. Nanoparticle technology and its implications in endodontics: a review. Biomater Res. 2020;24:21. 10.1186/s40824-020-00198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong J, Zou T, Lee AHC, Zhang C. The potential translational applications of nanoparticles in endodontics. Int J Nanomed. 2021;16:2087–106. 10.2147/IJN.S293518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, MacDonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73. 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 22.Silva CR, Babo PS, Gulino M, Costa L, Oliveira JM, Silva-Correia J, Domingues RMAA, Reis RL, Gomes ME. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018;77:155–71. 10.1016/j.actbio.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Mandakhbayar N, El-Fiqi A, Lee J-H, Kim H-W. Evaluation of strontium-doped nanobioactive glass cement for dentin-pulp complex regeneration therapy. ACS Biomater Sci Eng. 2019;5:6117–26. 10.1021/acsbiomaterials.9b01018. [DOI] [PubMed] [Google Scholar]
- 24.Chi Y, Liu T, Jin Q, Liu H. Extracellular vesicles carrying RUNX3 promote differentiation of dental pulp stem cells. Tissue Eng Regen Med. 2024;21:111–22. 10.1007/s13770-023-00578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raddi S, El Karmy B, Martinache O, Richert R, Colnot C, Grosgogeat B. Development of chlorhexidine-loaded lipid nanoparticles incorporated in a bioceramic endodontic sealer. J Endod. 2024. 10.1016/j.joen.2024.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Müller WEG, Boreiko A, Wang X, Krasko A, Geurtsen W, Custódio MR, Winkler T, Lukić-Bilela L, Link T, Schröder HC. Morphogenetic activity of silica and bio-silica on the expression of genes controlling biomineralization using SaOS-2 cells. Calcif Tissue Int. 2007;81:382–93. 10.1007/s00223-007-9075-4. [DOI] [PubMed] [Google Scholar]
- 27.Rajabnejadkeleshteri A, Kamyar A, Khakbiz M, Bakalani ZL, Basiri H. Synthesis and characterization of strontium fluor-hydroxyapatite nanoparticles for dental applications. Microchem J. 2020;153:104485. 10.1016/j.microc.2019.104485. [Google Scholar]
- 28.Mohabatpour F, Al-Dulaymi M, Lobanova L, Scutchings B, Papagerakis S, Badea I, Chen X, Papagerakis P. Gemini surfactant-based nanoparticles T-box1 gene delivery as a novel approach to promote epithelial stem cells differentiation and dental enamel formation. Biomater Adv. 2022;137:212844. 10.1016/j.bioadv.2022.212844. [DOI] [PubMed] [Google Scholar]
- 29.Sadek RW, Moussa SM, El Backly RM, Hammouda AF. Evaluation of the efficacy of three antimicrobial agents used for regenerative endodontics: an in vitro study. Microb Drug Resist. 2019;25:761–71. 10.1089/mdr.2018.0228. [DOI] [PubMed] [Google Scholar]
- 30.AlGazlan AS, Auda SH, Balto H, Alsalleeh F. Antibiofilm efficacy of silver nanoparticles alone or mixed with calcium hydroxide as intracanal medicaments: an ex-vivo analysis. J Endod. 2022;48:1294–300. 10.1016/j.joen.2022.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Shrestha S, Diogenes A, Kishen A. Temporal-controlled release of bovine serum albumin from chitosan nanoparticles: effect on the regulation of alkaline phosphatase activity in stem cells from apical papilla. J Endod. 2014;40:1349–54. 10.1016/j.joen.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Bellamy C, Shrestha S, Torneck C, Kishen A. Effects of a bioactive scaffold containing a sustained transforming growth factor-β1–releasing nanoparticle system on the migration and differentiation of stem cells from the apical papilla. J Endod. 