Abstract
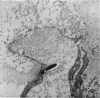
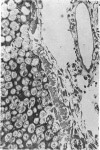
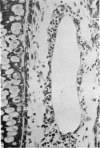
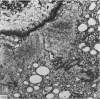
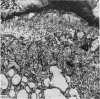
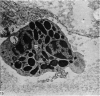
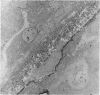
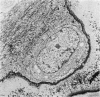
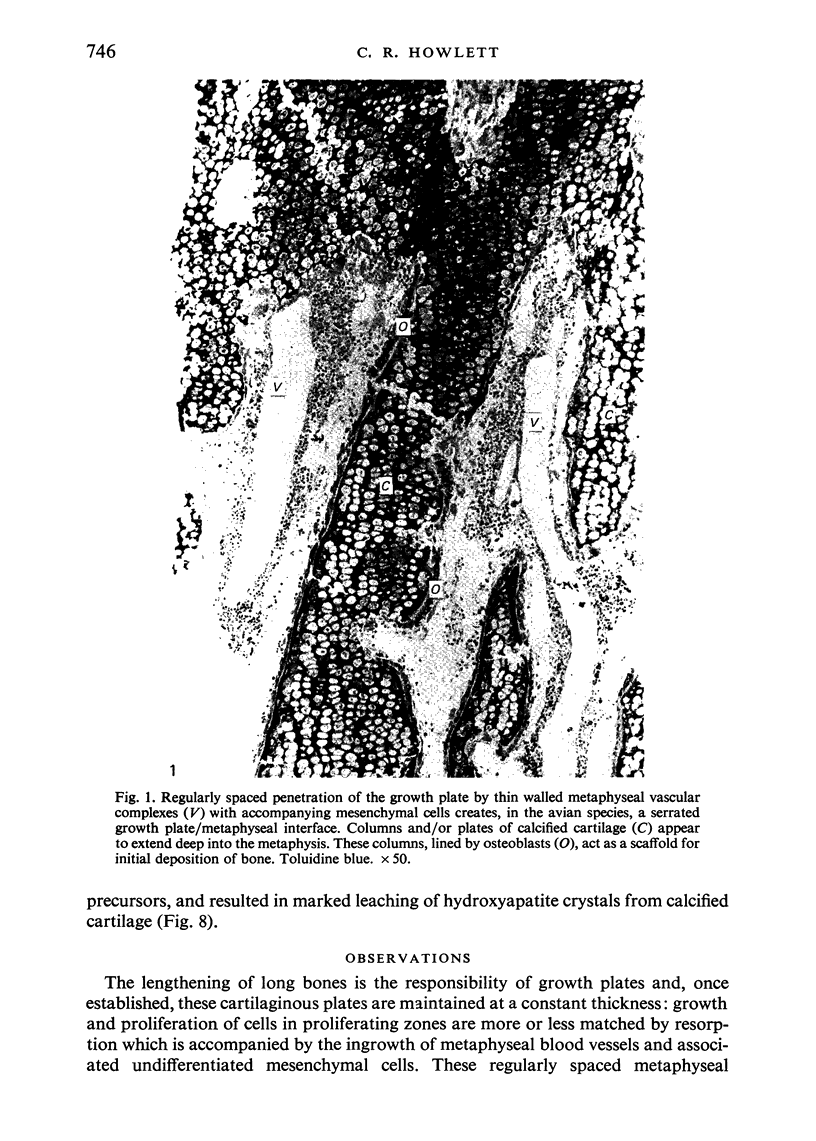
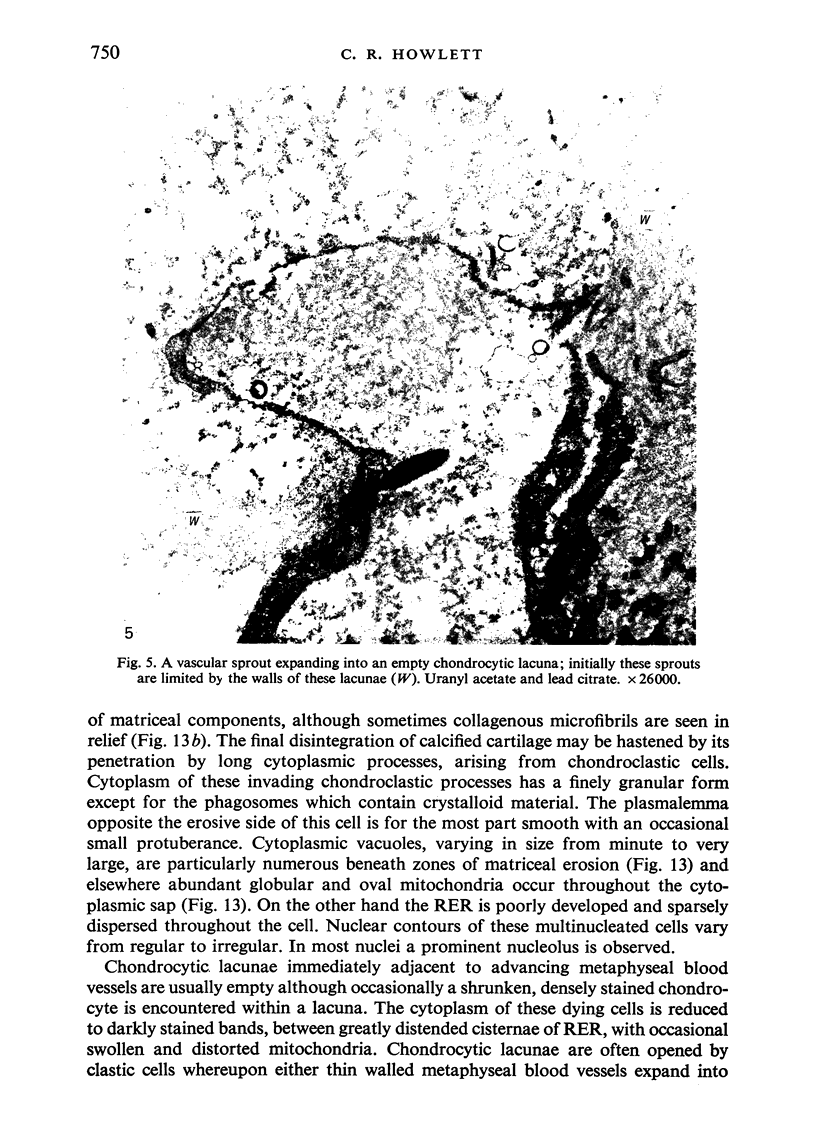
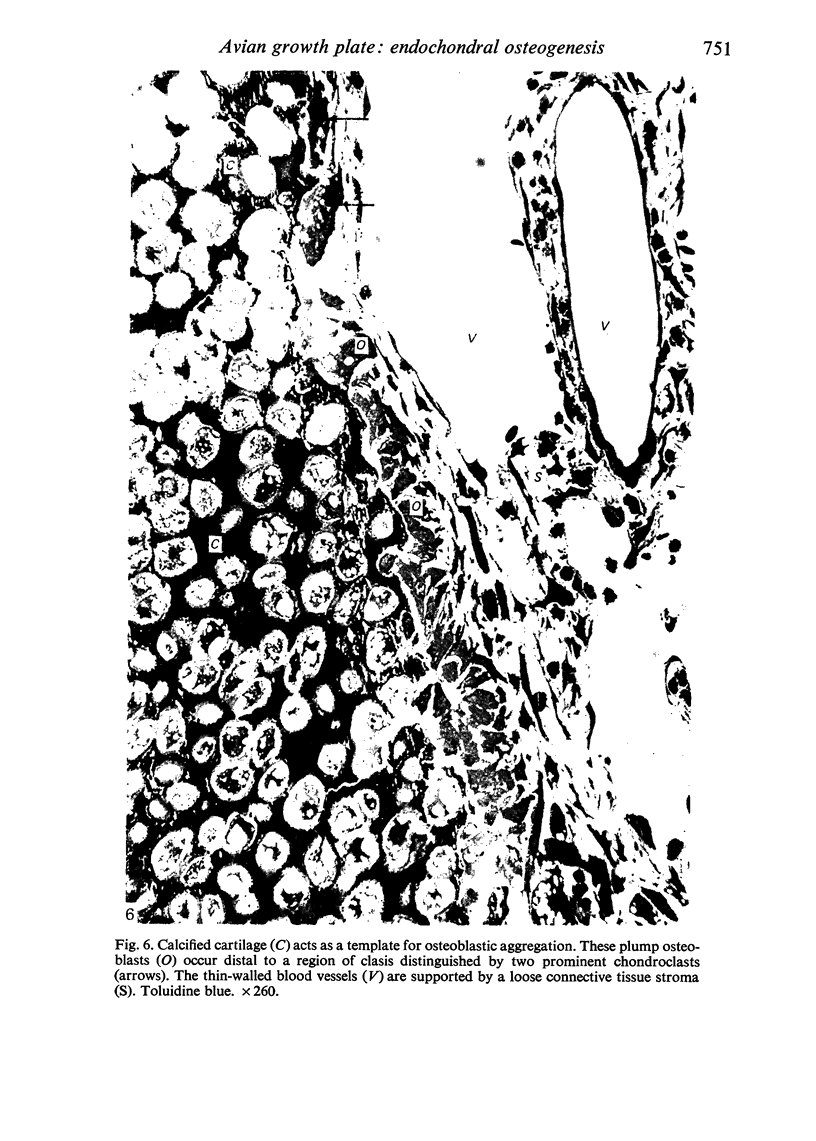
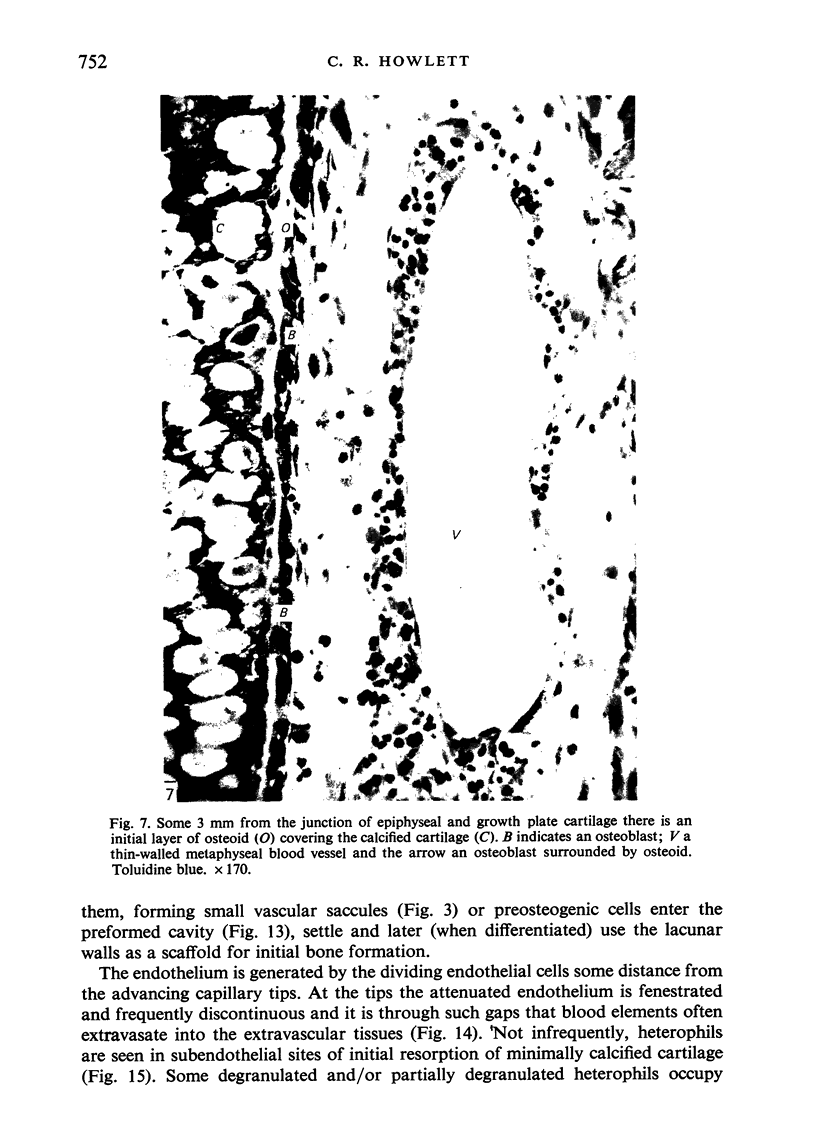
The ultrastructure of endochondral osteogenesis from the proximal end of tibias of 7 weeks old normal White Leghorn and broiler chickens is described. Little, if any, ultrastructural differences exists between these two strains of birds. In the growth plate, proliferation of chondrocytes and their matriceal production is approximately matched by resorption. Erosion of the metaphyseal aspect of the growth plate is accomplished under two sets of circumstances: firstly, when mineralization of the cartilaginous matrix is so scanty as to be discernible only by electron microscopy, and secondly, when calcification is gross and easily appreciated by light microscopy. The former process appears to be accomplished in the main by perivascular mononuclear cells and the latter, to a great extent, by chondroclasts. It appears that metaphyseal blood vessels expand, by saccular protrusions, into pre-existing spaces or