2016;42:1385–92. 10.1016/j.joen.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Shrestha S, Torneck CD, Kishen A. Dentin conditioning with bioactive molecule releasing nanoparticle system enhances adherence, viability, and differentiation of stem cells from apical papilla. J Endod. 2016;42:717–23. 10.1016/j.joen.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 34.Kukreti H, Li F-C, Singh K, Sodhi R, Kishen A. Efficacy of bioactive nanoparticles on tissue-endotoxin induced suppression of stem cell viability, migration and differentiation. Int Endod J. 2020;53:859–70. 10.1111/iej.13283. [DOI] [PubMed] [Google Scholar]
- 35.Karkehabadi H, Rahmati A, Abbasi R, Farmany A, Najafi R, Behroozi R, Rezaei-soufi L, Abbaspourrokni H. Effect of copper oxide nanoparticles and light-emitting diode irradiation on the cell viability and osteogenic/odontogenic differentiation of human stem cells from the apical papilla. BMC Oral Health. 2023;23:249. 10.1186/s12903-023-02916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung Y, Yoon J-Y, Patel KD, Lee H-H, Ma L, Kim J, Lee J-H, Shin J, Dev Patel K, Ma L, Lee H-H, Kim J, Lee J-H, Shin J, Patel KD, Ma L, Lee H-H, Kim J, Lee J-H, Shin J. Biological effects of tricalcium silicate nanoparticle-containing cement on stem cells from human exfoliated deciduous teeth. Nanomaterials. 2020;10:1–15. 10.3390/nano10071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J-H, Kang M-S, Mahapatra C, Kim H-W. Effect of aminated mesoporous bioactive glass nanoparticles on the differentiation of dental pulp stem cells. PLoS ONE. 2016;11:e0150727. 10.1371/journal.pone.0150727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Liao D, Sun G, Chu H. Odontogenesis and neuronal differentiation characteristics of periodontal ligament stem cells from beagle dog. J Cell Mol Med. 2020;24:5146–51. 10.1111/jcmm.15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan C, Li N, Xiao T, Ye X, Fu L, Ye Y, Xu T, Yu J. Extracellular vesicles from the inflammatory microenvironment regulate the osteogenic and odontogenic differentiation of periodontal ligament stem cells by miR-758-5p/LMBR1/BMP2/4 axis. J Transl Med. 2022;20:208. 10.1186/s12967-022-03412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoveizi E, Naddaf H, Ahmadianfar S, Gutmann JL. Encapsulation of human endometrial stem cells in chitosan hydrogel containing titanium oxide nanoparticles for dental pulp repair and tissue regeneration in male Wistar rats. J Biosci Bioeng. 2023;135:331–40. 10.1016/j.jbiosc.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Simila HO, Boccaccini AR. Sol-gel synthesis of lithium doped mesoporous bioactive glass nanoparticles and tricalcium silicate for restorative dentistry: comparative investigation of physico-chemical structure, antibacterial susceptibility and biocompatibility. Front Bioeng Biotechnol. 2023;11:1065597. 10.3389/fbioe.2023.1065597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Qu X, Xu C, Zhang Z, Qi G, Jin Y. Thermoplasmonic regulation of the mitochondrial metabolic state for promoting directed differentiation of dental pulp stem cells. Anal Chem. 2022;94:9564–71. 10.1021/acs.analchem.2c00288. [DOI] [PubMed] [Google Scholar]