those created by cellular erosion. Cones or plates of calcified cartilage, which extend into the metaphysis, act as a template for initial deposition of bone. Osteoblasts recently surrounded by osteoid have adjacent to their plasmalemma a pericellular sheath which is composed of ill-defined clumps of amorphous material as well as some incompletely aggregated collagenous fibrils. Beyond this sheath collagenous fibrils and fibres, with distinct periodicities (ranging from 57 to 69 nm) are observed. Towards the diaphysis the range of the periodicity of fibres in osteoid narrows to 64-69 nm.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. E., Parker J. Electron microscopy of the epiphyseal cartilage plate. A critical review of electron microscopy observations on enchondral ossification. Clin Orthop Relat Res. 1968 May-Jun;58:225–241. [PubMed] [Google Scholar]
- Anderson C. E., Parker J. Invasion and resorption in enchondral ossification. An electron microscopic study. J Bone Joint Surg Am. 1966 Jul;48(5):899–914. [PubMed] [Google Scholar]
- Brown J. N. The avian erythrocyte: a study of fixation for electron microscopy. J Microsc. 1975 Aug;104(3):293–305. doi: 10.1111/j.1365-2818.1975.tb04028.x. [DOI] [PubMed] [Google Scholar]
- CAMERON D. A. Erosion of the epiphysis of the rat tibia by capillaries. J Bone Joint Surg Br. 1961 Aug;43B:590–594. [PubMed] [Google Scholar]