- 43.Siddappa RHR, Bishop E, Ali A, Magalhaes M, Kishen A. Engineered immunomodulatory nanoparticles inhibit root resorption and ankylosis. J Endod. 2024;50(11):1579–92. 10.1016/j.joen.2024.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Walboomers XF, van den Dolder J, Yang F, Bian Z, Fan M, Jansen JA. Non-viral bone morphogenetic protein 2 transfection of rat dental pulp stem cells using calcium phosphate nanoparticles as carriers. Tissue Eng Part A. 2008;14:71–81. 10.1089/ten.a.2007.0102. [DOI] [PubMed] [Google Scholar]
- 45.Huang W, Yang J, Feng Q, Shu Y, Liu C, Zeng S, Guan H, Ge L, Pathak JL, Zeng S. Mesoporous bioactive glass nanoparticles promote odontogenesis and neutralize pathophysiological acidic pH. Front Mater. 2020;7:241. 10.3389/fmats.2020.00241. [Google Scholar]
- 46.Promta P, Chaiyosang P, Panya A, Laorodphun P, Leelapornpisid W, Imerb N. The evaluation of anti-osteoclastic activity of the novel calcium hydroxide biodegradable nanoparticles as an intracanal medicament. J Endod. 2024;50:667–73. 10.1016/j.joen.2024.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Huang C-Y, Huang T-H, Kao C-T, Wu Y-H, Chen W-C, Shie M-Y. Mesoporous calcium silicate nanoparticles with drug delivery and odontogenesis properties. J Endod. 2017;43:69–76. 10.1016/j.joen.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 48.He Y, Zhang Y, Hu F, Chen M, Wang B, Li Y, Xu H, Dong N, Zhang C, Hu Y, Lin Z, Peng Y, Ye Q, Luo L. Photosensitive hydrogels encapsulating DPSCs and AgNPs for dental pulp regeneration. Int Dent J. 2024;74:836–46. 10.1016/j.identj.2024.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu J, Bian H, Zhao Y, Guo J, Yao C, Liu H, Shen Y, Yang H, Huang C. Epigallocatechin-3-gallate/mineralization precursors co-delivery hollow mesoporous nanosystem for synergistic manipulation of dentin exposure. Bioact Mater. 2023;23:394–408. 10.1016/j.bioactmat.2022.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J-H, El-Fiqi A, Mandakhbayar N, Lee H-H, Kim H-W. Drug/ion co-delivery multi-functional nanocarrier to regenerate infected tissue defect. Biomaterials. 2017;142:62–76. 10.1016/j.biomaterials.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Machla F, Sokolova V, Platania V, Prymak O, Kostka K, Kruse B, Agrymakis M, Pasadaki S, Kritis A, Alpantaki K, Vidaki M, Chatzinikolaidou M, Epple M, Bakopoulou A. Tissue engineering at the dentin-pulp interface using human treated dentin scaffolds conditioned with DMP1 or BMP2 plasmid DNA-carrying calcium phosphate nanoparticles. Acta Biomater. 2023;159:156–72. 10.1016/j.actbio.2023.01.044. [DOI] [PubMed] [Google Scholar]
- 52.Jiang T, Miao S, Shen J, Song W, Tan S, Ma D. Enhanced effects of antagomiR-3074–3p-conjugated PEI-AuNPs on the odontogenic differentiation by targeting FKBP9. J Tissue Eng. 2023;14:20417314231184510. 10.1177/20417314231184512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin H-P, Tu H-P, Hsieh Y-P, Lee B-S. Controlled release of lovastatin from poly(lactic-co-glycolic acid) nanoparticles for direct pulp capping in rat teeth. Int J Nanomed. 2017;12:5473–85. 10.2147/IJN.S138410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li F, Wang P, Weir MD, Fouad AF, Xu HHK. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater. 2014;10:2804–13. 10.1016/j.actbio.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Gao X, Gong W, Zhang Z, Chen X, Dong Y. Odontogenic differentiation and dentin formation of dental pulp cells under nanobioactive glass induction. Acta Biomater. 2014;10:2792–803. 10.1016/j.actbio.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Al-Maula BH, Wally ZJ, Al-Magsoosi MJN, Dosh RH, Mustafa RM, Al-Nasrawi SJH, Alfutimie A, Haider J. Studying effects of calcium oxide nanoparticles on dentinogenesis in male wistar rats. Int J Dent. 2021;2021:1–9. 10.1155/2021/9983538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen B, Huang Y, Qiu T, Huo F, Xie L, Liao L, Tian W, Guo W. Reparative dentin formation by dentin matrix proteins and small extracellular vesicles. J Endod. 2021;47:253–62. 10.1016/j.joen.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 58.Imura K, Hashimoto Y, Okada M, Yoshikawa K, Yamamoto K. Application of hydroxyapatite nanoparticle-assembled powder using basic fibroblast growth factor as a pulp-capping agent. Dent Mater J. 2019;38:713–20. 10.4012/dmj.2018-198. [DOI] [PubMed] [Google Scholar]
- 59.Shayegan A, Atash R, Petein M, Vanden Abbeele A. Nanohydroxyapatite used as a pulpotomy and direct pulp capping agent in primary pig teeth. J Dent Child. 2010;77:77–83. [PubMed] [Google Scholar]
- 60.Wang D, Lyu Y, Yang Y, Zhang S, Chen G, Pan J, Tian W. Schwann cell-derived EVs facilitate dental pulp regeneration through endogenous stem cell recruitment via SDF-1/CXCR4 axis. Acta Biomater. 2022;140:610–24. 10.1016/j.actbio.2021.11.039. [DOI] [PubMed] [Google Scholar]
- 61.Zheng C, Hu X, Hua R, Ren X, Shi S, Hong X, Wang Y, Qiu L, Wu D, Cao T, Huang S, Zhao S, Pan Y. A cerium oxide loaded hyaluronic acid nanosystem remits glucose oxidative stress-induced odontoblasts mitochondrial apoptosis through regulation of PGAM5 pathway. ACS Appl Mater Interfaces. 2025. 10.1021/acsami.4c13484. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y, Song L, Li M, Peng H, Qiu X, Li Y, Zhu B, Liu C, Ren S, Miao L. Injectable CNPs/DMP1-loaded self-assembly hydrogel regulating inflammation of dental pulp stem cells for dentin regeneration. Mater Today Bio. 2024;24:100907. 10.1016/j.mtbio.2023.100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Zhou X, Zhai W, Cui J, Pan Z, Du L, Wen L, Ye R, Zhang B, Huang L, Li D, Wang C, Sun H. Novel L-(CaP-ZnP)/SA nanocomposite hydrogel with dual anti-inflammatory and mineralization effects for efficient vital pulp therapy. Int J Nanomed. 2024;19:6659–76. 10.2147/IJN.S464871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim E-C, Lim H-C, Nam OH, Kim M, El-Fiqi A, Yun H-M, Lee Y-M, Jin G-Z, Lee H-H, Kim H-W, Kim E-C. Delivery of dexamethasone from bioactive nanofiber matrices stimulates odontogenesis of human dental pulp cells through integrin/BMP/mTOR signaling pathways. Int J Nanomed. 2016;11:2557–67. 10.2147/IJN.S97846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niu X, Liu Z, Hu J, Rambhia KJ, Fan Y, Ma PX. Microspheres assembled from chitosan- graft -poly(lactic acid) micelle-like core-shell nanospheres for distinctly controlled release of hydrophobic and hydrophilic biomolecules. Macromol Biosci. 2016;16:1039–47. 10.1002/mabi.201600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osorio R, Rodríguez-Lozano FJ, Toledano M, Toledano-Osorio M, García-Bernal D, Murcia L, López-García S. Mitigating lipopolysaccharide-induced impairment in human dental pulp stem cells with tideglusib-doped nanoparticles: enhancing osteogenic differentiation and mineralization. Dent Mater. 2024;40:1591–601. 10.1016/j.dental.2024.07.012. [DOI] [PubMed] [Google Scholar]