- CAMERON D. A. The fine structure of bone and calcified cartilage. A critical review of the contribution of electron microscopy to the understading of osteogenesis. Clin Orthop Relat Res. 1963;26:199–228. [PubMed] [Google Scholar]
- Crissman R. S., Low F. N. A study of fine structural changes in the cartilage-to-bone transition within the developing chick vertebra. Am J Anat. 1974 Aug;140(4):451–469. doi: 10.1002/aja.1001400402. [DOI] [PubMed] [Google Scholar]
- Durkin J. F., Irving J. T., Heeley J. D. A comparison of the circulatory and calcification patterns in the mandibular condyle in the guinea pig with those found in the tibial epiphyseal and articular cartilages. Arch Oral Biol. 1969 Dec;14(12):1365–1371. doi: 10.1016/0003-9969(69)90252-0. [DOI] [PubMed] [Google Scholar]
- Ham K. N., Hurley J. V., Ryan G. B., Storey E. Localization of particulate carbon in metaphyseal vessels of growing rats. Aust J Exp Biol Med Sci. 1965 Oct;43(5):625–638. doi: 10.1038/icb.1965.47. [DOI] [PubMed] [Google Scholar]
- Howlett C. R. The fine structure of the proximal growth plate of the avian tibia. J Anat. 1979 Mar;128(Pt 2):377–399. [PMC free article] [PubMed] [Google Scholar]
- Höhling H. J., Steffens H., Stamm G. Transmission microscopy of freeze dried, unstained epiphyseal cartilage of the guinea pig. Cell Tissue Res. 1976 Mar 16;167(2):243–263. doi: 10.1007/BF00224331. [DOI] [PubMed] [Google Scholar]