- 67.Chang K, Chen R-S, Chang F-H, Chen M-H. Promoting dentinogenesis of DPSCs through inhibiting microRNA-218 by using magnetic nanocarrier delivery. J Formos Med Assoc. 2019;118:1005–13. 10.1016/j.jfma.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 68.Liu L, Shu S, Cheung GS, Wei X. Effect of miR-146a/bFGF/PEG-PEI nanoparticles on inflammation response and tissue regeneration of human dental pulp cells. Biomed Res Int. 2016;2016:3892685. 10.1155/2016/3892685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim G-H, Park Y-D, Lee S-Y, El-Fiqi A, Kim J-J, Lee E-J, Kim H-W, Kim E-C. Odontogenic stimulation of human dental pulp cells with bioactive nanocomposite fiber. J Biomater Appl. 2015;29:854–66. 10.1177/0885328214546884. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, Park Y-D, Bae W-J, El-Fiqi A, Shin S-H, Lee E-J, Kim H-W, Kim E-C. Effects of bioactive cements incorporating zinc-bioglass nanoparticles on odontogenic and angiogenic potential of human dental pulp cells. J Biomater Appl. 2015;29:954–64. 10.1177/0885328214550896. [DOI] [PubMed] [Google Scholar]
- 71.Moonesi Rad R, Pazarçeviren E, Ece Akgün E, Evis Z, Keskin D, Şahin S, Tezcaner A. In vitro performance of a nanobiocomposite scaffold containing boron-modified bioactive glass nanoparticles for dentin regeneration. J Biomater Appl. 2019;33:834–53. 10.1177/0885328218812487. [DOI] [PubMed] [Google Scholar]
- 72.Ahn JH, Kim I-R, Kim Y, Kim D-H, Park S-B, Park B-S, Bae M-K, Kim Y-I. The effect of mesoporous bioactive glass nanoparticles/graphene oxide composites on the differentiation and mineralization of human dental pulp stem cells. Nanomaterials. 2020;10:620. 10.3390/nano10040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corral Nunez C, Altamirano Gaete D, Maureira M, Martin J, Covarrubias C, Nunez CC, Gaete DA, Maureira M, Martin J, Covarrubias C, Corral Nunez C, Altamirano Gaete D, Maureira M, Martin J, Covarrubias C. Nanoparticles of bioactive glass enhance biodentine bioactivity on dental pulp stem cells. Materials. 2021;14:2684. 10.3390/ma14102684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee S-I, Lee E-S, El-Fiqi A, Lee S-Y, Kim E-C, Kim H-W. Stimulation of odontogenesis and angiogenesis via bioactive nanocomposite calcium phosphate cements through integrin and vegf signaling pathways. J Biomed Nanotechnol. 2016;12:1048–62. 10.1166/jbn.2016.2209. [DOI] [PubMed] [Google Scholar]
- 75.Karimi M, Hesaraki S, Alizadeh M, Kazemzadeh A. Effect of synthetic amorphous calcium phosphate nanoparticles on the physicochemical and biological properties of resin-modified glass ionomer cements. Mater Sci Eng, C. 2019;98:227–40. 10.1016/j.msec.2018.12.129. [DOI] [PubMed] [Google Scholar]
- 76.Xu HHK, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–9. 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y, Liu XM, Bi J, Yu S, Yang N, Song B, Chen X. Cell migration and osteo/odontogenesis stimulation of iRoot FS as a potential apical barrier material in apexification. Int Endod J. 2020;53:467–77. 10.1111/iej.13237. [DOI] [PubMed] [Google Scholar]
- 78.Azaryan E, Hanafi-Bojd MY, Alemzadeh E, Emadian Razavi F, Naseri M. Effect of PCL/nHAEA nanocomposite to osteo/odontogenic differentiation of dental pulp stem cells. BMC Oral Health. 2022;22:505. 10.1186/s12903-022-02527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yun H-M, Kang S-K, Singh RK, Lee J-H, Lee H-H, Park K-R, Yi J-K, Lee D-W, Kim H-W, Kim E-C. Magnetic nanofiber scaffold-induced stimulation of odontogenesis and pro-angiogenesis of human dental pulp cells through Wnt/MAPK/NF-κB pathways. Dent Mater. 2016;32:1301–11. 10.1016/j.dental.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 80.Han X, Tang S, Wang L, Xu X, Yan R, Yan S, Guo Z, Hu K, Yu T, Li M, Li Y, Zhang F, Gu N. Multicellular spheroids formation on hydrogel enhances osteogenic/odontogenic differentiation of dental pulp stem cells under magnetic nanoparticles induction. Int J Nanomed. 2021;16:5101–15. 10.2147/IJN.S318991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yun H-M, Lee E-S, Kim M, Kim J-J, Lee J-H, Lee H-H, Park K-R, Yi J-K, Kim H-W, Kim E. Magnetic nanocomposite scaffold-induced stimulation of migration and odontogenesis of human dental pulp cells through integrin signaling pathways. PLoS ONE. 2015;10:e0138614. 10.1371/journal.pone.0138614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Navidi G, Allahvirdinesbat M, Al-Molki SMM, Davaran S, Panahi PN, Aghazadeh M, Akbarzadeh A, Eftekhari A, Safa KD. Design and fabrication of M-SAPO-34/chitosan scaffolds and evaluation of their effects on dental tissue engineering. Int J Biol Macromol. 2021;187:281–95. 10.1016/j.ijbiomac.2021.07.104. [DOI] [PubMed] [Google Scholar]
- 83.Park S-M, Rhee W-R, Park K-M, Kim Y-J, Ahn J, Knowles JC, Kim J, Shin J, Jang T-S, Jun S-K, Lee H-H, Lee J-H. Calcium silicate-based biocompatible light-curable dental material for dental pulpal complex. Nanomaterials. 2021;11:1–13. 10.3390/nano11030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L, Yu Y, Joubert C, Bruder G, Liu Y, Chang C-C, Simon M, Walker SG, Rafailovich M. Differentiation of dental pulp stem cells on gutta-percha scaffolds. Polymers (Basel). 2016;8:193. 10.3390/polym8050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moonesi Rad R, Atila D, Akgün EE, Evis Z, Keskin D, Tezcaner A. Evaluation of human dental pulp stem cells behavior on a novel nanobiocomposite scaffold prepared for regenerative endodontics. Mater Sci Eng C. 2019;100:928–48. 10.1016/j.msec.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 86.Moonesi Rad R, Alshemary AZ, Evis Z, Keskin D, Altunbaş K, Tezcaner A. Structural and biological assessment of boron doped bioactive glass nanoparticles for dental tissue applications. Ceram Int. 2018;44:9854–64. 10.1016/j.ceramint.2018.02.230. [Google Scholar]
- 87.Zhu N, Wang D, Xie F, Qin M, Lin Z, Wang Y. Fabrication and characterization of calcium-phosphate lipid system for potential dental application. Front Chem. 2020;8:161. 10.3389/fchem.2020.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang S, Thiebes AL, Kreimendahl F, Ruetten S, Buhl EM, Wolf M, Jockenhoevel S, Apel C. Extracellular vesicles-loaded fibrin gel supports rapid neovascularization for dental pulp regeneration. Int J Mol Sci. 2020;21:4226. 10.3390/ijms21124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Effendi MC, Taufiq A, Bachtiar BM, Bachtiar EW, Herda E. The role of NMT induction on odontogenic proliferation and differentiation of dental pulp stem cells. Heliyon. 2021;7:e06598. 10.1016/j.heliyon.2021.e06598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim JK, Shukla R, Casagrande L, Sedgley C, Nör JE, Baker JR, Hill EE. Differentiating dental pulp cells via RGD-dendrimer conjugates. J Dent Res. 2010;89:1433–8. 10.1177/0022034510384870. [DOI] [PubMed] [Google Scholar]
- 91.Chuang Y-C, Chang C-C, Yang F, Simon M. Rafailovich, TiO2 nanoparticles synergize with substrate mechanics to improve dental pulp stem cells proliferation and differentiation. Mater Sci Eng C. 2021;118:111366. 10.1016/j.msec.2020.111366. [DOI] [PubMed] [Google Scholar]