- Jande S. S. Fine structural study of osteocytes and their surrounding bone matrix with respect to their age in young chicks. J Ultrastruct Res. 1971 Nov;37(3):279–300. doi: 10.1016/s0022-5320(71)80125-9. [DOI] [PubMed] [Google Scholar]
- Kalayjian D. B., Cooper R. R. Osteogenesis of the epiphysis: a light and electron microscopic study. Clin Orthop Relat Res. 1972;85:242–256. doi: 10.1097/00003086-197206000-00037. [DOI] [PubMed] [Google Scholar]
- Knese K. H. Osteoklasten, Chondraklasten, Mineraloklasten, Kollagenoklasten. Acta Anat (Basel) 1972;83(2):275–288. [PubMed] [Google Scholar]
- Kuettner K. E., Eisenstein R., Sorgente N. Lysozyme in calcifying tissues. Clin Orthop Relat Res. 1975 Oct;(112):316–339. [PubMed] [Google Scholar]
- Luk S. C., Nopajaroonsri C., Simon G. T. The ultrastructure of cortical bone in young adult rabbits. J Ultrastruct Res. 1974 Feb;46(2):184–205. doi: 10.1016/s0022-5320(74)80055-9. [DOI] [PubMed] [Google Scholar]
- Luk S. C., Nopajaroonsri C., Simon G. T. The ultrastructure of endosteum: a topographic study in young adult rabbits. J Ultrastruct Res. 1974 Feb;46(2):165–183. doi: 10.1016/s0022-5320(74)80054-7. [DOI] [PubMed] [Google Scholar]
- SCHOEFL G. I. STUDIES ON INFLAMMATION. III. GROWING CAPILLARIES: THEIR STRUCTURE AND PERMEABILITY. Virchows Arch Pathol Anat Physiol Klin Med. 1963 Nov 8;337:97–141. [PubMed] [Google Scholar]