- 92.Bashir SM, Ahmed Rather G, Patrício A, Haq Z, Sheikh AA, Shah MZUH, Singh H, Khan AA, Imtiyaz S, Ahmad SB, Nabi S, Rakhshan R, Hassan S, Fonte P. Chitosan nanoparticles: a versatile platform for biomedical applications. Materials. 2022;15:3521. 10.3390/ma15196521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdel-Moneim A, El-Shahawy A, Yousef AI, Abd El-Twab SM, Elden ZE, Taha M. Novel polydatin-loaded chitosan nanoparticles for safe and efficient type 2 diabetes therapy: in silico, in vitro and in vivo approaches. Int J Biol Macromol. 2020;154:1496–504. 10.1016/j.ijbiomac.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 94.Mohanbhai SJ, Sarwala MN, Gupta S, Shrimali N, Choudhury SR, Sharma SS, Guchhait P, Karmakar S. Colon targeted chitosan-melatonin nanotherapy for preclinical Inflammatory Bowel Disease. Biomater Adv. 2022;136:212796. 10.1016/j.bioadv.2022.212796. [DOI] [PubMed] [Google Scholar]
- 95.Coutinho AJ, Costa Lima SA, Afonso CMM, Reis S. Mucoadhesive and pH responsive fucoidan-chitosan nanoparticles for the oral delivery of methotrexate. Int J Biol Macromol. 2020;158:180–8. 10.1016/j.ijbiomac.2020.04.233. [DOI] [PubMed] [Google Scholar]
- 96.Pajares-Chamorro N, Chatzistavrou X. Bioactive glass nanoparticles for tissue regeneration. ACS Omega. 2020;5:12716–26. 10.1021/acsomega.0c00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–74. 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 98.El-Kady AM, Farag MM, El-Rashedi AMI. Bioactive glass nanoparticles designed for multiple deliveries of lithium ions and drugs: curative and restorative bone treatment. Eur J Pharm Sci. 2016;91:243–50. 10.1016/j.ejps.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 99.Azaryan E, Mortazavi-Derazkola S, Alemzadeh E, Emadian Razavi F, Yousefi M, Hanafi-Bojd MY, Naseri M. Effects of hydroxyapatite nanorods prepared through Elaeagnus angustifolia extract on modulating immunomodulatory/dentin–pulp regeneration genes in DPSCs. Odontology. 2023;111:461–73. 10.1007/s10266-022-00761-1. [DOI] [PubMed] [Google Scholar]
- 100.Hokmabad VR, Davaran S, Aghazadeh M, Alizadeh E, Salehi R, Ramazani A. Effect of incorporating Elaeagnus angustifolia extract in PCL-PEG-PCL nanofibers for bone tissue engineering. Front Chem Sci Eng. 2019;13:108–19. 10.1007/s11705-018-1742-7. [Google Scholar]
- 101.Chen JR, Lazarenko OP, Wu X, Kang J, Blackburn ML, Shankar K, Badger TM, Ronis MJ. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J Bone Miner Res. 2010;25:2399–411. 10.1002/jbmr.137. [DOI] [PubMed] [Google Scholar]
- 102.Zhang D, Leppäranta O, Munukka E, Ylänen H, Viljanen MK, Eerola E, Hupa M, Hupa L. Antibacterial effects and dissolution behavior of six bioactive glasses. J Biomed Mater Res A. 2010;93:475–83. 10.1002/jbm.a.32564. [DOI] [PubMed] [Google Scholar]
- 103.Yadav S, Yadav D, Kumar P, Yadav A, Nirala G, Yadav S. Bioactive glass for biomedical application: An overview. Defects Eng Electroceram Energy Appl. 2024. 10.1007/978-981-97-9018-0_12. [Google Scholar]
- 104.Kiarashi M, Bayat H, Shahrtash SA, Etajuri EA, Khah MM, AL-Shaheri NA, Nasiri K, Esfahaniani M, Yasamineh S. Mesenchymal stem cell-based scaffolds in regenerative medicine of dental diseases. Stem Cell Rev Rep. 2024;20(3):688–721. 10.1007/s12015-024-10687-6. [DOI] [PubMed] [Google Scholar]