- Savostin-Asling I., Asling C. W. Transmission and scanning electron microscope studies of calcified cartilage resorption. Anat Rec. 1975 Nov;183(3):373–391. doi: 10.1002/ar.1091830303. [DOI] [PubMed] [Google Scholar]
- Savostin-Asling I. Influence of incisor tooth development on osteoclast abundance and generation in the foetal rat mandible. Arch Oral Biol. 1974 Sep;19(9):793–800. doi: 10.1016/0003-9969(74)90167-8. [DOI] [PubMed] [Google Scholar]
- Schenk R. K., Spiro D., Wiener J. Cartilage resorption in the tibial epiphyseal plate of growing rats. J Cell Biol. 1967 Jul;34(1):275–291. doi: 10.1083/jcb.34.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk R. K., Wiener J., Spiro D. Fine structural aspects of vascular invasion of the tibial epiphyseal plate of growing rats. Acta Anat (Basel) 1968;69(1):1–17. doi: 10.1159/000143059. [DOI] [PubMed] [Google Scholar]
- TAKUMA S. Electron microscopy of cartilage resorption by chondroclasts. J Dent Res. 1962 Jul-Aug;41:883–889. doi: 10.1177/00220345620410042101. [DOI] [PubMed] [Google Scholar]
- Thyberg J. Electron microscopic studies on the initial phases of calcification in guinea pig epiphyseal cartilage. J Ultrastruct Res. 1974 Feb;46(2):206–218. doi: 10.1016/s0022-5320(74)80056-0. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Friberg U. Ultrastructure of the epiphyseal plate of the normal guinea pig. Z Zellforsch Mikrosk Anat. 1971;122(2):254–272. doi: 10.1007/BF00337633. [DOI] [PubMed] [Google Scholar]