- 105.Imanishi Y, Hata M, Matsukawa R, Aoyagi A, Omi M, Mizutani M, Naruse K, Ozawa S, Honda M, Matsubara T, Takebe J. Efficacy of extracellular vesicles from dental pulp stem cells for bone regeneration in rat calvarial bone defects. Inflamm Regen. 2021;41:12. 10.1186/s41232-021-00163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sui B, Wu D, Xiang L, Fu Y, Kou X, Shi S. Dental pulp stem cells: from discovery to clinical application. J Endod. 2020;46:S46–55. 10.1016/j.joen.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 107.Krivanek J, Adameyko I, Fried K. Heterogeneity and developmental connections between cell types inhabiting teeth. Front Physiol. 2017;8:376. 10.3389/fphys.2017.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. 10.1016/S0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 109.Melin M, Joffre-Romeas A, Farges JC, Couble ML, Magloire H, Bleicher F. Effects of TGFβ1 on dental pulp cells in cultured human tooth slices. J Dent Res. 2000;79:1689–96. 10.1177/00220345000790090901. [DOI] [PubMed] [Google Scholar]
- 110.Pedano MS, Li X, Jeanneau C, Ghosh M, Yoshihara K, Van Landuyt K, About I, Van Meerbeek B. Survival of human dental pulp cells after 4-week culture in human tooth model. J Dent. 2019;86:33–40. 10.1016/j.jdent.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 111.Elabd GM, Eldars W, Shamaa MS, Tawfik MA. Evaluation of the antibacterial effect of titanium dioxide nanoparticles combined with acrylic laminates for functional orthodontic appliances: a randomized controlled clinical trial. BMC Oral Health. 2024;24:20. 10.1186/s12903-023-03805-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farzanegan F, Shahabi M, Niazi AE, Soleimanpour S, Shafaee H, Rangrazi A. Effect of the addition of Chitosan and TiO2 nanoparticles on antibacterial properties of an orthodontic composite in fixed orthodontic treatment: a randomized clinical trial study. Biomed Phys Eng Express. 2021;7:045017. 10.1088/2057-1976/ac0609. [DOI] [PubMed] [Google Scholar]
- 113.Grace Pérez-Pacheco C, Rodrigues Fernandes NA, Primo FL, Tedesco AC, Bellile E, Retamal-Valdes B, Feres M, Rodrigues Guimarães-Stabili M, Rossa C. Local application of curcumin-loaded nanoparticles as an adjunct to scaling and root planing in periodontitis: randomized, placebo-controlled, double-blind split-mouth clinical trial. Clin Oral Investigat. 2021;25(5):3217–27. 10.1007/s00784-020-03652-3. [DOI] [PubMed] [Google Scholar]
- 114.Mathew CA, Veena HR, Shubha P, Daniel RA. Antimicrobial photocatalysis using bio-hydrothermally synthesized Zinc oxide nanoparticles in the management of periodontitis: a prospective split-mouth, double-blind, randomized, controlled clinical trial. J Appl Oral Sci. 2023;31:e20230271. 10.1590/1678-7757-2023-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fahim MM, Saber SEM, Elkhatib WF, Nagy MM, Schafer E. The antibacterial effect and the incidence of post-operative pain after the application of nano-based intracanal medications during endodontic retreatment: a randomized controlled clinical trial. Clin Oral Investig. 2022;26:2155–63. 10.1007/s00784-021-04196-w. [DOI] [PubMed] [Google Scholar]
- 116.Arafa MG, Mousa HA, Kataia MM, Shehabeldin M, Afifi NN. Functionalized surface of PLGA nanoparticles in thermosensitive gel to enhance the efficacy of antibiotics against antibiotic resistant infections in endodontics: a randomized clinical trial. Int J Pharm X. 2023;6:100219. 10.1016/j.ijpx.2023.100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Farshidfar N, Assar S, Amiri MA, Sahmeddini S, Hamedani S, Zarei M, Tayebi L. The feasible application of microfluidic tissue/organ-on-a-chip as an impersonator of oral tissues and organs: a direction for future research. Biodes Manuf. 2023;6:478–506. 10.1007/s42242-023-00235-5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.